Sir:
With interest but also concern we read the article on juvenile-like polyps in neurofibromatosis type 1 (NF-1) by Agaimy et al.1 They present 15 patients (four new and eleven from the literature) with NF-1 and one or more juvenile-like/inflammatory/hyperplastic gastrointestinal polyps. Most patients had only a single (7 patients), two (3 patients) or three (1 patient) polyps, but four patients had ten or more polyps.
Of note, none of the patients were genetically tested for juvenile polyposis syndrome (JPS). This is particularly important in the four patients fulfilling the WHO criteria for JPS (≥5 juvenile polyps).2 Germline mutations in the SMAD4 or BMPR1A genes are found in 50–60% of patients who fulfill the WHO criteria for JPS.3 Of course, the one to three inflammatory and hyperplastic gastrointestinal polyps in the upper and lower gastrointestinal tract of NF-1 patients may well represent sporadic (reactive) lesions unrelated to NF-1. Inflammatory and hyperplastic polyps are not uncommon in the general population. In addition, their arguments that cafe-au-lait spots have been reported in patients with JPS and that polyps in NF-1 have a “peculiar histology” lack specificity.4, 5
Our concern, based on the data presented by Agaimy et al., is that juvenile polyposis syndrome remains unrecognized in patients with NF-1. When clinicians believe that these polyps are part of the spectrum of NF-1, they may not investigate for JPS. Patients with unrecognized JPS receive suboptimal care, since this syndrome is associated with significant risk of gastrointestinal carcinoma requiring specific management. Therefore, we strongly recommend that patients fulfilling the WHO criteria for JPS be offered genetic testing and appropriate treatment for this syndrome.2
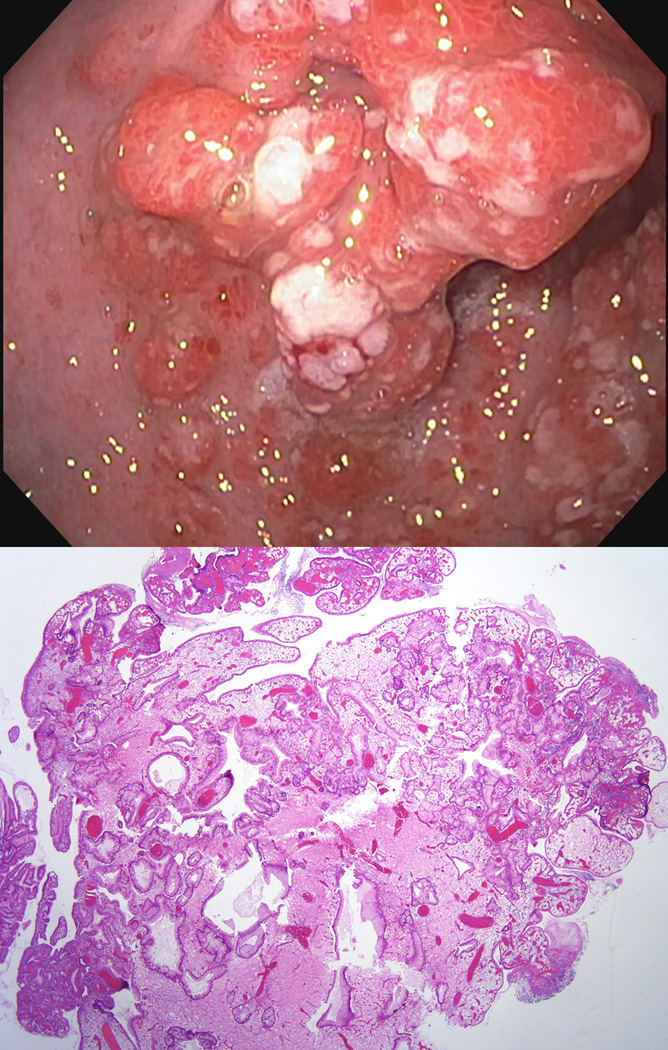
To support our concern we report a patient with both NF-1 and JPS. The patient was diagnosed with NF-1 at 2 years of age, based on presence of multiple café-au-lait spots, axillary freckling and one parent with NF-1. At later age, bilateral optic nerve gliomas, which were treated with radiation, and multiple cutaneous and subcutaneous neurofibromas developed. As an adult, genetic testing confirmed a germline mutation in the NF1 gene. At age 30 multiple colorectal juvenile/inflammatory polyps were diagnosed. Cumulatively, more than 27 colorectal polyps developed, of which at least 10 were histologically diagnosed as juvenile/inflammatory polyps, and 2 as tubular adenomas. In addition, multiple gastric hyperplastic polyps occurred, in this setting consistent with gastric juvenile polyps,4 11 of which were histologically confirmed. (Figure 1) Juvenile polyposis syndrome was confirmed in this patient by finding a germline mutation in the SMAD4 gene.3
Figure 1.
Endoscopic (top) and microscopic (bottom) pictures of a gastric juvenile polyp in the patient with both NF1 and JPS. Histology of gastric juvenile polyps is non-specific and these polyps are often indistinguishable from gastric hyperplasic polyps.
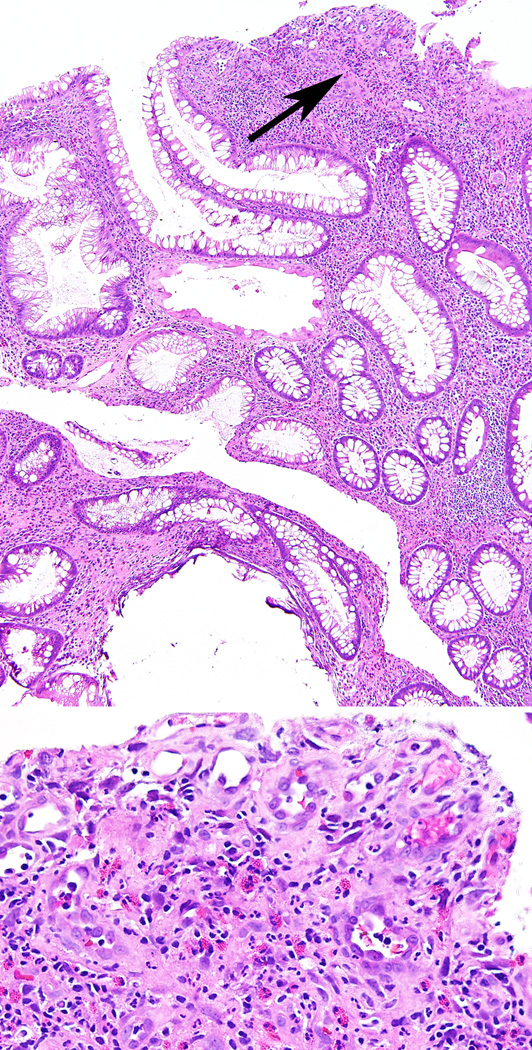
Agaimy et al. state that polyps in NF-1 are better classified as inflammatory-type polyps than juvenile-type polyps. We indeed agree that the term inflammatory polyp seems to be most appropriate for polyps in NF-1 patients with one or a few polyps since these may well be sporadic (reactive) lesions. Moreover, Agaimy et al. state that polyps in NF1 have a “peculiar histology” that differs from classic syndromic juvenile polyps. However, histology of juvenile polyps in JPS is variable and colorectal juvenile polyps and inflammatory polyps are histologically often indistinguishable. Therefore, without knowledge of the clinical context pathologists should be careful about the exact type or etiology of such polyps, since this may mislead clinicians in further patient management. In fact, some colonic polyps in the patient presented here were described as inflammatory polyps with granulation tissue and atypical stromal cells similar to some polyps in the report by Agaimy et al.1. (Figure 2) Furthermore, histology of gastric polyps in JPS is nonspecific and syndromic gastric polyps are often indistinguishable from sporadic gastric hyperplastic polyps.4 Of note, SMAD4 immunohistochemistry can be used as a first screening method in the molecular diagnosis of JPS. An underlying germline SMAD4 mutation is likely if reduced (compared with surrounding stroma) or absent SMAD4 expression is found in the epithelial component of a juvenile polyp. However, normal SMAD4 expression is less predictive of germline status.6
Figure 2.
Microphotograph of a small colonic juvenile polyp from the patient with NF1 and JPS showing expansion of the lamina propria with abundant inflammatory infiltrate and cystically dilated colonic crypts. In addition, there is surface erosion, granulation tissue and ‘atypical’ stromal cells, mainly representing reactive endothelial cells (arrow top panel and magnified in bottom panel).
To conclude, although an association between inflammatory polyps and NF-1 cannot be excluded, the conclusions made by Agaimy et al. are preliminary and may prevent appropriate medical care. The assumption that inflammatory/juvenile polyps are a specific gastrointestinal manifestation of NF-1 suggests no need to evaluate for the presence of an underlying polyposis syndrome in patients with NF-1 and inflammatory/juvenile-like polyps. Strengthened by our observation of a patient with both genetically proven NF-1 and JPS, we advise that patients fulfilling the WHO criteria for JPS should be offered appropriate management and genetic testing.2 This is not only important for patient management, in view of the gastrointestinal cancer risk in JPS and family counseling, but will also clarify the true association of intestinal polyps with NF-1.
Acknowledgments
Funding support: The John G Rangos Sr. Charitable Foundation; The Clayton Fund; NIH grant P50 CA62924;
Footnotes
Financial disclosures and/or conflicts of interest: The authors have nothing relevant to disclose.
References
- 1.Agaimy A, Schaefer IM, Kotzina L, et al. Juvenile-like (inflammatory/hyperplastic) mucosal polyps of the gastrointestinal tract in neurofibromatosis type 1. Histopathology. 2014;64:777–786. doi: 10.1111/his.12325. [DOI] [PubMed] [Google Scholar]
- 2.Syngal S, Brand RE, Church JM, et al. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol. 2015;110:223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hattem WA, Brosens LA, de Leng WW, et al. Large genomic deletions of SMAD4, BMPR1A and PTEN in juvenile polyposis. Gut. 2008;57:623–627. doi: 10.1136/gut.2007.142927. [DOI] [PubMed] [Google Scholar]
- 4.Lam-Himlin D, Park JY, Cornish TC, Shi C, Montgomery E. Morphologic characterization of syndromic gastric polyps. Am J Surg Pathol. 2010;34:1656–1662. doi: 10.1097/PAS.0b013e3181f2b1f1. [DOI] [PubMed] [Google Scholar]
- 5.Shah KN. The diagnostic and clinical significance of cafe-au-lait macules. Pediatr Clin North Am. 2010;57:1131–1153. doi: 10.1016/j.pcl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Langeveld D, van Hattem WA, de Leng WW, et al. SMAD4 immunohistochemistry reflects genetic status in juvenile polyposis syndrome. Clin Cancer Res. 2010;16:4126–4134. doi: 10.1158/1078-0432.CCR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]