Abstract
One of key roadblocks in UCNP development is its extremely limited choices of excitation wavelengths. We report a generic design to program UCNPs to possess highly tunable dye characteristic excitation bands. Using such distinctive properties, we were able to develop a new excitation wavelength selective security imaging. This work unleashed the greater freedom of the excitation wavelengths of the upconversion nanoparticles and we believe it is a game-changer in the field and this method will enable numerous applications that are currently limited by existing UCNPs.
In recent years, lanthanide-doped upconverting nanoparticles (UCNPs) have received a considerable amount of attention due to their ability to convert two or more low-energy pump photons at the NIR region to a higher-energy output emission at the ultra violet (UV), visible (Vis), and shorter wavelengths of NIR.1 Because of these unique optical properties, UCNPs have been applied in many areas including bio-sensing,2 in vivo imaging,3 drug delivery,4 photodynamic therapy,5 photoactivation,6 and solar cell development.7 In spite of these advances, one key roadblock in UCNP development is their extremely limited excitation wavelength choice. For example, the excitation wavelength of conventional Yb ion doped UCNPs are generally limited at 980 nm (matching the absorption of sensitizer Yb3+).8 Even we and other colleagues have recently developed Nd3+ and Yb3+ co-doped UCNPs to generate an additional excitation peak at 800 nm,9 these UCNPs remain limited by the intrinsically weak absorption coefficients and constitutional excitation peaks of the lanthanide sensitizers (Yb3+ and Nd3+). Such an inability to tailor excitation wavelength bands of the upconversion nanoparticles has been a major barrier in regard to numerous practical applications such as solar cell development, orthogonal bioimaging and photo-modulation.
Compared to these inorganic lanthanide ions, organic NIR dyes can be rationally designed through facile structural modification and derivation, to provide remarkable flexibility of tuning excitation wavelengths.10 In addition, NIR dyes have much higher absorption coefficients than the lanthanide sensitizers. However, to date, the only example in this regard is that, in 2012, Zou et al. reported that a NIR dye (IR-806 dye) can transfer the energy to UCNPs so as to amplify upconversion emissions via directly adding dyes into the solution of as-synthesized hydrophobic ligand coated UCNPs.11 Since then, surprisingly, the dye sensitization has not been further studied. IR-806 dye has been the only dye molecule reported and this method has not been successfully extended to any other dyes with different chemical structures. The highly tunable excitation advantages of NIR dyes have not been utilized in regard to UCNP. This inability is likely a result of the well-established knowledge that subtle changes in incoming molecule/ligand structures and reaction conditions can profoundly affect kinetics and thermodynamics of displacement reactions on ligand covered nanoparticles (NPs) surfaces.12 For example, in the above-mentioned method, the dye has to penetrate into the hydrophobic oleylamine ligand layer on UCNP surface and then displace some of them.
We reasoned that we can take advantage of hydrophobic organic ligand free UCNPs13 (i.e., removing the barrier on UCNP surface which affects the dye access) and largely selective NIR dyes as a series of sensitizers (or a set of antenna) to program the excitation bands of the UCNPs. We now report a generic design to create upconversion nanoparticles with NIR dye characteristic tunable excitation bands in which a series of near-infrared dye groups with systematically progressive absorption wavelengths are able to interact with a hydrophobic organic ligand free UCNP as customized “antennae” for programming excitation bands wavelengths of UCNPs (Scheme 1). Moreover, the excitation bands of UCNPs can be expanded by the inclusion of mixed dyes. Finally, using the distinctive properties of the engineered dye-sensitized UCNPs, we were able to develop a new excitation wavelength selective orthogonal security imaging.
Scheme 1.
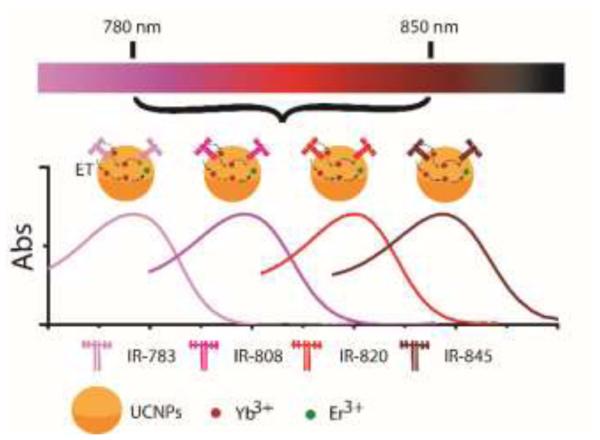
Schematic illustration of creating a set of UCNPs that feature NIR dye tuneable characteristic excitation wavelengths (ET: Energy transfer).
In this study, UCNPs (β-NaYF4: 20%Yb, 2%Er) with an average core diameter of 20 nm (Figure S1) were prepared using a literature method.14 Subsequently, the UCNPs were treated with nitrosonium tetrafluoroborate to remove hydrophobic organic ligands. To examine the feasibility of dye-sensitized UCNPs at different wavelength bands, a series of NIR dyes with systematically progressive absorption wavelengths were used. These included commercially available dyes (IR-783 and IR-820) and their respective carboxylate derivatives (IR-808 and IR-845) we synthesized. (Figure S2)
We first assessed the optimal concentrations of these IR-dyes (Figure S3). When these IR-dyes were titrated into the solution of UCNPs, we found that the upconversion emission intensity first increased which was consistent with an increase in overall absorption of the excitation energy. However, beyond a critical concentration, a further increase in the IR-dyes resulted in declining upconversion emission intensity, similar to that reported in the literature.11 The optimal IR-dyes: nanoparticles ratio is around 5 µmol/L : 0.1 µmol/L (dyes vs. UCNPs). The samples of the optimal ratio of IR-dyes coated nanoparticles were able to be isolated by centrifugation and the absorption spectra of the supernatants were subsequently measured. (Figure S4). No discernible free dyes were observed in the supernatant. Thus this result suggested that the dyes were quantitatively conjugated onto the UCNPs and the number of dyes that are conjugated on UCNP was able to be estimated 11,13 to be 50:1 dyes per particle. Except as noted, this ratio was used in all subsequent experiments.
As shown in Figure S5, at 750 nm excitation, the emission spectrum of all these IR-dyes extends well beyond 1000 nm. The overlap between the emission spectrum of IR-dyes and the excitation spectrum of the UCNP nanoparticles, allows for energy transfer from IR-dyes to the Yb3+ in the nanoparticles. The interaction of the IR-dyes with the nanoparticle was characterized by lifetime study. We observed that the lifetime of the IR-dye’s luminescence on the particle was shorter than that of the pure IR-dyes, indicating the occurrence of energy transfer from the IR-dyes to the nanoparticle (Figure 1).
Figure 1.
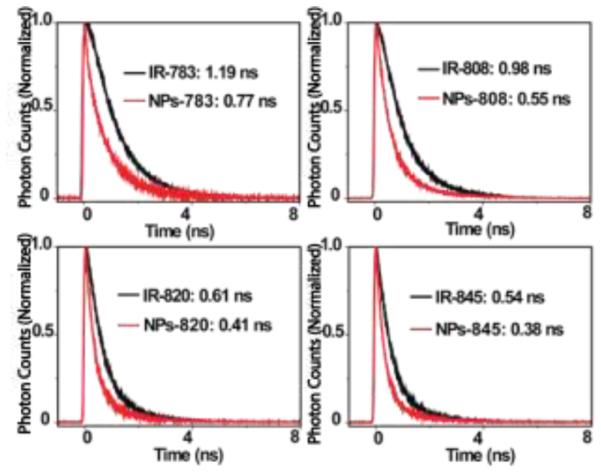
Time-resolved fluorescence of IR-dyes (5 µmol/L) and IR-dyes/β-NaYF4:20%Yb,2%Er NPs (5 µmol/L : 0.1 µmol/L) were respectively excited at 780 nm, 800 nm, 820 nm, 840 nm and detected at 820 nm, 835 nm, 850 nm, 865 nm by 150 fs pulsed 300 micron slit width laser. The decrease of fluorescence lifetime of IR-dyes upon of NPs indicated energy transfer from IR-dye to nanoparticles.
Furthermore, we studied the IR-dyes conjugation on the surface of the UCNPs by FT-IR spectra. The peak at 1550 cm−1 (C=C benzene skeleton vibration), the peak around 1400 cm−1 (C-H bending vibration), the peak at 1250 cm−1 (C-N stretching vibration), and the peak around 1170 cm−1, 1110 cm−1, 1035 cm−1 (S=O stretching vibration) were observed in the FTIR spectrum of IR-dyes-coated UCNPs (Figure S6). These results suggested that the IR-dyes had been coated on the surface of UCNPs.
The upconversion excitation spectrum was measured using a 2 mW calibrated wavelength-tunable laser. The total number of photons over the 500-685 nm range for the UCNPs that were collected was then plotted versus the excitation wavelength. It was found that all of these samples had distinguishable excitation bands that were recorded in the 720–1,000 nm spectral range. It was further found that there was an excellent match with the respective absorption peaks of the IR-dyes (Figure S7). When compared to the UCNPs alone, the different dye-coated UCNPs (denoted respectively as NPs-783, NPs-808, NPs-820, and NPs-845) exhibited shifted broad band excitation peaks around 790 nm, 810 nm, 830 nm, and 850 nm, respectively. The integrated spectral response of the IR-808 coated UCNPs was found to be approximately 200 times stronger than that of the non-sensitized nanoparticles. When compared to the non-sensitized nanoparticles (Figure 2), the IR-783 coated UCNPs exhibited 80 folds enhancement, the IR-820 coated UCNPs exhibited 70 folds enhancement and the IR-845 coated UCNPs exhibited 100 folds enhancement. As shown in Figure 3, this excitation wavelength selected upconversion can clearly be seen by the naked eye.
Figure 2.
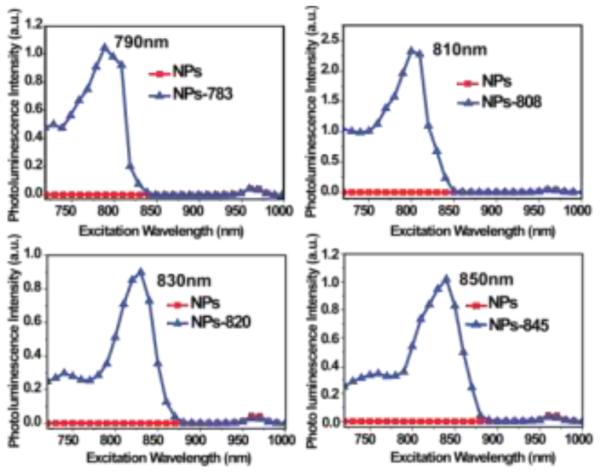
Excitation spectra of β-NaYF4:20%Yb,2%Er NPs and (a) IR-783, (b) IR-808, (c) IR-820, and (d) IR-845 IR-dye sensitized NPs.
Figure 3.
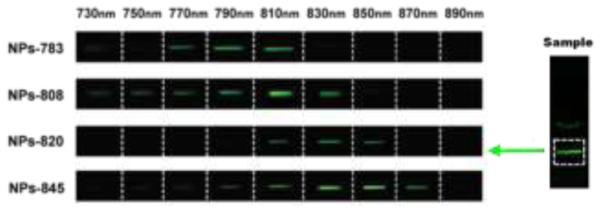
Photographic images of the corresponding solution samples taken under different excitation wavelengths from 730 nm to 890 nm. The right of the figure shows the portion of the light beam with each sample which was used for these images.
In addition, the emission spectra of the UCNPs that were irradiated at the corresponding excitation maxima (determined above: NPs-783 excited at 790 nm, NPs-808 excited at 810 nm, NPs-820 excited at 830 nm, NPs-845 excited at 850 nm) under the same power of 2 mW were recorded (Figure S8). The peak-to-peak ratio of non-sensitized and sensitized upconversion was about 1:18 (UCNPs:NPs-783), 1:35 (UCNPs:NPs-808), 1:14 (UCNPs:NPs-820), and 1:22 (UCNPs:NPs-845). Furthermore, to explore whether we can “mixing and matching” a pair of dyes, 0.25 µmol/L IR-783 and 0.25 µmol/L IR-845 dyes was mixed with 0.1 µmol/L UCNPs. As a result, we observed that, compared to the individual IR-dye coated nanoparticles, the nanoparticles of the mixed a pair of dyes (NPs/IR-783+IR-845) showed an expanded wavelength range (Figure 4).
Figure 4.
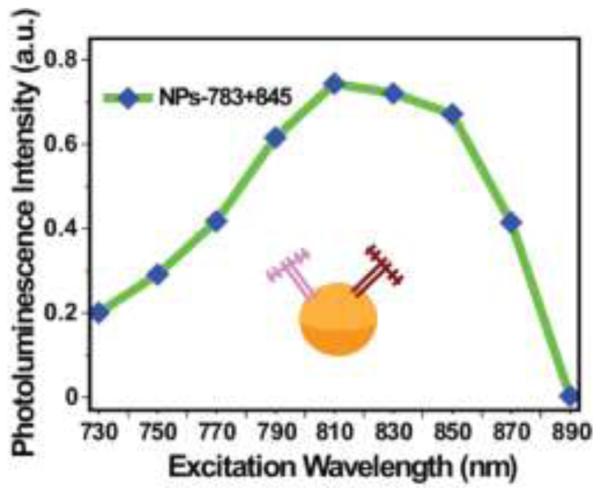
The experimental upconversion excitation spectrum of NPs with co-coated dyes (IR-783 and IR-845).
Since the eyes are sensitive in the visible range, while has no response to NIR light, the security detection application by taking advantage of NIR-to-Vis upconversion photoluminescence has gained considerable attentions. Indeed, an encrypted pattern that is invisible to human eyes, but flares up at a specific wavelength, providing a unique way to secure the object.2a,15 Nevertheless, to the best of our knowledge, existing security imaging applications in regard to UCNP-doped materials have used only single NIR excitation wavelength. We envision that IR-dye-coated nanoparticles with distinguishable excitation maxima would multiply the number of unique pieces of information that can be conveyed by conventional single wavelengths of UCNPs. To provide a proof-of-concept with respect to our system, we fabricated a traffic signal type logo of “GNO” bearing poly-urethane and poly-acrylic thin slab using a 3d-printer (Figure 5). We blended dye-UCNPs in PMMA and then loaded NPs-783 in the letter “G”, NPs-845 in the letter “N”, and NPs-783+845 co-coated nanoparticles in the letter “O”. These dye-UCNPs were solidified in PMMA after being remained overnight at room temperature (details in Supporting information). We subsequently imaged the slab under 790 nm and 850 nm CW laser beams. This generic pattern conveys manifold signals when illuminated by different wavelengths of lights, whereas single wavelength excitation yields a binary value (bright or dark). Under 790 nm excitation, “GO” was bright and “N” was dim. Under 850 nm excitation, “NO” was bright and “G” was dim. This versatility would also give UCNP a wide array of applications in regard to orthogonal imaging and photoactivation.
Figure 5.
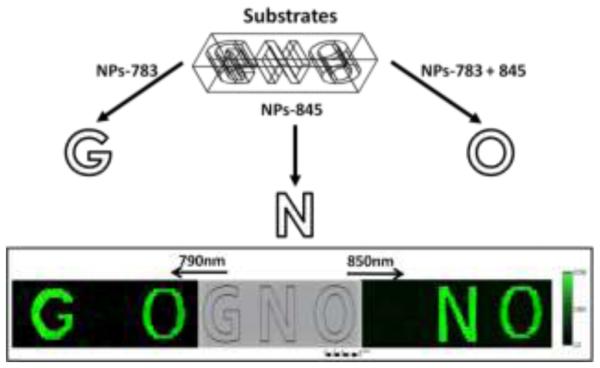
Schematic designs (top) of “GNO” bearing thin slab made by a 3D-printer. As shown on the bottom, excitation wavelength selective images were acquired under 790 nm (bottom, left) and 850 nm (bottom, right).
Conclusions
In conclusion, this report illustrates a readily replicable method of engineering the excitation wavelength of upconverting nanoparticles in the NIR region via utilizing a series of NIR dyes with systematically progressive absorption wavelengths. We demonstrated that these NIR dye molecules are able to act as a set of “antennae” to capture various bands of NIR wavelength photons for subsequent upconversion energy transfer. As a result, we can create a new set of UCNPs that feature NIR dye characteristic excitation wavelengths. Due to the modular nature of NIR dyes, the excitation band of UCNPs can also be expanded by mixing a pair of dyes. Finally, we provided proof–concept to engineer excitation wavelength of dye-sensitized UCNPs by applying it in orthogonal security imaging. This approach promises to provide a new generalized method to engineering upconversion nanoparticle excitations. This new strategy would be paradigm-shifting as it overcomes the key limitations of current UCNPs. We anticipate that our method will open a wide array of new opportunities in photonics and biophotonics such as orthogonal photoactivation and photostimulation as well as solar cell development.
Supplementary Material
Acknowledgements
This research was supported by the China Scholarship Council (CSC) and the start-up fund of the University of Massachusetts Medical School, a Worcester Foundation Mel Cutler Award, National Institutes of Health R01MH103133, Human Frontier Science Program, and a UMass CVIP award. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details for the synthesis, TEM, FTIR spectra, absorption and PL spectra. See DOI: 10.1039/x0xx00000x
References
- 1.(a) Zhou J, Liu Z, Li FY. Chem. Soc. Rev. 2012;41(3):1323–1349. doi: 10.1039/c1cs15187h. [DOI] [PubMed] [Google Scholar]; (b) Wang F, Liu XG. Chem. Soc. Rev. 2009;38(4):976–989. doi: 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]; (c) Wang F, Banerjee D, Liu YS, Chen XY, Liu XG. Analyst. 2010;135(8):1839–1854. doi: 10.1039/c0an00144a. [DOI] [PubMed] [Google Scholar]; (d) Su LT, Karuturi SK, Luo JS, Liu LJ, Liu XF, Guo J, Sum TC, Deng RR, Fan HJ, Liu XG, Tok AIY. Adv. Mater. 2013;25(11):1603–1607. doi: 10.1002/adma.201204353. [DOI] [PubMed] [Google Scholar]; (e) Haase M, Schafer H. Angew. Chem. Int. Edit. 2011;50(26):5808–5829. doi: 10.1002/anie.201005159. [DOI] [PubMed] [Google Scholar]; (f) Gorris HH, Wolfbeis OS. Angew. Chem. Int. Edit. 2013;52(13):3584–3600. doi: 10.1002/anie.201208196. [DOI] [PubMed] [Google Scholar]; (g) Zheng W, Huang P, Tu DT, Ma E, Zhou HM, Chen XY. Chem. Soc. Rev. 2015;44(6):1379–1415. doi: 10.1039/c4cs00178h. [DOI] [PubMed] [Google Scholar]
- 2.(a) Zhang F, Shi QH, Zhang YC, Shi YF, Ding KL, Zhao DY, Stucky GD. Adv. Mater. 2011;23(33):3775. doi: 10.1002/adma.201101868. [DOI] [PubMed] [Google Scholar]; (b) Wang LY, Yan RX, Hao ZY, Wang L, Zeng JH, Bao J, Wang X, Peng Q, Li YD. Angew. Chem. Int. Edit. 2005;44(37):6054–6057. doi: 10.1002/anie.200501907. [DOI] [PubMed] [Google Scholar]; (c) Zhou SY, Zheng W, Chen Z, Tu DT, Liu YS, Ma E, Li RF, Zhu HM, Huang MD, Chen XY. Angew. Chem. Int. Edit. 2014;53(46):12498–12502. doi: 10.1002/anie.201405937. [DOI] [PubMed] [Google Scholar]; (d) Huang P, Zheng W, Zhou SY, Tu DT, Chen Z, Zhu HM, Li RF, Ma E, Huang MD, Chen XY. Angew. Chem. Int. Edit. 2014;53(5):1252–1257. doi: 10.1002/anie.201309503. [DOI] [PubMed] [Google Scholar]
- 3.(a) Liu Q, Sun Y, Yang TS, Feng W, Li CG, Li FY. J. Am. Chem. Soc. 2011;133(43):17122–17125. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]; (b) Lim SF, Riehn R, Ryu WS, Khanarian N, Tung CK, Tank D, Austin RH. Nano Lett. 2006;6(2):169–174. doi: 10.1021/nl0519175. [DOI] [PubMed] [Google Scholar]; (c) Chen GY, Shen J, Ohulchanskyy TY, Patel NJ, Kutikov A, Li ZP, Song J, Pandey RK, Agren H, Prasad PN, Han G. ACS Nano. 2012;6(9):8280–8287. doi: 10.1021/nn302972r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Zhou W, Sun FF, Pan K, Tian GH, Jiang BJ, Ren ZY, Tian CG, Fu HG. Adv. Funct. Mater. 2011;21(10):1922–1930. [Google Scholar]; (b) Wang C, Cheng LA, Liu ZA. Biomaterials. 2011;32(4):1110–1120. doi: 10.1016/j.biomaterials.2010.09.069. [DOI] [PubMed] [Google Scholar]; (c) Hou ZY, Li CX, Ma PA, Li GG, Cheng ZY, Peng C, Yang DM, Yang PP, Lin J. Adv. Funct. Mater. 2011;21(12):2356–2365. [Google Scholar]
- 5.(a) Zhang P, Steelant W, Kumar M, Scholfield M. J. Am. Chem. Soc. 2007;129(15):4526–4527. doi: 10.1021/ja0700707. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tian G, Ren WL, Yan L, Jian S, Gu ZJ, Zhou LJ, Jin S, Yin WY, Li SJ, Zhao YL. Small. 2013;9(11):1929–1938. doi: 10.1002/smll.201201437. [DOI] [PubMed] [Google Scholar]; (c) Shan JN, Budijono SJ, Hu GH, Yao N, Kang YB, Ju YG, Prud'homme RK. Adv. Funct. Mater. 2011;21(13):2488–2495. [Google Scholar]; (d) Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. Nat. Med. 2012;18(10):1580–1586. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]
- 6.(a) Yang YM, Shao Q, Deng RR, Wang C, Teng X, Cheng K, Cheng Z, Huang L, Liu Z, Liu XG, Xing BG. Angew. Chem. Int. Edit. 2012;51(13):3125–3129. doi: 10.1002/anie.201107919. [DOI] [PubMed] [Google Scholar]; (b) Yan B, Boyer JC, Habault D, Branda NR, Zhao Y. J. Am. Chem. Soc. 2012;134(40):16558–16561. doi: 10.1021/ja308876j. [DOI] [PubMed] [Google Scholar]; (c) Jayakumar MKG, Idris NM, Zhang Y. P. Natl. Acad. Sci. USA. 2012;109(22):8483–8488. doi: 10.1073/pnas.1114551109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wang HQ, Batentschuk M, Osvet A, Pinna L, Brabec CJ. Adv. Mater. 2011;23(22-23):2675–2680. doi: 10.1002/adma.201100511. [DOI] [PubMed] [Google Scholar]; (b) van der Ende BM, Aarts L, Meijerink A. Phys. Chem. Chem. Phys. 2009;11(47):11081–11095. doi: 10.1039/b913877c. [DOI] [PubMed] [Google Scholar]; (c) de Wild J, Meijerink A, Rath JK, van Sark WGJHM, Schropp REI. Energ. Environ. Sci. 2011;4(12):4835–4848. [Google Scholar]
- 8.Li SF, Zhang M, Peng Y, Zhang QY, Zhao MS. J. Rare Earth. 2010;28(2):237–242. [Google Scholar]
- 9.(a) Xie XJ, Gao NY, Deng RR, Sun Q, Xu QH, Liu XG. J. Am. Chem. Soc. 2013;135(34):12608–12611. doi: 10.1021/ja4075002. [DOI] [PubMed] [Google Scholar]; (b) Wen HL, Zhu H, Chen X, Hung TF, Wang BL, Zhu GY, Yu SF, Wang F. Angew. Chem. Int. Edit. 2013;52(50):13419–13423. doi: 10.1002/anie.201306811. [DOI] [PubMed] [Google Scholar]; (c) Wang YF, Liu GY, Sun LD, Xiao J, Zhou JC, Yan CH. ACS Nano. 2013;7(8):7200–7206. doi: 10.1021/nn402601d. [DOI] [PubMed] [Google Scholar]; (d) Shen J, Chen GY, Vu AM, Fan W, Bilsel OS, Chang CC, Han G. Adv Opt. Mater. 2013;1(9):644–650. [Google Scholar]; (e) Li XM, Wang R, Zhang F, Zhou L, Shen DK, Yao C, Zhao DY. Sci. Rep-Uk. 2013:3. doi: 10.1038/srep03536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Xie XJ, Liu XG. Nat. Mater. 2012;(11):842–843. doi: 10.1038/nmat3426. [DOI] [PubMed] [Google Scholar]; (b) Umezawa K, Matsui A, Nakamura Y, Citterio D, Suzuki K. Chem-Eur J. 2009;15(5):1096–1106. doi: 10.1002/chem.200801906. [DOI] [PubMed] [Google Scholar]; (c) Samanta A, Vendrell M, Das R, Chang YT. Chem. Commun. 2010;46(39):7406–7408. doi: 10.1039/c0cc02366c. [DOI] [PubMed] [Google Scholar]; (d) Fabian J, Nakazumi H, Matsuoka M. Chem. Rev. 1992;92(6):1197–1226. [Google Scholar]
- 11.Zou WQ, Visser C, Maduro JA, Pshenichnikov MS, Hummelen JC. Nat. Photonics. 2012;6(8):560–564. [Google Scholar]
- 12.Hong R, Fernandez JM, Nakade H, Arvizo R, Emrick T, Rotello VM. Chem. Commun. 2006;(22):2347–2349. doi: 10.1039/b603988j. [DOI] [PubMed] [Google Scholar]
- 13.Dong AG, Ye XC, Chen J, Kang YJ, Gordon T, Kikkawa JM, Murray CB. J. Am. Chem. Soc. 2011;133(4):998–1006. doi: 10.1021/ja108948z. [DOI] [PubMed] [Google Scholar]; Zheng W, Zhou SY, Chen Z, Hu P, Liu YS, Tu DT, Zhu HM, Li RF, Huang MD, Chen XY. Angew. Chem. Int. Edit. 2013;52(26):6671–6676. doi: 10.1002/anie.201302481. [DOI] [PubMed] [Google Scholar]
- 14.Mai HX, Zhang YW, Si R, Yan ZG, Sun LD, You LP, Yan CH. J. Am. Chem. Soc. 2006;128(19):6426–6436. doi: 10.1021/ja060212h. [DOI] [PubMed] [Google Scholar]
- 15.(a) Meruga JM, Baride A, Cross W, Kellar JJ, May PS. J. Mater. Chem. C. 2014;2(12):2221–2227. [Google Scholar]; (b) Lee J, Bisso PW, Srinivas RL, Kim JJ, Swiston AJ, Doyle PS. Nat. Mater. 2014;13(5):524–529. doi: 10.1038/nmat3938. [DOI] [PubMed] [Google Scholar]; (c) Chen GY, Agren H, Ohulchanskyy TY, Prasad PN. Chem. Soc. Rev. 2015;44(6):1680–1713. doi: 10.1039/c4cs00170b. [DOI] [PubMed] [Google Scholar]; (d) Bunzli JCG, Eliseeva SV. Chem. Sci. 2013;4(5):1939–1949. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


