Abstract
Introduction
Patients with heart transplant are screened for silent graft rejection by recurrent endomyocardial biopsies. MRI can detect the presence of oedema non-invasively by quantitatively measuring changes of the transverse relaxation time T2 in the myocardium. Several monocentric studies have shown that T2 quantification could help detect graft rejection in a less invasive way. DRAGET is a national multicentre diagnostic study designed to prove that T2 quantification by MRI can detect graft rejection.
Methods and analysis
190 patients from 10 centres will undergo T2 quantification and endomyocardial biopsy, within 24 h, 4 to 6 times during the first year after transplantation. T2 will be computed by analysing a sequence of 10 images obtained from a short-axis slice. Specific phantoms will be used to calibrate the T2 quantification on each MR scanner to cope with the different equipment (different vendors, magnetic field strength, etc). Specific pads with known T2 will also be used during each examination and provide a quality check to cope with the different experimental conditions (temperature, etc). All MRI and biopsy data will be reinterpreted in our centre and reproducibility will be assessed. The primary outcome will be sensitivity and specificity of MRI. The secondary outcomes will be (1) prognostic values of T2, (2) reproducibility of each techniques, (3) number of adverse events during each procedures and (4) confidence of the physicians in T2.
Ethics and dissemination
Ethics approval has been obtained. The new MRI method will be disseminated at a national level and its practical usefulness will be assessed in centres not familiar with MRI T2 quantification. The ultimate aim of the DRAGET project is to replace a strategy based solely on biopsy with one based on a first-line MRI (with biopsy only when needed) for a more efficient and less invasive detection of rejection.
Trial registration numbers
ANSM 2014-A00848-39, NCT02261870.
Keywords: TRANSPLANT SURGERY
Strengths and limitations of this study.
This will be the first multicentric study to validate MRI T2 quantification as a possible diagnostic tool for heart transplant rejection.
This study uses specific phantoms and pad as calibration and quality check systems to cope with differences due to equipment and experimental conditions.
This multicentric study could not be randomised because the T2 quantification method had not yet been validated in a multicentre design (with different MR scanners, etc).
Introduction
Patients with heart transplant are constantly at risk of acute graft rejection because of partial histocompatibility.1 Approximately 20–40% of heart transplant recipients will experience at least one episode of acute rejection in the first postoperative year.2 The risk of allograft rejection is highest in the first 6 months and decreases sharply after 12 months.3 Rejection may lead to diastolic or systolic dysfunction, and may also lead to sudden cardiac death before the onset of symptoms, but the most common situation during the first year after transplantation is asymptomatic rejection. Therefore, efficient routine monitoring of this risk is mandatory. It relies on endomyocardial biopsies (EMB) which are performed about 15 times during the first year.1 These biopsies are usually performed, during day hospitalisation, through a large central venous catheter introduction system (>9 Fr) under radioscopy (irradiation). There are conflicting data on EMB variability and accuracy.4–6 The limited amount of tissue analysed induces a risk of false-negative results (true rejection with negative biopsies). Besides, a repetition of biopsies impairs the quality of life and exposes the patient to a 3% risk of adverse event (AE) per biopsy.7–10 The most serious AE is tamponade. Several alternative solutions have been proposed but have yet failed to be widely recognised. Echocardiography is one of those solutions. Standard parameters such as ejection fraction have too low sensitivity. More complex parameters such as diastolic function parameters are used by several teams11 but have limited reproducibility. Several teams have proposed using ventricular evoked responses that yield high negative predictive accuracy and prognostic value.12 However, owing to conflicting results and lack of sufficient prospective data, the routine use of this tool for rejection screening is not recommended.1
In 2001, our team proposed an MRI method, based on the detection of myocardial oedema by T2 quantification, to detect acute rejection. The acquisition lasts <20 min and the method is simple, reproducible and easily exportable. This method has been used locally in our centre and our results showed that: (1) MRI detected acute rejections with good sensitivity and specificity.1 13–15 Our method, with a threshold of 60 ms,15 detected every rejection (negative predictive value of 100%). (2) MRI provided a good prediction of rejection within a month,15 and often detected rejection at an earlier stage than biopsies (as soon as there is one locus of necrosis in the biopsy, which corresponds to the former grade 2). With a threshold of 65 ms, its positive predictive value for a rejection within a month was 93%.15 Consequently, MRI could be useful to better stratify the risk of future rejection.14 15 The International Society for Heart and Lung Transplantation (ISHLT) recently recognised the assets of this non-invasive technique and recommended a large multicentre study before it can be used instead of biopsies.1 In line with the conclusion of this report, the DRAGET project was designed (DRAGET is a French acronym for ‘Détection des Rejets Aigus de Greffe par Evaluation du T2’). It will assess the diagnostic value of MRI in a large multicentre setting and will study the predictive value of T2 quantification when biopsies are negative. We hypothesise that our study will prove that MRI, in the light of its good diagnostic performance, is a viable alternative to routine biopsies.
Study objectives
Our primary objective is to assess in a multicentre study the diagnostic performance (sensitivity and specificity) of myocardial T2 quantification with MRI to detect acute cellular rejection in comparison to the gold standard strategy based on EMB.
Our secondary objectives are:
To determine if myocardial T2 value can stratify the risk of rejection within months in patients with normal biopsy;
(a) To quantify complications with MRI and with biopsies. (b) To determine the magnitude of better tolerability of MRI over biopsies;
To assess interobserver reproducibility of T2 quantification with MRI and that of pathological grading of the biopsies;
To assess, at the end of the study, the level of confidence of the expert physicians of each centre concerning the use of T2 quantification as an alternative to routine biopsies.
Methods
Trial design
DRAGET is an ongoing multicentre prospective, open, transversal clinical trial with repeated measures.
Participant
Inclusion criteria
Patient with a heart transplant (discharge from intensive care/reanimation post transplantation)
Adult patient (age ≥18 years)
Who is to undergo ≥4 biopsy/MRI couples within 12 months after the transplant
Patient able to give informed consent
Non-inclusion criteria
Patients under a measure of legal protection
Contraindication to MRI: pacemaker, ferromagnetic foreign body, etc
Impossibility of undergoing MRI: claustrophobia, morbid obesity, hospitalisation in an intensive care unit, arrhythmia
Pregnancy
Settings
Patients are being recruited in the heart transplant services of the 10 participating centres (University hospital of Bordeaux, Grenoble, Lyon, Nancy, Nantes, Paris-HEGP, Paris-La Pitié Salpêtrière, Rennes, Strasbourg and Tours): every new patient with a heart transplant (once the patient is transferred out of the intensive care unit) will be considered for inclusion. DRAGET started on 6 January 2015 and the first participant was recruited on 18 February 2015. This trial is planned to be completed in 3 years. The trial sponsor is: Nancy University Hospital, Nancy, France.
Outcomes
Primary end point
The primary end point is the sensitivity and specificity of myocardial T2 assessed with MRI for the diagnosis of histological heart graft rejection (with 95% CI). Histological heart graft rejections will be documented by the presence of damaged myocytes (or cellular necrosis) in EMB (former grade 2, grade 2R and grade 3R). The pathological grading will be either confirmed by both pathologists (local and centralised interpretation), or will be decided by consensus between two pathologists if the local and centralised interpretations are different. The parameters taken into account for this objective will therefore be: T2 values and biopsy results.
Secondary end points
Incidence of histological or clinical rejection within months of an MRI/biopsy couple with normal biopsy (grade <2R). For this purpose, rejection will be defined with a clinical and histological combined end point: (a) acute rejection documented by the presence of damaged myocytes in EMB (former grade 2, grade 2R and grade 3R), or (b) marked decrease in the left ventricle ejection fraction (>10%), reversible after a subsequent increase in immunosuppressive treatment. Owing to the limits of confidence of the echocardiographic ejection fraction being close to 10%, a decrease in the left ventricle ejection fraction will be considered significant if it is above 15% for asymptomatic patients or above 10% with clinical signs (change of New York Heart Association (NYHA) class). The parameters taken into account for this objective will therefore be: T2 values, biopsy results, echocardiographic left ventricle ejection fraction, NYHA classification and significant increase in immunosuppressive treatment (eg, bolus)
(a) Number of major and minor AEs. Every AE will be collected during the inclusion of patients. Particular attention will be paid to expected complications of biopsies (pain, arterial punctures, vasovagal reactions, prolonged bleeding, arrhythmia or conduction abnormalities, perforation and pericardial effusion) and MRI (claustrophobia). During the last visit, a specific questionnaire will be proposed to the patient to collect his feelings towards MRI and his acceptance of this examination during his personal follow-up. (b) Physical and psychological distress assessed by the questionnaire using Likert scales. This questionnaire will be completed by the patient during the last visit
The 95% interobserver limits of agreement for T2 quantification and Cohen's κ coefficient for histological grading. MRI and biopsy slides will be sent to our centre. A second centralised interpretation of the images will be performed with the same software by physicians blinded for all patient data and previous quantification. A second centralised interpretation of the biopsies will be performed by qualified physicians blinded for all patient data and previous quantification. T2 values and histological grades from the first local interpretation and from the second centralised interpretation will be collected for this objective
The level of confidence of the expert physicians of each centre will be assessed by the questionnaire using Likert scales. This questionnaire will be completed by the study investigators at the end of the study.
Data collection
Phase 1a: MRI equipment calibration
MRI will be conducted using various MR scanners from various vendors depending on the centre. Owing to this high variability of hardware and software equipment, some amount of variability in the T2 values can be expected from one scanner to another. In order to obtain standardised T2 values comparable from one centre to another, a calibration of each scanner will be performed by members of our centre. This calibration will use a set of tubes with specific stable known T2 values covering the range useful for this purpose (50–90 ms). The aim of the calibration process will be to verify the reproducibility of the measurements with a given machine at a given temperature and to compute the transformation function that will be used to correct the measured T2 and to obtain a reference T2.
Phase 1b: radiologist training
Twenty-six cardiologists/radiologists have been identified in the 10 centres by the preliminary questionnaire and will be asked to run a e-learning program. The software will use a set of 10 previously recorded anonymised examinations so that each radiologist will be allowed to train himself. The physicians will be trained to register the 10 images to suppress the motion between each breath-hold scan and to delineate the appropriate region of interest as described below. They will be allowed to compare their registration and their regions of interest with the reference. The computed T2 value will also be compared with the reference value. The cardiologist/radiologist accreditation will be obtained when 10 successive T2 quantifications are comprised within the CI of the reference value.
Phase 2: inclusions
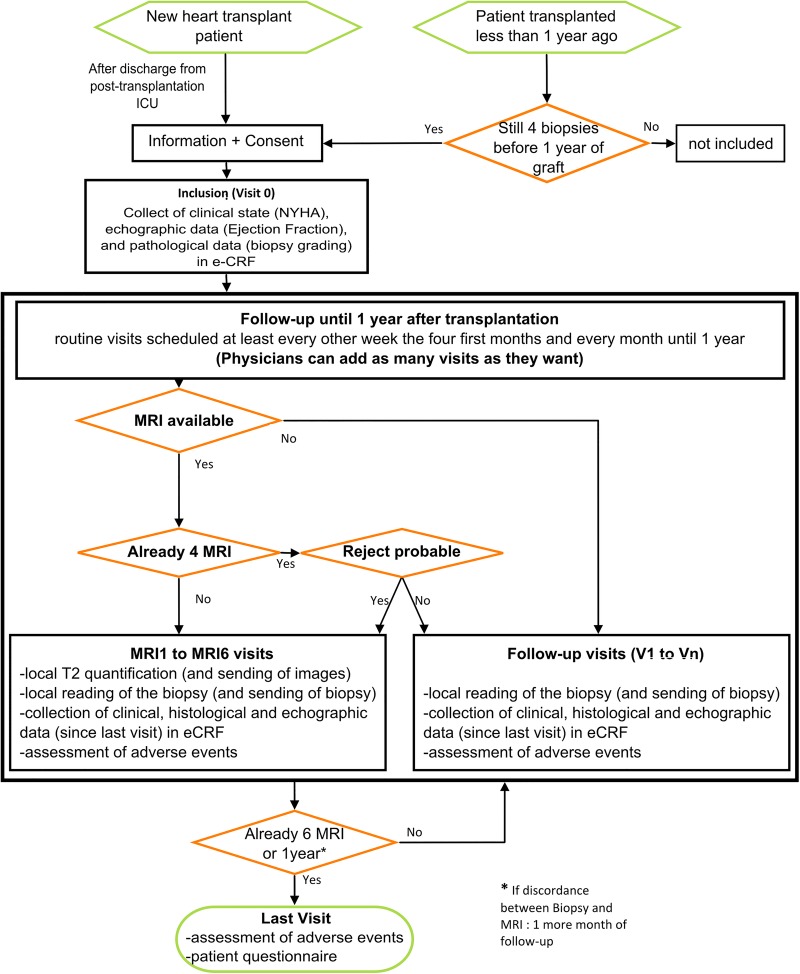
Seven hundred and fifty couples (MRI/biopsies) are required, and therefore 190 patients must be included. The duration of enrolment will be 24 months and the follow-up duration will be 13 months maximum (depending on the time of enrolment). The study flow chart is presented in figure 1 and table 1 summarises the actions performed during each visit.
Patients will be included during the initial visit called V0;
During the follow-up, data will be collected on a regular basis to document acute rejections (clinical data: NYHA stage, echocardiographic data: ejection fraction and pathological data: biopsies grading), as well as any AEs, during visits called V1-Vn;
Furthermore, 1–6 couples of MRI/biopsies will be acquired during visits called MRI1-MRI6;
At the end of the follow-up, a special visit called ‘Last Visit’ is scheduled with a questionnaire to the patient concerning his feeling about the use of MRI or biopsies in the follow-up of his graft and the possible complications that these examinations could induce.
Figure 1.
Flow chart of the study (eCRF, electronic case report form; ICU, intensive care unit; NYHA, New York Heart Association).
Table 1.
Patient follow-up summary
| Inclusion: V0 | Routine follow-up: V1-Vn | Visit with MRI: MRI1-MRI6 | Last visit | |
|---|---|---|---|---|
| Informed consent | X | |||
| Verification of inclusion and non-inclusion criteria | X | |||
| Medical history | X | |||
| Clinical examination | X | X | X | X |
| Biopsy | x | X | ||
| MRI | X | |||
| Sending MR examination (after 1st analysis) | X | |||
| Sending biopsies examinations | x | X | ||
| Collection of data in the electronic case report form: clinic, echography and histology (secondary objective 1) | X | X | X | |
| Retrieval of adverse events (secondary objective 2) | X | X | X | |
| Retrieval of serious adverse events | X | X | X | |
| Questionnaire on patient satisfaction | X |
X means always; x means sometimes according to the biopsy calendar.
The biopsy grades will be entered in the electronic case report form for each biopsy (even if no MRI examination was performed). The biopsies that are part of a visit with MRI (MRI1-MRI6) and that do follow a visit with discrepancy between an MRI and a biopsy will be sent to Nancy for a second centralised analysis. In case of significant disagreement between the local and centralised interpretations, a third interpretation will be performed and a consensus will be obtained between the last two interpretations.
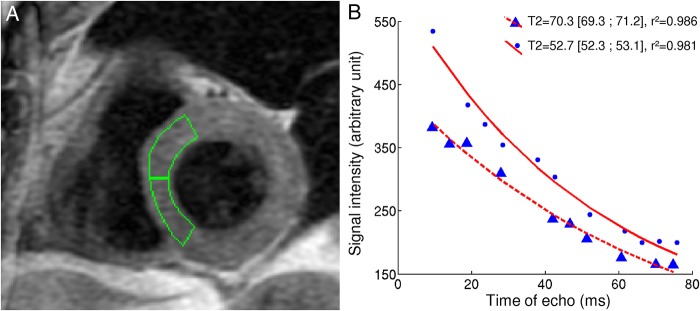
The MRI T2 quantification will be performed within 24 h of a biopsy (maximum delay between MRI and biopsy=24 h), four times during the year. Another two biopsy-MR couples can be added in case of high suspicion of acute rejection in order to increase the rejection prevalence in the study (the usual prevalence of moderate-to-severe rejection is close to 15% for a routine screening).1 MRI acquisitions will be performed according to the already described method13 15 based on conventional fast spin echo sequences and illustrated in figure 2. MRI will be performed, if possible, before the biopsy; otherwise, the radiologist will be kept blind to the biopsy results. The imaging protocol includes localisation sequences followed by the selection of a single mid-ventricular short-axis slice for T2 measurement. Slice thickness is 10 mm. This slice is acquired 10 times with different effective echo times (TE) ranging evenly from 9 to 80 ms. The sequence is a black blood double-inversion fast spin echo/turbo spin echo, with an echo train length/turbo factor of 16. The first inversion is non-selective and immediately followed by a selective slice inversion. The inter-TE is close to 4.5 ms. The repetition time (TR) is equal to two heartbeats. As TR is relatively short, complete recovery of the longitudinal magnetisation is not obtained. An inversion time of 485 ms is chosen to withdraw most of the blood-related signal.16 The main acquisition parameters are as follows: field of view 42×42 cm, acquisition matrix 256×160 and number of excitations equal to one. Images are obtained at the same phase of the cardiac cycle aiming for mid-diastole on sequential heartbeats using prospective ECG gating. Each image is recorded during an end-expiratory breath-hold of no more than 15 s. It is mandatory not to change the transmission and reception gains between two images. MRI acquisitions will be performed with an additional calibration pad positioned on the patient thorax (dedicated pad produced by the Nancy CIC-IT with stable and adapted T2). The pads will be composed of adapted gel. The pad concentration will be chosen to produce standard short T2 values. The gel will be packaged and sealed to ensure its stability.
Figure 2.
Illustration of our method to quantify myocardial T2. (A) Example of the positioning of the regions of interest for both septal segments. In this example, the physician chose to avoid the border of the myocardium to avoid error due to imperfect registration between the 10 images. (B) Examples of exponential fits for normal and high T2. Both curves comprise 10 points corresponding to the signal intensity within a given septal region of interest for each image acquired with 10 different echo times. Myocardium with higher T2 needs more time to decrease its signal intensity.
All local post-processing computations will be performed with dedicated software developed by the Nancy centre in Java language. T2 quantification will be performed once locally and a second time in Nancy. The pad T2 value will be used to detect potential technical issues during the acquisition and the examination will be discarded if such a situation occurs. Data will be archived in the Archimed database declared to the French authorities (CNIL declaration number: 1410005). The T2 quantification results will be communicated to every local investigator: during Phase 3, and each investigator will be asked to give his personal opinion about the usefulness of T2 quantification during the follow-up of patients with heart transplant. In case of disagreement between the local reading of a biopsy and the local T2 assessment (high T2 without histological rejection or histological rejection with low T2), the clinical and histological data during the next 30 days will be considered in the analysis. In this purpose, the results of the next biopsy (and the biopsy sample itself) will be collected. This biopsy will be reinterpreted in Nancy. If the patient is to be released from the study (after 12 months of study), the duration of the participation in the study will be increased by 1 month.
Phase 3: expert opinion
The opinion of the expert physicians of each centre, concerning the confidence they have in the MRI detection of rejection and their wish to use it for the routine follow-up of their patients, will be recorded by questionnaire at the end of the study.
Study calendar
Duration of recruitment: 24 months. Duration of participation of each patient: maximum 13 months (maximum 12 months+another month if the last MRI-biopsy couple shows a discrepancy).
Study population
Patients will be recruited in the heart transplant services of the participating centres: every new patient with a heart transplant (once the patient is transferred out of the intensive care unit) will be considered for inclusion as well as patients at the beginning of the first year of their graft (with still at least 4 EMB scheduled for routine follow-up). One hundred and ninety patients with heart transplant, older than 18 years, will be included. Since 10 centres are involved, there will be a mean of 19 patients per centre in 2 years which represents <1 per month per centre. The number of new patients with heart transplant in 2012 in these 10 centres was 255 (in 12 months). With a mean number of 4 MRI per patient, this should provide a sample of 750 biopsy/MRI couples. It is expected that several patients will leave the study before having undergone 4 MRI visits (eg, in case of premature death), but as several patients will have 5 or 6 MRI/biopsy couples, the total number of collected MRI/biopsy couples should reach 750. Indeed, to measure a sensitivity of 87% (conservative estimation based on both local studies: 89% in 200113 and 87% in 201215) with a precision of 5% (half-CI of sensitivity lower than 5%), with an α risk of 5% and a rejection prevalence of 30% (estimation based on both local studies with a similar design), the sample size should be 580 MRI-biopsy couples. To take into account the possibility that several MRI could be unreadable (bad fit during regression), we increased this number by 10% (in our local experience,15 7.5% of the examinations were discarded). To take into account the correlation due to the repetitions of observations (MRI/biopsies) with the same patients, we proposed another increase by 20%. Hence the total sample size of 750 MRI/biopsy couples.
This sample size will also allow a precise quantification of:
The specificity of the method (specificity estimation close to 75% based on both local studies) with a 5% precision;
The area under the receiver operating characteristic (ROC) curve (estimation of 0.8) with an α risk of 5% and a precision of 0.02.
There is no exclusion period during which participation in another study is prohibited, as soon as both protocols are compatible. A premature ending for a patient participation to the study will occur in three situations: (1) Withdrawal of consent. (2) Serious AE preventing further participation in the study. (3) Discovery of a non-inclusion criterion (eg, pregnancy during the study). No specific follow-up is organised for patients ending prematurely their participation in the study. The acute rejection monitoring of these patients will continue on the basis of the usual parameters (with or without MRI) as their local physicians judge necessary. When a patient withdraws his consent, the data acquired before the withdrawal will be available for the statistical analysis except in case of refusal by the patient. A procedure to replace patients ending prematurely their participation in the study will be implemented. No specific follow-up is organised for patients after their participation in the study. The acute rejection monitoring of these patients will continue on the basis of the usual parameters (with or without MRI) as their local physicians chose.
Statistics
The primary analysis of the study will be the determination of the MRI sensitivity to detect a cardiac graft rejection. The sensitivity will be calculated as the number of true positive tests (ie, positive MRI with a concurring positive biopsy) divided by the total number of positive biopsies. The cut-off which will be used to categorise positive and negative MRI tests will be 60 ms (after correction for the MR scanner calibration).
The specificity will be calculated as the number of truly negative tests (ie, negative MRI with a concurring negative biopsy) divided by the total number of negative biopsies.
The area under the ROC curve, which measures the overall predictive performance within all the spectrum of MRI values, will also be calculated. The isolated performance of MRI to predict cardiac graft rejection will be assessed. In addition to determining if MRI adds significant prediction to the usual clinical prediction of cardiac rejection, the difference between the C-index of the logistic regression model with cardiac graft rejection as an end point and usual clinical variables as covariates will be compared with the C-index of the same logistic model with MRI variables on top of other clinical variables. The information gain between the two nested models that predict cardiac graft rejection with either clinical variables or MRI in addition to clinical variables will also be assessed with the likelihood ratio test. Regarding the secondary end point 1, in the subset of patients with negative biopsies, the predictive performance of MRI with regard to a diagnosis of acute myocardial rejection within a month from the MRI will be further assessed. Sensitivity, specificity and area under the ROC curve will be determined. Again, the C-index difference and information gain related to the addition of MRI variables in addition to clinical variables will be determined. A survival analysis will not be used because the occurrence of the event rather than the timing of the event within 1 month is of importance here. Regarding the secondary end point 2a (proportion of AEs), χ2 test will be performed to assess differences in AE probability. Regarding the secondary end point 2b, we will use the Kruskal-Wallis test to determine if the overall physical and psychological distress scores are different across the two groups. Differences within subparts of the physical and psychological distress scores will be assessed with the Dunn post hoc test, which is a non-parametric post hoc test. The same statistical approach will be used for secondary end point 4. Regarding the secondary end point 3, 95% interobserver limits of agreement will be calculated for T2 quantification and Cohen's κ coefficient will be calculated for histological grading. The choice of the statistics has been driven by the nature of the variables: T2 is a continuous variable, whereas histology rating is a categorical variable. For T2 quantification, a Bland-Altman graph will also be provided. Patients who withdrew consent will not be analysed. Since MRI and biopsies will be performed, within 24 h of each other, a very low number of losses to follow-up will exist regarding the primary analysis. No imputations will be performed. Missing data will not be reconstructed. A p value smaller than 0.05 will be considered to be significant.
Discussion
This study protocol has two distinct characteristics:
It was designed to guarantee good quality control during the data acquisition process. Since we expected many vendors, fields and different versions of MRI scanners, we designed a calibration process to ensure that every T2 measured would be consistent. Every MRI acquisition will also include a control pad to detect any change in the process (due to the new software version, new radiologist or other confounding factors). Each acquisition and each biopsy will be interpreted twice, locally and in a centralised manner;
We chose to artificially increase the prevalence of rejection in order to obtain better statistical power. Therefore, every patient will have four MRI examinations, plus one or two more if the physicians suspect a rejection. With the same kind of rule, we reached a prevalence of 15% in our previous study.15
Ethics and dissemination
There is minimal risk to patients involved in this study as the only interventions are MRI examinations with no expected AEs. Patient data will be strictly anonymised. This trial was selected and granted by the national French clinical trial program (PHRC for ‘Programme Hospitalier de Recherche Clinique’) in 2013. The inclusions have started on January 2015. The study will lead to a dissemination of the T2 quantification method at a national level. The results of the study will also be published and could lead to a modification of the current ISHLT recommendation1 (such a study was recommended by the ISHLT).
Footnotes
Contributors: LB is the principal investigator and conceived the study. LB and AC designed the study and wrote the manuscript. FO designed the MRI registration and image treatment method and participated in the writing of the manuscript. NG helped in the design of the study as methodologist. JF significantly helped during the design of the study and participated in its design and coordination. All the authors read and approved the final manuscript.
Funding: This study is supported by a French government grant as part of the PHRCN-2013 program (National Program for Hospital Clinical Research) under the reference PHRCN13-15606N.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethics approval has been obtained from the institutional research ethics board (Comité de Protection des Personnes III—Hopital de Brabois—Vandoeuvre-les-Nancy) under the reference 2014-A00848-39. DRAGET was accepted by the French National Medical Security Agency (ANSM) on 3 July 2014.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Data sharing statement: The original protocol of the study is available.
References
- 1.Costanzo MR, Dipchand A, Starling R et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914–56. 10.1016/j.healun.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 2.Patel JK, Kobashigawa JA. Should we be doing routine biopsy after heart transplantation in a new era of anti-rejection? Curr Opin Cardiol 2006;21:127–31. 10.1097/01.hco.0000210309.71984.30 [DOI] [PubMed] [Google Scholar]
- 3.Rizeq MN, Masek MA, Billingham ME. Acute rejection: significance of elapsed time after transplantation. J Heart Lung Transplant 1994;13:862–8. [PubMed] [Google Scholar]
- 4.Winters GL, McManus BM. Consistencies and controversies in the application of the International Society for Heart and Lung Transplantation working formulation for heart transplant biopsy specimens. Rapamycin Cardiac Rejection Treatment Trial Pathologists. J Heart Lung Transplant 1996;15:728–35. [PubMed] [Google Scholar]
- 5.Stewart S, Winters GL, Fishbein MC et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710–20. 10.1016/j.healun.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 6.Marboe CC, Billingham M, Eisen H et al. Nodular endocardial infiltrates (Quilty lesions) cause significant variability in diagnosis of ISHLT Grade 2 and 3A rejection in cardiac allograft recipients. J Heart Lung Transplant 2005;24:S219–26. 10.1016/j.healun.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 7.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: a seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43–7. 10.1016/0735-1097(92)90049-S [DOI] [PubMed] [Google Scholar]
- 8.Huddleston CB, Rosenbloom M, Goldstein JA et al. Biopsy-induced tricuspid regurgitation after cardiac transplantation. Ann Thorac Surg 1994;57:832–6; discussion 836–7 10.1016/0003-4975(94)90184-8 [DOI] [PubMed] [Google Scholar]
- 9.Mielniczuk L, Haddad H, Davies RA et al. Tricuspid valve chordal tissue in endomyocardial biopsy specimens of patients with significant tricuspid regurgitation. J Heart Lung Transplant 2005;24:1586–90. 10.1016/j.healun.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Wong RC, Abrahams Z, Hanna M et al. Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant 2008;27:247–52. 10.1016/j.healun.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 11.Mena C, Wencker D, Krumholz HM et al. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiogr 2006;19:1295–300. 10.1016/j.echo.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 12.Schweiger M, Wasler A, Prenner G et al. The prognostic validity of the ventricular evoked response (VER) signals in cardiac transplantation. J Heart Lung Transplant 2005;24: 1730–5. 10.1016/j.healun.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 13.Marie PY, Angioï M, Carteaux JP et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol 2001;37:825–31. 10.1016/S0735-1097(00)01196-7 [DOI] [PubMed] [Google Scholar]
- 14.Usman AA, Taimen K, Wasielewski M et al. Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circ Cardiovasc Imaging 2012;5:782–90. 10.1161/CIRCIMAGING.111.971101 [DOI] [PubMed] [Google Scholar]
- 15.Bonnemains L, Villemin T, Escanye JM et al. Diagnostic and prognostic value of MRI T2 quantification in heart transplant patients. Transpl Int 2014;27:69–76. 10.1111/tri.12222 [DOI] [PubMed] [Google Scholar]
- 16.Bernstein MA, King KF, Zhou XJ. Handbook of MRI pulse sequences. Elsevier, 2004. [Google Scholar]