Abstract
Aims
This study is to examine the significance of the number and ratio of positive nodes in post neoadjuvant therapy pancreaticoduodenectomy (PD) for pancreatic ductal adenocarcinoma (PDAC).
Methods and results
Our study population consisted of 398 consecutive PDAC patients, who completed neoadjuvant therapy and PD between 1999 and 2012. Lymph node status was classified as ypN0 (node negative), ypN1 (1–2 positive nodes) and ypN2 (≥3 positive nodes) and correlated with disease-free survival (DFS) and overall survival (OS). The ypN0, ypN1 and ypN2 was present in 183 (46.0%), 117 (29.4%) and 98 (24.6%) patients respectively. Additionally, 162 (40.7%) had a lymph node ratio (LNR) ≤ 0.19 and 53 (13.3%) had a LNR > 0.19. Patients with ypN1 disease had shorter DFS and OS than those with ypN0 disease, but better DFS and OS than those with ypN2 disease (P<0.05). Similarly, patients with a LNR≤0.19 had better DFS and OS than those with a LNR≤0.19 (P<0.001). In multivariate analysis, both the number of positive nodes and LNR were independent prognostic factors for DFS and OS.
Conclusions
Subclassification of post-therapy node positive group into ypN1 (1–2 positive nodes) and ypN2 (≥ 3 positive nodes) should be incorporated into the AJCC staging of PDAC patients.
Keywords: Pancreatic cancer, Number of positive nodes, Lymph node ratio, Survival, Prognosis
INTRODUCTION
Pancreatic cancer remains a highly lethal disease with an estimated 46,420 newly diagnosed cases and 39,590 deaths in the United States in 20141. Approximately 80% of patients with pancreatic ductal adenocarcinoma (PDAC) have distant metastases or locally advanced disease at the time of diagnosis, with a 5-year survival of approximately 6%1. Despite significant improvements in operative techniques and treatment modalities, overall survival for patients with PDAC has not changed significantly in the past four decades1, 2.
Several factors have been shown to be associated with outcome following pancreaticoduodenectomy (PD) for PDAC. Along with tumor size, histologic grade, and resection margin status, the presence of lymph node metastasis is a well-known prognostic indicator for both disease-free and overall survival in patients with PDAC3–5. Recent studies have demonstrated the prognostic significance of total lymph node count, total number of positive lymph nodes, and lymph node ratio (LNR, the ratio of the number of positive lymph nodes to the total number of lymph nodes examined) in patients with PDAC who underwent PD. Several studies have shown that the examination of greater than 10 to 15 lymph nodes is associated with improved overall survival in patients with node negative disease6–8; however, other studies have failed to demonstrate this association9 and the experience with extended lymphadenectomy has not proven a survival benefit10–12. Among patients with PDAC who received either upfront surgery alone, or upfront surgery followed by adjuvant chemotherapy, both the number of lymph nodes involved by metastatic PDAC and LNR have been shown to be the predictors of survival13–15. In addition, some authors have reported that the LNR is a better predictor for survival than lymph node status alone16, 17. However, the prognostic significance of the number of positive lymph nodes and LNR in PDAC patients who received neoadjuvant therapy and subsequent PD is unclear. In our prior study of 240 PDAC patients, who received neoadjuvant therapy with subsequent PD, and 60 patients who underwent upfront surgery, we have shown that patients who received neoadjuvant therapy with subsequent PD had better overall survival and a lower frequency of lymph node metastasis than those who did not receive neoadjuvant therapy18. We also found that post-treatment AJCC stage and the total number of positive lymph nodes are independent prognostic factors for disease-free and overall survival18. In this study, we examine the prognostic significance of the number of positive lymph nodes and LNR in a cohort of 398 patients who received neoadjuvant therapy and underwent PD. Our results suggest the importance of classifying PDAC patients, with post-therapy node-positive disease, into ypN1 and ypN2 subgroups.
MATERIALS AND METHODS
Study Population, Patient Characteristics, and Follow-up
The study was approved by the institutional review board of The University of Texas M.D. Anderson Cancer Center. Three hundred ninety-eight consecutive patients, with histologically confirmed PDAC who received neoadjuvant therapy and underwent PD at our institution, between 1999 and 2012, were included in this study. Seventy-one patients (17.8%) received neoadjuvant fluoropyrimidine-based chemoradiation (group 1), 100 (25.1%) received neoadjuvant gemcitabine-based chemoradiation (group 2), 103 (25.9%) received systemic chemotherapy followed by gemcitabine-based chemoradiation (group 3), 106 (26.6%) received systemic chemotherapy followed by fluoropyrimidine-based chemoradiation (group 4) and the remaining 18 patients (4.5%) received neoadjuvant systemic chemotherapy alone (group 5). All patients underwent restaging evaluation after completion of neoadjuvant chemoradiation therapy. Pancreaticoduodenectomy was performed only in patients who had resectable disease, with no disease progression or metastasis, and had no contraindications to major abdominal surgery as previously reported19, 20. For this study, patients who underwent distal pancreatectomy, those who were determined to be unresectable after neoadjuvant therapy, and those who died of perioperative complications were excluded. To accurately evaluate the lymph node status in our study population, 13 patients with less than 12 lymph nodes examined were also excluded.
Clinical follow-up information was extracted from a prospectively maintained database at the Department of Surgical Oncology and verified by reviewing patient medical records and the U.S. Social Security Death Index. Recurrence status was updated at each follow-up clinic visit.
Pathologic Evaluation
A standardized system for the evaluation of PD specimens has been used at our institution since 1990. All cases were evaluated for tumor type, tumor size, differentiation, margin status, total number of lymph nodes, number of positive lymph nodes, post-treatment pathologic stage, and histopathologic tumor response grade (HTRG). The post-treatment pathologic staging was grouped according to the American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition21. HTRG was performed using the modified grading system of the College of American Pathologists (CAP), as reported in our previous study22. Lymph node ratio (LNR) was calculated as a ratio of the number of positive lymph nodes to the total number of lymph nodes examined. For statistical analyses, patients were first grouped according to lymph node status: patients with no lymph node metastasis (ypN0) and those with node positive disease. Patients with node positive disease were subclassified into ypN1 (1–2 positive lymph nodes) and ypN2 (≥ 3 positive lymph nodes). Separation into these categories was based on the median number of positive lymph nodes in the node positive group that gave the best separation of survival curves between the two groups. To identify the best cutoff value for LNR in our node-positive patient population, the LNR was initially stratified using the cutoff values of 25th percentile (0.06), 50th percentile (0.10), and 75th percentile (0.19) and then grouped into those with a LNR ≤0.19 and those with a LNR >0.19.
Statistical Analysis
Chi-square analysis or Fisher exact tests were used to compare categorical data and analysis of variance was used to compare continuous variables. Survival curves were constructed using the Kaplan-Meier method and the statistical significance of differences in survival was determined using the log-rank test. Disease-free survival (DFS) and overall survival (OS) were calculated as previously reported23. The prognostic significance of clinical and pathologic characteristics was determined using univariate Cox regression analysis. Cox proportional hazards models were fitted for multivariate analysis using a backward stepwise procedure. Statistical analysis was performed using Statistical Package for Social Sciences software (for Windows 22, SPSS Inc., Chicago, IL). A 2-sided significance level of 0.5 was used for all statistical analyses.
RESULTS
Patient Clinicopathologic Features
Our cohort consisted of 176 women and 222 men with a mean age of 63.5 years at the time of diagnosis (median, 64.1 years; range, 34.5–85.4 years). The average tumor size was 2.5 cm (range, 0 cm to 8.5 cm). Complete pathologic response (ypT0), ypT1, ypT2 and ypT3 were present in 9 (2.3%), 24 (6.0%), 9 (2.3%) and 356 (89.4%) respectively. The average number of lymph nodes identified was 24.4 (range, 12–68). Lymph node metastases were present in 215 (54.0%) patients. Among patients with node positive disease, the median number of lymph nodes involved by metastatic PDAC was 2.0 (range, 1–25). Within this group, 162 patients (75.3%) had a LNR of 0.19 (75th percentile LNR value) and 53 patients (24.7%) had a LNR > 0.19. Thirty-five patients (8.8%) had post-therapy pathologic stage IA or IB disease, 148 patients (37.2%) had stage IIA disease, and 215 patients (54.0%) had stage IIB disease. There were no patients with stage III or IV disease in this study. A total of 367 (92.2%) of patients had negative margins, while 31 (7.8%) had margin involvement by PDAC (R1). Clinicopathologic correlation of the number of positive lymph nodes and LNR in the 398 study patients are shown in Tables 1 and 2. Mean age at diagnosis was higher in patients with node negative disease than those with node positive disease. However, there was no difference between the subgroups within node positive group (P>0.05). Patients with ypN2 disease had more lymph nodes examined than those with ypN0 or ypN1 disease (P<0.001). The number of positive lymph nodes correlated significantly with ypT stage (P<0.001), AJCC stage (P<0.001), HTRG (P<0.001), and tumor recurrence (P<0.001). LNR correlated with ypT stage (P<0.001), AJCC stage (P<0.001), HTRG (P<0.001), resection margin status (p=0.02), and tumor recurrence (P<0.001).
Table 1.
Clinicopathological correlations with the number of positive lymph nodes
| Characteristics | ypN0 (%) | 1–2 positive lymph nodes (%) |
positive lymph nodes (%) |
P value |
|---|---|---|---|---|
| Gender | 0.13 | |||
| Female | 90 (51.1) | 50 (28.4) | 36 (20.5) | |
| Male | 93 (41.9) | 67 (30.2) | 62 (27.9) | |
| Age, years (Mean ± SD) | 65.2 ± 9.3 | 61.9 ± 9.7 | 62.1± 9.4 | 0.004 |
| Number of lymph nodes examined (Mean ± SD) | 23.1 ± 8.1 | 23.5 ± 9.4 | 27.9 ± 11.1 | <0.001 |
| Tumor differentiation | 0.13 | |||
| Well-Moderate | 118 (46.8) | 66 (26.2) | 68 (27.0) | |
| Poor | 65 (44.5) | 51 (34.9) | 30 (20.6) | |
| Pathologic tumor stage | <0.001 | |||
| ypT0, ypT1, ypT2 | 35 (83.3) | 6 (14.3) | 1 (2.4) | |
| ypT3 | 148 (41.6) | 111 (31.2) | 97 (27.2) | |
| Resection margin | 0.06 | |||
| Negative | 173 (47.1) | 109 (29.7) | 85 (23.2) | |
| Positive | 10 (32.3) | 8 (25.8) | 13 (41.9) | |
| AJCC stage | <0.001 | |||
| IA and IB | 35 (100) | 0 | 0 | |
| IIA | 148 (100) | 0 | 0 | |
| IIB | 0 | 117 (54.4) | 98 (45.6) | |
| HTRGa | <0.001 | |||
| CAP grade 0/1 | 43 (68.3) | 16 (25.4) | 4 (6.3) | |
| CAP grade 2/3 | 140 (41.8) | 101 (30.1) | 94 (28.1) | |
| Recurrenceb | <0.001 | |||
| None | 79 (63.7) | 33 (26.6) | 12 (9.7) | |
| Local | 35 (42.2) | 16 (19.3) | 32 (38.5) | |
| Distant | 69 (37.1) | 64 (34.4) | 53 (28.5) |
HTRG, histopathologic tumor response grade.
Recurrence data was not available for 5 patients.
Table 2.
Clinicopathological correlations with positive lymph node ratio (LNR)
| Characteristics | N0 (LNR = 0) (%) |
a (%) |
LNR > 0.19 (%) |
P value |
|---|---|---|---|---|
| Gender | 0.11 | |||
| Female | 90 (51.1) | 68 (38.6) | 18 (10.3) | |
| Male | 93 (41.9) | 94 (42.3) | 35 (15.8) | |
| Age, years (Mean ± SD) | 65.2 ± 9.3 | 62.0 ± 9.8 | 62.0 ± 8.9 | 0.004 |
| Number of lymph nodes examined (Mean ± SD) | 23.1 ± 8.1 | 25.9 ± 10.4 | 24.5 ± 10.5 | 0.024 |
| Tumor differentiation | 0.24 | |||
| Well-Moderate | 118 (46.8) | 96 (38.1) | 38 (15.1) | |
| Poor | 65 (44.5) | 66 (45.2) | 15 (10.3) | |
| Pathologic tumor stage | <0.001 | |||
| ypT0, ypT1, ypT2 | 35 (83.3) | 7 (16.7) | 0 (0) | |
| ypT3 | 148 (41.6) | 155 (43.5) | 53 (14.9) | |
| Resection margin | 0.02 | |||
| Negative | 173 (47.1) | 150 (40.9) | 44 (12.0) | |
| Positive | 10 (32.3) | 12 (38.7) | 9 (29.0) | |
| AJCC stage | <0.001 | |||
| IA and IB | 35 (100) | 0 | 0 | |
| IIA | 148 (100) | 0 | 0 | |
| IIB | 0 | 162 (75.3) | 53 (24.7) | |
| HTRGb | <0.001 | |||
| CAP grade 0/1 | 43 (68.3) | 17 (27.0) | 3 (4.7) | |
| CAP grade 2/3 | 140 (41.8) | 145 (43.3) | 50 (14.9) | |
| Recurrencec | <0.001 | |||
| None | 79 (63.7) | 39 (31.5) | 6 (4.8) | |
| Local | 35 (42.2) | 31 (37.3) | 17 (20.5) | |
| Distant | 69 (37.1) | 87 (46.8) | 30 (16.1) |
0.19 represents the 75th percentile value of lymph node ratio (LNR)
HTRG, histopathologic tumor response grade
Recurrence data was not available for 5 patients
Survival Analysis
The median follow-up time was 32.0 months (range, 7.6–177.5 months) in the overall study group and 63.0 months (range, 8.2 – 175.5 months) for patients who did not die from disease. At the time of last follow-up, 258 (64.8%) patients died of PDAC, 32 (8.0%) died of other causes, 19 (4.8%) patients were alive with disease, and 89 (22.4%) were alive with no clinical or radiographic evidence of disease. The median disease-free survival (DFS) was 15.7 months [95% confidence interval (CI): 12.4 –19.0 months] and median overall survival (OS) was 35.0 months (95% CI: 30.8 – 39.3 months). There was no significant difference in either DFS or OS amongst the different treatment groups (P>0.05).
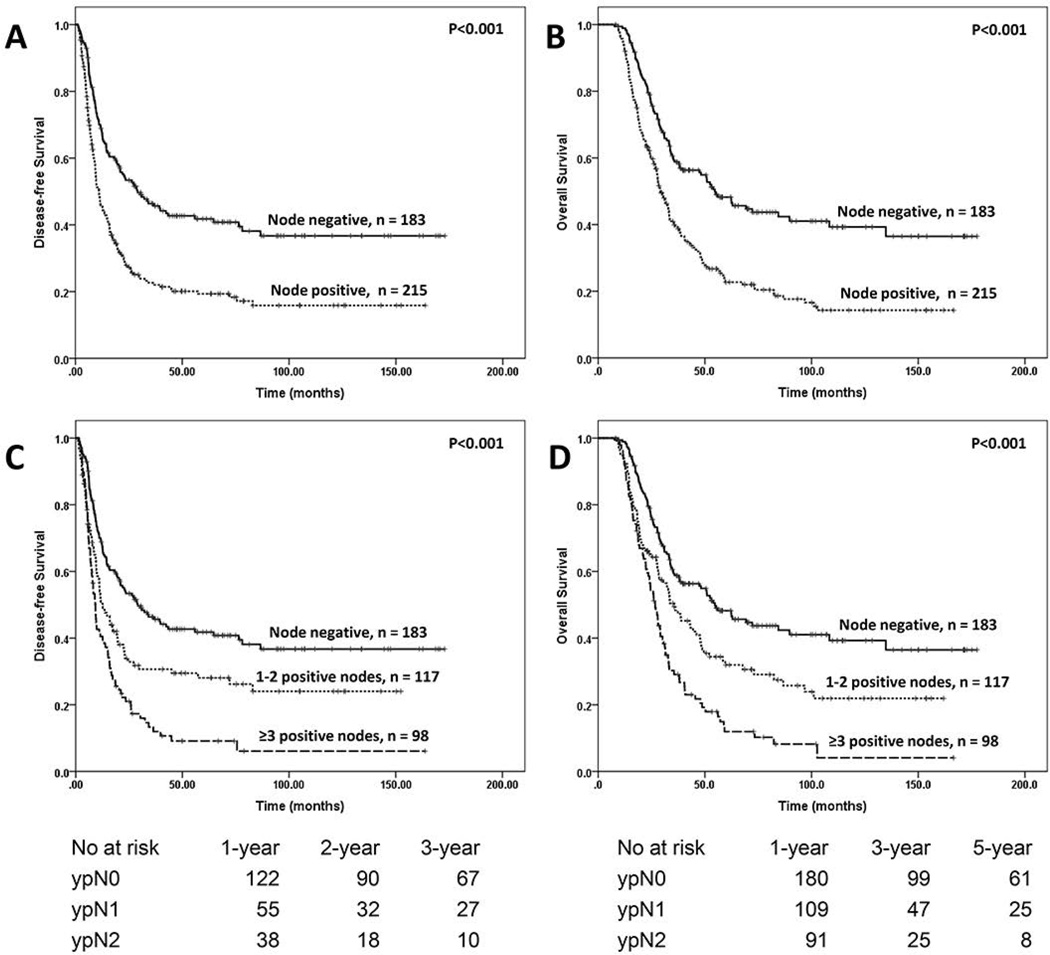
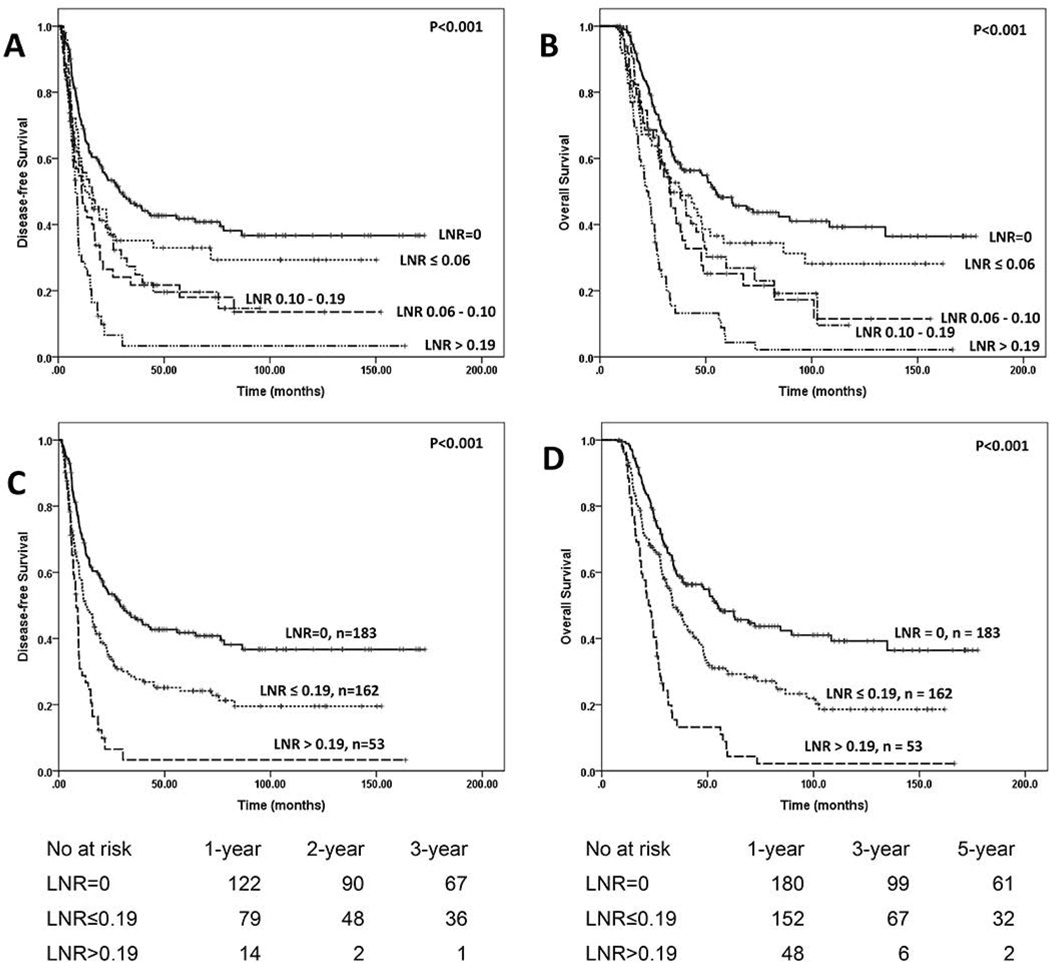
Patients with no lymph node metastases (ypN0) had significantly better DFS (29.1 months) and OS (54.7 months) than those with lymph node metastases (DFS: 11.0 months, P < 0.001 and OS, 29.2 months, P < 0.001, respectively, Fig. 1A & 1B). We further stratified node positive patients into two groups using the median number of positive lymph as a cutoff: patients with 1–2 positive lymph nodes (ypN1) and those with ≥3 positive lymph nodes (ypN2). Patients with ypN1 disease had a DFS of 12.7 months and an OS of 35.7 months compared to a DFS of 9.2 months (p=0.004) and OS of 26.7 months (P=0.001) in patients with ypN2 disease (Fig. 1C & 1D). To determine the best cutoff value for LNR, we first stratified the node-positive patients into four groups using the LNR cutoff values of 0.06 (25th percentile), 0.10 (50th percentile) and 0.19 (75th percentile). The Kaplan-Meier survival curves for DFS and OS are shown in Figures 2A and 2B respectively. There were no differences in either DFS (P=0.59) or OS (P=0.68) among the 3 groups with LNR values ≤0.19. However, patients with LNR>0.19 had shorter DFS (P=0.001) and OS (P<0.001) than the other three groups. We therefore stratified node positive patients into two groups: the group with a LNR ≤0.19 and those with a LNR >0.19. The median DFS and OS in patients with a LNR≤0.19 was 12.7 months and 33.6 months, respectively, compared to a DFS of 8.2 months (P<0.001, Fig. 2C) and OS of 22.1 months (P<0.001, Fig. 2D) in those with a LNR>0.19.
Figure 1.
A and B, Kaplan-Meier curves for disease-free and overall survival in patients with pancreatic ductal adenocarcinoma, stratified by lymph node status. C and D, Kaplan-Meier curves for disease-free and overall survival in patients with pancreatic ductal adenocarcinoma, stratified by the number of positive lymph nodes. The median number of positive lymph nodes in node positive groups, which gave the best separation of survival curves, was used as a cut off.
Figure 2.
A and B, Kaplan-Meier curves for disease-free and overall survival, in patients with pancreatic ductal adenocarcinoma, stratified by LNR using the 25th percentile (0.06), 50th percentile (0.10) and 75th percentile (0.19) values of LNR as a cutoff. There were no differences in either DFS (P=0.59) or OS (P=0.68) among the 3 groups with 0 < LNR ≤ 0.19.
C and D, Kaplan-Meier curves for disease-free and overall survival, in patients with pancreatic ductal adenocarcinoma, stratified by stratified by LNR using the 75th percentile (0.19) value of LNR as cut off. Patients with a LNR>0.19 had shorter DFS (P<0.001) and OS (P<0.001) than those with ypN0 and those with a LNR ≤ 0.19.
The results of univariate survival analysis are shown in Table 3. Tumor differentiation, ypT stage, AJCC stage, HTRG, the number of positive lymph nodes, and LNR were significant predictors for both DFS (P<0.01) and OS (P<0.01). The age at diagnosis correlated with DFS (P=0.02), but not OS (P=0.47). Resection margin status correlated with OS (P=0.005), but not DFS (P=0.07). In multivariate analysis, tumor differentiation, HTRG, LNR, and the number of positive lymph nodes were independent prognostic factors for both DFS and OS (Tables 4 and 5). Positive resection margin was a significant factor for overall survival only when the number of positive lymph nodes was used in multivariate analysis (P=0.04).
Table 3.
Univariate Cox Regression Analyses of Disease-free and Overall Survival in Relation to Clinicopathologic Features
| Characteristics | No. of patients |
Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age (years) | 398 | 0.99 (0.97 – 0.997) | 0.02 | 1.00 (0.98 – 1.01) | 0.47 |
| Gender | |||||
| Female (ref) | 176 | 1.00 | 1.00 | ||
| Male | 222 | 1.16 (0.91 – 1.48) | 0.22 | 1.20 (0.93 – 1.53) | 0.16 |
| Tumor differentiation | |||||
| Well-Moderate (ref) | 252 | 1.00 | 1.00 | ||
| Poor | 146 | 1.42 (1.11 – 1.81) | 0.005 | 1.51 (1.18 – 1.93) | 0.001 |
| Margins | |||||
| Negative (ref) | 367 | 1.00 | 1.00 | ||
| Positive | 31 | 1.48 (0.97 – 2.25) | 0.07 | 1.82 (1.20 – 2.76) | 0.005 |
| Pathologic tumor stage | |||||
| ypT0–ypT2 (ref) | 42 | 1.00 | 1.00 | ||
| ypT3 | 356 | 2.43 (1.49 – 3.97) | <0.001 | 2.46 (1.50 – 4.02) | <0.001 |
| AJCC Stage | |||||
| IA–IB (ref) | 35 | 1.00 | 1.00 | ||
| IIA | 148 | 2.35 (1.29 – 4.29) | 0.005 | 2.29 (1.25 – 4.19) | 0.007 |
| IIB | 215 | 3.98 (2.21 – 7.17) | <0.001 | 3.89 (2.16 – 7.00) | <0.001 |
| HTRG | |||||
| CAP grade 0/1 (ref) | 63 | 1.00 | 1.00 | ||
| CAP grade 2/3 | 335 | 1.99 (1.36 – 2.92) | <0.001 | 2.01 (1.37 – 2.95) | <0.001 |
| Number of positive lymph nodes | |||||
| 0 (ref) | 183 | 1.00 | 1.00 | ||
| 1–2 | 117 | 1.59 (1.19 – 2.13) | 0.002 | 1.58 (1.17 – 2.12) | 0.003 |
| 98 | 2.52 (1.88 – 3.37) | <0.001 | 2.59 (1.93 – 3.49) | <0.001 | |
| Lymph Node Ratio | |||||
| 0 (ref) | 183 | 1.00 | 1.00 | ||
| 0.19 | 162 | 1.70 (1.30 – 2.21) | <0.001 | 1.65 (1.25 – 2.16) | <0.001 |
| LNR > 0.19 | 53 | 3.36 (2.36 – 4.78) | <0.001 | 3.69 (2.60 – 5.23) | <0.001 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; ref, reference; HTRG, histopathologic tumor response grade; LNR, lymph node ratio
Table 4.
Multivariate Cox Regression Analysis of Disease-free and Overall Survival for the Number of Positive Lymph Nodes
| Characteristics | No. of patients |
Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age (years) | 398 | 0.99 (0.98 – 1.002) | 0.09 | ||
| Tumor differentiation | |||||
| Well-Moderate (ref) | 252 | 1.00 | 1.00 | ||
| Poor | 146 | 1.36 (1.06 – 1.74) | 0.02 | 1.55 (1.20 – 1.99) | 0.001 |
| Margins | |||||
| Negative (ref) | 367 | 1.00 | 1.00 | ||
| Positive | 31 | 1.33 (0.87 – 2.04) | 0.19 | 1.56 (1.02 – 2.37) | 0.04 |
| Pathologic tumor stage | |||||
| ypT0–ypT2 (ref) | 42 | 1.00 | 1.00 | ||
| ypT3 | 356 | 1.56 (0.92 – 2.65) | 0.10 | 1.56 (0.93 – 2.63) | 0.09 |
| HTRG | |||||
| CAP grade 0/1 (ref) | 63 | 1.00 | 1.00 | ||
| CAP grade 2/3 | 335 | 1.50 (1.01 – 2.24) | 0.047 | 1.44 (0.97 – 2.15) | 0.07 |
| Number of positive lymph nodes | |||||
| 0 (ref) | 183 | 1.00 | 1.00 | ||
| 1–2 | 117 | 1.38 (1.02 – 1.86) | 0.035 | 1.39 (1.02 – 1.88) | 0.04 |
| 98 | 2.16 (1.59 – 2.93) | <0.001 | 2.29 (1.68 – 3.11) | <0.001 | |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval; HTRG, histopathologic tumor response grade
Table 5.
Multivariate Cox Regression Analysis of Disease-free and Overall Survival for Lymph Node Ratio
| Characteristics | No. of patients |
Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age (years) | 398 | 0.99 (0.97 – 1.00) | 0.052 | ||
| Tumor differentiation | |||||
| Well-Moderate (ref) | 252 | 1.00 | 1.00 | ||
| Poor | 146 | 1.33 (1.04 – 1.71) | 0.02 | 1.54 (1.20 – 1.98) | 0.001 |
| Margins | |||||
| Negative (ref) | 367 | 1.00 | 1.00 | ||
| Positive | 31 | 1.17 (0.76 – 1.81) | 0.48 | 1.40 (0.91 – 2.14) | 0.13 |
| Pathologic tumor stage | |||||
| ypT0–ypT2 (ref) | 42 | 1.00 | 1.00 | ||
| ypT3 | 356 | 1.55 (0.91 – 2.63) | 0.11 | 1.52 (0.90 – 2.55) | 0.12 |
| HTRG | |||||
| CAP grade 0/1 (ref) | 63 | 1.00 | 1.00 | ||
| CAP grade 2/3 | 335 | 1.60 (1.08 – 2.38) | 0.02 | 1.63 (1.10 – 2.42) | 0.015 |
| Lymph Node Ratio | |||||
| 0 (ref) | 183 | 1.00 | 1.00 | ||
| 0.19 | 162 | 1.45 (1.10 – 1.90) | 0.009 | 1.45 (1.09 – 1.91) | 0.01 |
| LNR > 0.19 | 53 | 2.95 (2.05 – 4.25) | <0.001 | 3.47 (2.42 – 4.97) | <0.001 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval; HTRG, histopathologic tumor response grade; LNR, lymph node ratio
DISCUSSION
Although node metastasis is a well-established prognostic factor for PDAC patients who undergo upfront resection, the prognostic significance of the number of positive lymph nodes in patients who receive neoadjuvant therapy and PD remains unclear. Currently, there is no subclassification of the node positive group in the AJCC cancer staging manual (7th edition) for PDAC patients. Recently, Showalter et al. showed that patients with >3 positive nodes had worse DFS and OS than those with 0 or 1–3 lymph nodes in 538 PDAC patients who underwent resection followed by adjuvant chemoradiation. They did not, however, find a statistically significant difference in either DFS or OS between patients with 1–3 positive nodes and those with >3 positive nodes in multivariate analysis9. In another study of 696 untreated patients, House et al. found an inverse linear relationship between the number of positive nodes and median OS among patients with 1 to 7 positive lymph nodes24. Additionally, they found that the presence of two positive lymph nodes was the most significant point of separation in OS24. Consistent with these findings, we demonstrated that patients who received neoadjuvant therapy and PD and had ypN1 disease, had better DFS and OS than those with ypN2 disease, by univariate and multivariate analysis. The findings from this study are consistent with our prior study, which demonstrated that AJCC stage and the number of positive regional lymph nodes were independent prognostic factors for both DFS and OS, in patients who received neoadjuvant chemoradiation followed by PD18. We chose 2 positive nodes as the cut off for ypN1 and ypN2 in this study, instead of 3 positive nodes in previous study because 2 positive nodes provided the best separation of the survival curves for both DFS and OS among the different N groups. Among patients with carcinomas of gastrointestinal origin, the importance of the number of positive lymph nodes is well-recognized and this information is included in AJCC staging for the esophagus, stomach, small bowel, colon, rectum and appendix. This information has yet to be incorporated into the staging for PDAC. Based on our findings, we propose to subclassify the post-therapy node positive group into ypN1 (1–2 positive lymph nodes) and ypN2 (≥3 positive lymph nodes). In contrast, Slidell et al. showed that patients with 1, 2, or 3 positive lymph nodes had similar median overall survival, while those with 4–7 positive nodes had only a slightly worse OS, in a study of 4005 PDAC patients from the US Surveillance Epidemiology and End Result (SEER) registry25. However, 55.5% of cases in their study had fewer than 12 lymph nodes evaluated and 10.1% patients had no lymph nodes examined25. Previous studies have shown that a suboptimal number of lymph nodes examined is associated with poor survival in PDAC patients after resection8, 9, 24. To accurately evaluate node status, all patients included in this study had ≥12 lymph nodes examined. We did not find correlation between the total number of lymph nodes examined and either DFS or OS in our patient population.
Recent studies have emphasized the power of LNR in predicting prognosis for patients with PDAC. House et al. reported an inverse linear relationship between median OS and LNR for cases with LNR values up to 0.35 and that LNR, as a continuous variable, correlated significantly with OS. In their study, the best cutoff value was found to be 0.1824. Similar results were also reported by other studies8, 13, 26. Showalter et al. reported that increased LNR, either as a continuous or categorical variable, was associated with decreased OS and DFS26. Pawlik et al. found that LNR was a better predictor of outcome than the number of positive lymph nodes13. They reported that patients with a LNR value of 0 to 0.2 had a longer median survival (21.7 months) than those who had a LNR of 0.2 to 0.4 (15.3 months), or greater than 0.4 (12.8 months), in their node positive patients13. Although Slidell et al. did not find a significant correlation between the number of positive nodes and OS, their study did find that patients with a LNR of 0 to 0.2 had longer OS than those with a LNR of either 0.2 to 0.4, or greater than 0.425. Of note, a recent study showed that neoadjuvant therapy decreases LNR in patients with PDAC27. Using a median LNR of 0.14 as a cutoff, Roland et al. showed that PDAC patients who received neoadjuvant therapy and had a LNR≥0.15, had a shorter median OS and DFS, than those with a low LNR (LNR<0.15) or ypN0 disease. There was no difference in cancer-related deaths for patients with ypN0 disease and those with a low LNR (LNR<0.15) in their study27. Therefore, the optimal cutoff point for LNR in PDAC patients who received neoadjuvant treatment remains to be determined. In this study, we found that the 75th percentile value of LNR (0.19) was the best cutoff for our patient population. Patients with a LNR>0.19 had significantly shorter DFS and OS compared to those with a LNR≤0.19 and that LNR is an independent prognostic factor for both DFS and OS. Thus our study demonstrated that LNR is a key prognostic factor in PDAC patients who have received neoadjuvant therapy and PD.
Although, several studies have reported that LNR is superior to the number of positive lymph nodes in predicting survival in patients with PDAC, the median number of lymph nodes examined in these studies was highly variable, ranging from 7 to 2813, 24, 26, 27. In two large studies, the median numbers of nodes examined were 7 in Slidell’s study8 and 6 in patients with N0 disease in Schwarz’s study7. A low number of lymph nodes may understage disease by potentially missing the lymph node(s) that are involved by metastases. This may be the reason, in some studies, that the number of involved lymph nodes was not significantly correlated with survival and also explain why the LNR is often a better predictor of survival, particularly in studies with a low median lymph node count. At our institution, we have a standard approach to pancreaticoduodenectomy and we use a standardized protocol for the evaluation of all PD specimens. In this study, all patients had 12 or more lymph nodes examined. We found that both the number of positive lymph nodes and the LNR are independent prognostic indicators for DFS and OS. The advantage to using the number of positive lymph nodes is that this number is already contained within the surgical pathology report, whereas a baseline cutoff value for LNR still needs to be established.
In summary, our study demonstrated that the number and ratio of positive lymph nodes are independent prognostic factors for both DFS and OS in patients with PDAC who received neoadjuvant therapy followed by PD. We propose to subclassify the post-therapy node positive group into ypN1 (1–2 positive lymph nodes) and ypN2 (≥3 positive lymph nodes) in the staging of patients with PDAC who received neoadjuvant treatment.
ACKNOWLEDGMENTS
Laurice K. Fischer: Study design, data collection, pathology review, manuscript writing
Matthew H. Katz: Study design, data collection, manuscript editing
Sun M. Lee: Data collection and verification, pathology review
Li Liu: Data collection and verification, pathology review
Hua Wang: Data collection, statistical analysis, manuscript editing
Gauri R. Varadhachary: Study design, data collection and manuscript editing
Robert A. Wolff: Study design, data collection and manuscript editing
Jeffrey E. Lee: Study design, data collection and manuscript editing
Anirban Maitra: Study design, data collection and manuscript editing
Christina L. Roland: Data collection and manuscript editing
Jason B. Fleming: Study design, data collection and manuscript editing
Jeannelyn Estrella: Data collection and manuscript editing
Asif Rashid: Study design, data collection, statistical review, and manuscript editing
Huamin Wang: Study design, data collection, statistical analysis, pathology review, manuscript writing
Supported by the G. S. Hogan Gastrointestinal Cancer Research Fund and Khalifa Bin Zayed Al Nahyan Foundation Institute for Pancreatic Cancer Research at the University of Texas M.D. Anderson Cancer Center
Footnotes
There are no financial disclosures from all authors.
REFERENCES
- 1.National Cancer Institute SRP, Cancer Statistics Branch. Surveillance epidemiology and end results (seer) 1975 – 2011 [Google Scholar]
- 2.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. Jop. 2008;9:99–132. [PubMed] [Google Scholar]
- 3.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Annals of surgery. 2003;237:74. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. Journal of gastrointestinal surgery. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: Results, outcomes, and prognostic indicators. Journal of gastrointestinal surgery. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 6.Huebner M, Kendrick M, Reid-Lombardo KM, et al. Number of lymph nodes evaluated: Prognostic value in pancreatic adenocarcinoma. Journal of Gastrointestinal Surgery. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: Information from a large us population database. Annals of surgical oncology. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 8.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: A large, population-based analysis. Annals of surgical oncology. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 9.Showalter TN, Winter KA, Berger AC, et al. The influence of total nodes examined, number of positive nodes, and lymph node ratio on survival after surgical resection and adjuvant chemoradiation for pancreatic cancer: A secondary analysis of rtog 9704. International Journal of Radiation Oncology* Biology* Physics. 2011;81:1328–1335. doi: 10.1016/j.ijrobp.2010.07.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimura Y, Nagino M, Takao S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Journal of hepatobiliary-pancreatic sciences. 2012;19:230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 11.Sergeant G, Melloul E, Lesurtel M, DeOliveira ML, Clavien P-A. Extended lymphadenectomy in patients with pancreatic cancer is debatable. World journal of surgery. 2013;37:1782–1788. doi: 10.1007/s00268-013-2064-z. [DOI] [PubMed] [Google Scholar]
- 12.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the international study group on pancreatic surgery (isgps) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Robinson S, Rahman A, Haugk B, et al. Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. European Journal of Surgical Oncology (EJSO) 2012;38:333–339. doi: 10.1016/j.ejso.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006;33:240–245. doi: 10.1097/01.mpa.0000235306.96486.2a. [DOI] [PubMed] [Google Scholar]
- 16.Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, Hall RI. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World journal of surgery. 2010;34:768–775. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]
- 17.La Torre M, Cavallini M, Ramacciato G, et al. Role of the lymph node ratio in pancreatic ductal adenocarcinoma. Impact on patient stratification and prognosis. Journal of surgical oncology. 2011;104:629–633. doi: 10.1002/jso.22013. [DOI] [PubMed] [Google Scholar]
- 18.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–277. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 19.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 20.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 21.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A, editors. Ajcc cancer staging manual. New York: Springer; 2010. [Google Scholar]
- 22.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: A predictor for patient outcome. Cancer. 2012;118:3182–3190. doi: 10.1002/cncr.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage ii pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:3801–3811. doi: 10.1002/cncr.26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 25.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: A large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 26.Showalter TN, Zhan T, Anne PR, et al. The influence of prognostic factors and adjuvant chemoradiation on survival after pancreaticoduodenectomy for ampullary carcinoma. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011;15:1411–1416. doi: 10.1007/s11605-011-1518-6. [DOI] [PubMed] [Google Scholar]
- 27.Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]