SUMMARY
The BDNF receptor tyrosine kinase, TrkB, underlies nervous system function in both health and disease. Excessive activation of TrkB caused by status epilepticus promotes development of temporal lobe epilepsy (TLE), revealing TrkB as a therapeutic target for prevention of TLE. To circumvent undesirable consequences of global inhibition of TrkB signaling, we implemented a novel strategy aimed at selective inhibition of the TrkB-activated signaling pathway responsible for TLE. Our studies of a mouse model reveal that phospholipase Cγ1 (PLCγ1) is the dominant signaling effector by which excessive activation of TrkB promotes epilepsy. We designed a novel peptide (pY816) that uncouples TrkB from PLCγ1. Treatment with pY816 following status epilepticus inhibited TLE and prevented anxiety-like disorder yet preserved neuroprotective effects of endogenous TrkB signaling. We provide proof-of-concept evidence for a novel strategy targeting receptor tyrosine signaling and identify a therapeutic with promise for prevention of TLE caused by status epilepticus in humans.
INTRODUCTION
The epilepsies constitute a group of common, serious neurological disorders, among which temporal lobe epilepsy (TLE) is the most prevalent and is often devastating both because of its resistance to anticonvulsants and its associated behavioral disorders (Engel et al., 1998). Many patients with severe TLE experienced an episode of continuous seizure activity (status epilepticus, SE) years prior to the onset of TLE (French et al., 1993). Because induction of SE alone is sufficient to induce TLE in diverse mammalian species (Pitkanen, 2010), the occurrence of de novo SE is thought to contribute to development of TLE in humans.
Elucidating the molecular mechanisms by which an episode of SE induces lifelong TLE in an animal model will hopefully provide targets for preventive or disease modifying therapies (Loscher et al., 2013). Recent work identified a molecular mechanism required for induction of TLE by an episode of SE, namely, the excessive activation of a receptor tyrosine kinase, TrkB (Liu et al., 2013). A chemical-genetic method (Chen et al., 2005) was used to demonstrate that inhibition of TrkB signaling, initiated following SE and continued for two weeks, prevented both SE-induced TLE and anxiety-like behavior when tested one to two months after SE (Liu et al., 2013). The objective of the present work was to seek an inhibitor of TrkB signaling for prevention of epilepsy caused by SE. We report a novel strategy for inhibition of receptor tyrosine kinase signaling in vivo in which we identify the dominant effector by which TrkB activation promotes its epileptogenic consequences and demonstrate therapeutic effects of a unique peptide that uncouples TrkB from this effector while sparing neuroprotective effects of TrkB signaling.
RESULTS
Inhibition of TrkB kinase exacerbates SE-induced neuronal degeneration
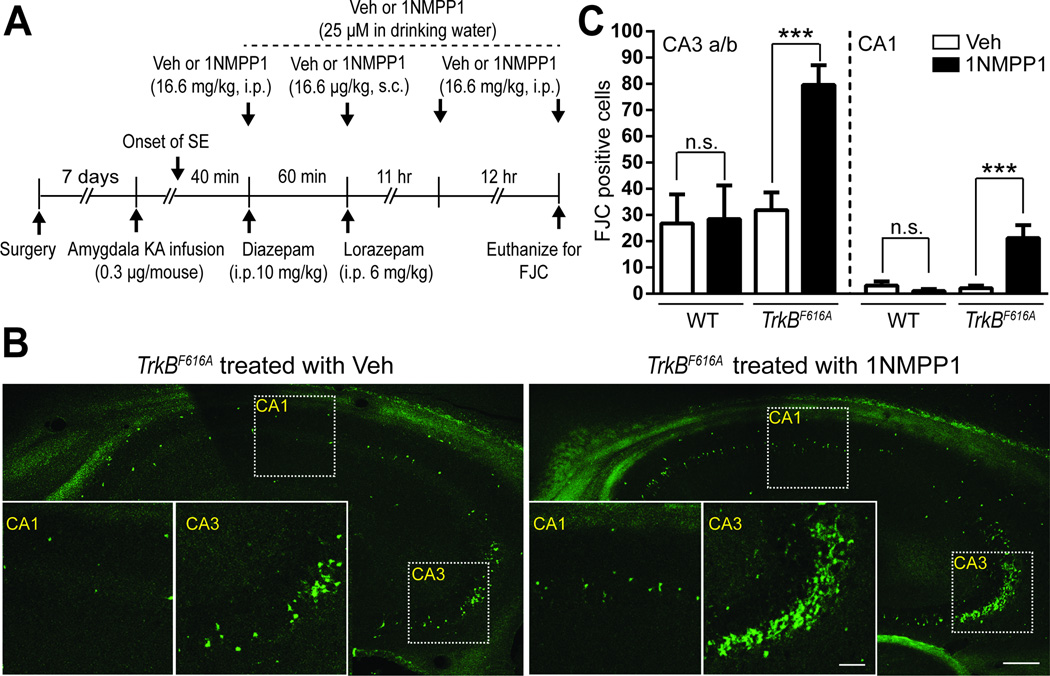
Although our prior work advanced TrkB kinase as a therapeutic target for prevention of SE-induced TLE, the pro-survival effects of TrkB signaling (Alcantara et al., 1997; Atwal et al., 2000) raised concern that inhibition of TrkB kinase might exacerbate SE-induced death of neurons (Henshall and Meldrum, 2012). To address this issue, we used Fluro-Jade C (FJC) staining of hippocampal sections from mice euthanized 24 hr following SE to quantify neuronal degeneration (Mouri et al., 2008). These experiments utilized a chemical-genetic method whereby administration of a small molecule, 1NMPP1, selectively inhibits TrkB kinase in TrkBF616A but not wild type (WT) mice (Figure 1A) (Chen et al., 2005). SE preferentially destroyed CA3 pyramidal cells as evident by FJC staining in sections from both WT and TrkBF616A animals treated with vehicle (Figures 1B and 1C), confirming results of Mouri et al (2008). Using doses demonstrated to inhibit SE-induced activation of TrkB (Liu et al., 2013), 1NMPP1 treatment of TrkBF616A mice produced three- to ten-fold increases in the number of FJC positive cells in the CA3 and CA1 pyramidal cell layers of hippocampus in comparison to vehicle treated controls (Figures 1B and 1C). Importantly, treatment of WT animals with 1NMPP1 following induction of SE produced no significant differences from vehicle (Figure 1C). In sum, these results demonstrate neuroprotective effects of TrkB kinase evident one day following SE and reveal a deleterious consequence of global inhibition of TrkB signaling in this context, namely exacerbation of neuronal degeneration.
Figure 1. Inhibition of TrkB kinase exacerbates SE-induced neuronal degeneration.
(A) Schematic of experimental design of assessment of neuronal degeneration 24 hr after SE induced by infusion of KA into amygdala. (B) Representative images of FJC staining in the hippocampus ipsilateral to KA infusion site in TrkBF616A mice treated with either DMSO (vehicle, Veh) or 1NMPP1; scale bar represents 200 µm. Insets: high-magnitude images of hippocampal CA1 and CA3 a/b subfields; scale bar represents 40 µm. (C) Counts of FJC positive cells within hippocampal subfield CA3 a/b or CA1 ipsilateral to infusion site of KA in WT or TrkBF616A mice treated with either Veh (WT, n=4; TrkBF616A, n=4) or 1NMPP1 (WT, n=6; TrkBF616A, n=4). Data are presented as mean ± SEM and analyzed using Two-way ANOVA with post hoc Bonferroni’s tests, ***p<0.001.
Antiseizure effect of limiting phospholipase Cγ1 signaling
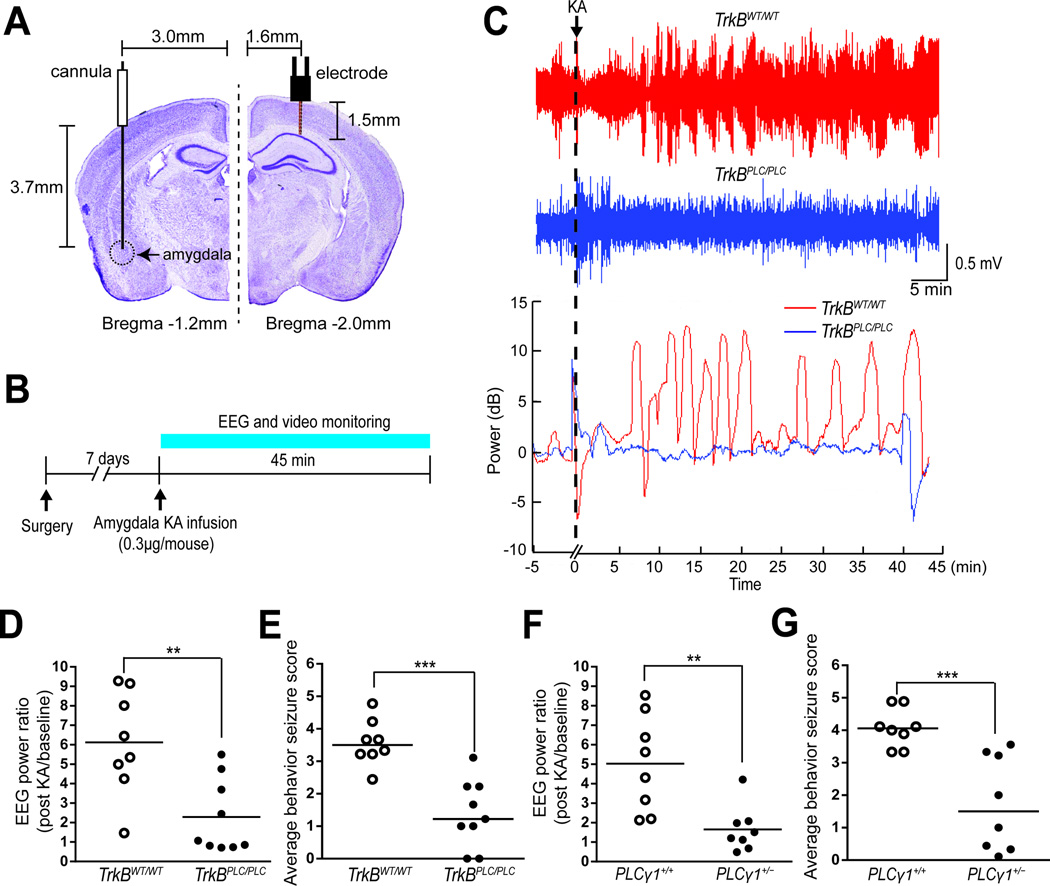
The untoward consequences of global inhibition of TrkB kinase led us to seek the signaling pathway by which SE-induced activation of TrkB promotes epilepsy. That is, if the downstream pathways mediating the deleterious and beneficial effects of TrkB signaling induced by SE were distinct, then selective inhibition of the deleterious pathway could be an attractive strategy for drug development. To begin to elucidate the signaling pathways underlying the anti-epileptic consequences of TrkB kinase inhibition, we asked whether inhibition of TrkB-mediated activation of phospholipase Cγ1 (PLCγ1) might inhibit seizures evoked by microinfusion of kainic acid (KA) into the amygdala of adult mice (Figures 2A and 2B). We initially studied TrkBPLC/PLC mice in which a phenylalanine is substituted for tyrosine at residue 816 of TrkB, thereby disrupting TrkB-mediated PLCγ1 signaling (Minichiello et al., 2002). Infusion of KA induced prolonged electrographic and convulsive motor seizures in each WT control mouse (Figures 2C, 2D, 2E and S1A–C). By contrast, the electrographic and convulsive motor seizures were markedly reduced in TrkBPLC/PLC mice (Figures 2C, 2D, 2E and S1A–C). Thus, genetically uncoupling TrkB from adaptor proteins and enzymes that bind the motif containing Y816 results in powerful antiseizure effects.
Figure 2. Antiseizure effects of inhibiting TrkB-mediated PLCγ1 signaling.
Schematics of sites of infusion cannula and recording electrode (A) and experimental design (B). (C) Representative EEG trace of TrkBWT/WT (upper row) or TrkBPLC/PLC (middle row) recording of 5 min prior to (baseline) and 45 min after KA infusion. The representative plots of log10 power analyses of EEG adjusted to baseline power prior to KA infusion is presented immediately below, using a similar time scale. Average EEG power normalized to baseline (D and F) and average behavioral seizure scores defined in legend of Figure S1 (E and G) were analyzed during 45 min following KA infusion in TrkBWT/WT (n=8) and TrkBPLC/PLC (n=9) mice (D and E) and in PLC+/+ (n=8) and PLCγ1+/− (n=8) mice (F and G). Data are presented from individual animals as well as mean (D and F) or median (E and G) and analyzed using Student’s t-test (D and F) or Mann-Whitney test (E and G), n=8–9, **p<0.01 and ***p<0.001.
Evidence that the pY816 motif of TrkB mediates the binding and activation of PLCγ1 suggested that the mechanism by which seizures were suppressed in TrkBPLC/PLC mice involved inhibition of PLCγ1 signaling in particular. That said, multiple adaptor proteins and enzymes can bind a given motif of an RTK. To test whether limiting signaling thru PLCγ1 mediates the antiseizure effects of the TrkBPLC/PLC mice, we examined responses to KA infusion in mice heterozygous for PLCγ1 (PLCγ1+/−) (He et al., 2014; Ji et al., 1997). Infusion of KA induced prolonged electrographic and convulsive motor seizures in each WT control mouse (Figures 2F, 2G and S1D–F). By comparison, the electrographic and convulsive motor seizures evoked by KA were reduced by 65–70% in PLCγ1+/− mice (Figures 2F, 2G and S1D–F). Thus the dominant mechanism underlying the antiseizure effects of the TrkBPLC/PLC mutation involves inhibition of PLCγ1 activation, thereby providing the rationale for identifying compounds that selectively uncouple PLCγ1 from activated TrkB.
pY816 peptide inhibits BDNF-mediated activation of PLCγ1
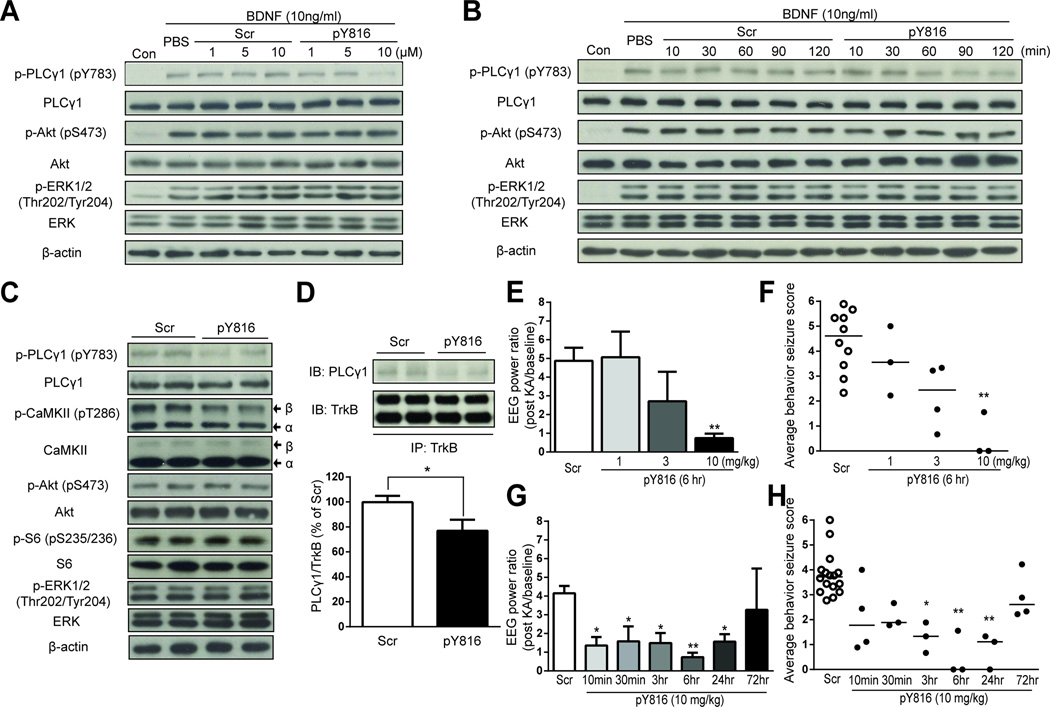
This led us to design a membrane-permeable peptide comprising HIV-1 Tat protein transduction domain and a sequence of TrkB which is required for binding of PLCγ1 to the motif of TrkB containing tyrosine 816 (pY816, YGRKKRRQRRR-LQNLAKASPVpYLDI) (Middlemas et al., 1994; Obermeier et al., 1993). An HIV-1 Tat conjugated to a randomly scrambled peptide (Scr, YGRKKRRQRRR-LYApYQLKIAPNDLS) served as a negative control. We first examined the concentration dependence and time course of pY816-mediated inhibition of TrkB-mediated PLCγ1 activation in cultured neurons in vitro. Preincubation of pY816 for 90 minutes prior to addition of BDNF produced a concentration-dependent inhibition of BDNF-mediated PLCγ1 activation (Figures 3A and S2A). Preincubation of pY816 at 10 µM for 60, 90, or 120 min prior to addition of BDNF produced marked inhibition of BDNF-mediated PLCγ1 activation (Figures 3B and S2B). Importantly, pY816-mediated inhibition was selective to PLCγ1 in that BDNF-mediated increases of p-Akt and p-ERK were not affected (Figures 3A and 3B, rows 3 and 5). With respect to in vivo actions, systemic infusion of pY816 reduced p-PLCγ1 (pY783) immunoreactivity by ~50% in comparison to Scr control at 3 hr (Figure 3C) and its inhibitory effects had dissipated at 72 hr as revealed by Western blots of hippocampal lysates (Figure S2C). The reduction of p-PLCγ1 (pY783) immunoreactivity was paralleled by a reduction of p-CaMKII immunoreactivity (Figures 3C and S2D), the predicted consequence of inhibiting TrkB-mediated activation of PLCγ1 (Blanquet and Lamour, 1997; Minichiello et al., 2002). Again the effects of pY816 were selective for PLCγ1 in that neither p-Akt nor p-S6 nor p-ERK was affected (Figure 3C, rows 5, 7, and 9). The inhibition of p-PLCγ1 immunoreactivity is likely due to pY816 binding PLCγ1 directly because PLCγ1 can be co-immunoprecipitated with pY816 in hippocampus following systemic infusion of pY816 (10 mg/kg, 3 hr) (Figure S3A). The binding of PLCγ1 by pY816 uncouples TrkB from PLCγ1 because systemic infusion of pY816 (10 mg/kg) significantly inhibited the coimmunoprecipitation of TrkB and PLCγ1 at 3 hr after peptide administration (Figure 3D). While pY816 may inhibit the interaction of PLCγ1 with other RTKs in addition to TrkB, the principal RTK of its inhibition in this model is TrkB because inhibition of TrkB kinase virtually eliminated PLCγ1 activation following SE (Figure S3B). Collectively, these experiments support the conclusion that pY816 selectively inhibits the activation of PLCγ1 by TrkB following SE in vivo.
Figure 3. pY816 peptide selectively inhibits PLCγ1 activation and inhibits chemoconvulsant-induced seizures.
Either PBS or various concentrations of Scr or pY816 peptide (A) were preincubated for various periods of time (B) with cultured neurons prior to addition of BDNF (100 ng/ml) (n=3). (A) and (B) present representative western blots of p-PLCγ1 (pY783), PLCγ1, p-Akt (pS473), Akt, p-ERK1/2 (Thr202/Tyr204), ERK and β-actin. (C) Scr or pY816 (10 mg/kg, i.v.) was injected into naïve mice (n=4) and animals were euthanized 3 hr later and hippocampal homogenates subjected to SDS-PAGE and western blotting. Compared to scrambled control peptide, pY816 peptide (10 mg/kg) reduces p-PLCγ1 (pY783) and p-CaMKII (pT286) but not p-Akt, p-S6 or p-ERK immunoreactivity. (D) Systemic infusion of pY816 peptide (10 mg/kg, i.v.) reduces co-immunoprecipitation of PLCγ1 with TrkB compared to Scr control in hippocampal homogenates isolated 3 hr after infusion (n=11 each). Systemic administration of pY816 at various doses (E and F, n=3–4) or at various intervals (G and H, n=3–4) prior to induction of seizures by infusion of KA into amygdala. Average EEG power normalized to baseline (E and G) and average behavioral seizure scores (F and H) were analyzed during 45 min following KA infusion. Scr treated controls were included in experiments examining effects of doses (total of 10, E and F) and time (total of 18, G and H). Data are presented as mean ± SEM and analyzed using Student’s t-test (D) or Two-way ANOVA with post hoc Bonferroni’s test (E and G) or from individual animals as well as median and analyzed using Kruskal-Wallis test followed by Dunn’s multiple comparisons test (F and H); *p<0.05 and **p<0.01.
pY816 peptide inhibits chemoconvulsant-induced seizures
The genetic evidence that uncoupling TrkB from PLCγ1 exerts antiseizure effects together with evidence that pY816 can inhibit PLCγ1 activation in vitro and in vivo led us to ask whether systemic administration of pY816 exerted antiseizure effects in the KA model. Towards this end, either pY816 or Scr was administered (i.v.) prior to KA infusion and both EEG and behavioral seizures were monitored for 45 min (Figure S2E). To optimize the effects, we asked how varying doses and intervals of pY816 administration affected seizures evoked by KA. Compared to control peptide, infusion of pY816 reduced electrographic and convulsive motor seizures in a dose- and time-dependent manner; inhibition approximating 90% was obtained with 10 mg/kg infused 6 hr prior to KA (Figures 3E–H and S2F–I). Inhibition was evident as early as 10 min following i.v. infusion and inhibition of 50–90% persisted for 24 hr, returning to control levels by 72 hr (Figures 3G, 3H, S2H and S2I). Together these results demonstrate that treatment with pY816 prior to infusion of KA inhibits the prolonged seizures induced by KA in a dose- and time-dependent manner.
pY816 peptide prevents SE-induced epilepsy
Usefulness of a preventive agent in a clinical setting requires it to be effective when administered following the SE. In the experiments described above, pY816 was administered prior to induction of SE by microinfusion of KA and suppressed the evoked seizures (i.e. anticonvulsant). A distinct question is whether administration of pY816 following chemoconvulsant-evoked SE prevents the resulting epilepsy (i.e. anti-epileptogenic).
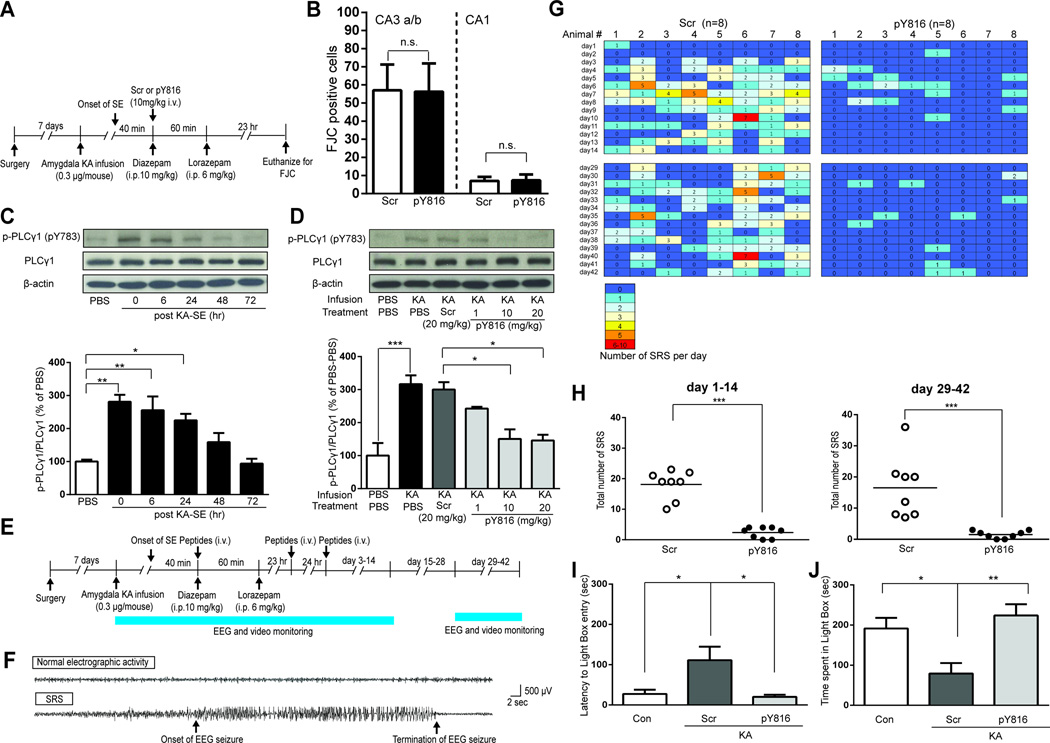
Prior to addressing this issue, we asked whether the neuroprotective effects of TrkB signaling are inhibited by treatment with pY816 administered following SE (Figure 4A). As observed previously (Figures 1B and 1C), SE led to destruction of CA3 and CA1 pyramidal cells as detected by FJC staining in sections from animals treated with Scr control peptide (Figure 4B). In contrast to inhibition of TrkB kinase, similar numbers of FJC stained neurons were detected in animals treated with pY816 (Figure 4B), demonstrating that pY816 did not interfere with the neuroprotective effects of endogenous TrkB signaling.
Figure 4. Treatment with pY816 following SE prevents epilepsy and anxiety-like behavior yet preserves neuroprotective effects of TrkB.
(A) Schematic of experimental design of assessment of neuronal degeneration 24 hr after SE induced by infusion of KA into amygdala. (B) Counts of FJC positive cells in hippocampal subfield CA3 a/b or CA1 ipsilateral to infusion site of KA in mice treated with Scr (n=7) or pY816 (n=8). (C) Representative western blot of hippocampal lysates of mice euthanized at various time points after the completion of SE (top); quantification of immunoreactivity of p-PLCγ1 (pY783) to PLCγ1 ratio is presented in bottom (n=3). (D) Representative western blots of hippocampal lysates of mice treated with PBS, Scr (20 mg/kg) or varying doses of pY816 (1, 10 and 20 mg/kg) immediately after completion of SE and euthanized 6 hr later (top); quantification of immunoreactivity of p-PLCγ1 (pY783) to PLCγ1 ratio is presented in bottom (n=3). (E) Schematic of experimental design of 3 days treatment of pY816 initiated after completion of SE. (F) Representative EEG recording of an electrographic seizure (bottom) and normal activity (top). (G) Heatmap presents number of spontaneous recurrent seizures (SRSs) detected each day during days 1–14 and days 29–42 after SE; each animal was treated with either Scr or pY816 (10 mg/kg, i.v.) immediately, 24 hr and 48 hr after completion of SE, n=8. (H) Total number of SRSs during days 1–14 or days 29–42 for each animal treated with either Scr or pY816 (n=8). (I and J) Anxiety-like behavior assessed by latency to enter light-compartment (I) and by time spent in lighted compartment (J). Mice undergoing infusion of PBS into amygdala served as controls (Con, n=7). Data are presented from individual animals as well as mean (H) or as mean ± SEM and analyzed using Student’s t-test in B and H or Two-way ANOVA with post hoc Bonferroni’s test in C, D, I and J. n.s. no significant difference, *p<0.05, **p<0.01, ***p<0.001.
We next sought to understand the time course of SE-induced activation of PLCγ1, the idea being that a critical period of enhanced activation following prolonged seizures may define a therapeutic window during which inhibition of TrkB-PLCγ1 signaling may prevent TLE. An episode of prolonged seizures induced a 2–3 fold increase of PLCγ1 activation that was maximal during the first 24 hr and returned to normal by 72 hr (Figure 4C). Importantly, injection of pY816 following the episode of SE inhibited PLCγ1 activation in a dose-dependent manner, inhibition approximating 75% with doses of 10 and 20 mg/kg (Figure 4D).
Next we asked whether treatment with pY816 (10 mg/kg, i.v.) initiated following termination of SE with diazepam and repeated 24 and 48 hr thereafter prevented SE-induced epilepsy. Spontaneous seizures were detected using continuous video-EEG recordings during days 1–14 and days 29–42 after SE (Figures 4E–H). Preventive effects of pY816 were evident during days 1–14 (Figures 4G and 4H). The latency to the onset of the first spontaneous seizure was delayed in pY816 treated animals compared to controls (Figure 4G, top panels). The initial spontaneous seizure was observed in 5 of 8 control animals within 3 days following prolonged seizures whereas only a single pY816 infused animal exhibited a seizure in this interval. A striking reduction (86%) in the number of spontaneous seizures was observed in pY816 compared to Scr control animals during the two weeks immediately following SE (Figures 4G and 4H). Additional video-EEG recordings were conducted during a two week period (days 29–42) initiated approximately four weeks after the last dose of pY816. Once again, a marked reduction (90%) in the number of spontaneous seizures was observed in pY816 compared to Scr control animals (Figures 4G and 4H). Among the eight animals treated with pY816, two exhibited no seizures and the remaining six animals exhibited only 1–3 seizures. By contrast, control animals exhibited 7–36 spontaneous recurrent seizures during this same interval (Figures 4G–4H). Importantly, hippocampal damage, a pathological hallmark of TLE, was attenuated by pY816 treatment when assessed months after SE (Figures S4A and S4B).
Patients with epilepsy commonly exhibit anxiety disorders and anxiety-like behavior has been identified in the current model and was found to be prevented by transient inhibition of TrkB kinase commencing after SE (Liu et al., 2013). We therefore asked whether this behavioral abnormality induced by SE can also be prevented by treatment with pY816. Following completion of video-EEG recording during days 29–42, anxiety-like behavior was assessed using the light-dark emergence test (Bourin and Hascoet, 2003). In comparison to controls, mice undergoing SE followed by treatment with Scr exhibited a prolonged latency to enter the lighted compartment and spent less time in the lighted compartment (Figures 4I and 4J). By comparison to the Scr controls, mice undergoing SE followed by treatment with pY816 exhibited a significantly reduced latency to enter the lighted compartment and spent increased time in the lighted compartment (Figures 4I and 4J). Similarities in locomotor activity in an open field between two groups undergoing SE excluded differences in spontaneous activity as a confounding variable in the light-dark emergence results (data not shown). Collectively, these results demonstrate that treatment with pY816 for three days commencing after SE prevents SE-induced anxiety-like behavior as assessed by the light-dark emergence test.
Meaningful interpretation of the beneficial effects of pY816 treatment requires that the insult of SE be similar in the Scr control and pY816 groups. Behavioral and EEG features of the SE prior to treatment with Scr or pY816 revealed similarity of the insult in the two groups (S4C–E). The anticonvulsant effects of pY816 when administered prior to SE raised the question as to whether treatment with pY816 administered after diazepam might suppress seizures and thereby minimize the SE insult. Detailed behavioral and EEG analyses for 48 hours following SE, including visual review by blinded reviewers (Figures 4G top two rows, S4F and S4G) and computerized measures of EEG power (Figure S4H) revealed no detectable anticonvulsant effects of pY816 when administered following diazepam.
DISCUSSION
Previous work utilizing a chemical-genetic approach revealed that transient inhibition of the receptor tyrosine kinase, TrkB, prevented epilepsy and anxiety-like disorder caused by a brief episode of SE (Liu et al., 2013). Here we sought an inhibitor of TrkB signaling for prevention of epilepsy caused by SE. We used genetically modified mice and a novel biologic together with biochemical and electrophysiological methods in studies of an animal model. Global inhibition of TrkB signaling by targeting its kinase activity exacerbated neuronal death induced by SE assessed one day afterwards, this untoward consequence leading us to seek a TrkB downstream signaling pathway promoting epilepsy. A combination of genetic approaches and a novel biologic, pY816, revealed PLCγ1 to be the dominant signaling effector by which SE-induced TrkB activation promotes its epileptogenic consequences.
Clinical observations together with studies of animal models support the conclusion that an episode of SE contributes to the emergence of severe TLE years later (Annegers et al., 1987; Pitkanen, 2010; Tsai et al., 2009). The seizure free latent period provides a therapeutic window for intervention aimed at preventing development of TLE. The transiently enhanced activation of PLCγ1 induced by SE in the current model largely preceded onset of recurrent seizures (Figures 4C and 4G upper left). This provided a window of opportunity during which to intervene with pY816. Notably, only three doses of pY816 were administered following SE, the last of which was given 48 hr after the insult. The inhibition of spontaneous recurrent seizures assessed 4–5 weeks after the last treatment is not likely due to residual pY816 because the antiseizure effects of pY816 persisted less than three days after treatment (Figures 3G, 3H, S2H and S2I). The likelihood that pY816 will be effective in additional models of SE-induced TLE is strengthened by the fact that activation of TrkB and PLCγ1 is detected in other SE models (He et al., 2010). Whether TrkB signaling promotes epileptogenesis following other insults such as stroke or trauma is uncertain. The prevention of SE-induced abnormalities in the light-dark emergence test raises the possibility that pY816 may also exert anti-anxiogenic effects, a possibility warranting study with additional tests of anxiety-like behavior.
The diversity of diseases caused by excessive RTK signaling led to development of inhibitors, more than a dozen of which are in current clinical use (Lemmon and Schlessinger, 2010). These are either small molecules that target the cytoplasmic kinase domain or monoclonal antibodies that target the extracellular domain, limiting ligand-mediated activation. While clearly useful, one limitation is that global inhibition of RTK signaling may have both desirable and undesirable consequences. Our studies reveal an undesirable consequence of global inhibition of TrkB kinase signaling, namely exacerbation of neuronal destruction detected one day following SE. This adds to prior work revealing neuroprotective effects of TrkB signaling, both in vitro and in vivo (Alcantara et al., 1997; Atwal et al., 2000; Nagahara et al., 2009; Wu and Pardridge, 1999), an effect likely mediated by signaling downstream of Y515 of TrkB (Atwal et al., 2000). This untoward consequence of global inhibition of TrkB signaling led us to identify PLCγ1 as the key downstream signaling effector mediating the unwanted consequences of TrkB activation and design of a peptide to selectively inhibit the disease-causing pathway. The mechanism by which the peptide exerts its beneficial effects likely involves its binding an SH2 domain of PLCγ1 (Figure S3A), thereby preventing the binding to and activation of PLCγ1 by TrkB. This underlies the selectivity of pY816-mediated inhibition of PLCγ1 and its downstream activation of CaMKII (Blanquet and Lamour, 1997; Minichiello et al., 2002) relative to p-Akt, p-S6 kinase, and p-ERK in vivo (Figure 3C). Among diverse RTKs, the variation of sequences flanking a core phosphotyrosine binding motif that binds the SH2 domain of a protein such as PLCγ1 may result in different binding affinities of different RTKs for the SH2 domain containing protein (Koch et al., 1991; Songyang et al., 1993). While pY816 may inhibit the interaction of PLCγ1 with other RTKs in addition to TrkB, we provide evidence that TrkB is the principal RTK promoting PLCγ1 activation following SE in this model (Figure S3B). Importantly, the limited duration of treatment with pY816 required for its beneficial effects is likely to minimize untoward consequences of limiting activation of PLCγ1 by TrkB or other RTKs.
To the best of our knowledge, the strategy of treating a disease model in vivo by uncoupling an RTK from a signal transducer has not been successfully implemented. One reason may lie in the many tyrosine autophosphorylation sites within the cytoplasmic domain of an RTK such as the epidermal growth factor receptor (Lemmon and Schlessinger, 2010), each of which can recruit different SH2 and PTB domain-containing adaptor proteins, the redundancy perhaps precluding inhibition of a single signaling pathway by disrupting a single point of contact of the RTK with an effector. By contrast, the relative paucity of identified tyrosine autophosphorylation sites within the cytoplasmic domain of TrkB (Huang and Reichardt, 2003) may reduce redundancy and enhance the feasibility of this strategy for TrkB and hopefully additional RTKs. Peptide inhibitors of protein-protein interactions provide an attractive option both because of their size (pY816 is 3,167 daltons) and because available knowledge of structural components required for the interaction of the RTK and the effector facilitates design. Importantly, the sequence of pY816 is identical in mouse and human, raising the possibility that pY816 itself or a derivative thereof may provide a therapeutic for prevention of TLE caused by SE in humans.
EXPERIMENTAL PROCEDURES
Experimental procedures are provided in Supplemental information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Richard D. Mooney for critical assessment of the manuscript and Wei-Hua Qian for animal breeding and genotyping. Y.Z.H was supported by CURE Taking Flight Award. This work was supported by NS056217 from NINDS (J.O.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and four Supplemental Figures.
AUTHOR CONTRIBUTIONS
B.G. designed and performed experiments, interpreted data, and wrote the manuscript. Y.Z.H performed experiments, interpreted data, and wrote the manuscript. X.P.H performed experiments and interpreted data. R.B.J and W.J performed video-EEG reading and data analyses. J.O.M designed and supervised research, interpreted data and wrote the manuscript.
REFERENCES
- Alcantara S, Frisen J, del Rio JA, Soriano E, Barbacid M, Silos-Santiago I. TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. J Neurosci. 1997;17:3623–3633. doi: 10.1523/JNEUROSCI.17-10-03623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316:493–498. doi: 10.1056/NEJM198702263160901. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Blanquet PR, Lamour Y. Brain-derived neurotrophic factor increases Ca2+/calmodulin-dependent protein kinase 2 activity in hippocampus. J Biol Chem. 1997;272:24133–24136. doi: 10.1074/jbc.272.39.24133. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Engel J, Williamson P, Wieser H-G. Mesial Temporal Lobe Epilepsy. In: Engel J, Pedley TA, editors. Epilepsy : a comprehensive textbook. Philadelphia: Lippincott-Raven: 1998. pp. 2417–2426. [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- He XP, Pan E, Sciarretta C, Minichiello L, McNamara JO. Disruption of TrkB-mediated phospholipase Cγ signaling inhibits limbic epileptogenesis. J Neurosci. 2010;30:6188–6196. doi: 10.1523/JNEUROSCI.5821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XP, Wen R, McNamara JO. Impairment of kindling development in phospholipase Cγ1 heterozygous mice. Epilepsia. 2014;55:456–463. doi: 10.1111/epi.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall DC, Meldrum BS. Cell death and survival mechanisms after single and repeated brief seizures. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annual Review of Biochemistry. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G. Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2999–3003. doi: 10.1073/pnas.94.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CA, Anderson D, Moran MF, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Gu B, He XP, Joshi RB, Wackerle HD, Rodriguiz RM, Wetsel WC, McNamara JO. Transient Inhibition of TrkB Kinase after Status Epilepticus Prevents Development of Temporal Lobe Epilepsy. Neuron. 2013;79:31–38. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. Nature Reviews Drug Discovery. 2013;12:757–776. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- Middlemas DS, Meisenhelder J, Hunter T. Identification of TrkB autophosphorylation sites and evidence that phospholipase Cγ1 is a substrate of the TrkB receptor. J Biol Chem. 1994;269:5458–5466. [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, Matsushima S, Taki W, Henshall DC. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature medicine. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A, Halfter H, Wiesmuller KH, Jung G, Schlessinger J, Ullrich A. Tyrosine 785 is a major determinant of Trk--substrate interaction. The EMBO journal. 1993;12:933–941. doi: 10.1002/j.1460-2075.1993.tb05734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A. Therapeutic approaches to epileptogenesis--hope on the horizon. Epilepsia. 2010;51(Suppl 3):2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Tsai MH, Chuang YC, Chang HW, Chang WN, Lai SL, Huang CR, Tsai NW, Wang HC, Lin YJ, Lu CH. Factors predictive of outcome in patients with de novo status epilepticus. QJM. 2009;102:57–62. doi: 10.1093/qjmed/hcn149. [DOI] [PubMed] [Google Scholar]
- Wu D, Pardridge WM. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:254–259. doi: 10.1073/pnas.96.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.