Abstract
Clinical data indicate that cutaneous burn injuries covering greater than ten percent total body surface area are associated with significant morbidity and mortality, where pulmonary complications, including acute respiratory distress syndrome (ARDS), contribute to nearly half of all patient deaths. Approximately 50% of burn patients are intoxicated at the time of hospital admission, which increases days on ventilators by three-fold, and doubles length of hospital admittance, compared to non-intoxicated burn patients. The most common drinking pattern in the United States is binge drinking, where one rapidly consumes alcoholic beverages (4 for women, 5 for men) in 2 hours and an estimated 38 million Americans binge drink, often several times per month. Experimental data demonstrate a single binge ethanol exposure prior to scald injury, impairs innate and adaptive immune responses, thereby enhancing infection susceptibility and amplifying pulmonary inflammation, neutrophil infiltration, and edema, and is associated with increased mortality. Since these characteristics are similar to those observed in ARDS burn patients, our study objective was to determine whether ethanol intoxication and burn injury and the subsequent pulmonary congestion affects physiological parameters of lung function using non-invasive and unrestrained plethysmography in a murine model system. Furthermore, to mirror young adult binge drinking patterns, and to determine the effect of multiple ethanol exposures on pulmonary inflammation, we utilized an episodic binge ethanol exposure regimen, where mice were exposed to ethanol for a total of 6 days (3 days ethanol, 4 days rest, 3 days ethanol) prior to burn injury. Our analyses demonstrate mice exposed to episodic binge ethanol and burn injury have higher mortality, increased pulmonary congestion and neutrophil infiltration, elevated neutrophil chemoattractants, and respiratory dysfunction, compared to burn or ethanol intoxication alone. Overall, our study identifies plethysmography as a useful tool for characterizing respiratory function in a murine burn model and for future identification of therapeutic compounds capable of restoring pulmonary functionality.
Keywords: Lung, Alcohol, Burn, Plethysmography, Neutrophils, Cytokines
Introduction
Burn injury is associated with significant morbidity and mortality, with greater than 10% of patients succumbing to their injuries when the burn size exceeds 10% of their total body surface area (ABA, 2014). Clinical data suggest that 42% of the mortality observed in burn patients is due to pulmonary complications (Achauer, Allyn, Furnas, & Bartlett, 1973; Phillips & Cope, 1962). Severe burn, even in the absence of inhalation injury, is a common predisposing factor for the development of Acute Respiratory Distress Syndrome (ARDS) (Liffner, Bak, Reske, & Sjoberg, 2005; Turnage et al., 2002). ARDS is associated with rigid lungs, hypoxemia, and bi-lateral infiltrates in chest radiographs (Ashbaugh, Bigelow, Petty, & Levine, 1967). Overall, respiratory dysfunction in burn patients is characterized by shallow breathing and fluid accumulation in the lung interstitium, leading to heightened vascular resistance and an increased effort to breathe (Achauer et al., 1973; Turnage et al., 2002). A net result of insufficient gas exchange underlies the large percentage of burn fatalities due to pulmonary complications.
Binge alcohol drinking is an increasingly prevalent activity, affecting an estimated 38 million adults in the United States (CDC, 2012). It is characterized by either the number of alcoholic drinks one consumes in 2 hours (4 for women, 5 for men) or by a blood alcohol concentration of 0.08%. Interestingly, approximately 50% of burn patients are under the influence of alcohol at the time of hospital admission (Grobmyer, Maniscalco, Purdue, & Hunt, 1996; Silver et al., 2008). Intoxicated burn patients have three times as many days on ventilators and an overall twice as long hospital stay compared to burn patients who were not intoxicated (Hadjizacharia et al., 2011; Silver et al., 2008). This leads to an increased risk of pulmonary complications that predisposes burn patients to multiple organ failure, with the lungs preceding all other organs, as well as a higher chance of mortality (Ciesla et al., 2005; Hollingsed, Saffle, Barton, Craft, & Morris, 1993). Notably, the vast majority of intoxicated burn patients are binge drinkers and not chronic dependent drinkers (Howland & Hingson, 1987; Schermer, 2006; Smith & Kraus, 1988). Experimental models of binge alcohol intoxication and burn injury have demonstrated alterations in innate and adaptive immunity that result in the marked immune dysfunction, greater susceptibility to infection, and amplified pulmonary inflammation (Bird, Morgan, Ramirez, Yong, & Kovacs, 2010; Bird, Zahs, et al., 2010; Choudhry et al., 2000; Faunce, Gregory, & Kovacs, 1997, 1998; Kawakami, Switzer, Herzog, & Meyer, 1991; Messingham, Faunce, & Kovacs, 2002; Murdoch, Brown, Gamelli, & Kovacs, 2008; Murdoch, Karavitis, Deburghgraeve, Ramirez, & Kovacs, 2011; Patel, Faunce, Gregory, Duffner, & Kovacs, 1999). Previously, our laboratory established that in a mouse model of single-dose binge ethanol exposure and burn injury there is amplified neutrophil infiltration, alveolar wall thickening, and edema in the lungs when alcohol precedes burn injury (Bird, Morgan, et al., 2010; Bird, Zahs, et al., 2010; Chen et al., 2013; Chen et al., 2014; Patel et al., 1999). Since ARDS is associated with similar pulmonary characteristics in humans, including increased neutrophil infiltration, capillary permeability and pulmonary edema (Dancey et al., 1999; Liffner et al., 2005; Steinvall, Bak, & Sjoberg, 2008), the objective of our study was to determine whether intoxication and burn injury and the resulting histological pulmonary congestion affects physiological parameters of lung function using a murine model system.
In these studies, we used non-invasive and unrestrained plethysmography to examine the impact of pulmonary congestion caused by burn injury on breathing patterns and respiratory function (Irvin & Bates, 2003). The Center for Disease Control (CDC) has reported 1 in 6 adults binge drink at least 4 times a month. Additionally, weekend binge drinking is a pattern observed in many cultures (Lundqvist, Alling, Knoth, & Volk, 1995). To mirror this drinking pattern, our laboratory used a mouse model of episodic binge ethanol intoxication prior to burn injury (adapted from (Callaci et al., 2004), (Przybycien-Szymanska, Mott, & Pak, 2011; Przybycien-Szymanska, Rao, & Pak, 2010; Qin et al., 2014; Vaagenes et al., 2015)) to assess respiratory physiology and gain an understanding of the effect of intoxication on lung function after burn injury.
Overall, our analyses identify plethysmography as a useful tool for characterizing respiratory function in a murine model of ethanol intoxication and burn injury. Our studies demonstrate that episodic binge ethanol intoxication prior to burn causes increased pulmonary neutrophil infiltration. The timing of neutrophil accumulation in the lung parallels the heightened levels of lung neutrophil chemoattractants and is associated with respiratory dysfunction, which likely contributes to diminished survival rates.
Materials and Methods
Mice
Male (C57BL/6) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used at 8-10 weeks old. Mice were housed in sterile micro-isolator cages under specific pathogen-free conditions in the Loyola University Medical Center Comparative Medicine facility. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee. Mice weighing between 22 to 27 g were used in these studies.
Murine Model of Binge Ethanol and Burn Injury
A murine model of episodic binge ethanol intoxication and burn injury was employed using intraperitoneal injections as described previously (Faunce et al., 1997; Messingham, Fontanilla, Colantoni, Duffner, & Kovacs, 2000; Qin et al., 2014). Animals were given ethanol (1.2g/kg) or saline vehicle at a dose designed to elevate the blood alcohol concentration (BAC) to 150 mg/dL at 30 min after ethanol exposure (Murdoch et al., 2008). This dose of 150 μl of 20% (v/v) ethanol solution or saline control was given daily for 3 days consecutively, mice were given 4 days without ethanol, and then given 3 additional daily ethanol doses. Thirty minutes following the final ethanol exposure, when the BAC was 150 mg/dl, the mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine) and their dorsums shaved. The mice were placed in a plastic template exposing 15% of the total body surface area and subjected to a scald injury in a 92-95°C water bath or a sham injury in room-temperature water (Faunce et al., 1997). The scald injury results in an insensate, full-thickness burn (Faunce et al., 1999). The mice were then resuscitated with 1.0 ml saline and allowed to recover on warming pads. All experiments were performed between 8 and 9 am to avoid confounding factors related to circadian rhythms. Animals were either euthanized at 24 hours or survival was measured out to 7 days post-injury.
Plethysmography
Pulmonary function was assessed at 24 hours post-injury by using barometric plethysmography (Buxco Research Systems). BAC levels had returned to baseline undetectable levels at this time point (Karavitis, Murdoch, Gomez, Ramirez, & Kovacs, 2008). Mice were placed in an unrestrained whole body barometric plethysmography chamber and allowed to acclimate to the environment before lung function parameters (Chen et al., 2014)were recorded for 10 minutes on a breath-by-breath basis. Enhanced pause (Penh), breath frequency (f), tidal volume (TVb) and minute volume (MVb) were analyzed.
Histopathologic Examination of the Lungs
The upper right lobe of the lung was inflated with 10% formalin and fixed overnight as described previously (Patel et al., 1999), embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Sections were evaluated using light microscopy (Zeiss AxioVert, Zeiss, Thorndale, CA) and histology photographs were taken at 1000x magnification. To measure pulmonary congestion, photographs were taken in a blinded fashion of 10 high power fields (400X) per animal and analyzed using the Java-based imaging program ImageJ (National Institutes of Health, Bethesda, MD). The images were converted to binary to differentiate lung tissue from air space and then analyzed for the percent area covered by lung tissue in each field of view as described previously (Chen et al., 2013). Neutrophils were counted in a blinded fashion in 10 high power fields (400X).
Measuring Neutrophil Counts by Flow Cytometry
The upper left lung lobe was removed and cut into small pieces with a razor. The lung tissue was then transferred to a C-tube (Miltenyi Biotec, Auburn, CA) and processed using digestion buffer containing 1mg/ml of Collagenase D and 0.1 mg/ml DNase I (Roche, Indianapolis, IN) in HBSS and a GentleMACS dissociator (Miltenyi Biotec), according to manufacturer's instructions. The homogenates were then filtered through 70 um nylon cell strainers to obtain a single cell suspension. Red blood cells were lysed using ACK lysis buffer (Life Technologies, Grand Island, NY). Cells were counted using trypan blue to exclude dead cells. To assess neutrophil numbers, 1×106 lung cells were first incubated with anti-CD16/32 (clone 93,eBioscience, San Diego, CA) to block unspecific binding to the Fcy II/III receptor. Cells were then immunostained with rat anti-mouse antibodies: CD45 e780 (clone30-F11, eBioscience), CD11b e450 (clone M1/70, eBioscience), and Ly6G (Gr-1) PE Cy7-conjugated (clone RB6-8C5, eBioscience). Antibody incubation was carried out for 30 minutes at 4°C. Cells were washed and fixed as described (Boehmer, Goral, Faunce, & Kovacs, 2004; Murdoch et al., 2011). Samples were run on a BD Fortessa cytometer (BD Biosciences, San Jose, CA). Data analysis was performed using Flow Jo FCS analysis software (Tree Star Inc., Ashland, OR).
Cytokine Analysis of Lung Homogenates
The right middle lung lobe was snap-frozen in liquid nitrogen and then homogenized in 1 ml of BioPlex cell lysis buffer according to manufacturer's protocol (BioRad, Hercules, CA). The homogenates were filtered and analyzed for cytokine production using a BioPlex multiplex bead array. The results were normalized to total protein using the BioRad protein assay (BioRad) (Bird, Zahs, et al., 2010; Chen et al., 2013).
Statistical Analysis
Statistical comparisons were made between the sham vehicle, sham ethanol, burn vehicle, and burn ethanol treatment groups. One-way analysis of variance (ANOVA) was used with Tukey's post-hoc test and values were considered statistically significant when p < 0.05. Data is reported as mean values ± the standard error of the mean (SEM). The Gehan-Breslow-Wilcoxon test was used to generate comparisons between burn groups in the survival study and Pearson's correlation test was used to generate the correlation coefficient between neutrophil numbers and Penh, breath frequency, tidal volume, and minute volume.
Results
Binge Ethanol Intoxication Decreases Survival after Burn Injury
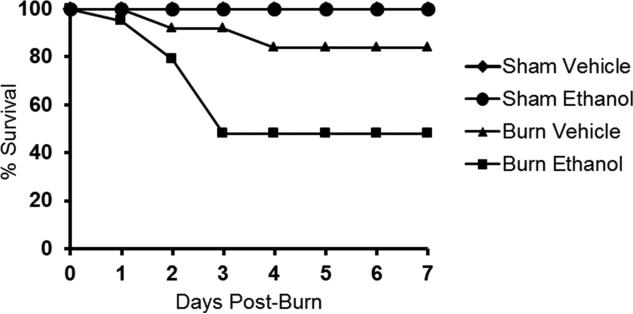
Mice exposed to episodic binge ethanol intoxication and burn injury were monitored for survival out to 7 days post-injury. Both sham groups had 100% survival regardless of ethanol intoxication (Figure 1). Survival of burn-injured mice exposed to ethanol was significantly lower (48% survival) (p < 0.05) when compared to burn-injured mice not exposed to ethanol (84% survival) (p < 0.05). Observed mortality in the combined injury group occurred within 72 hours of injury, with 5% succumbing by 24 hours, 16% mortality between 24 and 48 hours and the majority of death, 31%, occurring between 48 and 72 hours. This suggests there is a therapeutic window between time of injury and 24 hours to potentially prevent mortality when ethanol precedes burn injury.
Figure 1. Survival of mice exposed to ethanol prior to burn injury.
Mice were exposed to ethanol or saline as a control for a total of 6 days (3 days ethanol, 4 days rest, 3 days ethanol) prior to burn injury and monitored for survival out to 7 days post-injury. p < 0.05, burn ethanol compared to burn vehicle by Gehan-Breslow-Wilcoxon test. Data were combined from 2 independent experiments and are expressed as percent survival. N = 4 (sham groups), n = 10-19 (burn groups).
Intoxication Reduces Lung Function in Mice Subjected to Burn Injury
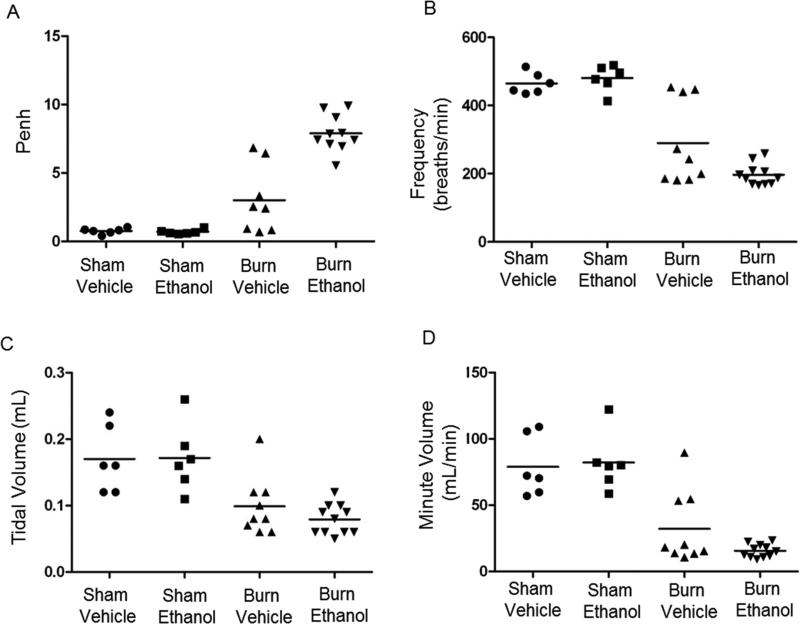
Lung function was assessed 30 minutes prior to animals being euthanized at 24 hours post-injury. Enhanced pause (Penh), a measure of bronchoconstriction and airway resistance, revealed that animals that were intoxicated at the time of burn injury had an average Penh of 8, compared to sham groups with a Penh of less than 1 and burn injury alone with an average Penh of 3 (Figure 2A). When ethanol intoxication precedes burn injury, this corresponds with an 8-fold increase in airway resistance, compared to sham groups (p < 0.05), and a 2.5-fold increase, compared to burn injury alone (p < 0.05). Moreover, our data reveal that animals with a Penh of 7 or above are at a greater risk for mortality. The frequency of breaths per minute was also significantly decreased in both burn groups. Burn injury alone had a 39% decrease in the average number of breaths per minute compared to shams (p < 0.05), while intoxicated and burn-injured mice had a 59% decrease compared to shams (p< 0.05) and a 33% decrease compared to burn alone (p < 0.05) (Figure 2B). Additionally, tidal volume, the amount of air that is inhaled and exhaled in a single breath, was decreased in both burn groups compared to sham groups (p < 0.05) (Figure 2C). Minute volume, the amount of air inhaled and exhaled per minute, was also decreased in both burn groups (Figure 2D). Compared to shams, burn injury alone had a 60% decrease in the amount of air per minute (p<0.05), while intoxicated and burn-injured mice had an 80% decrease (p<0.05). Furthermore, combined injury had a 52% decrease compared to burn alone, though this did not reach statistical significance. These data indicate burn injury alone induces abnormal breathing patterns, however, when intoxication precedes burn injury, there is a dramatic increase in airway resistance and a pronounced reduction in lung function. Overall, intoxicated and burn injured animals have a shallow, slower breathing rate than burn injury alone, indicative of overall respiratory dysfunction.
Figure 2. Effects of ethanol intoxication on post-burn lung function.
Mice were placed in an unrestrained whole body barometric plethysmography chamber and lung function parameters were recorded for 10 minutes. Penh, p < 0.05 burn ethanol versus all groups. Breath frequency, p < 0.05 burn ethanol versus all groups, p < 0.05 burn vehicle versus sham groups. Tidal volume, p < 0.05 burn ethanol compared to sham groups, p < 0.05 burn vehicle compared to sham groups. Minute volume, p < 0.05 burn ethanol compared to sham groups, p < 0.05 burn vehicle compared to sham groups. Data were combined from 2 independent experiments. Data points shown as individual animals. N = 6-11 animals per group.
Elevated Pulmonary Neutrophil Infiltration and Congestion after Intoxication and Burn Injury
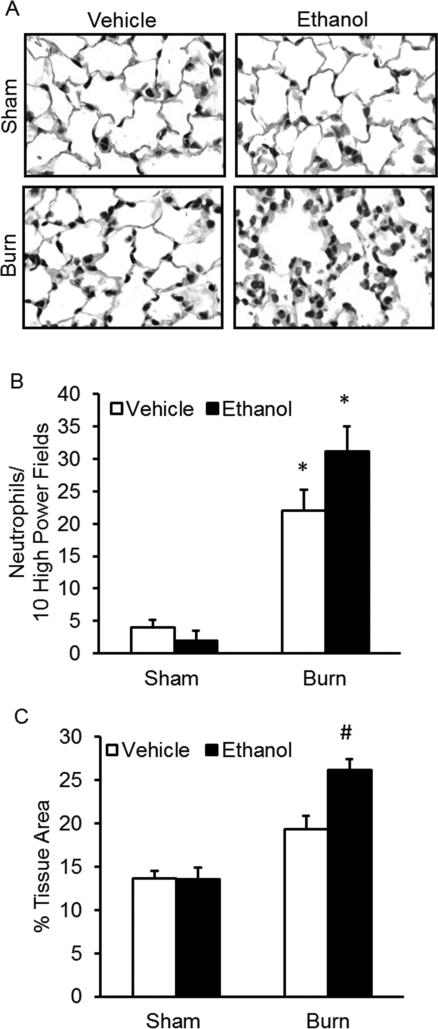
Similar to the single-dose binge ethanol and burn injury results previously reported by our laboratory, (Bird, Morgan, et al., 2010; Bird, Zahs, et al., 2010; Chen et al., 2013; Patel et al., 1999) histochemical analyses of sectioned lung tissue demonstrated that episodic binge ethanol intoxication prior to burn injury results in increased cellularity and alveolar wall thickening 24 hours post-injury (Figure 3A-C). Neutrophils were counted by light microscopy as previously described (Patel et al., 1999). When intoxication preceded burn injury, there was a 10-fold increase compared to sham groups (p < 0.05) and an approximate 1.5-fold increase compared to burn alone (Figure 3B). Additionally, these neutrophils were localized to the interstitium and not the alveolar space. Pulmonary congestion was quantified using imaging software to measure the area of lung tissue in 10 high power fields per animal and is reported as a percentage of the entire field of view. Intoxicated and burn injured animals had a significant increase in tissue area compared to all other groups (p < 0.05), correlating to a decrease in air space and an increase in pulmonary congestion (Figure 3C). Notably, these changes were isolated to the distal airways. There were no apparent changes in the larger airways.
Figure 3. Histological assessment of the lungs.
Lungs were sectioned and stained with H&E and assessed for cellular infiltration and alveolar wall thickening. A) Representative sections from each treatment group are shown at 1000×. B) Neutrophils were counted by light microscopy in H&E-stained lung sections 24 hours after intoxication and burn injury. Data are shown as the total number of neutrophils in 10 high power fields (400×). C) Quantification of pulmonary congestion 24 hours after injury. * p < 0.05 compared to sham groups; # p < 0.05 compared to all groups. Data are presented as mean values ± SEM. N = 3-6 animals per group.
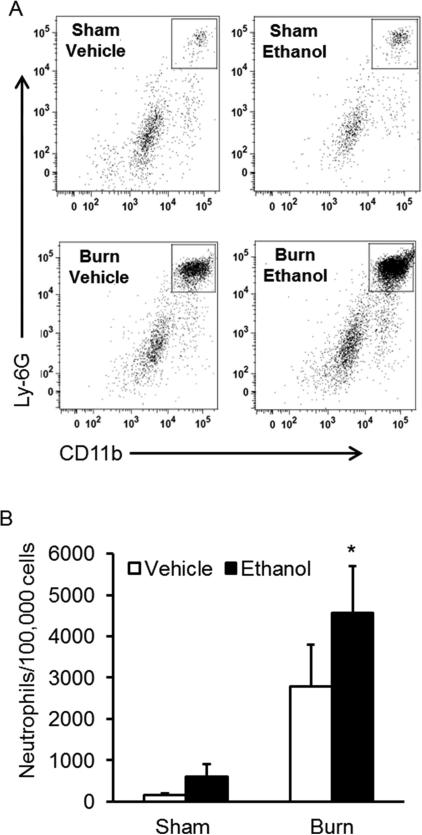
To further demonstrate that neutrophils were the key cell type contributing to the escalated cellularity, flow cytometry was performed. Flow cytometric analysis of cellular subsets in the lung revealed dramatically increased CD45+CD11b+Ly6G+ neutrophil cell counts in intoxicated and burn injured mice at 24 hours post-injury, compared to sham and isolated burn injury alone (p < 0.05) (Figures 4A and 4B). Mice exposed to the combined insult had a 7-fold increase (p < 0.05) in neutrophil numbers when compared to both sham groups and a 1.5-fold increase when compared to burn-injured animals without ethanol intoxication, confirming that neutrophils contribute to increased cellularity in the lungs of intoxicated and burn-injured mice.
Figure 4. Flow cytometry analysis of neutrophils in lung isolates.
Lung tissue was obtained 24 hours post-injury from all treatment groups. Tissue was digested into a single cell suspension and cells were analyzed by flow cytometry. A) Representative gating for Ly-6G+CD11b+ neutrophils (box) of CD45+ cells (data not shown) from all four treatment groups. B) Absolute number of neutrophils in lung isolates. * p < 0.05 versus sham groups. Data are presented as mean of total neutrophils from 100,000 lung cells per group ± SEM. N = 3-6 animals per group.
It was also observed that the increase in the number of neutrophils corresponded to an increase in Penh and decreases in breath frequency, tidal volume and minute volume. Pearson's correlation coefficient was used to determine if there was a linear relationship between the number of infiltrating neutrophils and lung function measurements. We found a positive correlation between the number of neutrophils and Penh (r = 0.9597) and negative correlations between neutrophil numbers and breath frequency (r = −0.9866), tidal volume (r = −0.9777) and minute volume (r = −0.9784). These data emphasize the potential use of plethysmography as an instrument to determine the effect of neutrophil accumulation in lung tissue on overall lung function.
Ethanol Intoxication Before Burn Injury Enhances Pulmonary KC and Macrophage Inflammatory Protein - 2 (MIP-2) Levels
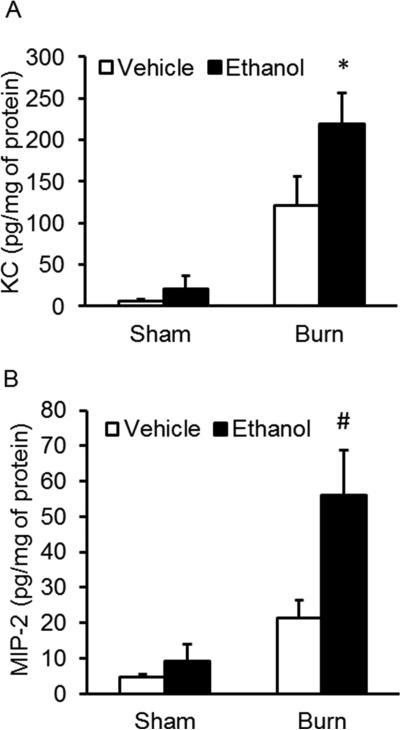
Since we observed increased neutrophil infiltration, we next sought to examine levels of the neutrophil chemoattractants, KC and MIP-2. Similar to our single-dose binge ethanol and burn injury findings (Bird, Morgan, et al., 2010; Bird, Zahs, et al., 2010; Patel et al., 1999), the upregulation of neutrophil chemokines KC and MIP-2 highlight a mechanism behind the increase in pulmonary neutrophil accumulation in episodic binge ethanol exposed and burn-injured mice (Figure 4). Combined injured animals had an 11-fold more KC than both sham groups (p < 0.05) and 1.8-fold more KC than burn injury alone. Additionally, MIP-2 was significantly upregulated compared to all treatment groups, with a 6-fold increase in MIP-2 compared to sham groups (p < 0.05) and a 2.5-fold increase compared to burn injury alone (p < 0.05).
Discussion
Intoxication at the time of burn injury increases bacterial translocation from the intestines into the lymphatic system and bloodstream. This may induce heightened hepatic production of interleukin-6 (IL-6) that circulates to the vascular bed of the lungs and initiates pulmonary inflammation and congestion (Chen et al., 2014; Choudhry et al., 2004; Emanuele et al., 2009; Kavanaugh et al., 2005; Zahs, Bird, Ramirez, Choudhry, & Kovacs, 2013; Zahs et al., 2012). Prior to this study, it was not known how multiple doses of ethanol would affect burn-induced pulmonary inflammation. Ethanol intoxication alone has been shown to suppress immune responses in many organs, including the lungs (Gamble, Mason, & Nelson, 2006; Goral, Choudhry, & Kovacs, 2004; Happel & Nelson, 2005; Karavitis, Murdoch, Deburghgraeve, Ramirez, & Kovacs, 2012). Whether episodic ethanol intoxication before burn injury would yield heightened or diminished pulmonary inflammatory effects had yet to be determined. It was also not known how intoxication prior to injury affects lungs function. Here, we have demonstrated that episodic binge ethanol prior to burn injury causes heightened pulmonary inflammatory responses and congestion, paralleling single-dose binge ethanol exposure. Importantly, we have found that these histologic inflammatory responses cause physiological derangements in intoxicated and burn-injured animals, including increased airway resistance and abnormal breathing patterns. These findings highlight an underlying mechanism contributing to the drastic mortality rate observed in mice exposed to both ethanol and burn injury.
Non-invasive, unrestrained whole-body plethysmography is a convenient method to evaluate normal breathing patterns in mice (Glaab, Taube, Braun, & Mitzner, 2007). Unlike invasive methods using a tracheotomy and mechanical ventilation to study plethysmography, non-invasive techniques do not require anesthesia or surgery. This significantly reduces stress and allows individual animals to be assessed multiple times, a useful feature for time course studies (J. H. Bates & Irvin, 2003; Glaab et al., 2007; Milton, Dickinson, Jenkin, & Lim, 2012). In non-invasive plethysmography, Penh is a dimensionless parameter that has been viewed as a measure of bronchoconstriction and airway resistance, though several studies claim Penh is an unreliable measure of respiratory mechanics (Adler, Cieslewicz, & Irvin, 2004; Hoymann, 2007). However, other studies suggest Penh should be used as a measure of overall lung function, highlighting changes in respiratory breathing patterns (J. Bates et al., 2004; Glaab et al., 2007; Verheijden, Henricks, Redegeld, Garssen, & Folkerts, 2014). Verheijden, et. al. recently compared non-invasive plethysmography (Penh) to invasive plethysmography (Resistance) in a severe model of allergic airway inflammation. Their data revealed an increase in both Penh and resistance with severe allergic airway inflammation. Notably, a Penh of 8 corresponded to a significant increase in resistance, compared to controls, suggesting that in severe models of inflammation, Penh is a reflective measure of resistance. Additionally, it was determined an increase in Penh paralleled an increase in bronchoalveolar lavage cells in the airways (Verheijden et al., 2014), furthering supporting our data that pulmonary congestion with intoxication and burn injury leads to functional lung complications.
We observed that ethanol intoxication at the time of injury leads to a longer pause between breaths (Penh), correlating with a decrease in the frequency of breaths per minute. With this data, one would expect this slower breathing pattern, an indicator of increased lung resistance, would result in deeper breaths, represented by an increase in tidal volume. In contrast, we observed a decrease in tidal volume and minute volume, an indication that the mice are not able to compensate for the increase in lung resistance. This breathing pattern may mirror agonal breathing, which is characterized by infrequent, shallow breaths and has been observed in severely injured humans. This pattern is precipitated by hypoxemia regardless of arterial carbon dioxide levels or pH, which may be the case in our injured animals since agonal breathing in humans is typically viewed as the terminal stage of respiration, occurring immediately before death (Guntheroth & Kawabori, 1975). When the plethysmography measurements were taken at 24 hours in our experiments, majority of the animals appeared either moribund or lethargic in the plethysmography chamber. With 47% of death occurring between 24 and 72 hours, the breathing pattern we detected in mice at 24 hours strongly suggests it can be characterized as agonal breathing, indicating a mechanism behind the large percentage of mortality in the following 48 hours.
The location of the dorsal burn injury in this model could also restrict chest wall movement during both the inflammatory and the wound healing phases, promoting the observed decrease in breaths per minute, overall leading to decreased gas exchange. Humans with chest, back or torso circumferential burns have breathing patterns with rapid shallow breaths, not slow shallow breaths, as we have observed in our model (Achauer et al., 1973). These differences, however, may be attributed to the immediate care provided to human burn patients. Torso circumferential burns can limit respiration and require a chest escharotomy where incisions are made in the burn wound, releasing constricted and non-elastic damaged tissue, to restore chest movement. The mice in our model do not receive circumferential burns, removing the need for escharotomy in the model, but the injury location may still lead to an altered breathing pattern. Tidal breathing is usually interrupted every few minutes by a deep breath or yawn, which is needed to expand underinflated alveoli. Restricted chest wall movement can cause burn patients to lose this ability. Without this interruption in tidal volume, the alveoli eventually collapse in a process known as atelectasis, resulting in decreased blood flow, oxygen exchange and the ability of the lungs to expand (Achauer et al., 1973; McCutcheon, 1953). Our data demonstrate that the loss of lung compliance with burn injury, even in the absence of inhalation injury, is further decreased when intoxication precedes burn injury. The restricted chest movement and subsequent collapse of alveoli may also explain the differences in breathing patterns in our mouse model, in comparison to human burn patients. However, the 3-fold increase in Penh with burn alone does not correspond to significantly increased mortality rates. This suggests ethanol, not the location of the burn, leads to impaired lung function and elevated mortality rates.
In conclusion, these data emphasize the importance of immediate burn care treatment within the few hours after injury, to stabilize breathing patterns and to maintain respiratory function, especially when patients are intoxicated at the time of injury. Noninvasive, unrestrained whole-body plethysmography will be a useful tool in future animal studies to help identify therapeutic reagents that can improve respiratory function in all burn patients.
Highlights.
A higher rate of mortality is observed with intoxication and burn injury
Non-invasive plethysmography demonstrates lung dysfunction in ethanol and burn injury
Increased lung congestion with ethanol and burn corresponds to decreased function
Figure 5. Pulmonary neutrophil chemokine levels in lung tissue.
Lung homogenates from all four treatment groups were analyzed for levels of A) KC and B) MIP-2. * p < 0.05 versus sham groups; # p < 0.05 versus all treatment groups. Data are presented as the mean in picograms per milligram of protein ± SEM. N = 3-6 animals per group.
Acknowledgements
Research reported in the publication was supported by the National Institutes of Health (NIH), R01 AA012034 (EJK), R01 GM115257 (EJK), T32 AA013527 (EJK), Department of Defense W81XWH-10-2-0172 (RHK), F31 AA022566 (JAS), F32 AA021636 (BJC) and F30 AA022856 (MMC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Work was also supported by the Dr. Ralph and Marian C. Falk Medical Research Trust. The authors would like to give special thanks to Mary Brown and Jessica Remus for technical assistance and Patricia Simms for technical assistance in experiments involving flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABA. American Burn Association National Burn Repository: 2014 Report. Chicago, IL.: 2014. [Google Scholar]
- Achauer BM, Allyn PA, Furnas DW, Bartlett RH. Pulmonary complications of burns: the major threat to the burn patient. Ann Surg. 1973;177(3):311–319. doi: 10.1097/00000658-197303000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol (1985) 2004;97(1):286–292. doi: 10.1152/japplphysiol.00821.2003. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, Sly P. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol. 2004;31(3):373–374. doi: 10.1165/ajrcmb.31.3.1. [DOI] [PubMed] [Google Scholar]
- Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol (1985) 2003;94(4):1297–1306. doi: 10.1152/japplphysiol.00706.2002. doi: 10.1152/japplphysiol.00706.2002. [DOI] [PubMed] [Google Scholar]
- Bird MD, Morgan MO, Ramirez L, Yong S, Kovacs EJ. Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. J Burn Care Res. 2010;31(4):652–660. doi: 10.1097/BCR.0b013e3181e4c58c. doi: 10.1097/BCR.0b013e3181e4c58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MD, Zahs A, Deburghgraeve C, Ramirez L, Choudhry MA, Kovacs EJ. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcohol Clin Exp Res. 2010;34(10):1733–1741. doi: 10.1111/j.1530-0277.2010.01260.x. doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75(2):342–349. doi: 10.1189/jlb.0803389. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcohol Clin Exp Res. 2004;28(1):182–191. doi: 10.1097/01.ALC.0000108661.41560.BF. doi: 10.1097/01.ALC.0000108661.41560.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Vital Signs: Binge Drinking Prevalence, Frequency, and Intensity Among Adults. Morbidity and Mortality Weekly Report. 2012;61(1):14–19. [PubMed] [Google Scholar]
- Chen MM, Bird MD, Zahs A, Deburghgraeve C, Posnik B, Davis CS, Kovacs EJ. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol. 2013;47(3):223–229. doi: 10.1016/j.alcohol.2013.01.004. doi: 10.1016/j.alcohol.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM, Zahs A, Brown MM, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. Am J Physiol Gastrointest Liver Physiol. 2014;307(7):G711–718. doi: 10.1152/ajpgi.00185.2014. doi: 10.1152/ajpgi.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Messingham KA, Namak S, Colantoni A, Fontanilla CV, Duffner LA, Kovacs EJ. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21(3):239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33(3):199–208. doi: 10.1016/j.alcohol.2004.05.004. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138(4):749–757. doi: 10.1016/j.surg.2005.07.020. discussion 757-748. doi: 10.1016/j.surg.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Dancey DR, Hayes J, Gomez M, Schouten D, Fish J, Peters W, Stewart TE. ARDS in patients with thermal injury. Intensive Care Med. 1999;25(11):1231–1236. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, Emanuele MA, Morgan MO, Sulo D, Yong S, Kovacs EJ, Callaci JJ. Ethanol potentiates the acute fatty infiltration of liver caused by burn injury: prevention by insulin treatment. J Burn Care Res. 2009;30(3):482–488. doi: 10.1097/BCR.0b013e3181a28df3. doi: 10.1097/BCR.0b013e3181a28df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62(6):733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10(2):135–140. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ. Neutrophil chemokine production in the skin following scald injury. Burns. 1999;25(5):403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- Gamble L, Mason CM, Nelson S. The effects of alcohol on immunity and bacterial infection in the lung. Med Mal Infect. 2006;36(2):72–77. doi: 10.1016/j.medmal.2005.08.010. doi: 10.1016/j.medmal.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res. 2007;8:63. doi: 10.1186/1465-9921-8-63. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J Leukoc Biol. 2004;75(3):553–559. doi: 10.1189/jlb.0703350. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil. 1996;17(6 Pt 1):532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56(6):1371–1377. doi: 10.1172/JCI108217. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjizacharia P, O'Keeffe T, Plurad DS, Green DJ, Brown CV, Chan LS, Rhee P. Alcohol exposure and outcomes in trauma patients. Eur J Trauma Emerg Surg. 2011;37(2):169–175. doi: 10.1007/s00068-010-0038-5. doi: 10.1007/s00068-010-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–432. doi: 10.1513/pats.200507-065JS. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Hollingsed TC, Saffle JR, Barton RG, Craft WB, Morris SE. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166(6):592–596. doi: 10.1016/s0002-9610(05)80662-2. discussion 596-597. [DOI] [PubMed] [Google Scholar]
- Howland J, Hingson R. Alcohol as a risk factor for injuries or death due to fires and burns: review of the literature. Public Health Rep. 1987;102(5):475–483. [PMC free article] [PubMed] [Google Scholar]
- Hoymann HG. Invasive and noninvasive lung function measurements in rodents. J Pharmacol Toxicol Methods. 2007;55(1):16–26. doi: 10.1016/j.vascn.2006.04.006. doi: 10.1016/j.vascn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cell Immunol. 2012;274(1-2):61–71. doi: 10.1016/j.cellimm.2012.02.002. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Murdoch EL, Gomez CR, Ramirez L, Kovacs EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. J Interferon Cytokine Res. 2008;28(7):413–422. doi: 10.1089/jir.2007.0111. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh MJ, Clark C, Goto M, Kovacs EJ, Gamelli RL, Sayeed MM, Choudhry MA. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31(3):290–296. doi: 10.1016/j.burns.2004.09.021. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Switzer BR, Herzog SR, Meyer AA. Immune suppression after acute ethanol ingestion and thermal injury. J Surg Res. 1991;51(3):210–215. doi: 10.1016/0022-4804(91)90096-5. [DOI] [PubMed] [Google Scholar]
- Liffner G, Bak Z, Reske A, Sjoberg F. Inhalation injury assessed by score does not contribute to the development of acute respiratory distress syndrome in burn victims. Burns. 2005;31(3):263–268. doi: 10.1016/j.burns.2004.11.003. doi: 10.1016/j.burns.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Alling C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment. Alcohol Alcohol. 1995;30(6):737–748. [PubMed] [Google Scholar]
- McCutcheon FH. Atmospheric respiration and the complex cycles in mammalian breathing mechanisms. J Cell Physiol. 1953;41(2):291–303. doi: 10.1002/jcp.1030410207. [DOI] [PubMed] [Google Scholar]
- Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28(3):137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- Messingham KA, Fontanilla CV, Colantoni A, Duffner LA, Kovacs EJ. Cellular immunity after ethanol exposure and burn injury: dose and time dependence. Alcohol. 2000;22(1):35–44. doi: 10.1016/s0741-8329(00)00100-2. [DOI] [PubMed] [Google Scholar]
- Milton PL, Dickinson H, Jenkin G, Lim R. Assessment of respiratory physiology of C57BL/6 mice following bleomycin administration using barometric plethysmography. Respiration. 2012;83(3):253–266. doi: 10.1159/000330586. doi: 10.1159/000330586. [DOI] [PubMed] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–510. doi: 10.1165/rcmb.2013-0086MA. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch EL, Brown HG, Gamelli RL, Kovacs EJ. Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. J Burn Care Res. 2008;29(2):323–330. doi: 10.1097/BCR.0b013e3181667599. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- Murdoch EL, Karavitis J, Deburghgraeve C, Ramirez L, Kovacs EJ. Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock. 2011;35(4):403–410. doi: 10.1097/SHK.0b013e31820217c9. doi: 10.1097/SHK.0b013e31820217c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PJ, Faunce DE, Gregory MS, Duffner LA, Kovacs EJ. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am J Respir Cell Mol Biol. 1999;20(6):1229–1237. doi: 10.1165/ajrcmb.20.6.3491. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- Phillips AW, Cope O. Burn therapy. II. The revelation of respiratory tract damage as a principal killer of the burned patient. Ann Surg. 1962;155:1–19. doi: 10.1097/00000658-196201000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Pak TR. Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE) PLoS One. 2011;6(10):e26647. doi: 10.1371/journal.pone.0026647. doi: 10.1371/journal.pone.0026647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am J Physiol Endocrinol Metab. 2010;298(2):E320–328. doi: 10.1152/ajpendo.00615.2009. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Hamilton JL, Bird MD, Chen MM, Ramirez L, Zahs A, Makowski L. Adipose inflammation and macrophage infiltration after binge ethanol and burn injury. Alcohol Clin Exp Res. 2014;38(1):204–213. doi: 10.1111/acer.12210. doi: 10.1111/acer.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer CR. Alcohol and injury prevention. J Trauma. 2006;60(2):447–451. doi: 10.1097/01.ta.0000196956.49282.91. doi: 10.1097/01.ta.0000196956.49282.91. [DOI] [PubMed] [Google Scholar]
- Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008;29(5):784–789. doi: 10.1097/BCR.0b013e31818481bc. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Kraus JF. Alcohol and residential, recreational, and occupational injuries: a review of the epidemiologic evidence. Annu Rev Public Health. 1988;9:99–121. doi: 10.1146/annurev.pu.09.050188.000531. doi: 10.1146/annurev.pu.09.050188.000531. [DOI] [PubMed] [Google Scholar]
- Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns. 2008;34(4):441–451. doi: 10.1016/j.burns.2007.10.007. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Turnage RH, Nwariaku F, Murphy J, Schulman C, Wright K, Yin H. Mechanisms of pulmonary microvascular dysfunction during severe burn injury. World J Surg. 2002;26(7):848–853. doi: 10.1007/s00268-002-4063-3. doi: 10.1007/s00268-002-4063-3. [DOI] [PubMed] [Google Scholar]
- Vaagenes IC, Tsai SY, Ton ST, Husak VA, McGuire SO, O'Brien TE, Kartje GL. Binge ethanol prior to traumatic brain injury worsens sensorimotor functional recovery in rats. PLoS One. 2015;10(3):e0120356. doi: 10.1371/journal.pone.0120356. doi: 10.1371/journal.pone.0120356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijden KA, Henricks PA, Redegeld FA, Garssen J, Folkerts G. Measurement of airway function using invasive and non-invasive methods in mild and severe models for allergic airway inflammation in mice. Front Pharmacol. 2014;5:190. doi: 10.3389/fphar.2014.00190. doi: 10.3389/fphar.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock. 2013;39(4):373–379. doi: 10.1097/SHK.0b013e318289d6c6. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G705–712. doi: 10.1152/ajpgi.00157.2012. doi: 10.1152/ajpgi.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]