Abstract
Background
Multiple sclerosis (MS) predominantly affects women with a sex ratio of 3:1 in contrast with a 1:1 sex ratio seen in pre-pubertal onset. Thus, puberty may influence MS risk differentially in males and females. How puberty may be associated with MS clinical features and disease course remains unknown.
Objective
To determine the association of menarche with disease course in girls with MS.
Methods
This is a longitudinal retrospective study from the UCSF Regional Pediatric MS Center database. We categorized patients by time of disease onset: pre-menarche, peri-menarche and post-menarche. Poisson regression models were used for within subject relapse analyses offset by follow-up time.
Results
Seventy six girls were included (pre-menarche onset=17; peri-menarche onset=9; post-menarche onset=50). Age of menarche was similar in all groups (Kruskal-Wallis p=0.19). Relapse rate was the same in all three groups during first 2 years of follow-up. In girls with follow-up overlapping at least two time periods, within-subject analyses showed increased relapses during peri-menarche compared to post-menarche period (adjusted IRR=8.5, 95%CI 2.5–28.7, p=0.001).
Conclusion
Pubertal status may influence MS course at least in female patients. Understanding how puberty influences MS clinical features may offer new insights in important factors regulating disease processes.
Introduction
Multiple sclerosis (MS) onset occurs in childhood in about 5% (1,2) and before 10 years of age in 0.2 to 0.7% of all MS cases (2). That MS is more common in women and pregnancy is a major disease modifier, point at the role of sex hormones (3). The effect of other dramatic hormonal changes such as those occurring during puberty on MS course is largely unknown.
Younger age at menarche may be associated with increased risk for adult MS (4) while MS onset in adults may be delayed in those with later menarche (5). In pediatric MS, the female to male ratio varies with age of onset from 1:1 in younger patients approaching adult ratio of 2–3:1 after age 11 suggesting a critical role of puberty (6). The few reports on puberty and MS risk have been generated from retrospective studies of patients with adult-onset MS (4,6,7) and as such have faced recall bias to assess age of puberty when questioning adults in their 30s or 40s. A recent longitudinal study evaluated the association of puberty and clinical presenting features in pediatric MS using the same age cut-off for all patients although age of puberty varies substantially between individuals and genders (8).
First, we aimed to determine differences in disease course and presentation in pre-menarche, peri-menarche and post-menarche onset females with pediatric MS, classified by time of disease onset relative to menarche. Second, we performed within-subject analyses in those with MS onset pre- or peri-menarche to determine if the variation in disease course in a given patient can be explained by association with menarche.
Methods and subjects
Study design
This is a retrospective study of patients seen at the University of California San Francisco (UCSF) pediatric MS center between January 2006 and June 2014.
Standard protocol approvals, registration and patient consents
This study was approved by the UCSF Committee on Human Research and is part of an ongoing cohort study in pediatric MS. Written informed consent was obtained from parents and patients aged 18 years and older. Assent was also obtained from all patients younger than 18 years of age.
Puberty definition
Puberty is a physiological process that encompasses several years. Although Tanner stages are used to define various phases of puberty (9,10), this tool is in fact poorly reliable as it is usually completed by the patient or the parents and the scoring is not very consistent over time (11). We used onset of menarche as a surrogate for puberty. We therefore included only female patients since there is not an equivalent milestone of puberty in males. We split time of first demyelinating event into three epochs relative to menarche: 1)pre-menarche (defined as any time occurring at least 6 months before menarche), 2) peri-menarche (defined as within six months before or after menarche) to take in account various hormonal changes over time, and 3)post-menarche (defined as at least 6 months after menarche).
Study subjects
We included female patients who fulfilled recently published criteria for pediatric MS (13). We collected date of menarche at the time of visits in our standard clinic questionnaire. We excluded patients with other demyelinating disease or with a history of use of any hormonal therapy that could interfere with onset and physiology of normal puberty.
Clinical and demographic data
We prospectively collected clinical data at the time of their routine clinical visits (date of first demyelinating event, presenting symptoms, cerebrospinal fluid (CSF) results, neurological exam as measured by EDSS and body mass index measured at their first clinical visit).
Date of first demyelinating event was defined as the date of onset of first neurological symptoms of MS. First clinical visit was defined as first visit to UCSF pediatric MS center. Race and ethnicity were self-reported according to NIH definitions. Race was dichotomized into whites and non-whites. Patients with mixed race were considered non-white. Ethnicity was dichotomized into Hispanic and non-Hispanic. Relapses were defined according to prior publications as new neurological symptoms localizing to the CNS and lasting for at least 24 hours after a remission of 30 days or more since the previous attack in the absence of an infection or fever (12). Pseudo-relapses were not included. The first demyelinating event was not included as a relapse to avoid an artificially elevated relapse rate in those with shorter follow-up duration.
Statistical analyses
We used Fisher’s exact test to compare dichotomous outcomes and Kruskal-Wallis test to compare continuous non-parametric outcomes across the three groups. We used Poisson regression models with random intercepts to evaluate number of relapses offset by time from first demyelinating event for across group analyses. Only the first two-year follow-up data were used as for longer durations regression-to-the-mean can occur. In addition, we did not want to contaminate pre- and peri-menarche data with subsequent epochs.
We also used mixed effects Poisson regression models with random intercepts to accommodate the repeated measures gathered per subject and to provide estimates of within-subject associations of time period with relapses offset by follow up time. This analysis allows comparison of relapses within a subject based on its occurrence pre-, peri- or post-menarche. This analysis only allows comparison in subjects with events in at least two time periods; therefore only patients with pre-menarche (who reached menarche) and peri-menarche onset were included (n=17). As this is a within-subject analysis we only adjusted for time on disease-modifying therapy during each epoch.. All analyses were completed using Stata/SE12.1 (Statacorp, College Station, TX). A two sided p-value of less than 0.05 was considered significant.
Results
Patient characteristics
Ninety-two female patients with pediatric-onset MS seen at the UCSF regional pediatric MS clinic between January 2006 and July 2014 were identified from the pediatric MS database in which all cases seen are entered prospectively. Seventy-six girls fulfilled inclusion criteria. We excluded 16 of 92 patients: 10 for missing menarche data; four for unconfirmed diagnosis of MS, one for primary progressive MS and one for precocious puberty complicated by hormonal abnormalities on multiple hormonal therapies.
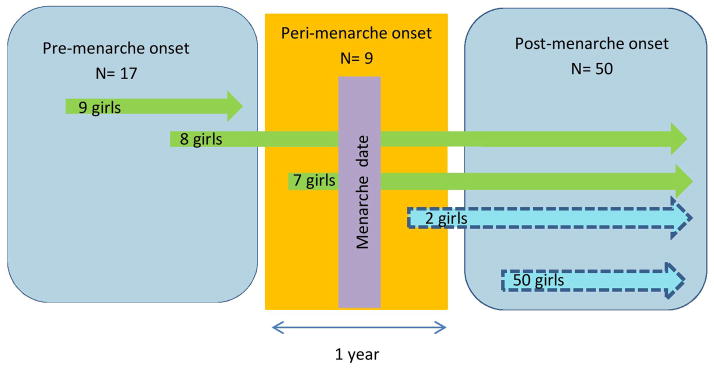
There were 17 girls (22%) with pre-menarche MS onset, nine (12%) with peri-menarche onset (i.e. within six months of menarche) and 50 (66%) with post-menarche onset (Figure 1). Of the 17 pre-menarche onset patients, nine had not yet experienced menarche at the time of this analysis. Of the 76 girls with pediatric MS, 31 (42%) were Hispanic and 40 (52%) were White (Table 1); non-whites included two (3%) American Indian/ Alaska Native, 12 (16%) Asian, seven (9%) African American, and 11 (15%) were of mixed race. Race was missing for four girls (5%).
Figure 1. Menarche and time of disease onset in girls with pediatric multiple sclerosis at the University of California, San Francisco.
This graph shows the three time epochs of menarche; pre-menarche, peri-menarche and post-menarche. The time of their first demyelinating event is represented by the beginning of the arrow. A total of 17 girls had pre-menarche onset of their first demyelinating event, of which nine girls have not yet experienced menarche at the time of their last visit. Eight girls with pre-menarche onset were followed up through peri-menarche period and post-menarche period. A total of nine girls had onset of symptoms within six months of menarche (peri-menarche); Seven before and two after. The majority of our patients (50 girls) had their first demyelinating event after menarche.
Table 1.
Demographics are described in pre-, peri- and post-menarche epochs by first demyelinating event onset.
| Total n=76 |
Pre-menarche onset n=17 |
Peri-menarche onset n= 9 |
Post-menarche onset n=50 |
|
|---|---|---|---|---|
|
| ||||
| Median age at first demyelinating event, (IQ range) | 14.2 (4, 17) | 4.9 (4, 8) | 13.1 (12, 14) | 15.2 (13, 17) |
|
| ||||
| Median age of menarche in those who reached menarche (IQ range) | 12.1 (9.5,14.5) | 12 (11.9, 12.1) | 13.5 (12, 13.9) | 12.1 (9.5, 14.3) |
| Hispanic | 11.9 (9.5, 13.5) | 12 (9.8, 12.5) | 13.5 (11.9, 14.5) | 11.5 (9.5, 13.5) |
| White | 12.2 (9.5, 14.4) | 12 (11.2, 12.5) | 13.5 (11.9, 14.4) | 12.3 (9.5, 13.5) |
|
| ||||
| Hispanic, n (%)a | 31/73 (42) | 8 (47) | 3 (33) | 20/47 (43) |
|
| ||||
| White, n (%) | 40 (52) | 8 (47) | 7 (78) | 25 (50) |
|
| ||||
| Mean body mass indexb ± SD | 26.4 ± 7.3 | 20.7 ± 6.2 | 28.5 ± 10.6 | 27.8 ± 6 |
|
| ||||
| Median disease duration at end of follow up in years, (IQ range) | 3.7 (0.4, 10.5) | 5.6 (2.9, 7.7) | 5.5 (5.4, 5.9) | 2.7 (0.4, 7.4) |
|
| ||||
| Median time from first demyelinating event to first clinic visit, months (IQ range) | 5.3 (0.6, 52) | 8.8 (2.8, 52) | 5.4 (3.6, 22.5) | 4.2 (1, 26.4) |
|
| ||||
| Treatment with disease-modifying therapy, n (%) | 68 (89) | 15 (88) | 8 (89) | 45 (90) |
|
| ||||
| First disease-modifying therapy used, n (%) | ||||
| Injectables | 65 (86) | 15 (94) | 8 (89) | 42 (84) |
| Dimethylfumarate | 1 (1) | 0 | 0 | 1 (2) |
| Natalizumab | 2 (3) | 0 | 0 | 2 (4) |
| None | 8 (10) | 2 (12) | 1 (11) | 5 (10) |
|
| ||||
| Lumbar puncture performed, n (%) | 64 (84) | 17 (100) | 7 (78) | 40 (80) |
|
| ||||
| Presence of at least 2 oligoclonal bands in cerebrospinal fluidc, n/total available (% ) | 39/53 (74) | 5/14 (36) | 5/6 (83) | 29/33 (88) |
Ethnicity was unknown in 3 post-menarche subjects.
Body mass index was missing in 1 subject.
Presence of oligoclonal bands in those with results of cerebrospinal fluid analysis.
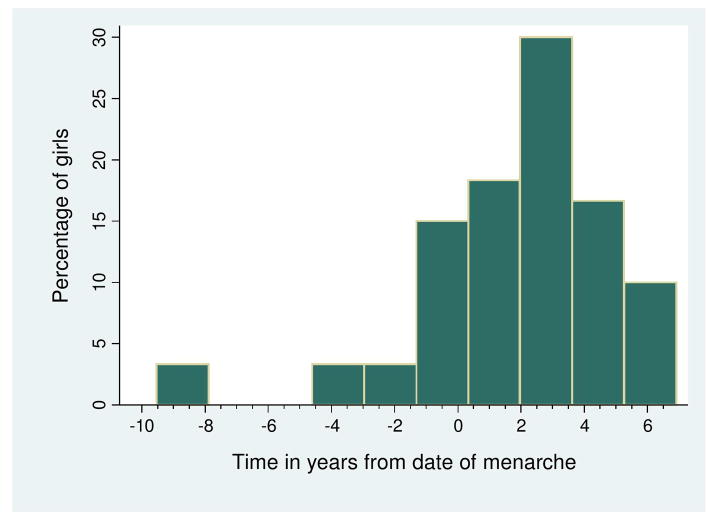
Median age of patients at first demyelinating event was 4.9 years (IQ range 4, 8) in the pre-menarche, 13.1 years (IQ range 12, 14) in the peri-menarche and 15.2 years (IQ range 13, 17) in the post-menarche groups (Table 1). For those who reached this milestone, median age of menarche was 12.1 (IQ range 9.5, 14.5) (Table 1). Median age of menarche was similar between the three groups (Kruskal-Wallis test p=0.19). For those who reached menarche, median disease onset was 2.01 years (IQ range −8, 5.5) after menarche (Figure 2).
Figure 2. Time of first demyelinating event from menarche.
This is a histogram showing the time in years of the first demyelinating event and onset of menarche in 60 girls with pediatric MS. Nine patients who have not yet reached menarche are not included. Median time of first demyelinating event was 2.2 years after menarche. Time less than zero indicates onset prior to menarche.
Presenting symptoms of first demyelinating event
There were differences in first demyelinating symptoms between pre-, peri and post-menarche onset patients. Post-menarche onset patients tended to present with sensory symptoms compared to peri- and pre-menarche girls (48% versus 33% and 18%, p=0.08, Fisher’s exact test, Table 2). Cognitive changes at the time of first symptoms were less common in post-menarche onset compared to peri- and pre-menarche onset girls (4% versus 22% and 41%, p=0.001, Fisher’s exact test, Table 2).
Table 2.
Presenting symptom in pre-, peri- and post-menarche onset girls with multiple sclerosis
| Presenting neurological system | Pre-menarche onset, n=17 (%) | Peri-menarche onset, n= 9 (%) | Post-menarche onset, n= 50 (%) | P-valuea |
|---|---|---|---|---|
| Visual | 7 (41) | 2 (22) | 21 (42) | 0.60 |
| Pyramidal | 6 (35) | 1 (11) | 13 (26) | 0.49 |
| Brainstem | 5 (29) | 2 (22) | 22 (44) | 0.35 |
| Sensory | 3 (18) | 3 (33) | 24 (48) | 0.08 |
| Cerebellar | 6 (35) | 2 (22) | 13 (26) | 0.73 |
| Bowel and bladder | 2 (12) | 0 | 4 (8) | 0.67 |
| Cognitive/cerebral | 7 (41) | 2 (22) | 2 (4) | 0.001 |
| More than one system | 9 (53) | 3 (33) | 30 (60) | 0.35 |
Fisher’s exact test.
Relapse rate analysis between menarche groups
For between group analyses we compared relapse rates across the three epochs according to time of onset. Due to differences in follow up time (Table 1) we limited our analyses to only include the first two years of follow-up. Because race and ethnicity have been associated with both age of menarche and relapses (14); they were adjusted for in Model B (Supplementary Table 1). While the use of disease modifying therapy (DMT) is not known to be associated with menarche, and BMI is only associated with menarche (14), they may strongly modify the relapse rate and we included them in the third model, (Supplementary Table 1). There were no differences noted in incidence rate ratios between groups in both unadjusted and adjusted models used (Supplementary Table 1).
Within-patient longitudinal relapse analysis
There were a total of 229 relapses captured across all patient epochs (Table 3). To better understand how going through menarche influences relapses, we performed within-subject analyses. However, not all relapse data were used for comparison. Since cases with post-menarche onset (n=50) and those with pre-menarche MS onset who have not yet experienced menarche (n=9) have only one epoch to contribute (and thus were not informative); the within-patient analysis is limited to 17 patients with at least two epochs after disease onset.
Table 3.
Relapse data from patients with at least 3 months duration in each epoch stratified by the epoch the relapse occurred.
| Pre-menarche relapses | Peri-menarche relapses | Post-menarche relapses | |
|---|---|---|---|
| Total relapses captured (n= 229) | 22 | 15 | 192 |
| Total patients contributing to relapses | 15 | 14 | 59 |
| Median time during the epoch, years (IQ range) | 3.6 (2.3 – 5.6) | 1 (1 – 1) | 5.4 (3.9 – 7.5) |
| Median annualized relapse rate (IQ range) | 0 (0 – 0.7) | 1 (1 – 1) | 0.51 (0.3 – 0.8) |
IQ: interquartile range
The peri-menarche epoch was associated with a higher relapse rate compared to the post-menarche epoch in both unadjusted (IRR = 10.9, 95% CI 4–30; p<0.001, Table 4) and adjusted for time on DMT analyses (IRR = 8.5, 95% CI 2.5–28.7); p<0.001, Table 4). There was a lower relapse risk in pre- compared to peri-menarche epoch in unadjusted models (IRR= 0.1, 95% CI 0.03–0.4, p=0.001); but not when adjusted for time on DMT (IRR= 0.4, 95% CI 0.05–3.2, p= 0.4).
Table 4.
Unadjusted and adjusted incidence rate ratios in pre- and peri-menarche epochs compared to post-menarche epoch performed by mixed effects poisson regression models.
| Pre-menarche | Peri-menarche | |||
|---|---|---|---|---|
| IRR (95% CI) | p-value | IRR (95% CI) | p-value | |
| Unadjusted | 1.1 (0.3, 4.4) | 0.9 | 10.9 (4, 30) | <0.001 |
| Adjusted | 3.5 (0.4, 31) | 0.3 | 8.5 (2.5, 28.7) | 0.001 |
IRR: Incidence rate ratio; DMT: Disease-modifying therapy
Discussion
We report clinical demographics in pediatric onset girls relative to before, during and after menarche. There are no prior studies describing these associations specifically within the peri-menarche time period in pediatric MS. We report that within-patient relapse rate showed a spike in relapse risk only during the peri-menarche epoch. While this is based on a relatively small number of patients, it is of interest and suggests that specific hormonal changes occurring around menarche may contribute to increased disease activity at least transiently. This is important to recognize when making treatment decisions in these patients.
In contrast with our within-patients’ findings, relapse risk in patients with pre-, peri- and post menarche onset suggested no differences in unadjusted and adjusted analyses using data from the first 2 years of follow-up. This is in line with a recent study in pediatric MS, which reported similar annualized relapse rate in pre- and post-pubertal patients using different definitions: pre-pubertal patients were defined as those under 11 years and post-pubertal as those with age of onset from 14 to 16 years (8). However, the peri-pubertal time period was not included in their analyses.
We describe the temporal association between pediatric MS onset and puberty in girls using patient-specific date of menarche, a biological milestone, instead of age cut-off. Our data allowed us to address the effects of the peri-menarche period. We also adjusted for BMI and use of DMT which are potentially important modifiers of relapse rate.
It is possible that the finding of increased relapses in peri-menarche epoch is only noted on the within-group analysis because of many complex variables that play a role in MS disease course and activity. Within-patient analyses compared the relapses within the same patient and minimized the variability in disease course that is known to occur across MS patients. Using a cut off time period of two years in our between-group analysis allowed us to examine more closely the potential temporal association with the epoch of interest at the expense of not using relapse data beyond two years.
In our study, menarche age was not statistically different between pre-, peri- and post-menarche onset groups. Although age at menarche may influence the risk of adult MS and its time of onset (5), disease onset in pre-pubertal girls suggests other precipitating or inducing factors. Median age of menarche in a recent United States survey study was 12.4 years which is in line with our findings (median age 12.1) (15).
Puberty is a physiological developmental event that is associated with major hormonal and immunologic changes. Leptin, an acute phase reactant, acts as a permissive factor for the onset of puberty likely through its effects on luteinizing hormone release hormone secretory hormones of the hypothalamus which orchestrates the hormonal changes of puberty (17,18).
During infancy and early childhood, luteinizing hormone release hormone (LHRH) pulse generator is suppressed. Dis-inhibition of LHRH pulse generator at the time of puberty results in pulsatile LHRH discharges which influences increased stimulation of luteinizing hormone (LH) and follicle stimulating hormone (FSH). During the different stages of puberty, LH peaks continue to rise, whereas FSH does not and puberty is precipitated by an increased LH to FSH ratio (19).
Leptin levels rise just prior to puberty regardless of gender and continue to rise during puberty in girls but not in boys (20,21). This difference may be due to inhibitory effects of testosterone secretion on leptin (22). Leptin promotes pro-inflammatory processes thought to be associated with MS (23,24). Interestingly, leptin-deficient mice are resistant to induction of experimental autoimmune encephalomyelitis, the animal model of MS (25). Our findings of a peak in disease onset two years after menarche (Figure 2) are also consistent with the role of puberty and sex hormones in the complex immunological processes occurring in MS.
The strengths of our study include the clear identification of date of menarche in patients who were prospectively followed at our center. Although the majority of the patients presented after menarche and menarche date was collected in that case retrospectively after MS onset, the short time elapsed between menarche onset and time of information collection and the presence of their parents at the visit reduced recall bias. We also have carefully adjusted analyses for factors known to be associated with relapse rate such as race, BMI and use of DMT.
Some of our study limitations include the limited sample size in the younger age groups and the restriction to girls which limits the generalizability to all children with pediatric-onset MS. In addition, we cannot rule out that the younger patient group may have under reported mild relapses due to lack of awareness related to age. However, the fact that the within-patient relapse rate was similar in pre- and post-menarche epochs in contrast with the spike around menarche argues against this bias. A potential bias could include better adherence to DMT in younger children for whom parents administer the medication, we do not have adherence data in our patient sample to investigate more comprehensively this potential bias. Finally, future studies should benefit from use of hormonal levels to better define pubertal status.
As seen with pregnancy, puberty may influence MS course. Peri-menarche period could represent a higher risk for relapses. Understanding how puberty influences MS clinical features in both sexes may offer insights in important factors regulating disease processes. Utilizing multi-center data would improve sample size limitations. Future studies will benefit from measurements of potential biomarkers of the hormonal changes of puberty such as leptin and sex hormones.
Supplementary Material
Acknowledgments
Dr. Lulu is funded by a Sylvia Lawry fellowship award from the National Multiple Sclerosis Society (NMSS) (FP 1784-A-1). This study is funded by a fellowship research grant from Questcor (A122760). The pediatric MS clinic is funded by the NMSS. Dr. Graves is supported by the Foundation for the CMSC and NIH BIRCWH program (5K12HD052163). Dr. Waubant is supported by the NIH (R01NS071463).
Footnotes
Author contributions:
Sabeen Lulu designed the study, performed data entry, statistical analysis and interpreted the results. Dr. Lulu wrote the first draft of the paper.
Jennifer Graves contributed to study design, statistical analysis and results interpretation. Dr. Graves revised manuscript for intellectual content.
Emmanuelle Waubant supervised study design, statistical analysis and interpretation of results. Dr. Waubant revised manuscript for intellectual content.
Contributor Information
Jennifer Graves, Email: Jennifer.Graves@ucsf.edu.
Emmanuelle Waubant, Email: Emmanuelle.Waubant@ucsf.edu.
References
- 1.Krupp LB, Banwell B, Tenembaum S International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007 Apr 17;68(16 Suppl 2):S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 2.Renoux C, Vukusic S, Mikaeloff Y, Edan G, Clanet M, Dubois B, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007 Jun 21;356(25):2603–2613. doi: 10.1056/NEJMoa067597. [DOI] [PubMed] [Google Scholar]
- 3.Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004 Jun;127(Pt 6):1353–1360. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- 4.Ramagopalan SV, Valdar W, Criscuoli M, DeLuca GC, Dyment DA, Orton SM, et al. Age of puberty and the risk of multiple sclerosis: a population based study. Eur J Neurol. 2009 Mar;16(3):342–347. doi: 10.1111/j.1468-1331.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 5.Sloka JS, Pryse-Phillips WE, Stefanelli M. The relation between menarche and the age of first symptoms in a multiple sclerosis cohort. Mult Scler. 2006 Jun;12(3):333–339. doi: 10.1191/135248506ms1267oa. [DOI] [PubMed] [Google Scholar]
- 6.Tintore M, Arrambide G. Early onset multiple sclerosis: the role of gender. J Neurol Sci. 2009 Nov 15;286(1–2):31–34. doi: 10.1016/j.jns.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Sloka JS, Pryse-Phillips WE, Stefanelli M. The relation between menarche and the age of first symptoms in a multiple sclerosis cohort. Mult Scler. 2006 Jun;12(3):333–339. doi: 10.1191/135248506ms1267oa. [DOI] [PubMed] [Google Scholar]
- 8.Huppke B, Ellenberger D, Rosewich H, Friede T, Gartner J, Huppke P. Clinical presentation of pediatric multiple sclerosis before puberty. Eur J Neurol. 2014 Mar;21(3):441–446. doi: 10.1111/ene.12327. [DOI] [PubMed] [Google Scholar]
- 9.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970 Feb;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969 Jun;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmangles JC, Lappe JM, Lipaczewski G, Haynatzki G. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol Metab. 2006 Mar;19(3):213–221. doi: 10.1515/jpem.2006.19.3.213. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011 Feb;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013 Sep;19(10):1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 14.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008 Feb;121( Suppl 3):S208–17. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 15.Chumlea WC, Schubert CM, Roche AF, Kulin HE, Lee PA, Himes JH, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003 Jan;111(1):110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 16.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005 Jun;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 17.Matkovic V, Ilich JZ, Badenhop NE, Skugor M, Clairmont A, Klisovic D, et al. Gain in body fat is inversely related to the nocturnal rise in serum leptin level in young females. J Clin Endocrinol Metab. 1997 May;82(5):1368–1372. doi: 10.1210/jcem.82.5.3917. [DOI] [PubMed] [Google Scholar]
- 18.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997 Feb;138(2):855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 19.Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB., Jr Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990 Nov;71(5):1251–1258. doi: 10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 20.Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab. 1997 Apr;82(4):1066–1070. doi: 10.1210/jcem.82.4.3878. [DOI] [PubMed] [Google Scholar]
- 21.Roemmich JN, Clark PA, Berr SS, Mai V, Mantzoros CS, Flier JS, et al. Gender differences in leptin levels during puberty are related to the subcutaneous fat depot and sex steroids. Am J Physiol. 1998 Sep;275(3 Pt 1):E543–51. doi: 10.1152/ajpendo.1998.275.3.E543. [DOI] [PubMed] [Google Scholar]
- 22.Horlick MB, Rosenbaum M, Nicolson M, Levine LS, Fedun B, Wang J, et al. Effect of puberty on the relationship between circulating leptin and body composition. J Clin Endocrinol Metab. 2000 Jul;85(7):2509–2518. doi: 10.1210/jcem.85.7.6689. [DOI] [PubMed] [Google Scholar]
- 23.Matarese G, Procaccini C, De Rosa V. The intricate interface between immune and metabolic regulation: a role for leptin in the pathogenesis of multiple sclerosis? J Leukoc Biol. 2008 Oct;84(4):893–899. doi: 10.1189/jlb.0108022. [DOI] [PubMed] [Google Scholar]
- 24.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998 Jan;12(1):57–65. [PubMed] [Google Scholar]
- 25.Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001 May 15;166(10):5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.