Abstract
Background
Individuals with functional gastrointestinal disorders (FGIDs) report experiencing trauma more often than healthy controls, but little is known regarding psychophysical correlates.
Purpose
Test the hypothesis that adolescents and young adults with FGIDs since childhood and a trauma history (n = 38) would exhibit heightened temporal summation to thermal pain stimuli, an index of central sensitization, and greater clinical symptoms compared to patients with FGIDs and no trauma history (n = 95) and healthy controls (n =135).
Methods
Participants completed self-report measures, an experimental pain protocol, and psychiatric diagnostic interview as part of a larger longitudinal study.
Results
FGID+Trauma patients exhibited greater temporal summation than FGID+No Trauma patients and healthy controls. Additionally, FGID+Trauma patients exhibited greater gastrointestinal and non-gastrointestinal symptom severity, number of chronic pain sites, and disability.
Conclusions
Assessing for trauma history in patients with FGIDs could identify a subset at risk for greater central sensitization and pain-related symptoms.
Keywords: central sensitization, functional abdominal pain, chronic pain, trauma, temporal summation
Individuals with functional gastrointestinal disorders (FGIDs) report having experienced a psychological trauma (e.g., sexual and/or physical abuse or assault) more often than patients with organic gastrointestinal diseases or healthy individuals (1-3). A variety of psychosocial and biological mechanisms have been proposed to account for high rates of FGIDs in individuals exposed to interpersonal trauma. Traumatic events may increase risk for FGIDs by altering appraisals of bodily symptoms (e.g., catastrophizing), enhancing reliance on maladaptive coping styles (e.g., avoidance), or by triggering the onset of psychiatric conditions known to impact pain sensitivity, such as posttraumatic stress disorder (PTSD) or major depressive disorder (4). Individuals exposed to trauma also tend to exhibit heightened autonomic and hypothalamic-pituitary-adrenal (HPA) responses to physical and psychological stressors. This elevated stress reactivity could amplify distress and contribute to greater functional disability in the context of GI symptoms (5). Finally, the experience of psychological trauma may impact risk for FGIDs by altering corticolimbic pain modulation (6) as well as visceral and central sensitization (7).
Heightened central sensitization, characterized by increased responsiveness to painful stimuli resulting from alterations within the central nervous system (CNS), has been observed in patients with FGIDs (8) and represents one possible mechanism linking FGIDs and trauma. Central sensitization can be indexed through a pattern of increased perceived pain intensity in response to repetitive stimulation administered at a constant intensity, termed temporal summation (9). While evidence for enhanced temporal summation to evoked pain has been found in FGID and other chronic pain populations (10, 11), the relation of trauma to temporal summation of pain stimuli is unknown in individuals with FGIDs.
Past work on associations between trauma exposure and laboratory pain responses is inconsistent. Trauma-exposed individuals with chronic pain tend to exhibit enhanced evoked pain sensitivity to laboratory pain relative to similar individuals without trauma exposure (12-14). However, in populations without chronic pain, trauma-exposed individuals generally exhibit diminished pain sensitivity compared to those without trauma exposure (15, 16). Existing laboratory studies have largely been restricted to pain tolerance and threshold outcomes, which are influenced by a combination of the level of nociceptive sensory input, descending inhibition, and ascending facilitation (i.e. central sensitization)(17). Assessment of temporal summation may provide a somewhat more specific index of central sensitization (11).
A Medline search reveals only two published studies that have specifically assessed temporal summation in trauma-exposed individuals. Both studies were of individuals without chronic pain (18, 19). Fillingim and Edwards (19) found adults with a history of childhood abuse showed less temporal summation compared to adults with no prior abuse, a pattern similar to other laboratory evoked pain findings of individuals with a trauma history in the absence of chronic pain (15, 16). Moeller-Bertram et al.(18) attempted to isolate the effects of PTSD on pain sensitivity by comparing temporal summation in a veteran population with PTSD and combat exposed controls. Veterans with PTSD exhibited significantly increased temporal summation compared to combat controls, consistent with a model in which trauma-related central sensitization might enhance vulnerability to development of chronic pain disorders. However, neither of these studies allowed for the examination of relations between trauma-exposure and temporal summation in the context of clinically significant chronic pain, such as that in pain-related FGIDs.
The present study extends the literature by assessing temporal summation (to thermal pain stimuli), clinical correlates, and trauma history (i.e. history of physical or sexual abuse/assault) in adolescents and young adults with pain-related FGIDs who have experienced functional abdominal pain since childhood. We hypothesized that FGID patients with a history of trauma would exhibit heightened temporal summation of pain compared to FGID patients with no trauma history and healthy controls. Further, we hypothesized that FGID patients with a history of trauma would exhibit greater symptoms and impairment--possible clinical indicators of central sensitization--compared to FGID patients with no trauma history and healthy controls.
Methods
Participants
FGID patients
This study was part of a larger prospective study of long-term health outcomes of consecutive new patients in a pediatric gastroenterology clinic evaluated for functional abdominal pain (duration ≥ three months) when they were between the ages of 8 and 17(20, 21). Baseline characteristics of the patients are reported elsewhere (22-24). Participants were eligible for the current study if, at the time of follow-up, they met the Rome III criteria for a pain-related FGID (25). Additional eligibility criteria included: at least four years elapsed since initial study enrollment, no evidence of significant organic disease found in the initial medical evaluation for functional abdominal pain, no significant chronic disease (e.g., multiple sclerosis, celiac disease, inflammatory bowel disease) at follow-up, and completion of a psychiatric diagnostic interview at follow-up. The FGID sample was comprised of 133 patients assessed an average of 8.68 years (SD = 3.36) after initial evaluation (72.2% female; 94.7% Caucasian; mean age = 20.5 years).
Control participants
Control participants in this study had participated in control groups drawn from local schools for the original studies when they were between the ages of 8 and 16 years (20, 21). To be eligible for the original studies, participants had to have no chronic illness and no abdominal pain in the month preceding initial study participation. For the current study, eligibility criteria included: no current FGID diagnosis, at least four years elapsed since initial study enrollment, and no other significant chronic disease. Control participants who reported a history of trauma during the psychiatric interview were excluded from primary analyses of this study due to the small sample size of this subgroup (n = 11, 9 of whom completed experimental pain testing). The final Control sample was comprised of 135 participants assessed an average of 7.46 years (SD = 2.35) after initial evaluation (51.1% female; 96.2% Caucasian; mean age = 18.42 years).
Procedure
Participants answered questions about their health, current chronic pain symptoms, and FGID status during a telephone interview. They were then invited to participate in the experimental portion of the study during which they completed thermal evoked pain testing and a psychiatric diagnostic interview. Participants unable to attend the experimental portion completed the psychiatric diagnostic interview over the phone; these participants were included in analyses of trauma prevalence and clinical characteristics, but were excluded from analyses involving experimental pain. Procedures of the study were approved by the Vanderbilt University Institutional Review Board and all participants gave informed consent or assent prior to participation in the study.
Measures
The Rome III Diagnostic Questionnaire (25) is a self-report measure used to identify individuals who meet the Rome III symptom criteria for FGIDs. For the current study, only the 24-items assessing symptoms associated with abdominal pain-related FGIDs (irritable bowel syndrome, functional dyspepsia, abdominal migraine, and functional abdominal pain) were administered.
The Anxiety Disorders Interview Schedule-IV (ADIS)
Adult Lifetime and Child and Parent Versions (26, 27) is a semi-structured interview designed to assign DSM-IV psychiatric diagnoses. The ADIS was used in this study to assess for anxiety and depressive disorders, as well as a history of sexual or physical trauma. The adult version was used for participants ages 18 and older. The child version incorporates both parent- and child-report into a combined score.
Coding of Trauma
Exposure to physical and sexual assault/abuse was determined by the posttraumatic stress disorder module of the ADIS. Participants described each traumatic event and indicated the age at which it occurred. A research assistant listened to audio-recordings of each clinical interview, and coded each incident as sexual or physical trauma in accordance with the criteria proposed by Leserman and colleagues (28, 29). Traumatic events were defined as sexual in nature if they included (a) unwanted touching of the subject’s sexual organs with hands, mouth, or objects, (b) having the subject touch the perpetrator’s sexual organs or anus with hands, mouth, or objects, or (c) having the subject participate in vaginal or anal intercourse against their will. Traumatic events were defined as physical in nature if they included (a) life threat through being physically attacked with or without a weapon, with the intent to kill or seriously injure, or (b) other physical assaults such as being kicked, hit, beat up, bit, or burned by another person outside of normal disciplinary spanking or children fighting. Single events were coded as sexual assault or physical assault, and recurrent events were coded as sexual abuse or physical abuse. The term “any trauma” is used hereafter to refer any of these four types of event.
The Children’s Somatization Inventory (CSI) assesses the severity of somatic symptoms over the past two weeks (30). For each item, participants rate “How much were you bothered by (symptom)?” during the past 2 weeks using a 5-point scale ranging from “not at all” (0) to “a whole lot” (4). For this study, we utilized two subscales: GI symptoms (9 items, e.g. abdominal pain, nausea, constipation, diarrhea, bloating) and non-GI symptoms (26 items, e.g., dizziness, back pain, headaches, sore muscles) (31). Items were summed to yield total scores for each subscale. Alpha reliabilities of the subscales for the measure in this study were .81 and .86 for GI and non-GI symptoms, respectively.
The Persistent Pain Questionnaire (PPQ) was used to assess history and location of non-abdominal chronic pain (32). Participants were asked to identify locations of both current and lifetime chronic pain based on standard body locations described by the International Association for the Study of Pain. For each site, participants were asked if they had experienced chronic pain daily or almost daily for the past 3 months. If they endorsed pain at any site, they were asked to rate their current pain intensity at that site on a scale of 0-100. Participants were considered to have current non-abdominal chronic pain if they rated current pain at a site as ≥ 30. The total number of sites was computed, yielding a total score between 0 and 7, which reflected the number of non-abdominal chronic pain sites having an intensity of 30 or greater (33).
The State-Trait Anxiety Inventory, Trait Form (STAI-T) (34) assesses the extent to which individuals typically experience 20 anxiety symptoms, each rated on a 4-point response scale. Items were summed, yielding a total score between 20 and 80. Alpha reliability of the measure in this study was .94.
The Center for Epidemiological Studies Depression Scale- Revised (CESD-R)(35) assesses 20 depression symptoms experienced over the past week, derived from the American Psychiatric Association Diagnostic and Statistical Manual, Fourth Edition, each rated on a 4-point response scale. The items were summed, yielding a total score between 0 and 60. Alpha reliability of the measure in this study was .89.
The Functional Disability Inventory (FDI) assesses difficulty in physical and psychosocial functioning due to physical health (36). Participants reported how much difficultly they would have performing 15 activities due to their physical health on a 5-point scale ranging from 0 to 4. The items were summed, yielding a total score between 0 and 60. Alpha reliability for the FDI was .88 in this study.
The Hollingshead Index of Social Status was used to assess socioeconomic status based on both occupation and level of education (37). For adolescent participants (< 18 years of age) parent occupation and level of education were utilized. Higher index scores indicate higher socioeconomic status.
Temporal summation of evoked pain
Temporal summation procedures used a standardized oscillating thermal stimulation protocol (10, 38). Consistent with a protocol developed by Fillingim and Edwards (19), a sequence of ten heat pulses with a 48°C target stimulus intensity was applied to the ventral forearm with a Medoc TSA-II Neurosensory Analyzer using commercially available software (TPS-CoVAS version 3.19, Medoc Inc.). Each pulse was 0.5 seconds in duration, started at a temperature of 40°C, with sequential pulses administered at a frequency of 0.4 Hz. Electrophysiological studies indicate that wind-up in the dorsal horn of the spinal cord is elicited by pulse frequencies as low as 0.2 Hz, with maximal wind-up observed at 1–2 Hz (9, 39, 40). Participants were asked to verbally rate the pain intensity experienced shortly after the peak of every heat pulse within the sequence on a 0-100 numerical rating scale (0 = “No Pain” and 100 = “Worst Possible Pain”). Similar protocols have demonstrated chronic pain-related differences in temporal summation between healthy controls and both chronic low back pain patients (38) and functional abdominal pain patients (10). Following the procedures of Hastie et al. (41), the difference between the maximum pain rating within the series of 10 stimuli and the first pain rating was utilized to index temporal summation; thus positive values indicated greater temporal summation.
Statistical Analyses
All analyses were conducted using IBM SPSS version 20. Chi-square and independent samples t-tests were used to examine differences between groups on sample characteristics. Because FGID patients and controls differed significantly by age and gender, subsequent analyses controlled for these two variables. Based on our a priori hypothesis that FGID patients with a trauma history would experience the highest levels of temporal summation, we divided our sample into three groups: healthy controls (n = 100), FGID with no trauma history (FGID+NoTrauma; n = 63), and FGID with trauma history (FGID+Trauma; n = 21). ANCOVA analyses, controlling for age and gender, assessed differences in clinical characteristics and temporal summation between these three groups. To evaluate the appropriateness of the ANCOVA model, we tested the assumption of homogeneity of regression slopes by fitting a model containing main effects of the group variable and each covariate as well as group-by-covariate interaction terms (42). For models where the interaction term was non-significant (p > .05), the assumption was met. All analyses met the assumption of homogeneity of regression slopes with the exception of two: anxiety X age and depression X age. Thus, ANCOVAs examining group differences for anxiety and depression were excluded from the paper because of this assumption violation. Post-hoc comparisons on the adjusted means with Bonferroni corrections for multiple comparisons were used to evaluate differences between the pairs of groups on these measures. Because mood and anxiety symptoms have both shown associations with temporal summation in the literature (43), follow-up ANCOVA analyses were used to examine whether controlling for mood and anxiety symptoms changed the relation between trauma history, FGID, and temporal summation. Both anxiety X temporal summation and depression X temporal summation met the assumption for homogeneity of regression lines upon formal analysis and were appropriate covariates for this model.
Results
Table 1 shows the sample characteristics as well as trauma history by group. Over a fourth, 28.6% (38/133), of FGID patients reported either a sexual or physical trauma.
Table 1.
Participant characteristics
| Controls (n = 135) |
FGID+No Trauma (n = 95) |
FGID+Trauma (n = 38) |
|
|---|---|---|---|
| Age, M ± SD | 18.43 ± 2.84a | 19.88 ± 3.96b | 22.05 ± 3.99c |
| Sex, n (%): | |||
| Male | 66 (48.9)a | 30 (31.6)b | 7 (18.4)b |
| Female | 69 (51.1)a | 65 (68.4)b | 31 (81.6)b |
| Race, n (%): | |||
| Caucasian | 128 (96.2) | 90 (96.8) | 34 (89.5) |
| African-American | 1 (0.8) | 3 (3.2) | 2 (5.3) |
| Other | 4 (3.0) | 0 (0.0) | 2 (5.3) |
| SES (Hollingshead), M ± SD | 37.31 ± 12.25 | 40.41 ± 11.79 | 39.78 ± 13.89 |
| Type of trauma, n (%): | |||
| Physical assault | 0 (0) a | 0 (0) a | 16 (12.0) b |
| Physical abuse | 0 (0) a | 0 (0) a | 6 (4.5) b |
| Sexual assault | 0 (0) a | 0 (0) a | 21 (15.8) b |
| Sexual abuse | 0 (0) a | 0 (0) a | 3 (2.3) b |
| Number of trauma types experienced, n (%): | |||
| One type of trauma | 0 (0) a | 0 (0) a | 31 (23.3) b |
| Two or more types of trauma | 0 (0) a | 0 (0) a | 7 (5.3) b |
| Age of first experienced trauma | N/A | N/A | 12.26 ± 5.50 |
Note. Within rows, values with different superscripts differ significantly at p < .05.
Trauma and Temporal Summation of Evoked Pain
Temporal summation analyses were conducted for participants who completed the laboratory portion of the study (n = 184). Groups did not significantly differ on ratings of the first stimuli in the ten pulse series (F[2,183] = 0.56, p > 0.1). For mean ratings for the ten pulses by group, see Figure 1. ANCOVA results revealed significant differences between groups on temporal summation (F[2,179] = 4.13, p = .018, ; Figure 2). Post-hoc comparisons on age and gender adjusted means with a Bonferroni correction for multiple comparisons revealed that, as hypothesized, the FGID+Trauma group exhibited significantly greater temporal summation (adjusted mean = 24.09, SE = 3.10) compared to both the FGID+ No Trauma (adjusted mean = 14.82, SE = 1.76; p = .027), and Control groups (adjusted mean = 14.35, SE = 1.41; p = .018). The results of analyses examining temporal summation remained significant even when anxiety and mood symptoms were included as covariates (F[2,175] = 4.20, p = .016, ).
Figure 1.
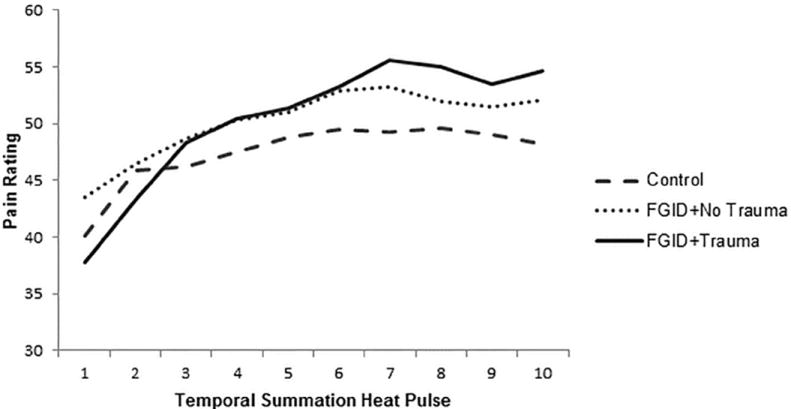
Mean pain ratings for ten heat pulses at 48° by FGID and trauma groups
Figure 2.
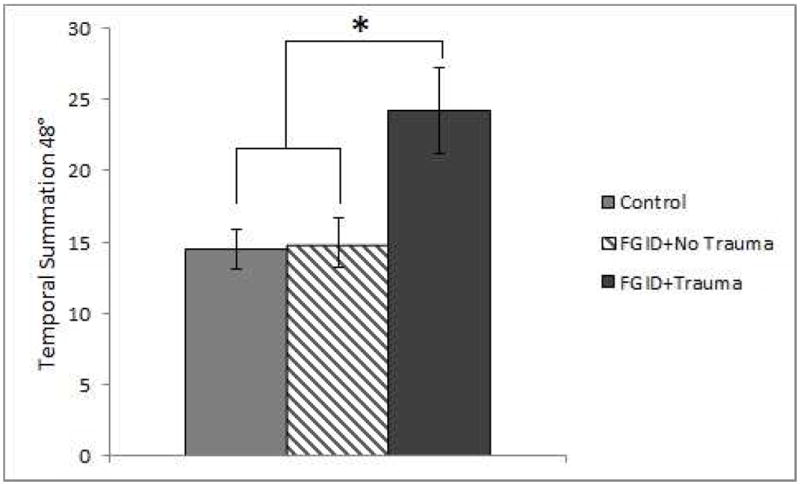
Temporal summation at 48° target temperature by FGID and trauma groups
Note. * p < .05
Trauma and Clinical Characteristics
Table 2 presents estimated marginal means and standard errors (adjusted for age and gender) for each self-report measure by group. Results of ANCOVAs (controlling for age and gender) revealed significant differences between groups on GI symptom severity (F[2,263] = 74.53, p < .001, ), non-GI symptom severity, (F[2,263] = 25.76, p < .001, ), number of non-abdominal chronic pain sites (F[2,259] = 8.94, p < .001, ), and functional disability (F[2,263] = 29.89, p < .001, ). Specifically, the FGID+Trauma group exhibited significantly greater GI and non-GI symptom severity, more non-abdominal chronic pain sites, and greater functional disability than the FGID+No Trauma and Control groups (p’s < .05 for all pairwise comparisons).
Table 2.
Adjusted means ± standard errors by FGID and trauma groups for clinical characteristics
| Variable | Control (n = 131 - 135) |
FGID+ No Trauma (n = 92 - 95) |
FGID+ Trauma (n = 37 - 38) |
|---|---|---|---|
| GI symptoms (CSI) | 1.91 ± .31a | 6.63 ± .36b | 9.04 ± .59c |
| Non-GI symptoms (CSI) | 5.67 ± .65a | 10.79 ± .75b | 15.20 ± 1.25c |
| # of non-abdominal chronic pain sites (PPQ) | .26 ± .08a | .50 ± .10a | 1.04 ± .16b |
| Disability (FDI) | 1.89 ± .49a | 5.09 ± .57b | 10.15 ± .94c |
Note. Within rows, values with different superscripts differ significantly at p < .05.
Discussion
FGID patients with a history of physical or sexual assault/abuse exhibited a greater degree of temporal summation to evoked thermal pain stimuli compared to both FGID patients and healthy controls without a trauma history. These findings are consistent with previous studies showing enhanced pain responsiveness in chronic pain patients with a history of trauma (12-14). However, these findings go beyond prior work by suggesting that the combination of chronic pain and trauma may be particularly toxic in part due to an association with heightened central sensitization, as reflected in elevated temporal summation. Indeed, FGID patients with a history of trauma were noted in this study to report greater GI and non-GI symptom severity, more non-abdominal chronic pain sites, and greater disability than FGID patients without a history of trauma. The findings of the current work might help shed light on the frequent clinical observations of an association between trauma history and dysfunctional chronic pain adaptation, as reflected in the relatively high prevalence of patients seeking chronic pain specialty care reporting a trauma history (44, 45).
The relation between traumatic stress and FGIDs is likely influenced by a variety of psychosocial and biological risk factors beyond central sensitization alone. Cognitive theories of PTSD emphasize the potential for traumatic life events to alter information and emotion processing such that fear networks in memory become stable, generalized and easily-accessed (46). Posttraumatic alterations in appraisals (e.g., greater perceived threat) and behaviors (e.g., avoiding potential triggers of trauma-related memories) can interfere with the recovery process by preventing habituation of stress response systems (47, 48). Enduring stress response dysregulation, in turn, increases risk for negative physical and mental health outcomes (49, 50). Similar alterations in stress response systems, including the HPA axis and serotonergic systems, may also occur in “central sensitivity syndromes,” a term coined to describe overlapping chronic pain conditions that involve central sensitization (51). Future studies should assess features of the stress response (e.g., HPA activity) throughout experimental pain protocols to determine whether enhanced stress reactivity and/or delayed stress recovery may contribute to the impact of trauma history on the experience of FGIDs.
This study has limitations that must be considered when interpreting the findings. First, the cross-sectional design does not allow testing temporal relations among trauma exposure, enhanced temporal summation, and FGID. It is therefore impossible to determine whether trauma led to enhanced temporal summation that then contributed to development of FGID, whether FGID in the context of trauma jointly led to elevated temporal summation, or rather, whether trauma alone was sufficient to increase temporal summation. Conclusions regarding the latter possibility were limited by the small number of control participants in this study reporting a history of trauma (n=9 with temporal summation data), who were excluded from analyses because of insufficient statistical power to detect differences relative to the other study groups in a meaningful way. Findings that FGID alone was not associated with elevated temporal summation compared to controls are nonetheless consistent with some aspect of a trauma contributing to enhanced central sensitization, a conclusion supported by one prior study (18). Prospective studies are needed to evaluate possible patterns of temporal sequencing. Additionally, the use of change scores to quantify temporal summation is a potential limitation. There is still considerable debate regarding the optimal methodological approach for determining temporal summation. We elected to examine change scores to facilitate between-study comparisons because these are among the most commonly reported outcome measures in the literature (52).
In conclusion, individuals with chronic FGIDs and a history of physical or sexual assault/abuse exhibited significantly higher levels of temporal summation to evoked laboratory pain, as well as more severe clinical pain and disability as compared to those with chronic pain and no history of physical or sexual assault/abuse. Assessing for trauma history in patients with FGIDs could help identify a subset at risk for greater central sensitization and pain-related symptoms. Individuals with prior trauma are at increased risk of developing posttraumatic stress disorder in response to future traumatic life events (53) and cognitive behavioral therapy targeting posttraumatic stress disorder in individuals with chronic pain reduces pain severity (54). However, little is known regarding whether traditional pain-management programs also alleviate posttraumatic stress symptoms (55), Given this evidence, the present findings suggest that clinicians managing individuals with FGID and history of trauma should be particularly vigilant regarding the potential need for integrative treatment strategies especially following retraumatization. Further research is needed to determine appropriate interventions for this population exhibiting enhanced central sensitization, clinically-significant pain, and trauma histories.
Acknowledgments
This study was funded by R01 HD23264 (L.S. Walker, principal investigator), R01 DA031726 (S. Bruehl, principal investigator), Vanderbilt Kennedy Center (P30 HD15052), Vanderbilt Digestive Disease Research Center (DK058404), and the Vanderbilt CTSA (1 UL1 RR024975) from the National Center for Research Resources, National Institutes of Health.
Footnotes
COI and Ethical Adherence
Conflict of Interest: The authors declare that they have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 2.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390.e383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afari N, Ahumada SM, Wright LJ, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76:2–11. doi: 10.1097/PSY.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt BA, Sikes RW. The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Prog Brain Res. 2000;122:223–235. doi: 10.1016/s0079-6123(08)62141-x. [DOI] [PubMed] [Google Scholar]
- 7.Leserman J, Drossman DA. Relationship of abuse history to functional gastrointestinal disorders and symptoms: some possible mediating mechanisms. Trauma Violence Abuse. 2007;8:331–343. doi: 10.1177/1524838007303240. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Verne GN. New insights into visceral hypersensitivity—clinical implications in IBS. Nature Reviews Gastroenterology and Hepatology. 2011;8:349–355. doi: 10.1038/nrgastro.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 10.Dengler-Crish CM, Bruehl S, Walker LS. Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain. 2011;152:802–808. doi: 10.1016/j.pain.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–323. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander RW, Bradley LA, Alarcon GS, et al. Sexual and physical abuse in women with fibromyalgia: association with outpatient health care utilization and pain medication usage. Arthritis Care Res. 1998;11:102–115. doi: 10.1002/art.1790110206. [DOI] [PubMed] [Google Scholar]
- 13.Scarinci IC, McDonald-Haile J, Bradley LA, Richter JE. Altered pain perception and psychosocial features among women with gastrointestinal disorders and history of abuse: a preliminary model. Am J Med. 1994;97:108–118. doi: 10.1016/0002-9343(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 14.Fillingim RB, Maixner W, Sigurdsson A, Kincaid S. Sexual and physical abuse history in subjects with temporomandibular disorders: relationship to clinical variables, pain sensitivity, and psychologic factors. J Orofac Pain. 1997;11:48–57. [PubMed] [Google Scholar]
- 15.Granot M, Somer E, Zisman-Ilani Y, et al. Characteristics of response to experimental pain in sexually abused women. Clin J Pain. 2011;27:616–622. doi: 10.1097/AJP.0b013e3182132963. [DOI] [PubMed] [Google Scholar]
- 16.Kraus A, Geuze E, Schmahl C, et al. Differentiation of pain ratings in combat-related posttraumatic stress disorder. Pain. 2009;143:179–185. doi: 10.1016/j.pain.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Moeller-Bertram T, Strigo IA, Simmons AN, et al. Evidence for acute central sensitization to prolonged experimental pain in posttraumatic stress disorder. Pain Medicine. 2014;15:762–771. doi: 10.1111/pme.12424. [DOI] [PubMed] [Google Scholar]
- 19.Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21:387–397. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- 20.Walker LS, Garber J, Smith CA, Van Slyke DA, Claar RL. The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. J Consult Clin Psychol. 2001;69:85–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Walker LS, Smith CA, Garber J, Van Slyke DA. Development and validation of the Pain Response Inventory for Children. Psychological Assessment. 1997;9:392–405. [Google Scholar]
- 22.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012;153:1798–1806. doi: 10.1016/j.pain.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dengler-Crish CM, Horst SN, Walker LS. Somatic complaints in childhood functional abdominal pain are associated with functional gastrointestinal disorders in adolescence and adulthood. J Pediatr Gastroenterol Nutr. 2011;52:162–165. doi: 10.1097/MPG.0b013e3181ec1d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horst S, Shelby G, Anderson J, et al. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drossman DA, Corazziari E, Delvaux M, et al. Rome III: the Functional Gastrointestinal Disorders. Third Edition. McLean, VA: Degnon Associates; 2006. [Google Scholar]
- 26.Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM-IV: Child and parent version. New York, NY: Oxford University Press Inc.; 1996. [Google Scholar]
- 27.Dinardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Adult Lifetime Version. New York, NY: Oxford University Press Inc.; 1994. [Google Scholar]
- 28.Leserman J, Drossman DA, Li Z. The reliability and validity of a sexual and physical abuse history questionnaire in female patients with gastrointestinal disorders. Behav Med. 1995;21:141–150. doi: 10.1080/08964289.1995.9933752. [DOI] [PubMed] [Google Scholar]
- 29.Leserman J, Li Z, Drossman DA, et al. Impact of sexual and physical abuse dimensions on health status: development of an abuse severity measure. Psychosom Med. 1997;59:152–160. doi: 10.1097/00006842-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Walker LS, Beck JE, Garber J, Lambert W. Children’s Somatization Inventory: Psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;34:430–440. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012;153:1798–1806. doi: 10.1016/j.pain.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruehl S, France CR, France J, Harju A, al’Absi M. How accurate are parental chronic pain histories provided by offspring? Pain. 2005;115:390–397. doi: 10.1016/j.pain.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150:568–572. doi: 10.1016/j.pain.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spielberger CD, Gorsuch RL, Lushene R. Manual for the State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press; 1970. [Google Scholar]
- 35.Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish M, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Vol. 3. Mahwah, NJ: Lawrence Erlbaum; 2004. pp. 363–377. [Google Scholar]
- 36.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollingshead AB. Four factor index of social status. New Haven: Yale University; 1975. [Google Scholar]
- 38.Chung OY, Bruehl S. The impact of blood pressure and baroreflex sensitivity on wind-up. Anesth Analg. 2008;107:1018–1025. doi: 10.1213/ane.0b013e31817f8dfe. [DOI] [PubMed] [Google Scholar]
- 39.Schouenborg J. Functional and topographical properties of field potentials evoked in rat dorsal horn by cutaneous C-fibre stimulation. J Physiol. 1984;356:169–192. doi: 10.1113/jphysiol.1984.sp015459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrero JF, Cervero F. Changes in nociceptive reflex facilitation during carrageenan-induced arthritis. Brain Res. 1996;717:62–68. doi: 10.1016/0006-8993(95)01585-x. [DOI] [PubMed] [Google Scholar]
- 41.Hastie BA, Riley JL, 3rd, Robinson ME, et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. 2. Mahwah, NJ: Erlbaum; 2004. [Google Scholar]
- 43.Rhudy JL, Martin SL, Terry EL, et al. Using multilevel growth curve modeling to examine emotional modulation of temporal summation of pain (TS-pain) and the nociceptive flexion reflex (TS-NFR) Pain. 2012;153:2274–2282. doi: 10.1016/j.pain.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 44.Moeller-Bertram T, Keltner J, Strigo IA. Pain and post traumatic stress disorder - review of clinical and experimental evidence. Neuropharmacology. 2012;62:586–597. doi: 10.1016/j.neuropharm.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 46.Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behav Ther. 1989;20:155–176. [Google Scholar]
- 47.Chemtob C, Roitblat HL, Hamada RS, Carlson JG, Twentyman CT. A cognitive action theory of post-traumatic stress disorder. J Anxiety Disord. 1988;2:253–275. [Google Scholar]
- 48.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 49.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 50.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 51.Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Anderson RJ, Craggs JG, Bialosky JE, et al. Temporal summation of second pain: variability in responses to a fixed protocol. Eur J Pain. 2013;17:67–74. doi: 10.1002/j.1532-2149.2012.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 54.Beck JG, Coffey SF, Foy DW, Keane TM, Blanchard EB. Group cognitive behavior therapy for chronic posttraumatic stress disorder: an initial randomized pilot study. Behav Ther. 2009;40:82–92. doi: 10.1016/j.beth.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Beck JG, Clapp JD. A different kind of co-morbidity: Understanding posttraumatic stress disorder and chronic pain. Psychol Trauma. 2011;3:101–108. doi: 10.1037/a0021263. [DOI] [PMC free article] [PubMed] [Google Scholar]


