Abstract
The skin harbors a variety of resident leukocyte subsets that must be tightly regulated to maintain immune homeostasis. Hair follicles are unique structures in the skin that contribute to skin dendritic cell homeostasis via chemokine production. We demonstrate that CD4+ and CD8+ skin resident memory T cells (TRM), responsible for long-term skin immunity, resided predominantly within the hair follicle epithelium of unperturbed epidermis. TRM tropism for the epidermis and follicles was herein termed epidermotropism. Hair follicle-derived IL-15 was required for CD8+ TRM, and IL-7 for CD8+ and CD4+ TRM, to exert epidermotropism. The lack of either cytokine impaired hapten-induced contact hypersensitivity responses. In a model of cutaneous T cell lymphoma, epidermotropic CD4+ TRM lymphoma cell localization depended on hair follicle-derived IL-7. These findings implicate hair follicle-derived cytokines as regulators of malignant and non-malignant TRM cell tissue residence and suggest they may be targeted therapeutically in inflammatory skin disease and lymphoma.
Hair follicles are unique structures in the mammalian skin that provide a niche for keratinocyte and melanocyte stem cells1,2. Various leukocyte subsets localize with hair follicles3. We recently demonstrated that hair follicles recruit skin dendritic cells to sites of minor trauma4. Keratinocytes in the hair follicle infundibulum and isthmus produce the chemokines CCL2 and CCL20, recruiting myeloid cells after physical perturbation. A subset of keratinocytes in the suprabasal layer of the follicular bulge region expressed CCL8. CCL8 prevented local Langerhans cell (LC) accumulation, a mechanism that may protect bulge stem cells from excessive leukocyte infiltration4. These data establish that hair follicles actively promote immune homeostasis.
T cells that reside in peripheral tissues have been described in the recent years, and their importance is now well-established5–9. Skin resident memory T cells (TRM) display an effector memory phenotype and are generated after immunological insults such as viral infection6,8. In the context of infection, CD4+ memory T cells accumulate primarily in the dermis, whereas CD8+ TRM accumulate within the epidermis7. Both T cell subsets exhibited tropism to the hair follicles7. Skin TRM express CD69 and CD10310. CD69 suppresses sphingosine-1-phosphate receptor 1 expression, preventing T cells from emigrating from lymphoid organs or other tissues into the circulation11,12. CD103 mediated retention of T cells in the skin likely occurs via adhesion to E-cadherin13. The non-migratory nature of CD8+ TRM has been established in parabiotic mice8, and CD4+ TRM in human skin engrafted onto immunodeficient mice14.
Infiltration of T cells into the epidermis is a prominent feature in both inflammatory and neoplastic human diseases including graft-versus host disease, drug eruptions, and cutaneous T cell lymphoma (CTCL). In fixed drug eruption, CD8+ T cells attack the epidermis to cause keratinocyte cell death in the presence of the drug(s)15. After clinical resolution, CD8+ T cells with a memory phenotype persist within the epidermis15,16. The majority of CTCL is caused by CD4+ lymphoma cells17. In the classic form, mycosis fungoides, lymphoma cells of a TRM phenotype infiltrate the epidermis including the follicular epithelium18,19 and slowly accumulate and proliferate to form tumors. Such epidermis- and follicle-infiltrating T cells are termed epidermotropic T cells20.
Given the importance of TRM in conferring long-term immunological memory6,8 and regulatory functions13,21,22, elucidation of mechanisms that support long-term persistence in the skin may provide insight into T cell homeostasis in health and disease. Herein, we demonstrate that the epidermotropism of TRM is supported by the hair follicle-derived cytokines, IL-7 and IL-15.
RESULTS
CD4+ and CD8+ T cells in steady state epidermis
To characterize TRM in the epidermis, we prepared vertical sections of frozen skin samples taken from unmanipulated adult C57BL/6J mice, and visualized CD4+ and CD8+ T cells using immunofluorescence microscopy. We routinely observed small numbers of both CD4+ and CD8+ T cells in follicular epithelium (Fig. 1a). Visualization of the basement membrane via integrin α6 staining confirmed that both CD4+ and CD8+ T cells resided within the follicular epithelium (Supplementary Fig. 1a). Staining epidermal sheets revealed that CD8+ T cells were present in both hair follicles and the interfollicular epidermis, whereas CD4+ T cells were localized exclusively around hair follicles (Supplementary Fig. 1b). In flow cytometry analysis of epidermal cell suspensions, exclusion of LC and dendritic epidermal T cells enabled the identification of small numbers of CD4+ and CD8+ T cells, consistent with the immunofluorescence microscopy results (Fig. 1b). The number of CD4+ T cells in the epidermis was comparable to that in the dermis (Fig. 1c). CD8+ T cells were found exclusively in epidermal cell suspensions (Fig. 1c)7. Contamination of dermal leukocytes into the epidermal preparation and vice versa appeared unlikely (Supplementary Fig. 1c,d).
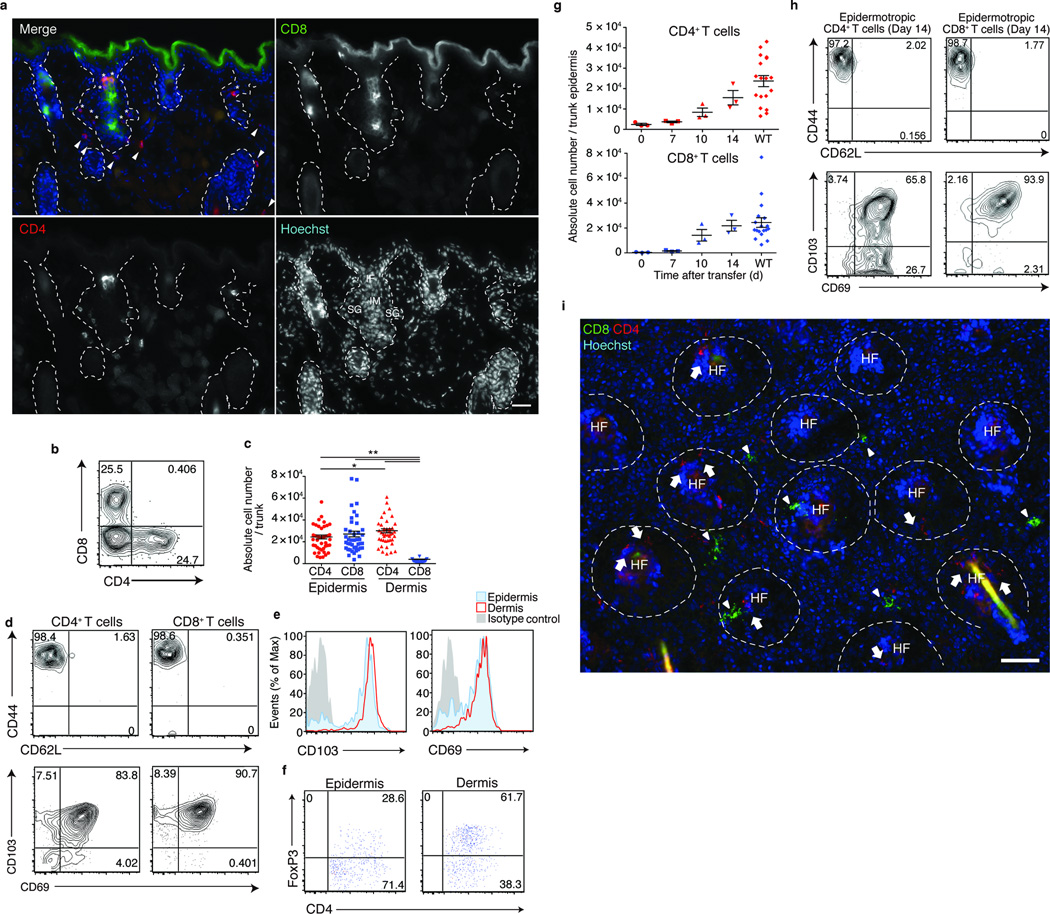
Figure 1. Epidermotropic CD4+ and CD8+ TRM associate with hair follicles.
(a) Skin sections from WT mice, stained as indicated. Dotted lines delineate hair follicles. Asterisk and arrowheads depict CD4+ T cells in epidermis and dermis, respectively. Scale bar, 50 µm. (b) Flow cytometry analysis of CD45+CD3+MHCll−TCRγδ− epidermal cells from WT mice. Numbers in quadrants represent percentage among the gated subset (c) T cell numbers in the epidermis and dermis. Each symbol represents individual mice; horizontal lines depict the mean and error bars indicate s.e.m. *P < 0.05, **P < 0.01 (unpaired two-tailed Student’s t-test). (d) CD44 and CD62L, and CD103 and CD69 expression on T cells in the epidermis. (e) Expression of CD103 and CD69 expression on CD4+ T cells in the epidermis (red) and dermis (blue) with isotype controls (grey). (f) Frequency of CD4+FoxP3+ Treg cells in the epidermis and dermis. (g) Number of donor T cells in Rag2−/− recipient epidermis monitored via flow cytometry at indicated dates after adoptive transfer of WT splenocytes. (h) Epidermotropic T cells from (g) were analyzed via flow cytometry as in (d), 14 d after transfer. Shown are CD45+CD3+MHCll−TCRγδ− CD4+ (left panels) or CD8+ (right panels) populations. (i) Epidermal sheets from mice as in (g), day 14 after transfer, were stained as indicated. Dashed lines delineate hair follicles. Scale bar, 100 µm. Data are from one experiment representative of three independent experiments with three mice per group (a,b,d–k) or from ten independent experiments with a total of 42 mice (c).
Phenotypic analysis suggests most of the CD4+ and CD8+ T cells are effector memory T cells, as assessed by CD44 and CD62L expressions, and are resident cells, by CD103 and CD69 co-expression (Fig. 1d). CD4+ T cells in the epidermis expressed CD103 at slightly lower levels than those in the dermis (Fig. 1e,f). Furthermore, the ratio of CD4+ FoxP3+ regulatory TRM were lower in the epidermis than in the dermis, suggesting that organization of CD4+ TRM in the two skin compartments may be differentially regulated. In this manuscript, memory T cells that reside in the epidermis and the follicles will be referred to as epidermotropic TRM not to indicate their entity as a distinct T cell subset, but solely to describe their localization in the skin.
TRM associate with hair follicles during epidermal entry
TRM are generated during skin infection and accumulate not only at sites of primary inoculation, but also at distant sites8,23. To model this distribution of TRM, we adoptively transferred WT splenocytes into Rag2−/− mice. After transfer into a lymphopenic environment, donor T cells undergo homeostatic proliferation, and we hypothesized that such T cells might distribute to peripheral tissues including epidermis and skin. Indeed, CD4+ and CD8+ T cells appeared in the epidermis of Rag2−/− recipients 10 days after transfer, and by day 14 their numbers reached levels detectable in WT mice (Fig. 1g). Donor T cells displayed effector memory and resident phenotypes (Fig. 1h), and thus represent epidermotropic TRM.
Visualization of CD4+ and CD8+ TRM during active epidermal repopulation revealed close association with hair follicles (Fig. 1i). CD4+ TRM initially appeared within the dermis and accumulated around hair follicles in frozen sections at day 7 after transfer and then distributed within the epidermis and dermis thereafter (Supplementary Fig. 1e). In contrast to CD4+ TRM, CD8+ TRM appeared directly within the interfollicular epidermis, and then accumulated around the hair follicles (Supplementary Fig. 1e,f). Thus, the anatomical mode of entry for CD4+ and CD8+ TRM appears to be distinct.
Hair follicle keratinocytes express il15 and il7
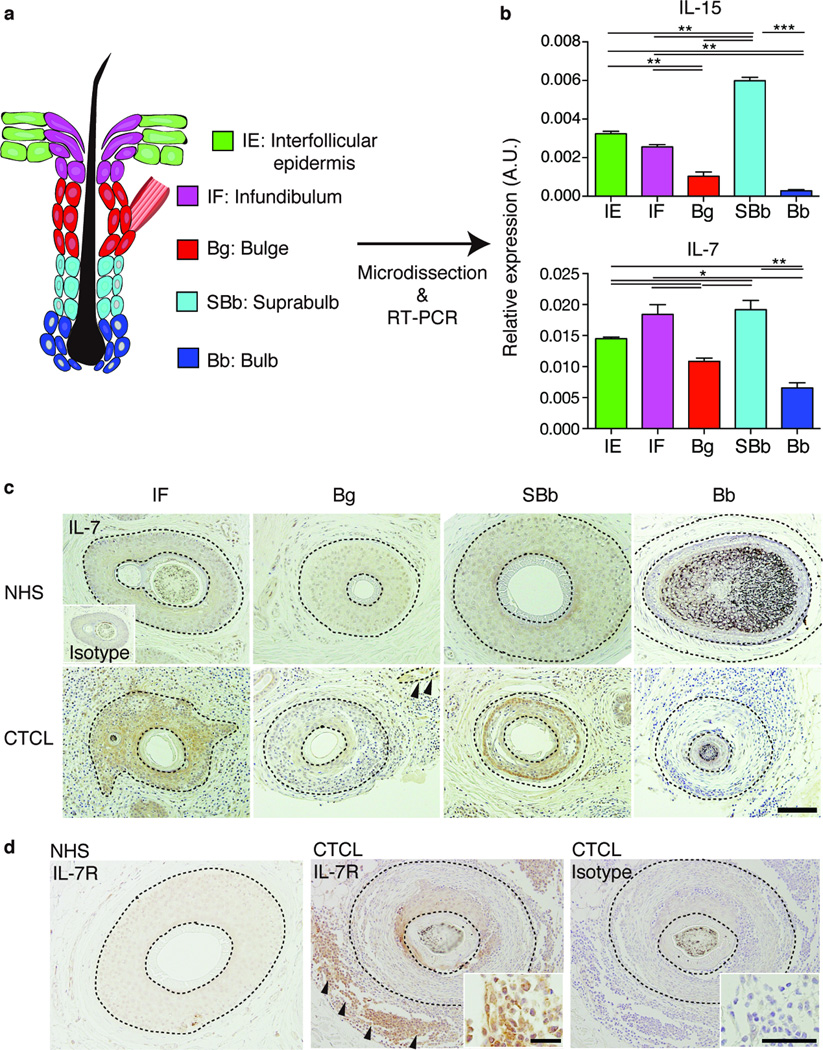
IL-15 and IL-7 are important cytokines that enable the generation and maintenance of memory T cells24,25. Our previous study revealed that hair follicle keratinocyte subsets exhibit distinct chemokine expression profiles4. To determine if hair follicle keratinocytes expressed mRNA encoding IL-15 and IL-7, we sorted epidermal keratinocytes into those from interfollicular epidermis, infundibulum, isthmus, basal layer bulge and suprabasal layer bulge4 (Fig. 2a). Similar to previously described patterns of chemokine expression4, real-time PCR analysis revealed that both il15 and il7 mRNA were predominantly expressed by keratinocytes in the infundibulum and isthmus (Fig. 2b). Because most of the mouse pelage hair follicles are in telogen, we studied the vibrissae to determine if cytokines were expressed during anagen. The transient portions of anagen hair follicles did not express il7, but the bulb expressed low levels of il15 (Supplementary Fig. 2a). We also analyzed cytokine mRNA expression in major leukocyte subsets in the epidermis, and found that LC, but not dendritic epidermal T cells, expressed il15 (Supplementary Fig. 2b). Both leukocyte subsets lacked il7 expression (data not shown).
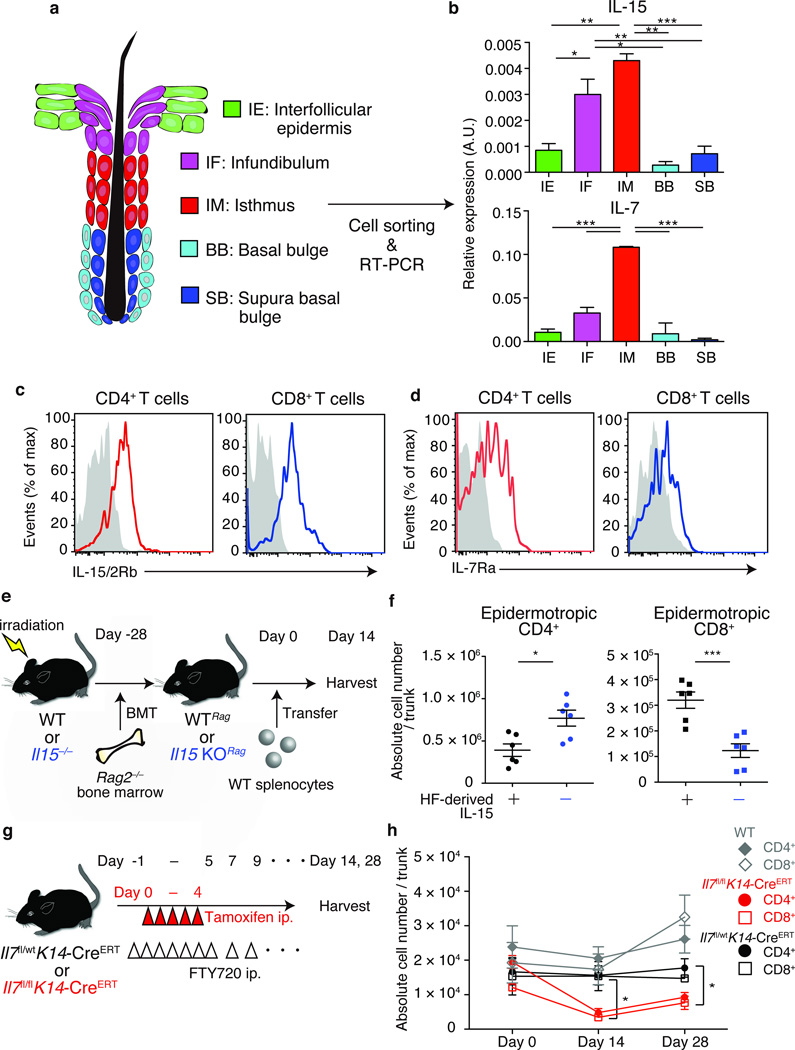
Figure 2. Epidermotropic TRM require hair follicle-derived cytokines.
(a) Schematic representation of mouse hair follicle keratinocytes in telogen. Keratinocytes were sorted into five subsets based on cell-surface marker expressions. (b) Real-time PCR analysis for il15 and il7 expressions by hair follicle keratinocyte subsets, presented in arbitrary units (A.U.), relative to Actb expression. (c) IL-15/2 receptor β (IL-15/2Rβ) and (d) IL-7 receptor α (IL-7Rα) expression by TRM, assessed via flow cytometry, gated on the CD45+CD3+MHCll−TCRγδ− population (n = 3). (e) Experimental scheme for the analysis of TRM dependence on hair follicle-derived IL-15. WT or il15−/− mice were reconstituted with Rag2−/− bone marrow (WTRag or Il15 KORag), and were transferred with WT splenocytes. (f) Epidermotropic TRM numbers in recipient WTRag or il15 KORag mice (n = 6). Symbols represent individual mice. (g) Experimental scheme for analyzing TRM dependence on hair follicle-derived IL-7. Indicated mice were treated with tamoxifen i.p. on days 0 to 4. FTY720 was injected i.p. from day -1 for 7 consecutive days and every other day thereafter, until tissue harvest. (h) Epidermotropic TRM numbers after tamoxifen-induced ablation of hair follicle IL-7 (n = 3). Data are representative of three (b–d) or two (f, h) independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired two-tailed Student’s t-test). Horizontal lines depict the mean, and error bars indicate s.e.m. in all graphs.
Epidermotropic TRM require hair follicle-derived cytokines
Analysis of CD4+ and CD8+ TRM via flow cytometry revealed that both T cell subsets expressed IL-15/2Rβ and IL-7Rα (Fig. 2c,d). Given the unavailability of an animal model enabling conditional ablation of IL-15, we generated bone marrow (BM) chimeric mice to determine if epidermotropic TRM were influenced by hair follicle-derived cytokines. Reconstitution of WT or Il15−/− mice with BM from Rag2−/− mice led to the generation of lymphopenic mice that either expressed (WTRag) or lacked (Il15 KORag) IL-15 in peripheral tissues including skin (Fig. 2e). We then transferred WT splenocytes into these BM chimeric mice and analyzed the numbers of epidermotropic TRM at day 14 after transfer (Fig. 2e). The numbers of epidermotropic CD4+ TRM were slightly increased in recipient Il15 KORag mice, whereas epidermotropic CD8+ TRM numbers were reduced (Fig. 2f). The numbers of CD4+ and CD8+ T cells in the spleen were comparable in recipient Il15 KORag and WTRag mice (Supplementary Fig. 2c), indicating that the difference in the numbers of epidermotropic CD8+ TRM was due to the loss of IL-15 in skin. The numbers of epidermotropic CD8+ TRM were 10-fold higher than that of unmanipulated WT mice. Upregulation of IL-15 mRNA expression in keratinocytes in lethally irradiated mice may contribute to this (Supplementary Fig. 2d).
Because LC also expressed IL-15 mRNA, we studied the effect of LC depletion on epidermotropic TRM utilizing two different models. Neither constitutive loss nor depletion of LC affected the numbers of epidermotropic TRM (Supplementary Fig. 3a–c). Thus, hair follicle-derived IL-15 is crucial for the maintenance of epidermotropic CD8+ TRM.
A different approach was taken to evaluate the contribution of hair follicle-derived IL-7 because T cells failed to undergo homeostatic proliferation when transferred into Il7 KORag mice (data not shown). We crossed K14-CreERT mice26 with Il7-floxed mice to generate mice in which ablation of IL-7 in epidermis could be specifically induced in the skin of adult mice via tamoxifen injection (Supplementary Fig. 2e). Because of the potential for a continuous influx of newly generated TRM that could alter total cell numbers during and after IL-7 ablation, we treated mice with FTY720, a sphingosine-1-phosphate receptor 1 inhibitor that inhibits lymphocyte egress from lymph nodes, thereby preventing the influx of endogenous TRM into the epidermis (Fig. 2g). Epidermal ablation of IL-7 reduced the numbers of both CD4+ and CD8+ TRM 14 days after loss of IL-7, an effect that persisted for at least 28 days (Fig. 2h). The numbers of splenic T cells remained unaffected by the loss of IL-7 in the skin (Supplementary Fig. 2f). Therefore, hair follicle-derived IL-7 is required for both CD4+ and CD8+ TRM to persist in the epidermis.
Impaired CHS responses in the absence of IL-7 and IL-15
Impaired anatomical localization of TRM during the steady state in the absence of hair follicle-derived IL-15 and IL-7 might affect subsequent immune responses in the skin. To address this, we induced contact hypersensitivity (CHS) with a hapten in mice that lacked hair follicle-derived cytokines. To analyze the effect of IL-15 deficiency in the context of CD8+ TRM, we generated WTRag and Il15 KORag mice via BM transplantation (Fig. 2e). These BM chimeric mice were injected with CD8+ T cells obtained from skin draining lymph nodes of 1-fluoro-2,4-dinitrobenzene (DNFB)-sensitized mice (Fig. 3a). Transferred CD8+ T cells are expected to undergo homeostatic proliferation and distribute to peripheral tissues including skin in WTRag, but not in Il15 KORag mice (Fig. 1h, 2e). Recipient mice were then challenged with DNFB and ear-swelling responses were monitored. Ear swelling and lymphocytic infiltration were reduced in the absence of tissue-derived IL-15 (Fig. 3b,c).
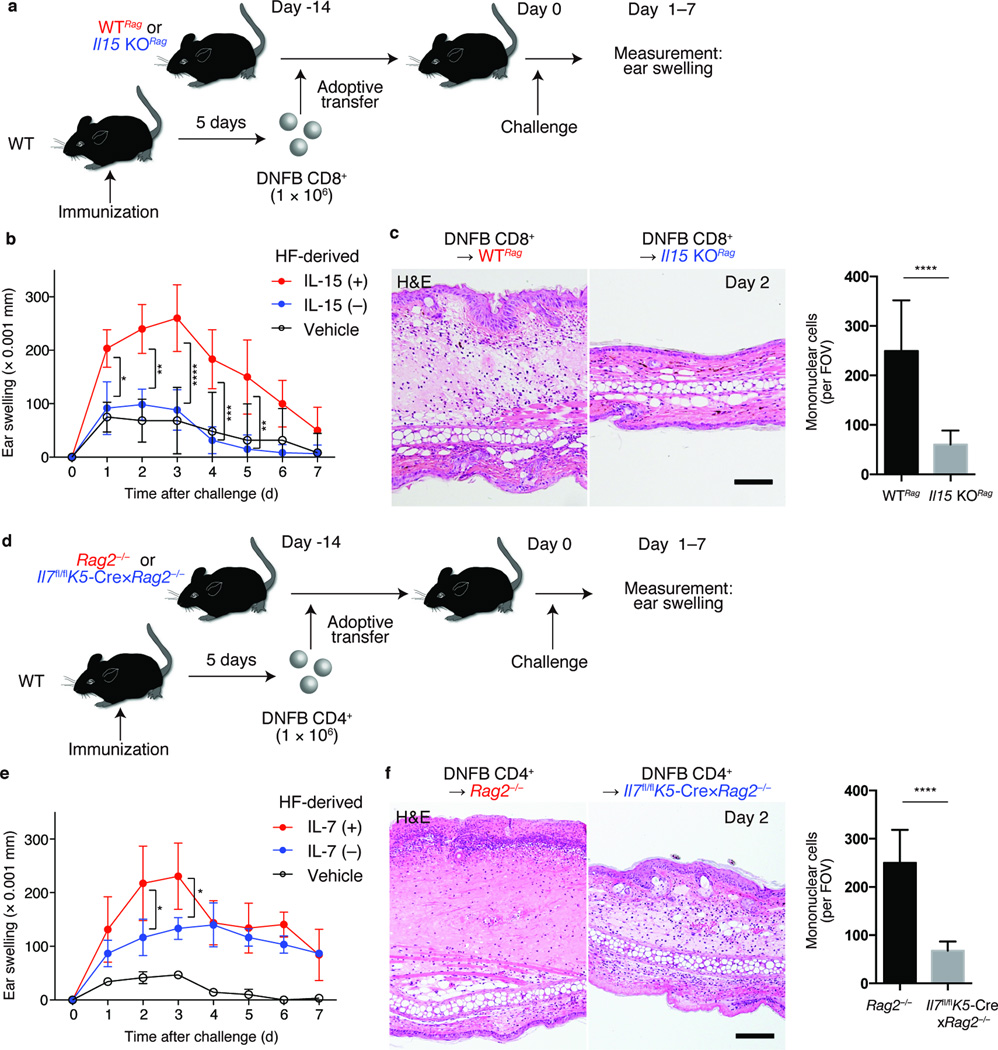
Figure 3. Impaired contact hypersensitivity responses in the absence of hair follicle-derived cytokines.
(a) Experimental scheme for the analysis of CD8+ TRM-mediated CHS in the absence of peripheral tissue (hair follicle)-derived IL-15. CD8+ T cells from DNFB (DNFB CD8+) or vehicle-immunized (Vehicle CD8+) WT mice were transferred into indicated lymphopenic mice. 14 days later, recipients were challenged with DNFB on their ears, and ear-swelling responses were measured. (b) Ear thickness (Δmm) of DNFB CD8+-transferred WTRag (red), Il15 KORag (blue), and vehicle CD8+-transferred WTRag (black) (n = 3). (c) Hematoxylin and eosin staining of ear sections from indicated mice, day 2 after challenge and quantification of skin-infiltrating mononuclear cells. (d) Experimental scheme for analyzing CD4+ TRM-mediated CHS in the absence of hair follicle-derived IL-7. DNFB CD4+ or vehicle CD4+ were adoptively transferred into indicated lymphopenic mice. (e) Ear thickness (Δ mm) of DNFB CD4+-transferred Rag2−/− (red), Il7fl/flK5-Cre×Rag2−/− (blue), or vehicle CD4+-transferred Rag2−/− (black) (n = 3). (f) Hematoxylin and eosin staining of ear sections from indicated mice, day 2 after challenge, and quantification of skin-infiltrating mononuclear cells. Data are from one experiment, representative of two independent experiments with three mice per group in each (b, c, e, f). Cell counts represent pools of three fields of view per section (100×) from three mice per group (c, f). Scale bar, 100 µm (c, f). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (two way ANOVA).
The role of hair follicle-derived IL-7 and TRM was assessed in the context of CD4+ T cell-mediated CHS. To generate lymphopenic mice that constitutively lack IL-7 in the epidermis, K5-Cre mice and Il7-floxed mice27 were each crossed to the Rag2−/− background (Il7fl/fl;K5-Cre×Rag2−/− mice). CD4+ T cells isolated from skin draining lymph nodes of DNFB-sensitized WT mice were transferred into Il7fl/flK5-Cre×Rag2−/− mice or control Rag2−/− mice. Recipient mice were challenged with DNFB 14 days after transfer and ear-swelling responses were assessed (Fig. 3d). Ear swelling and lymphocytic infiltration were reduced in Il7fl/flK5-Cre×Rag2−/− mice on days 2 and 3 after challenge (Fig. 3e,f). This transient effect might reflect the generation and influx of newly generated recipient TRM after DNFB challenge.
A CD4+ T cell lymphoma model with skin involvement
Epidermotropism of T cells is a histological hallmark of CTCL. In particular, lymphoma cells in mycosis fungoides exhibit a TRM phenotype18, 19,28. IL7 expression is increased in CTCL skin29. Whether lymphoma cells also require hair follicle-derived cytokines remains unclear. Thus, we extended our TRM repopulation model in Rag2−/− mice by generating a novel lymphoma model.
Mutations in, or up regulation of, the oncogene MYC30,31, as well as mutations in the tumor suppressor gene INK4A/ARF have been implicated in human lymphoma and CTCL17,30. Ink4a/Arf−/− mice are prone to tumor development including T cell lymphoma32,33. Ink4a/Arf−/− progenitor B cells transduced with Myc generate B cell lymphoma34. Taking advantage of these previous findings, we isolated CD4+ T cells from Ink4a/Arf−/− mice and retrovirally transduced them with Myc (Fig. 4a). Approximately 50% of T cells were transduced, as determined by GFP expression (Myc+Ink4a/Arf−/− CD4+ T cells) (Fig. 4b). Recipient Rag2−/− mice developed erythroderma (redness and fine scaling of the entire skin surface) approximately three weeks after transfer of Myc+Ink4a/Arf−/− CD4+ T cells (Fig. 4c). Flow cytometry analysis revealed increased numbers of epidermotropic CD4+ T cells (Fig. 4d), the majority of which expressed Myc-GFP (Fig. 4e) with increased IL-7Rα expression (Fig. 4e).
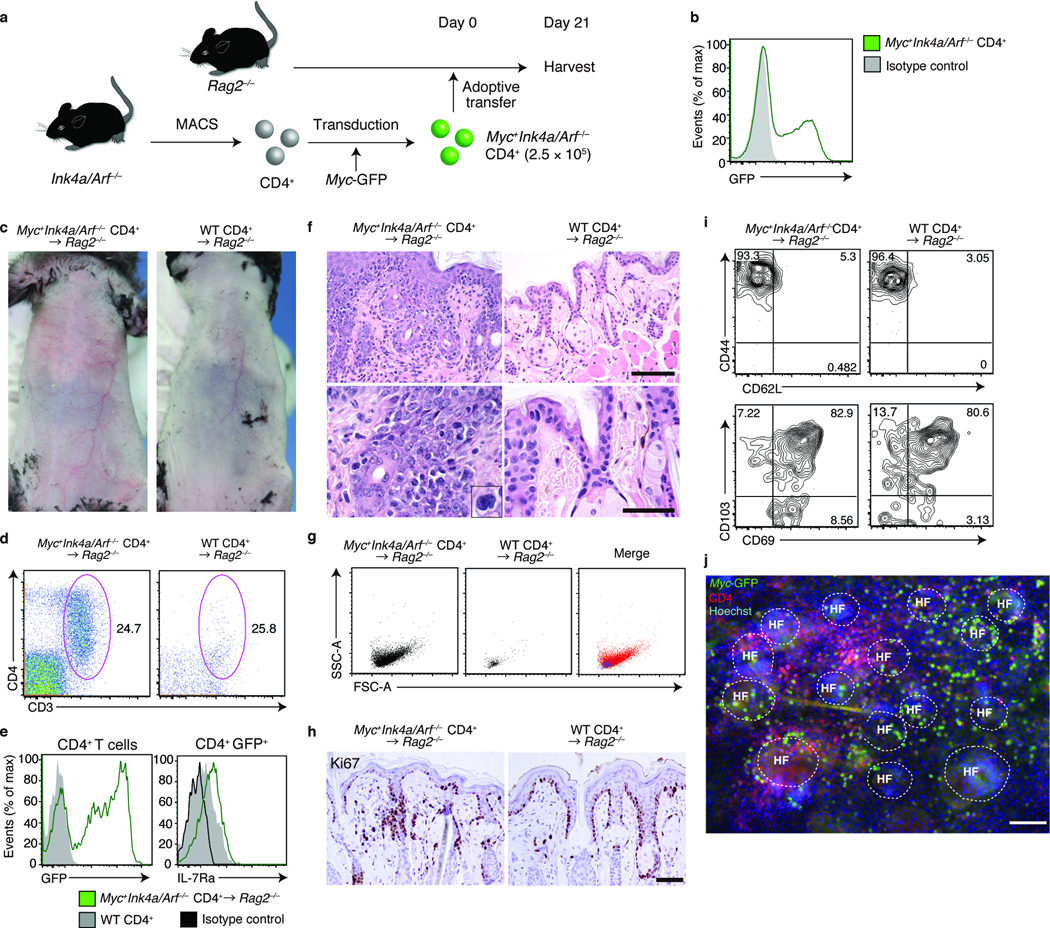
Figure 4. Generation of a model of T cell lymphoma with skin involvement.
(a) Experimental scheme for a CD4+ T cell lymphoma model with skin involvement. CD4+ T cells isolated from Ink4a/Arf−/− mice were retrovirally transduced with Myc-GFP (Myc+Ink4a/Arf−/− CD4+) and were adoptively transferred into Rag2−/− mice. (b) Flow cytometry analysis of Myc-GFP expression on Myc+Ink4a/Arf−/− CD4+ T cells before transfer. (c) Skin phenotype of Myc+Ink4a/Arf−/− CD4+-transferred (left panel) or WT CD4+-transferred Rag2−/− mice mice (right panel) (n = 3). (d) Flow cytometry analysis of CD3+CD4+ epidermotropic cells in indicated mice, for (e) expression of GFP and IL-7Rα, (g) forward and side scatter profiles, (i) CD44 versus CD62L and CD103 versus CD69 expression. Gated on CD45+MHCll−TCRγδ− (d,g), CD45+MHCll−TCRγδ− CD3+CD4+ (left panel in e, i and j) or CD45+MHCll−TCRγδ− CD3+CD4+GFP+ populations (right panel in e) (n = 3). (f) Hematoxylin and eosin staining of lip sections from indicated mice and (h) immunohistochemical staining for Ki-67 (n = 3). (j) Immunofluorescence microscopy of epidermal sheets, visualized for Myc-GFP, CD4 and Hoechst (n = 3). Scale bar, 100 µm. Data are from one experiment representative of three independent experiments with three mice per group in each.
Histology revealed epidermotropism of lymphocytes with large, atypical nuclei, recapitulating that of human CTCL (Fig. 4f)17. Consistently, flow cytometry analysis revealed that Myc+Ink4a/Arf−/− CD4+ T cells were enlarged in size (Fig. 4g). Infiltrating lymphocytes expressed Ki-67, demonstrating that they were proliferative (Fig. 4h). Epidermotropic CD4+ T cells were of the TRM phenotype (Fig. 4i) and accumulated around the hair follicles (Fig. 4f,j). Collectively, Myc+Ink4a/Arf−/− CD4+ T cells transferred into Rag2−/− mice infiltrated skin and epidermis exhibiting histologic features of human CTCL. These mice also exhibited lymphoma in the lymph nodes and spleen, and died within 10 weeks after transfer (Supplementary Fig. 4), thereby also recapitulating an aspect of Sezary’s syndrome, a leukemic subtype of CTCL30.
Epidermotropism in CTCL depends on IL-7
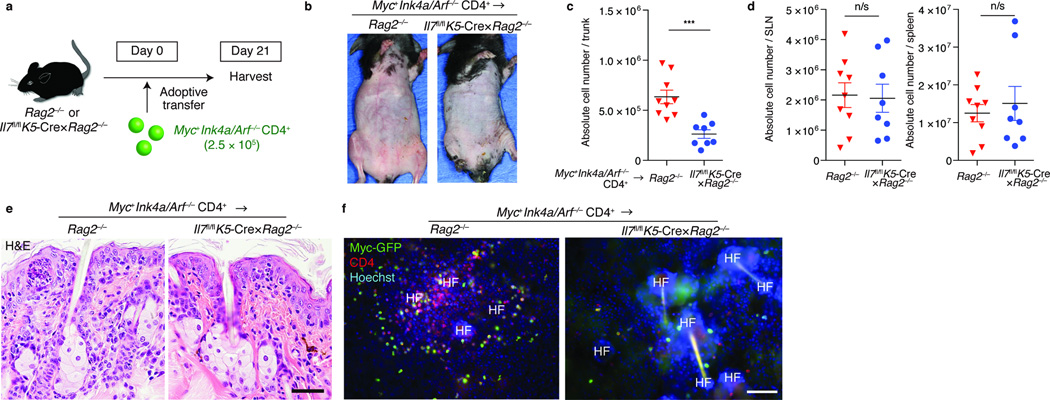
To determine whether CD4+ lymphoma cells also relied on hair follicle-derived IL-7, we transferred Myc+Ink4a/Arf−/− CD4+ T cells into Rag2−/− or Il7fl/flK5-Cre×Rag2−/− mice (Fig. 5a). In contrast to Rag2−/− mice, Il7fl/flK5-Cre×Rag2−/− mice did not develop erythroderma (Fig. 5b). Furthermore, the absolute numbers of epidermotropic lymphoma cells were reduced in the absence of hair follicle-derived IL-7 (Fig. 5c), whereas significant differences were not observed in the spleen and lymph nodes (Fig. 5d). Histologic analysis of control Rag2−/− mice revealed abundant epidermotropic lymphocytes with epidermal thickening, but histological changes were minimal in Il7fl/flK5-Cre×Rag2−/− mice (Fig. 5e). Visualization of CD4+ T cells in epidermal sheets confirmed these findings (Fig. 5f). Thus, epidermotropic CD4+ TRM require continued hair follicle-derived IL-7 after neoplastic transformation.
Figure 5. CD4+ TRM lymphoma cells depend on hair follicle-derived IL-7 to exhibit epidermotropism.
(a) Experimental scheme. Myc+Ink4a/Arf−/− CD4+ T cells were adoptively transferred into Rag2−/− or Il7fl/flK5-Cre×Rag2−/− mice. Recipient mice were harvested approximately three weeks after transfer. (b) Skin phenotype of recipient mice of indicated genotype (n = 3). Quantification of (c) epidermotropic TRM or (d) T cells in skin-draining LNs (SLN) and spleen, assessed at day 21 after adoptive transfer (n = 8–9). ***P < 0.001 (unpaired two-tailed Student’s t-test). (e) Histology of skin sections from indicated mice, stained for hematoxylin and eosin, and (f) immunofluorescence microscopy of ear epidermal sheets of indicated mice, visualized for Myc-GFP, CD4 and Hoechst, assessed day 21 after adoptive transfer (n = 3). Scale bar, 100 µm. Data are from one experiment representative of three independent experiments with two to three mice per group in each (b,e,f) or an accumulation of three independent experiments (c,d).
In CTCL, IL-7 is upregulated in human hair follicles
To examine if our findings could be extended to humans, we studied human hair follicles. Whereas hair follicles in the trunk skin of mice and humans are mostly in telogen, the majority of those in human scalp are in anagen. The terminus end is called the bulb, and the portion between the bulb and the stem cell-containing bulge is referred to as the suprabulb (Fig. 6a and Supplementary Fig. 5). We dissected hair follicles from normal human scalp into interfollicular epidermis, infundibulum, bulge, suprabulb and bulb, and obtained RNA from each of these sites (Fig. 6a)4 and performed real-time PCR for IL15 and IL7. IL15 was predominantly expressed by hair follicle keratinocytes in the suprabulb, and IL7 expression was highest in the infundibulum and suprabulb (Fig. 6b).
Figure 6. IL-7 and IL-15 expression in human hair follicles from normal scalp and cutaneous T cell lymphoma.
(a) Schematic representation of human hair follicle keratinocytes. (b) Real-time PCR analysis for IL15 and IL7 expression at distinct sites in the hair follicle. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired two-tailed Student’s t-test). (c) Immunohistochemistry of scalp sections from normal humans (NHS) (n = 3 samples from three subjects) and from subjects with CTCL (n = 6 samples from 3 subjects), stained with an anti-IL-7 antibody or with an isotype control. Scale bar, 100 µm. Arrowheads indicate arrector pili muscle (reflecting the bulge region). (d) Immunohistochemistry of scalp sections from patients with CTCL, stained with an anti-IL-7R antibody or with an isotype control (n = 6 samples from three subjects). Scale bar, 100µm in low power field, 50 µm in high power field. Dashed lines delineate hair follicles. Arrowheads indicate IL-7R-positive lymphoma cells. Data are from one experiment representative of three (b,c) or two (d) independent experiments.
We additionally evaluated IL-7 expression in hair follicles from normal human scalp and lesional scalp skin of patients with CTCL. Faint staining for IL-7 was detected in normal human hair follicles via immunohistochemistry. IL-7 expression was apparent in a CTCL subject, in which the staining was detected in the infundibulum and the suprabulbar area (Fig. 6c), consistent with the real-time PCR data from normal human scalp. In other patients, IL-7 staining was increased not only in hair follicle keratinocytes, but also in keratinocytes in the interfollicular epidermis (Supplementary Fig. 6).
Consistent with our observations in mice, IL-7R expression by T cells was increased in lesional CTCL epidermis in comparison to that on T cells in normal human scalp skin (Fig. 6d and Supplementary Fig. 5). IL-7R expression in lesional keratinocytes in CTCL appears to be increased, although the significance of this has yet to be determined.
Discussion
Epidermotropism of TRM, both non-malignant and malignant, is supported by the hair follicle-derived cytokines IL-7 and IL-15. Previous studies have focused on TRM biology in the context of viral infections. We studied the requirement of epidermotropic TRM during steady state by utilizing non-malignant and malignant models. The lack of hair follicle-derived cytokines led to the failure of both CD4+ and CD8+ TRM to persist in skin. Impaired CHS responses in the absence of hair follicle-derived cytokines highlight the importance of homeostatic organization of TRM prior to inflammation.
The requirement of IL-7 and IL-15 in the generation and maintenance of memory T cells is well-established24,25, but the fate of TRM in peripheral tissues that are deprived of these cytokines remains unclear. In particular, whether the cells undergo cell death or migration deserves further attention in the context of lymphoma.
The tropism that CD4+ TRM exhibit to the hair follicles demonstrated in this study is compatible and complementary to a previous report in which approximately 30% of virus-specific CD4+ memory T cells were found to associate with the hair follicles after herpes simplex virus skin infection7. To date, TRM in the skin has mainly been characterized in the context of CD8+ T cells. Although CD4+ memory T cells may well be comparable to CD8+ TRM as skin residents based on surface marker expression and past reports13,22, thorough studies on CD4+ memory T cells as bona fide TRM has yet to be performed. Our observation that epidermal and dermal TRM have subtle differences in CD103 expression and the higher ratio of CD4+ FoxP3+ TRM in the dermis suggests that CD4+ TRM in these skin compartments might be regulated via distinct mechanisms.
In conclusion, we have demonstrated the importance of hair follicle-derived IL-7 and IL-15 in TRM homeostasis in the epidermis which represent attractive therapeutic targets in inflammatory skin diseases and malignant lymphoma, the concept of which may also be relevant in other peripheral tissues.
ONLINE METHODS
Mice
C57 BL/6J (CD45.2) mice and Il15−/− mice were purchased from CLEA Japan. C57 BL/6J Rag2−/− mice were purchased from the Central Institute for Experimental Animals (Tokyo, Japan). K14-CreERT mice were purchased from Jackson Laboratory (ME, U.S.A.)26. Ink4a/Arf−/− mice (B6.129-Cdkn2atm1Rdp) were from Mouse Models of Human Cancers Consortium (NCI-Frederick)33. Il7-floxed mice, K5-Cre mice (kindly provided by Junji Takeda, Osaka University), Langerin-DTA mice and Langerin-DTR mice (kindly provided by Daniel H. Kaplan, University of Minnesota and Björn E. Clausen, Johannes Gutenberg University of Mainz, respectively) were generated as previously described27,35–37. Only female mice 6 to 12 weeks of age were used for experiments. All mice were bred and housed in a specific pathogen-free condition. All animal procedures and study protocols were approved by the Keio University Ethics Committee for Animal Experiments.
Human tissue
All samples for this study were obtained according to protocols approved by the Institutional Review Board of Keio University (protocol number 2003-0057) or NCI, NIH (protocol number 96-C-0102). Informed consent was obtained from all subjects prior to the acquisition of the skin tissues. Two sets of normal human scalp samples for gene analysis and formalin-fixed scalp samples for histopathological examination were obtained from excess normal skin that resulted from surgical removal of benign, subcutaneous skin tumors. Formalin-fixed scalp samples from subjects with CTCL were biopsy specimen obtained from lesional skin for diagnosis.
Preparation of epidermal sheets
Epidermal sheets were prepared as before4 with slight modification. Briefly, ears were split into dorsal and ventral halves with forceps. Thioglycolic acid-containing Hair Removing Body Cream Epilat (Kracie) was used to remove hair in some experiments. Ear halves were incubated for 15 minutes at 37°C on 3.8% ammonium thiocyanate (Wako Pure Chemical Industries) in phosphate buffer (pH 7.0). Epidermal sheets were manually detached from the dermis under a dissecting microscope (Olympus).
Preparation of epidermal and dermal cell suspensions
Epidermal and dermal cell suspensions were prepared as before4 with slight modification. Briefly, shaved whole trunk skin was harvested from appropriate animals and, after removal of subcutaneous tissues with forceps, was floated with epidermal side up onto 10 mL of Trypsin-EDTA solution containing 5 mL of 0.25% Trypsin (Nacalai tesque) and 5 mL of 0.05% Trypsin-0.53 mM EDTA • 4Na (Nacalai tesque) at 37°C for less than 30 minutes in 10 cm dish. Epidermis and dermis were separated manually with forceps. Dermis was cut into small chips manually with scissors and further incubated in 4 mL of RPMI containing 0.03% Liberase TL Research grade (Roche Applied Science) and 200 U/mL of DNase (Wako Pure Chemical Industries) for 60 minutes at 37°C shaking with rotation at 200 rpm (Bioshaker, Taitec). Epidermal and dermal cells were then suspended in 5% FCS in PBS, washed and filtered through Cell strainer (BD Falcon).
Antibodies for immunofluorescence microscopy and flow cytometry analysis for mouse samples
Anti-mouse CD4 monoclonal antibody (clone GK1.5, BioLegend) was used in conjugated forms pre-labeled with PE/Cy7 or APC or labeled in house with Alexa Fluor 568 (Invitrogen). Anti-mouse CD8 monoclonal antibody (clone 53.6.7, BioLegend) was used in conjugated forms pre-labeled with FITC, APC, APC/Cy7 or AmCyan or labeled in house with Alexa Fluor 647 (Invitrogen). The following pre-labeled monoclonal antibodies and polyclonal antibodies, all obtained from BioLegend unless otherwise stated, were used for immunofluorescence microspcopy and flow cytometry: CD3ε (clone 145-2c11), TCRγδ (clone GL3), MHC II (anti-IA/IE; clone M5/114.15.2), CD34 (clone RAM34, eBioscience), CD44 (clone IMF7), CD45 (clone 30F-11), CD49f (anti-integrinα6; clone GoH3), CD62L (clone MEL-14), CD69 (clone H1.2F3), CD103 (clone 2E7), CD122 (anti-IL-15/2Rβ; clone 5H4), CD127 (anti-IL-7Rα; clone SB/199), EpCAM (clone G8.8), Ly-6A/E (anti-Sca-1; clone E13-161.7), Antibodies that were labeled in-house were stored at 0.5mg/ml concentration. All primary antibodies for flow cytometry were diluted 1:200 with the exception of clone G8.8, which was used at 1:800 dilution. For immunofluorescence microscopy, primary antibodies were used at 1:100 dilution.
Primary antibodies were detected, if needed, with Alexa Fluor labeled secondary antibodies (anti-Fluorescein/Oregon Green, A11096 or anti-Green Fluorescent Protein, A21311; both from Life Technologies) at 1:200 dilution. Anti-mouse CD16/32 (clone 93, BioLegend) was routinely used (1:100 dilution) to block Fcγ receptors prior to staining.
Immunofluorescence microscopy
Staining of epidermal sheets and frozen skin sections was performed as before4 with slight modification. Briefly, sheets and sections were fixed in acetone for 5 minutes at −20°C or in 4% paraformaldehyde (Wako Pure Chemical Industries) in PBS for 15 minutes at room temperature, and were rehydrated or washed in PBS for 5 minutes. They were blocked in 3% dry milk (Morinaga) in PBS with 5% goat serum for at least an hour at room temperature. For intracellular staining, 0.2% Triton X-100 (Sigma-Aldrich) was added in blocking buffer. Primary antibodies were diluted in blocking buffer and incubated overnight at 4°C. After washing, primary antibodies were detected with appropriate secondary antibodies and nuclei were visualized with Hoechst 33258 (Invitrogen). Mouse lips were routinely used for vertical sections because this provided wide view of vertical and horizontal sections of both pelage hair and vibrissae. Images were mostly observed with Zeiss Axio Observer. Z1 with or without Apotome (Carl Zeiss), collected with the Axiovision software (ver. 4.8). Adjustments of levels, if needed, were performed on Photoshop CS 5.1 (Adobe), where controls were also treated identically.
Flow cytometry analysis and cell sorting
Data were collected utilizing FACS Canto II (BD Biosciences) and analyzed with Flow Jo (Tree star). Non-viable cells were omitted using propidium iodide (Sigma-Aldrich) staining or Live/Dead cell (Invitrogen) pre-staining for fixed and permeabilized cells before primary antibody staining. Fluorescence activated cell sorting of hair follicle keratinocyte subsets was performed with FACSAria II (BD Biosciences) or MoFlow (Beckman Coulter), during which cells were directly sorted into TRIzol LS (Invitrogen) and divided into five subsets based on the expression of cell-surface markers as described before4; interfollicular epidermis (MHCII−CD45−Sca-1+ EpCAMlow), infundibulum (MHCII−CD45−Sca-1+ EpCAMint), isthmus (MHCII−CD45−Sca-1−EpCAMhi), basal bulge (MHCII−CD45−Sca-1−CD34+ integrin α6+) or suprabasal layer bulge (MHCII−CD45−Sca-1−CD34+ integrin α6−). Sorted cells were further processed for RNA extraction.
Isolation of human hair follicles for gene analysis
Samples taken from human scalp were dissected as previously described4. Briefly, scalp samples were dissected into five anatomical areas (interfollicular epidermis, infundibulum, bulge, suprabulb and bulb) under a dissecting microscope, and were then incubated overnight at 4°C with 1,500 U/mL of Dispase II (Godo Syusei) in DMEM to remove non-epidermal components.
Real-time PCR
Cytokine expressions of hair follicle keratinocyte subsets were analyzed as previously described4. Briefly, total mRNA was purified from FACS-sorted epidermal keratinocyte population, using an RNeasy Micro Kit (QIAGEN). cDNA was synthesized using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen), and then real-time PCR analysis was performed using Power SYBR Green PCR Master Mix (Applied Biosystems) and StepOne Real-Time PCR system (Applied Biosystems), according to the manufacturer’s protocol. All primers (Supplementary Table 1) were designed using Primer Express software (Applied Biosystems) and reactions were conducted under the following cycling conditions: 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. Normalization of mRNA expression was performed based on the expression of β-Actin utilizing cycling threshold (ΔCT) method and the amount of PCR product was calculated based on 2−ΔCT (ref. 38). Results were presented as ± standard deviations.
TRM repopulation model
Spleens from 8-week-old C57BL/6J mice were harvested and homogenized on sterile silicone meshes and were then filtered through Cell Strainer (BD Falcon). Cells were washed in 5% FCS in PBS, centrifuged, and resuspended in PBS to obtain whole splenocyte single cell suspension. 5 × 106 splenocytes in 200 µl PBS were adoptively transferred i.v. into 6 to 10-week-old lymphopenic recipients.
Generation of bone marrow chimeras
C57BL/6J WT or Il15−/− mice were lethally irradiated (950 rad) and were transferred i.v. with 2 × 106 total bone marrow cells from Rag2−/− mice on the following day to generate lymphopenic bone marrow chimeric mice (WTRag or Il15 KORag), which were then used as recipients in TRM repopulation experiments.
FTY720 and tamoxifen treatment
Il7fl/flK14-CreERT or Il7fl/wtK14-CreERT mice were injected i.p. with 1 mg of tamoxifen (Cayman) in 100 µL of sunflower oil (Nacalai tesque)/day for 5 consecutive days (day 0 to 4)39. 30 µg of FTY720 (Cayman) was injected i.p. from day -1 for 7 consecutive days8 and every other day thereafter, until tissues were harvested.
FTY720 and diphtheria toxin (DT) treatment
For in vivo depletion of LCs, Langerin-DTR mice (or control WT mice) were injected i.p. with 500 ng of DT40 (Sigma, USA) in 200 µL of sterile PBS at days 0 and 7. 30 µg of FTY720 (Cayman) was injected i.p. from day -1 for 7 consecutive days and every other day thereafter, until tissues were harvested.
Hapten-induced contact hypersensitivity
WT donor mice were sensitized as described41 with slight modification. Briefly, 60 µL of 0.5% 1-fluoro-2,4-dinitrobenzene (DNFB) in vehicle (olive oil: acetone = 1:4) or vehicle alone was topically applied to the shaved-trunk skin, ears and paws of WT mice. 5 days after sensitization, 1 × 106 CD4+ or CD8+ T cells, which were purified via MACS cell separation system (Miltenyi Biotec) from skin-draining lymph nodes, were adoptively transferred into lymphopenic recipients. 2 weeks after transfer, the ears were challenged with 10 µL of 0.3% DNFB. Ear swelling, (ear thickness on day x) − (basal ear thickness on day 0), was measured in a blinded fashion by an investigator that was not involved in the study.
T cell lymphoma model with skin involvement
Mouse c-Myc was cloned into the retroviral vector pMXs-IG, which was transfected and transduced as described with slight modification34,42. Briefly, the pMX-based vector (Myc-GFP) was transfected into a packaging cell line, Plat-E43, using FuGENE HD Transfection Reagent (Roche Applied Science) and then the viral supernatant was collected after 48 hours of incubation. CD4+ T cells, which were collected and enriched from spleen and skin-draining lymph nodes of Ink4a/Arf−/− mice by positive selection utilizing the MACS cell separation system, were cultured in complete medium (RPMI 1640 containing 10% FCS, 2 mM L-glutamine, 1 mM pyruvate, 50 U/mL penicillin, 50 µg/mL streptomycin, 0.05 mM 2-ME) supplemented with 100 U/mL hIL-2 and 2.5µg/mL Con A at 37°C for 24 hours. Flow through cells that were collected after CD4+ T cell enrichment were irradiated (3,000 rad) and used as feeder cells for co-culture with CD4+ T cells. The activated CD4+ T cells were then resuspended in retroviral supernatant and centrifuged (3,000 rpm, 1 hour, 30°C). After incubation at 37°C for four hours, the retroviral supernatant was removed, and the cells were further cultured in complete medium supplemented with 100 U/mL hIL-2 at 37°C for 44 hours. The whole bulk of cultured T cells were transferred i.v. into Rag2−/− or Il7fl/flK5-Cre×Rag2−/− mice. To avoid inter-experimental variability due to subtle differences in transduction efficiency, the number of transferred cells was normalized to contain 2.5 × 105 Myc-GFP+ CD4+ cells. Equivalent numbers of CD4+ T cells from WT splenocytes were transferred i.v. into Rag2−/− mice. To address the contribution of hair follicle-derived IL-7, the bulk of transduced T cells, numbers normalized as above, were transferred into Rag2−/− or Il7fl/flK5-Cre×Rag2−/− mice.
Preparation of transverse sections
4-mm punch biopsy specimens were sliced at four levels44; just beneath the epidermis (infundibulum), sebaceous glands attachment (bulge), between sebaceous glands attachment and bulb (suprabulb), and bulb. Tissues were sectioned and a single slide containing 5µm-disks from four different layers were analyzed for histology or immunohistochemistry (Supplementary Fig. 4).
Histopathological and immunohistochemical analysis of human and mouse skin sections
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at a thickness of 5 µm. Sections were paraffin-depleted and rehydrated in a graded series of ethanol solutions. For histology, sections were stained with hematoxylin and eosin. For immunohistochemistry staining for human IL-7 expression, sections were washed with PBS and treated with 3% H2O2, before incubation with the following primary antibody: rabbit anti-IL-7 polyclonal antibody (clone sc-7921, Santa Cruz Biotechnology) (1:100). For IL-7R expression, sections were autoclaved at 120°C for 1 minute in 5 mM EDTA buffer (pH 8.0) and were allowed to cool at room temperature before incubation with the following primary antibody: rabbit anti-IL-7R polyclonal antibody (clone sc-25475, Santa Cruz Biotechnology) (1:100). Immune complexes were detected by using the ImmPRESS REAGENT KIT (Vector Laboratories) with 3, 3’-diaminobenzidine, and sections were counterstained with hematoxylin. For immunohistochemistry of mouse samples for Ki-67 expression, sections were subjected to 20 minutes microwave treatment in citrate buffer (pH 7.0) and were allowed to cool at room temperature. Non-specific binding was blocked in 3% dry milk PBS with 5% goat serum for 1 hour at room temperature, before incubation with the following primary antibody: rabbit anti-Ki67 monoclonal antibody (clone SP6, Thermo Scientific Lab Vision) (1:100), and then washed, and bound antibodies were detected with Histofine Simple Stain MAX PO (Nichirei Corporation). Secondary antibodies were further visualized with ImmPACT DAB (Vector Laboratories), and sections were counterstained with hematoxylin. Images were collected via an inverted microscope (BX41, Olympus), equipped with a digital camera (DP20, Olympus).
Statistics
Statistical significance was calculated with an unpaired two-tailed Student’s t test or a two-way ANOVA using GraphPad Prism 6 (GraphPad software). The values presented are expressed as the means +/− standard error of the mean (s.e.m.). Variances were similar between groups in all experiments as determined by the F test using GraphPad Prism 6. For all statistical analyses, data were considered significant when P ≤ 0.05 (*), P ≤ 0.01 (**) or P ≤ 0.001 (***). The animal experiments were not randomized. The investigators were not blinded to allocation during experiments and analyses unless otherwise indicated.
Supplementary Material
ACKNOWLEDGMENTS
We thank Manabu Ohyama for helpful discussion on human hair follicles, N. Sakai, K. Eguchi, and S. Sato for assistance (Department of Dermatology, Keio University School of Medicine), Heidi H. Kong (Dermatology Branch, CCR, NCI, NIH) on human CTCL, Y. Madokoro (Department of Pathology, Keio University School of Medicine) for human CTCL immunohistochemical staining, T. Kitamura (The University of Tokyo) for providing the retroviral vector pMXs-IG and Plat-E cells. This work was supported by the Japan Society for the Promotion of Science, The Kanae Foundation for the Promotion of Medical Science, JSID’s Fellowship Shiseido Award, and the U.S. National Institutes of Health (NIH) NCI Intramural Research Programs.
Footnotes
AUTHOR CONTRIBUTIONS
T.A. and K.N. conceived and designed all experiments. Experiments were performed by T.A. with the assistance of T.K.; E.S. and H.S. provided Ink4a/Arf−/− mice and assisted with retroviral transduction; T.Y. assisted with immunohistochemical staining; K.I. provided Il7-floxed mice; S.P. provided human CTCL samples; M.A discussed data and provided administrative support; K.N. guided the project and T.A. and K.N. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura EK, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 3.Christoph T, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagao K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 10.Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 11.Shiow LR, et al. CD69 acts downstream of interferon-[alpha]/[beta] to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 12.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating Memory T Cells Are a Unique Subset of CD4+ T Cells with a Distinct Phenotype and Migratory Pattern. J Immunol. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suffia I, Reckling SK, Salay G, Belkaid Y. A Role for CD103 in the Retention of CD4+CD25+ Treg and Control of Leishmania major Infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez Rodriguez R, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiohara T, Moriya N. Epidermal T cells: their functional role and disease relevance for dermatologists. J Invest Dermatol. 1997;109:271–275. doi: 10.1111/1523-1747.ep12335465. [DOI] [PubMed] [Google Scholar]
- 16.Mizukawa Y, et al. Direct evidence for interferon-gamma production by effector-memory-type intraepidermal T cells residing at an effector site of immunopathology in fixed drug eruption. Am J Pathol. 2002;161:1337–1347. doi: 10.1016/s0002-9440(10)64410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sezary syndrome. Lancet. 2008;371:945–957. doi: 10.1016/S0140-6736(08)60420-1. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767–771. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark RA, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelson RL. Cutaneous T cell lymphoma: mycosis fungoides, Sezary syndrome, and other variants. J Am Acad Dermatol. 1980;2:89–106. doi: 10.1016/s0190-9622(80)80385-9. [DOI] [PubMed] [Google Scholar]
- 21.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez Rodriguez R, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariotti S, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 24.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr Opin Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang B, et al. Role of hepatocyte-derived IL-7 in maintenance of intrahepatic NKT cells and T cells and development of B cells in fetal liver. J Immunol. 2012;189:4444–4450. doi: 10.4049/jimmunol.1201181. [DOI] [PubMed] [Google Scholar]
- 28.Dorfman DM, Shahsafaei A. CD69 expression correlates with expression of other markers of Th1 T cell differentiation in peripheral T cell lymphomas. Hum Pathol. 2002;33:330–334. doi: 10.1053/hupa.2002.32215. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka K, et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;107:2440–2445. doi: 10.1182/blood-2005-03-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinet B, Salgado R. Mycosis fungoides and Sezary syndrome. Methods Mol Biol. 2013;973:175–188. doi: 10.1007/978-1-62703-281-0_11. [DOI] [PubMed] [Google Scholar]
- 31.Kanavaros P, et al. Mycosis fungoides: expression of C-myc p62 p53, bcl-2 and PCNA proteins and absence of association with Epstein-Barr virus. Pathol Res Pract. 1994;190:767–774. doi: 10.1016/S0344-0338(11)80423-1. [DOI] [PubMed] [Google Scholar]
- 32.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer Res. 1999;59:2217–2222. [PubMed] [Google Scholar]
- 33.Serrano M, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 34.Sugihara E, et al. Ink4a and Arf are crucial factors in the determination of the cell of origin and the therapeutic sensitivity of Myc-induced mouse lymphoid tumor. Oncogene. 2012;31:2849–2861. doi: 10.1038/onc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 35.Tarutani M, et al. Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc Natl Acad Sci U S A. 1997;94:7400–7405. doi: 10.1073/pnas.94.14.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KK, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Li M, et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 40.Ouchi T, et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J Exp Med. 2011;208:2607–2613. doi: 10.1084/jem.20111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fyhrquist N, Wolff H, Lauerma A, Alenius H. CD8+ T cell migration to the skin requires CD4+ help in a murine model of contact hypersensitivity. PLoS One. 2012;7:e41038. doi: 10.1371/journal.pone.0041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi H, et al. Desmoglein 3-specific CD4+ T cells induce pemphigus vulgaris and interface dermatitis in mice. J Clin Invest. 2011;121:3677–3688. doi: 10.1172/JCI57379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 44.Evaluating and Describing Transverse (Horizontal) Sections. An Atlas of Hair Pathology with Clinical Correlations. :17–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.