Abstract
Respiratory complications of rickets may be life-threatening particularly in developing countries. A 7-month-old boy presented with recurrent infections, seizures, failure to thrive, wheezing and respiratory distress progressing to global respiratory failure. Several antimicrobial regimens, bronchodilators and corticosteroids resulted in only short-term improvement. He was transferred from Cape Verde to a third-care hospital in Portugal. He was hypotonic and undernourished, with respiratory anguish and classical skeletal signs of rickets, despite vitamin D supplementation. Hypocalcaemia, normal phosphate levels and normal vitamin D status 25(OH)D3 and 1.25(OH)2D3) pointed to vitamin D-dependent rickets type II. Treatment with high doses of calcium and calcitriol allowed progressive respiratory, musculoskeletal and neurological recovery. Although respiratory manifestations of rickets were described many years ago, the present case raises relevant issues about the level of diagnostic support, the risk of complications and how they should be assessed and monitored.
Background
Vitamin D plays an important role in extraskeletal health (cardiovascular regulation processes, growth, cellular differentiation, oncogenesis prevention and modulation of immune response), besides calcium–phosphorus homoeostasis and bone metabolism regulation.1 Physiological concentrations of the active form, 1.25(OH)2D3 (calcitriol), has been shown to induce the human cathelicidin microbial peptide responsible for macroautophagy and subsequent killing of infectious agents including Mycobacterium tuberculosis and HIV1.2 This activated form of vitamin D plays a simultaneous role in regulating inflammatory responses by inhibiting lymphocyte proliferation and activation, differentiation and survival of dendritic cells.1
The ‘rachitic lung’ was described in the 60s and 70s of the last century, and was associated with nutritional deficiency, particularly in premature infants.3 4 In recent years, anecdotal reports of cases of chronic lung disease related to rickets were mainly due to vitamin D dietary deficiency associated with darker skin pigmentation, reduced solar exposure and exclusive breast feeding.5 6 In developed countries, respiratory symptoms associated with vitamin D deficiency are rarely described or are a consequence of iatrogenesis or genetic defects.
Despite these descriptions, cases of rickets with severe compromise of organs not directly involved in the metabolic processes, such as chronic lung disease, are now rare.
We present a case of late diagnosis of vitamin D-resistant rickets that raises relevant issues about the level of diagnostic support, the risk of complications, and how such cases should be assessed and monitored.
Case presentation
A 7-month-old African boy was admitted to Hospital Agostinho Neto, Cape-Verde, with fever, wheezing, shortness of breath and hypoxaemia. Family history was unremarkable and gestation uneventful. He had been admitted to hospital monthly, from the age of 3 months, for bronchiolitis, gastroenteritis, failure to thrive, seizures and pneumonia associated with oral candidiasis.
No aetiological agent for infections was found; tests were negative for Toxoplasma gondii, rubella, cytomegalovirus, Plasmodium, and HIV1 and 2, as was a Venereal Disease Research Laboratory (VDRL) test. Several empiric wide-spectrum antimicrobial regimens resulted in short-term improvement, response to bronchodilators and corticosteroids was insubstantial, and respiratory distress progressed uneventfully to global respiratory failure, requiring long-term oxygen therapy. Despite the absence of epidemiological or laboratory evidence for tuberculosis, the patient was started on triple tuberculosis medication, without improvement.
Owing to the clinical and radiographic evidence of rickets, calcium and cholecalciferol supplementation (1334UI 3id) was initiated. Cardiac examination was normal. The baby failed to thrive despite a well-tolerated high caloric ingestion of 195 mL/kg/day. He presented developmental delay; he was hypotonic, unable to sit and had poor vocalisations, but he did stare, and could visually follow objects and recognise familiar faces; additionally, he could transfer objects, exhibited a pincer grasp and explored objects orally.
At 10 months of age, he was transferred to Santa Maria Hospital, Portugal. On admission, he presented tachypnoea (80–90 cpm), hypoxaemia (SpO2<93%, FiO2 0.4), severe chest retractions, nasal flaring, an expiratory moan, and an insistent cough accompanied by desaturation and tachycardia. Prolonged expiratory time and bilateral crackles were heard. He presented a bell-shaped thorax, wide anterior fontanelle and frontal bossing.
He had dark curly hair with no apparent anomalies. Episodic tremor of the superior right limb, preserving intentional movement, was identical to previously interpreted seizures. Small cervical, axillar and inguinal lymphadenopathies were palpable. He had significant growth compromise (weight as P50 for 1.5 months and length as P50 for 4 months for Center for Disease Control and Prevention scales).
Investigations
Laboratory tests revealed iron-deficiency anaemia with haemoglobin 10.1 g/dL (11.1–14.1 g/dL), no inflammatory pattern and normal renal function.
Basic screening for primary (lymphocyte subpopulations, immunoglobulins, CH50, total IgE) and acquired immunodeficiency (serological tests for HIV1/2) was negative.
Non-serotyped Haemophilus influenzae and extended-spectrum-β-lactamase-producing Klebsiella pneumoniae multiresistant (sensitive only to carbapenems) were isolated in initial sputum cultures and the boy was treated with meropenem, co-trimoxazole and azithromycin for 10 days. Subsequent cultures of bronchial specimens, blood, urine and gastric aspirate were negative including for acid-fast bacilli.
Tests for cystic fibrosis were negative and so was the 24 h pH monitoring for gastro-oesophageal reflux.
Thorax CT scan showed a bell-shaped thorax, diminished pulmonary complacency, prominent chondrocostal junctions, coarse bone trabeculation and pathological rib fractures, ground-glass opacities and atelectasis of the lung parenchyma, and absence of bronchiectasis and of mediastinal lymphadenopathies (figure 1).
Figure 1.
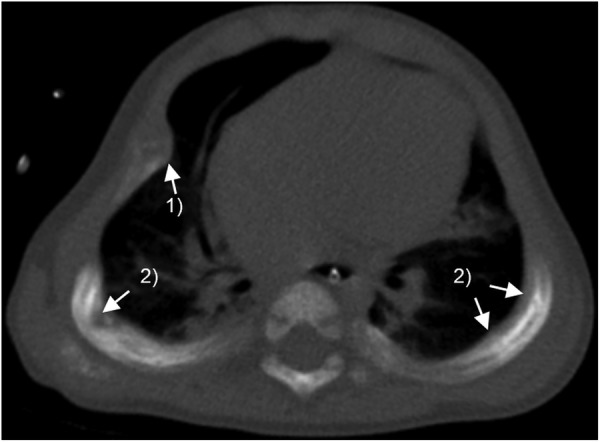
Thorax CT scan of the patient at 11 months of age: compromise of thorax expansibility and diffuse abnormal density and coarse trabeculation of the bone. (1) Enlarged costochondral junctions (‘rachitic rosary’) and (2) Pathological rib fractures.
Bone radiographs confirmed widening and fraying metaphyseal regions of the long bones, mainly in the proximal humerus; radius and ulna old fractures and incipient dorsolumbar scoliosis (figure 2).
Figure 2.

Thorax radiograph of the patient at 10 months of age showing a bell-shaped thorax. (1) Areas of consolidation and atelectasis. (2) Widening, loss of definition and fraying of metaphysis. (3) Enlarged costochondral junctions with a beading aspect (‘rachitic rosary’) and (4) Healing fractures.
Further investigation of phosphorus–calcium metabolism revealed hypocalcaemia (serum calcium 7.9 mg/dL (9–11), ionised calcium 0.86 mmol/L (1.13–1.32)), normal phosphate levels, parathyroid hormone 409.6 pg/mL (14–72), alkaline phosphatase 1673 U/L (<462), high urinary phosphate (144 mg/dL) and diminished phosphate tubular reabsorption (64.4%). Vitamin D status, both 25(OH)D3 and 1.25(OH)2D3 revealed no deficits (with 25(OH)D3 supplementation 1800 UI/day). Evaluation of teeth revealed no abnormalities.
A geneticist examination identified no dimorphism or syndromic disease. CYP27B1 and vitamin D receptor (VDR) gene sequencing revealed no mutations known to cause disease.
EEGs were normal.
Treatment
The infant was initiated on calcium gluconate 10% (4 mL three times per day, approximately 110 mg Ca/day) and calcium carbonate 1 g/d (approximately 400 mg Ca/day), 25(OH)D3 (maximum 3600 UI/day) and 1.25(OH)2D3 (progressively increased to 0.5 µg/day). Treatment was adjusted according to clinical evolution and laboratorial results with withdrawal of 25(OH)D3 and maintenance of 1.25(OH)2D3 0.25 µg/day (6 days/week). Tuberculosis medication was stopped soon after arrival and broad-spectrum antibiotics adjusted to the antimicrobial tests from cultures. Bronchodilator and steroids were discontinued and reinitiated only on exacerbations; a gradual decline in oxygen demand to 1 L/min by nasal cannula was noticed. Sodium valproate was stopped.
Outcome and follow-up
Skeletal (figures 3A–D) and thoracic deformation gradually improved, so did the respiratory distress, allowing withdrawal from oxygen after 9 months (the baby was 18 months of age). The baby was discharged at 15 months of age and re-evaluated in outpatient. There was a significant progress in growth (at 19 months his weight corresponded to P50 for 14 months and length to P50 for 6 months) and a notable tonus recovery was seen with achievement of important motor milestones, such as the ability to walk independently by 18 months.
Figure 3.
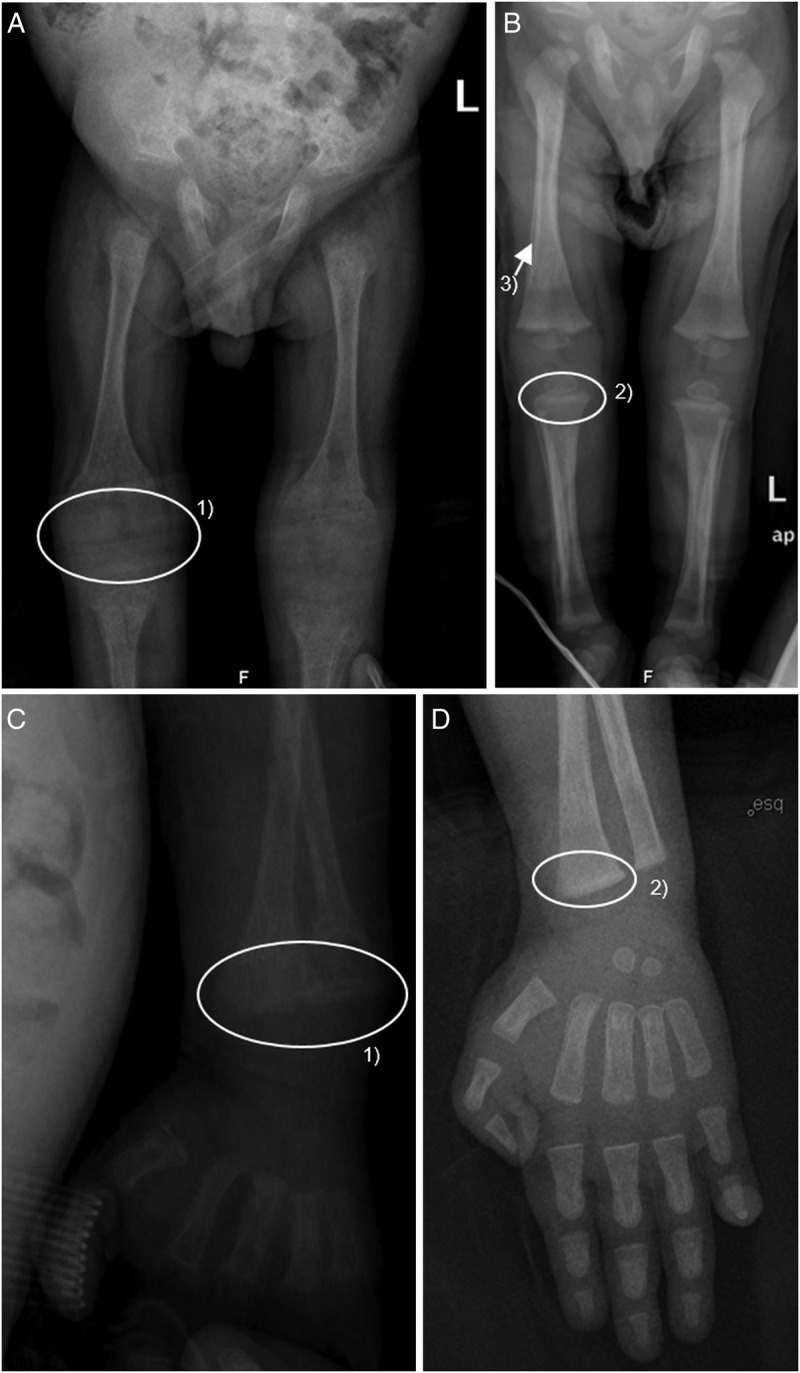
(A) and (C): Radiographic skeletal signs of rickets at 11 months of age. (1) Widening, loss of definition, cupping and fraying of metaphysis. (B) and (D): Skeletal radiographs of the patient at 15 months of age, evidencing signs of recovery with instituted therapy. (2) Persistence of widening of metaphysis, but recovery of its definition and (3) Signs of periosteal reaction evidencing signs of new bone deposition.
Discussion
The child's severe lung disease demanded evaluation for chronic lung diseases including cystic fibrosis, tuberculosis and immunodeficiency or lung disease secondary to gastro-oesophageal reflux. All of these were discarded.
Clinical, radiological and laboratory findings were diagnostic for hypocalcaemic rickets. This condition could be secondary to nutritional deficiency and could explain the hypocalcaemic seizures this child presented in the first year of life, and the delayed achievement of motor milestones due to decreased muscle tone and recurrent infection.7 8 Despite having several risk factors for nutritional vitamin D deficiency (late preterm, dark skin, low sun exposure due to multiple hospital admissions6), normal–high serum 1.25(OH)2D3 suggests a genetic aetiology: vitamin D-dependent rickets type I or II, both autosomal recessive disorders. Vitamin D-dependent rickets type I is caused by inactivating mutations in the CYP27B1 gene (which encodes the enzyme 1α-hydroxylase), resulting in deficient conversion of 25(OH)D3 to the active form 1.25(OH)2D3; type II is caused by mutations in the VDR, resulting in end-organ resistance to vitamin D action.
As this boy was under 25(OH)D3, that could not be withheld to measure basal serum levels of 1.25(OH)2D3, interpretation of laboratory results and the distinction between these two forms of vitamin D-dependent rickets turned particularly difficult. Furthermore, genetic tests did not identify mutations in VDR and CYP27B1 genes that could account for this phenotype.
The absence of 1.25(OH)2D3 deficit under supplementation with 25(OH)D3 1800 UI/day (indicating that the conversion of 25(OH)D3 to the active form 1.25(OH)2D3 was not completely compromised), and the patient's clinical and laboratory evolution, requiring supplementation at very high doses of calcium and calcitriol, suggested vitamin D-dependent rickets type II. However, alopecia (present in about two-thirds of patients with vitamin D-dependent rickets type II) was not present.9 Moreover, considering the patient's remarkable recovery under treatment with relatively small doses of calcitriol (0.5 μg/day), as opposed to the very high doses (up to 30–60 μg/day) sometimes required to treat vitamin D-dependent rickets type II, vitamin D-dependent rickets type I could not be categorically excluded.
Adequate correction of phosphorus–calcium metabolism resulted in improvement of respiratory, musculoskeletal, neurological and growth recovery and infection control, indicating rickets as the origin of these multisystem manifestations.
This case is an important reminder that—in additional to the skeletal manifestations—anaemia, hypotonia, increased risk of infectious diseases, hypocalcaemic seizures and respiratory distress can all be associated with rickets.6 Decreased muscle tone and recurrent infections contribute to the delayed achievement of motor milestones. Anaemia may result from recurrent infections, associated nutritional deficits and myelofibrosis derived from vitamin D deficiency.10
The aetiology of respiratory disease in the context of rickets has been classically attributed to osteodystrophy of the thoracic cage, compression of lung parenchyma, and hypocalcaemic myopathy of the diaphragm and intercostal muscles, leading to impaired clearance and respiratory infections.11 However, recent findings suggest that vitamin D's immune-regulatory effects are also important contributors.12
Vitamin D deficiency is re-emerging in several parts of the globe, including in Europe and the USA; a problem possibly aggravated by trends for so called ‘natural’ diets and lifestyles.13
By enabling rapid sequencing of large stretches of DNA base pairs spanning the entire exome or targeting a panel of genes of interest, next-generation sequencing technology may provide the answers that current genetic tests could not. However, currently, in our practice, this innovative technology is still expensive and reserved only for circumstances in which the result could offer a practical benefit for the patient.
Learning points.
Neither is rickets in children from tropical countries always nutritional, nor is nutritional rickets extinct in developed countries.
In additional to classical skeletal signs, hypocalcaemic rickets may present with extraskeletal signs, including respiratory distress and failure, anaemia, decreased muscle tone, delay in achievement of motor milestones, recurrent infections and hypocalcaemic seizures.
Next-generation sequencing is a promising technology in cases of disease of genetic aetiology in which classical genetic tests are unable to identify the mutations responsible for disease.
Acknowledgments
The authors would like to acknowledge the assistance provided by Dr Juliette Dupont, geneticist in the Department of Paediatrics, Santa Maria Hospital, was greatly appreciated.
Footnotes
Contributors: ASCF was responsible for acquiring the information, drafting of the manuscript and subsequent revision, with help from all the coauthors. SL was responsible for the initial and current care of the patient in his home country. ARS and CS were responsible for the nephrological investigation and follow-up. LL was responsible for the imaging studies, and their interpretation and selection. LL, ARS, CS and TB provided clinical input into the article. TB was responsible for the original idea, final approval of the manuscript and respiratory follow-up of the patient.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Christakos S, DeLuca HF. Minireview: Vitamin D: is there a role in extraskeletal health? Endocrinology 2011;152:2930–6. 10.1210/en.2011-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog 2012;8:e1002689 10.1371/journal.ppat.1002689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.[No authors listed] Rickets, jaundice, and late onset respiratory distress in premature babies. BMJ 1977;2:78–9. 10.1136/bmj.2.6079.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasgow JF, Thomas PS. Rachitic respiratory distress in small preterm infants. Arch Dis Child 1977;52:268–73. 10.1136/adc.52.4.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr 2004;50:364–8. 10.1093/tropej/50.6.364 [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui TS. Respiratory distress: a rare presentation of rickets. J Ayub Med Coll Abbottabad 2006;18:67–8. [PubMed] [Google Scholar]
- 7.Pedrosa C, Ferraria N, Limbert C et al. Hypovitaminosis D and severe hypocalcaemia: the rebirth of an old disease. BMJ Case Rep 2013;2013:pii:bcr2012007406 10.1136/bcr-2012-007406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nield LS, Mahajan P, Joshi A et al. Rickets: not a disease of the past. Am Fam Physician 2006;74:619–26. [PubMed] [Google Scholar]
- 9.Malloy PJ, Feldman D. Genetic disorders and defects in vitamin D action. Endocrinol Metab Clin North Am 2010;39:333–46. 10.1016/j.ecl.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.al-Eissa YA, al-Mashhadani SA. Myelofibrosis in severe combined immunodeficiency due to vitamin D deficiency rickets. Acta Haematol 1994;92:160–3. 10.1159/000204211 [DOI] [PubMed] [Google Scholar]
- 11.Tiwari S, Kumar R, Singla S et al. Congenital rickets presenting as refractory respiratory distress at birth. Indian J Pediatr 2014;81:800–2. 10.1007/s12098-013-1099-3 [DOI] [PubMed] [Google Scholar]
- 12.Chesney RW. Vitamin D and The Magic Mountain: the anti-infectious role of the vitamin. J Pediatr 2010;156:698–703. 10.1016/j.jpeds.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Le Louer B, Lemale J, Garcette K et al. [Severe nutritional deficiencies in young infants with inappropriate plant milk consumption]. Arch Pediatr 2014;21:483–8. 10.1016/j.arcped.2014.02.027 [DOI] [PubMed] [Google Scholar]


