Abstract
Diabetic retinopathy is a major cause of vision loss in diabetic patients. Currently, there is a need for making decisions using intelligent computer algorithms when screening a large volume of data. This paper presents an expert decision-making system designed using a fuzzy support vector machine (FSVM) classifier to detect hard exudates in fundus images. The optic discs in the colour fundus images are segmented to avoid false alarms using morphological operations and based on circular Hough transform. To discriminate between the exudates and the non-exudates pixels, colour and texture features are extracted from the images. These features are given as input to the FSVM classifier. The classifier analysed 200 retinal images collected from diabetic retinopathy screening programmes. The tests made on the retinal images show that the proposed detection system has better discriminating power than the conventional support vector machine. With the best combination of FSVM and features sets, the area under the receiver operating characteristic curve reached 0.9606, which corresponds to a sensitivity of 94.1 % with a specificity of 90.0 %. The results suggest that detecting hard exudates using FSVM contribute to computer-assisted detection of diabetic retinopathy and as a decision support system for ophthalmologists.
Keywords: Diabetic retinopathy, Colour fundus images, Hard exudates, Laws texture energy measures, Fuzzy support vector machine
Introduction
Diabetic retinopathy is a complication of diabetes that is caused by damage to the small blood vessels of the retina. Blindness is an outcome of diabetic retinopathy, and it occurs in 80 % of all diabetic patients [1]. DR is the leading cause of blindness in people between the age of 20 and 60. However, early detection and prompt treatment could prevent the vision loss caused by DR. Premature detection of DR is a challenging task, because patients with DR will have no symptoms until vision loss develops. Hence, person with diabetes should have a comprehensive retina screening once in every year. Colour fundus photography documents the retina of the eye with specially designed cameras, which is considered as a sensitive means of detecting early signs of DR when examined by the ophthalmologists. DR is a progressive disease; it can progress from nonproliferative diabetic retinopathy (NPDR) to proliferative diabetic retinopathy (PDR). NPDR is the earliest stage of DR, while PDR is an advanced stage. Yearly screening of the eye is done by examining the eye fundus of the diabetic patients looking for any signs of NPDR. It has been shown that fundus photography is the most accurate means of screening for retinopathy [2]. According to the clinical findings done by Viswanath and Murray [3], in most developing countries, there are very few specialists to examine diabetic patients by ophthalmologists in screening programmes.
NPDR commonly known as background retinopathy is an early detection of DR. The DR starts with a mild NPDR, which usually does not affect the vision. When vision is affected, it is the result of leaking of fluid in the blood vessels called exudates (EXs). Exudates can be hard exudates (yellow spots seen in the retina) and soft exudates (pale yellow or white areas with ill-defined edges). In this article, an intelligent computer-aided DR detection system is developed for detecting the exudates in the fundus images. An automated tool can be used for assisting the ophthalmologists in the detection process, which gives attention to diabetic patients in receiving effective treatment. The screening tool utilises intelligent computer algorithms to automatically analyse the features of DR and detect early signs of pathology.
With the advent of medical imaging and artificial intelligence, automated screening methods are investigated by many researches. A method was presented by Geir E. Oien in 1995 [4], which uses an automated system based on modern digital image processing which needs less human intervention and will yield more consistent results. Maria Garcia et al. [5] applied a radial basis function classifier for detecting hard exudates in retinal images. In the first stage, the luminosity and the contrast of the images were normalised to avoid the intra-image variation caused by inadequate illumination and poor quality of the image. Then segmentation is done by making use of the local properties of the exudates and combining global and local histogram thresholding methods. The author used a total of 24 features extracted from the segmented regions, and a feature selection is done based on linear regression to select the relevant features for specific problem; to classify the regions as exudates or non-exudates, the selected significant 12 features were given as input to the radial basis function network. The performance was tested on a database of 67 retinal images of varying colour, size and quality.
Alireza et al. [6] investigated a computational intelligence approach for automatic detection and identification of exudates pathologies. As a pre-processing step, colour normalisation and contrast enhancement are done. Images are segmented based on the colour represented in Luv colour space, and a fine segmentation is done using fuzzy c-means (FCM) clustering algorithm. Appropriate features are selected using genetic algorithm from the segmented regions, and classification of exudates is done using neural network for a dataset of 300 manually labelled images.
Gwenole et al. [7] evaluated a computer-aided diagnostic system for automatically detecting abnormalities in colour retinal mages. The bright lesions were detected by employing pixel classification to group pixels that are likely inside the bright lesion. The pixel classification approach used a K-nearest neighbour classifier, and 31 features were extracted using Gaussian filter bank. Haniza et al. [8] proposed an automated system for detecting hard exudates in fundus images. For the purpose of diagnosis, the fundus images were segmented using FCM clustering and segments the background region outside of the eyeball, the blood vessels, the healthy background retina, the low intensity exudates and high intensity exudates and tested with 15 images achieving a true positive fraction of 97.8 %. The segmented regions are subjected to edge detection by Prewitt operations to identify the soft and hard exudates. Finally, an inverse surface thresholding is used to separate exudates and optic disc from the background.
Niemeijer et al. [9] proposed a pixel-based method for differentiating exudates, drusen and cotton wool spots and tested with 300 retinal images achieving a sensitivity of 95.5 % for exudates detection. The probability of each pixel is calculated, and the pixel with higher probability is taken as a lesion pixel. Based on the cluster characteristics, clusters were classified as exudates, drusen and cotton wool spots. Usher et al. [10] employed a neural network-based classification on the candidate regions obtained using recursive region growing and adaptive intensity thresholding. A matched filter has been used for diagnosing proliferative diabetic retinopathy by Lei Zhang [11]. A local vessels cross-section analysis using double-sided thresholding is used for reducing the false positive alarms. Carla Agurto et al. [12] used an amplitude modulation frequency modulation for discriminating between normal and pathological retinal images.
From the above, we can see that the previous methods either have a less classification accuracy for hard exudates detection, or that they are not a fully automated system. The previous methods did not consider texture features as an important feature on hard exudates, while the complexity of hard exudates detection requires us to identify them more accurately by considering multiple feature characteristics.
DR may cause several abnormalities in the retina like microneurysms (MAs), haemorrhages (HMs), hard exudates and soft exudates (cotton-wool spots). Among these pathologies, exudates are the prime markers of DR causing vision loss [13]. The main goal of this article is to develop an intelligent decision-making system that automatically detects the hard exudates in the retinal images using intelligent fuzzy support vector machine classifier. The hard exudates detection is formulated into three stages. First, the retinal images are subjected to segmentation of optic disc. In this step, the retinal images are subjected to morphological and edge-based operation. Then the colour features are extracted from the image. Retinal images taken have variable quality, colour and brightness; the optic disc segmentation will reduce the false alarms in all the images. In the second phase, colour and texture features are extracted from the retinal images. The colour-based features are extracted from the RGB components and opponent colour space of the original retinal image. In addition to colour features, textures feature extracted from the histogram-equalised image are considered for hard exudates detection. The feature set is given as input to the intelligent fuzzy classifier to classify the exudates and non-exudates pixel with high accuracy.
In recent years, researches on using kernel function in machine learning algorithms are used in many medical applications with support vector machine (SVM) being an important one [14]. SVM is sensitive to noise and outliers, because SVM assumes all data points have the same importance in classification problems [15]. SVM does not have the ability to deal with such data points. Choosing an optimal separating hyperplane in training patterns is difficult due to uncertainty in making decisions. Fuzzy approaches are widely applied for uncertain problems [16]. Fuzzy support vector machine (FSVM) is applied for classification of exudates in DR to deal with the effects of noise and outliers. FSVM highly integrates with fuzzy data and have a good generalisation power [17]. The proposed detection scheme employs a fuzzy membership function to estimate the class membership of the features that contain exudates and non-exudates. The discriminating ability of the colour and texture features and the performance of the FSVM classifier are evaluated using the receiver operating characteristic (ROC) curve.
Materials and Methods
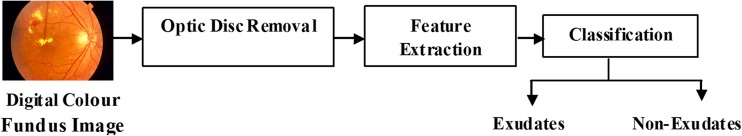
In this section, the database used and the methods that are used for hard exudates detection is discussed. Figure 1 shows the overall block diagram of the proposed diabetic retinopathy detection system.
Fig. 1.
The proposed diabetic retinopathy detection system model
Database Description
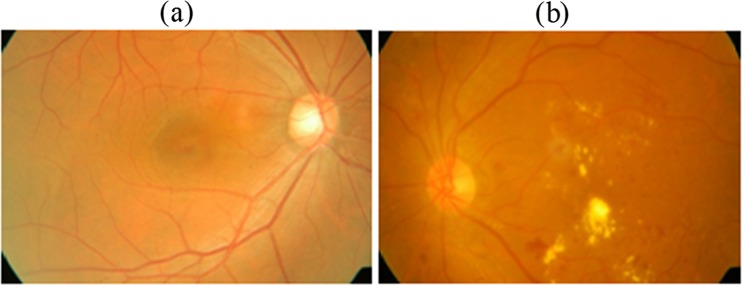
The digital colour fundus image database utilised in this paper was collected from medical diagnostics centre. The images were taken in a Canon CR6-45NM retinal camera and were stored in JPEG format. The Canon CR6-45NM digital imaging system is a non-mydriatic retinal camera equipped with a Canon EOS 10D digital camera back. A total of 200 retinal images with varying colour, brightness and quality were collected from diabetic patients. Of the 200 images in the database, 75 retinal images are of patients with no pathologies and remaining 125 contain pathologies (exudates). The images are of 2240 × 1488 dimensions with resolution of 96 dpi. The age of the diabetic patients from whom retinal images were captured is from 20 to 60. For all the individuals whose fundus retina images used in this manuscript has given consent to publish the case details, and IRB approved this study for further processing using computer-aided diagnosis system. All the images in the database were graded by trained ophthalmologists for evaluating the performance of the proposed intelligent classifier. The example of retinal images with pathology and without pathology (normal) is shown in Fig. 2.
Fig. 2.
Example colour fundus image. a Normal retinal image. b Retinal image with exudates
Optic Disc Removal
Optic disc (OD) removal is an important stage in developing a decision-making system for automatic diagnosis of retinal pathologies. The removal process requires OD localization and then segmenting the optic disc. In this paper, segmentation of OD is performed using morphological and edge-based techniques and used circular Hough transform for OD segmentation followed by Arturo Aquino et al. [18].
Feature Extraction
For the classification phase, the input to the FSVM classifier is given in the form of colour and texture features. A complete representation of true exudates is identified by considering the 29 features of which, 4 features are obtained by the colour component of the retinal image and remaining 25 features are obtained using Laws texture energy measures [19–21]. The retinal images are taken in different imaging conditions, and to get the best features from the images, colour histograms are computed. Colour information is a human subjective visual characteristic, and it is a widely used visual feature in recognising objects. The colour histograms are built from the retinal images in the RGB colour space. The RGB values are measured, and the normalised rg values is computed as [22]
| 1 |
| 2 |
The opponent colour space is used as a feature set for better perception of colour. The two components are taken from the colour space, the luminance channel (O1) and the red-green (referred as G-R) channel (O2). The opponent colour spaces are defined by [22]
| 3 |
| 4 |
The retinal images cannot be analysed by using colour features alone, because the colour histogram representation depends on the colour of the object that is studied, not depending on the texture features. In this paper, we combine both colour and texture features for classifying exudates in retinal images. The texture features are obtained using Laws texture energy measure and distinctive physical composition of image surface [19]. Laws texture energy measures uses fitter masks to extract different texture descriptors in retinal images. They measure texture properties by assessing average grey level, spot, wave, ripple and edges which can be used for classification. The labelled texture descriptors are derived from a 3 × 3 masks and 5 × 5 masks. The 5 × 5 masks can be combined to form 2D convolution kernels. The five vectors are given below.
These masks are convoluted with the retinal image, and the texture energy statistics are calculated which forms the texture features. The resultant is a set of grey scale images.
A nonlinear windowing operation is performed with a window size of 15 × 15. The proposed classification system is a pixel-based classification. Every pixel of the convoluted image is replaced by comparing with the local neighbourhood based on sum of the neighbouring pixel. The texture energy measure Ek(i, j) is given as,
| 5 |
Fk, is the result of filtering with kth mask. The images obtained after windowing operation are normalised using min-max normalisation [23]. The 25 texture features obtained from the retinal images for exudates detection in diabetic patients are analysed, and the discriminating ability of these features were tested using FSVM classifier.
Classification Using FSVM
Support vector machine is a constructive learning algorithm based on the statistical learning theory and the Vapnik-Chervonenkis (VC) dimensions [24]. For nonlinear classifier models, SVM maps the input vector x into a higher dimensional feature space ℋ, and an optimal separating hyperplane is constructed. A nonlinear transformation φ(x) is done for mapping, using the suitable basis function, and then uses the linear model in the feature space, which is called the kernel function. The SVM classification is written in the following form;
| 6 |
where parameter w is the weight factor, and b is the bias, which are determined from training data set. SVM finds the support vectors that define the separators giving the widest separation of classes. The merit of support vector machine is that by a kernel function K(xi, yi), which is the inner product in the feature space, it tries to make the training data linear reparability in the high-dimension feature space, thus achieve nonlinear reparability in the input space. A training sample (xi, yi) is a support vector if it holds yiwTφ(x) + b ≥ 1. Let Sk be the support vector and k ∈ [1, K], and the SVM function becomes
K(.,.) represents the kernel function to represent the effect of nonlinear mapping φ(.) in classification of hard exudates. A radial basis function network is used as a kernel function for classification of hard exudates with σ the width of the network mapping the input space to a higher dimensional feature vector and is given as,
| 7 |
The strategy is to map the training data to the higher dimensional feature space and include most of them into a hypersphere of minimum size. That is, the task is to minimise the following objective function.Minimise,
| 8 |
where C is the regularisation parameter controlling the tradeoffs between margin maximisation and classification error. E is called the slack variable that is related to classification errors in SVM.
Fuzzy SVM, which was proposed by Lin and Wang [17], applies a fuzzy membership function to each input data of SVM. In SVM, the training process is very sensitive to those outliers in the training dataset which are far away from their own class [15, 16]. The data points in the training set are assigned with a membership and sum of the deviations weighted by their membership. This will reduce the effect of outliers or noises, and if one data point is detected as outlier, then it is assigned with a low membership value, so their impacts to total error sum decreases. The training set is transferred into a fuzzy training set, and the classification problem is modelled as,
| 9 |
Where fi is the fuzzy membership and fi ∈ [0, 1]. In the above objective function, when fi is small, then the data point is less important, and the term fiEi will become small. The error term Ei is scaled by the membership value fi. The solution of the optimization problem in Eq. (9) can be found by finding the saddle point of the Lagrangian.
Where αi and βi are the non-negative Lagrange multipliers. Differentiating L with respect to w, b, Ei and setting the result to zero, we obtain,
It is noted from the above equation that ai corresponds to a training data point xi. The main idea of FSVM is that if the input is detected as an outlier, we reduce one input’s membership such that its contribution to total error term is decreased. In such way, we expect the new fuzzy machine to make full use of data and achieve better generalisation ability.
Experimental Results and Discussion
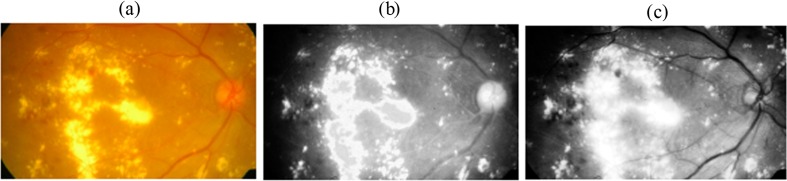
The proposed FSVM classifier was implemented using the proposed approach as shown in Fig. 1. The colour fundus images were collected from DR screening centres taken using Canon CR6-45NM digital imaging system. The proposed intelligent system first segments the optic disc in the retinal image. Optic disc imposes false alarms and decreases the accuracy by increasing the misclassification rate. Then, features are extracted for pixel-based classification. The pixel-based classification system will assign one or more classes to individual pixels in the image. In this work, the pixel feature classification system will assign the pixels to either exudates class or to non-exudates class. The intelligent classification system uses multiple features by extracting colour features and texture features from the retinal image. The histogram of R and G component and the opponent colour space components are used as colour features. The texture features based on laws texture descriptors (average grey level, spot, wave, ripple and edge) are taken from pixel and its surrounding. The intelligent FSVM classifier operates in two phases: (1) training phase, the classifier learns to correctly classify the exudates and non-exudates with a known output and (2) testing phase, the classification system classifies previously unseen images. The main goal of the proposed intelligent decision-making classifier is to separate the valid hard exudates from false alarms. A training set consisting of randomly selected 75 retinal images with and without pathologies and 200 images were used for testing the classifier. The testing was done on images in the whole database (200 images). The FSVM classifier was trained based on the 29 feature for each pixel. Figure 3 shows the automated analysis of retinal images and shows the R and G channels. Figure 3b, c shows the red and green colour space features of the retinal image, respectively.
Fig. 3.
Automated feature analysis of the colour fundus image. a Colour fundus image. b R component. c G component
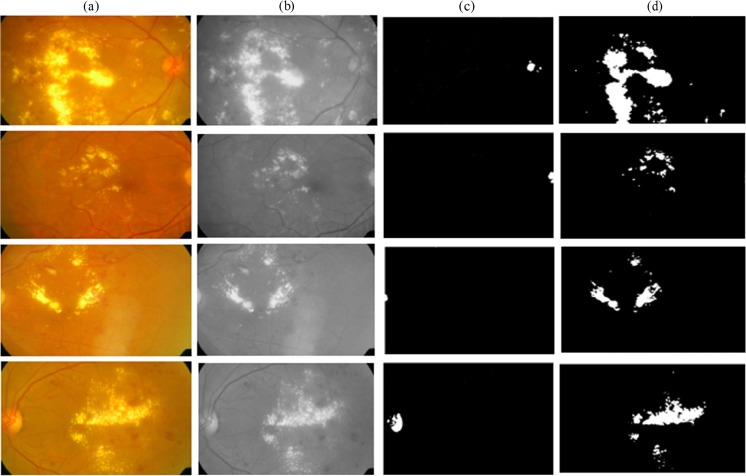
In the texture feature extraction stage, the input image is divided into 15 × 15 overlapping subregions. The subregions are convoluted with a mask, and the sum of the subregions was used as an input to the FSVM classifier. Since the exudates can be of varying size, intensity and brightness taking colour and texture features offers a better solution for automatic detection of pathologies and is shown in Fig. 4.
Fig. 4.
Detection of hard exudates using FSVM classifier. a Original colour fundus image. b Grey scale image. c Optic disc segmentation. d Hard exudates detection
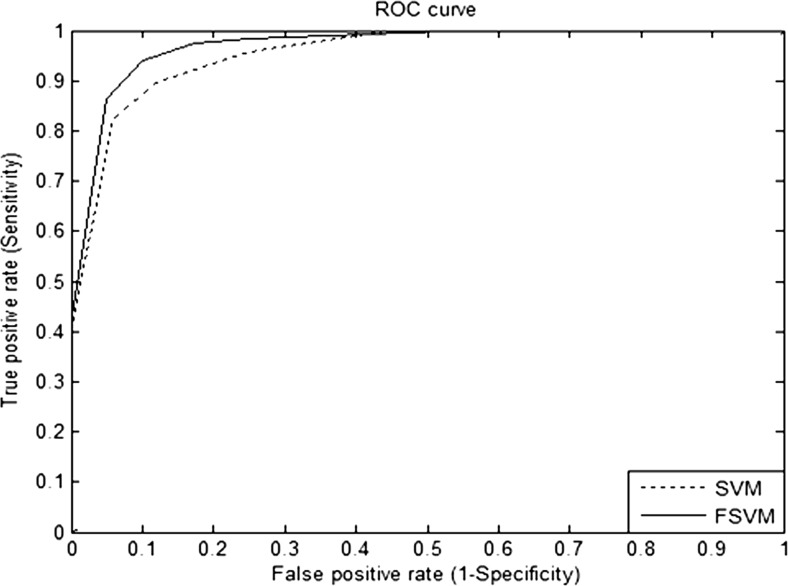
The concept of fuzzy set theory is incorporated with the SVM model. The fuzzy parameters used in this work are constrained to a logistic regression membership function [25] given by Eq. (10). The membership value for the membership functions is assigned with a value in the interval of [0, 1]. In the FSVM classifier, the regularisation parameter is set as C = 10, σ2 = 1 for radial basis function kernel. These parameters are set using trial and error method. The performance of the proposed classifier is evaluated using the receiver operating characteristic (ROC) curve. Figure 5 shows the ROC curve of the proposed approach and the conventional SVM classifier.
| 10 |
Fig. 5.
ROC curve for SVM and FSVM classifier
For evaluation of detection performance, the number of true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN) should be taken into consideration. In this way, the true positive rate can be plotted against the false positive rate. Each decision threshold results in a corresponding operating point on the curve. Such a curve is referred as receiver operating characteristic (ROC) curve [26]. TP, TN, FP and FN are the four different possible outcomes of a single prediction for two classes exudates (+1) and non-exudates (−1). The values taken for the detection performance is calculated by considering pixel by pixel classification. The total number of pixels in the test data is estimated, and the correctly classified number of pixels is evaluated; based on that, the sensitivity and specificity for the ROC curve is measured. The overall performance of detection systems has been measured and reported in terms of classification accuracy which is the percentage of diagnostic decisions that proved to be correct. In characterising the accuracy of a diagnostic screening test, the ROC curve of the test provides much more information about how the test performs than just a single estimate of the test’s sensitivity and specificity. A high-performance AZ value is achieved with the hyper parameters C = 10 and σ2 = 1. The ROC is drawn by choosing different values for hyper parameters C and σ2, and this is chosen as the operating point. The real-time fundoscopic images are taken using the same retinal camera. Hence, the retinal images will not show more variations from images to images. In case of the SVM classifier, the AZ value obtained was 0.84533. When fuzzy is applied to the SVM, the AZ value obtained was 0.9552, which is higher than the SVM classifier. In order to evaluate the laws texture features on FSVM classifier, additional metrics like sensitivity, specificity, classification accuracy, Youden’s index and misclassification rate are considered. Youden’s index (J), developed by W. J. Youden [27], is a single statistic that captures the performance of a classification test.
The result emphasises the potential of FSVM algorithm to be used as a classifier for exudates detection in retinal images. Table 1 demonstrates the performance of the classifier using various summary measures. The performance of the FSVM classifier with an accuracy of 93.0 % is better than the conventional SVM classifier. A sensitivity of 94.1 % is achieved using the proposed method with the specificity of 90.0 %. The FSVM classifier has a misclassification rate of 0.07 which is less when compared to the other classifier models. It was of more interest to focus the experimentation on trying to improve the classification rate by focussing on fuzzy membership values. Consequently, by using fuzzy parameter for SVM, the classification accuracy is improved drastically.
Table 1.
Performance measures for SVM and FSVM classifiers
| Classifiers/metrics | Performance measures | |
|---|---|---|
| SVM | FSVM | |
| Sensitivity (%) | 89.5 | 94.1 |
| Specificity (%) | 88.2 | 90.0 |
| Accuracy (%) | 89.2 | 93.0 |
| AUC | 0.9426 | 0.9606 |
| Youden’s index | 0.7775 | 0.8407 |
| Misclassification rate | 0.108 | 0.07 |
Detection of hard exudates is a difficult and challenging task, since the newly formed blood vessel is tiny and not clearly visible. FSVM have a great potential to be applied in automatic detection of hard exudates in retinal images by reducing the misclassification rate. In experimental analysis, getting an accurate suspected region in retinal image is a crucial issue. As expected in the experiment, the texture and the colour features obtained from the original image removes the fluctuations caused by blood vessels, varying image quality and illumination. Since the FSVM combines the fuzzy membership characteristic with the supervised learning of conventional support vector machine, the proposed detection algorithm based on the FSVM worked very well and obtained a robust performance. The corresponding performance results demonstrated in Table 1 shows FSVM is higher in terms of sensitivity, specificity, accuracy, AUC, Youden’s index and misclassification rate. A global solution is found by using FSVM algorithm increased the detection accuracy.
Conclusion
In this paper, we investigated a novel method for the detection of hard exudates in colour fundus images. The pathology caused by diabetic retinopathy is detection using an intelligent decision support system. The colour and texture features are used as feature sets for discriminating between the exudates and non-exudates pixels. First, the optic disc is segmented using a morphological and edge detection process. Second, colour and laws texture energy measures are applied to obtain texture features from the retinal area. Then, an intelligent classifier—fuzzy SVM—is used to detect the pathological regions in the colour fundus images. The proposed intelligent decision support system can be used by the ophthalmologists as a second reader which detects the subtle signs of hard exudates at early stage. The performance evaluation demonstrates that the result of the FSVM classifier is generally better than that of the conventional SVM classifier.
Contributor Information
T. Jaya, Email: jayatcsiit@gmail.com
J. Dheeba, Phone: +91-9442009711, Email: deeps_3u4@yahoo.com
N. Albert Singh, Phone: +91-9442111776, Email: albertsingh@rediffmail.com.
References
- 1.National Institute of Health: Facts about diabetic retinopathy disease. National Eye Institute Health Information, No. 03–2171, 2009
- 2.Phillips R, Forrester J, Sharp P. Automated detection and quantification of retinal exudates. Graefe Arch Clin Exp Ophthalmol. 1993;231:90–94. doi: 10.1007/BF00920219. [DOI] [PubMed] [Google Scholar]
- 3.Viswanath K, Murray McGavin DD. Diabetic retinopathy: clinical findings and management. Community Eye Health. 2003;16(46):21–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Oien GE, Osnes P: Diabetic retinopathy: automatic detection of early symptoms from retinal images. In Proc, Norw. Signal Process. Symposium, pp. 135–140, 1995
- 5.Garcia M, Sanchez CI, Poza J, Lopez MI, Hornero R. Detection of hard exudates in retinal images using a radial basis function classifier. Biomed Eng Soc. 2009;37(7):1448–1463. doi: 10.1007/s10439-009-9707-0. [DOI] [PubMed] [Google Scholar]
- 6.Osareh A, Shadgar B, Markham R. A computational-intelligence-based approach for detection of exudates in diabetic retinopathy images. IEEE Trans Inf Technol Biomed. 2009;13(4):535–545. doi: 10.1109/TITB.2008.2007493. [DOI] [PubMed] [Google Scholar]
- 7.Quellec G, Russell SR, Abramoff MD. Optimal filter framework for automated, instantaneous detection of lesions in retinal images. IEEE Trans Med Imaging. 2011;30(2):523–533. doi: 10.1109/TMI.2010.2089383. [DOI] [PubMed] [Google Scholar]
- 8.Yazid H, Arof H, Isa HM. Automated identification of exudates and optic disc based on inverse surface thresholding. J Med Syst. 2012;36:1997–2004. doi: 10.1007/s10916-011-9659-4. [DOI] [PubMed] [Google Scholar]
- 9.Niemeijer M, Ginneken BV, Russell SR, Suttorp M, Abramoff MD. Automatic detection and differentiation of drusen, exudates and cotton wool spots in digital colour fundus photographs for diabetic retinopathy diagnosis. Invest Ophthalmol Vis Sci. 2007;48:2260–2267. doi: 10.1167/iovs.06-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usher D, Dumskyj M, Himaga M, Williamson T, Nussey S, Boyce J. Automated detection of diabetic retinopathy in digital retinal images: a tool for diabetic retinopathy screening. Diabetes Med. 2004;21:84–90. doi: 10.1046/j.1464-5491.2003.01085.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Li Q, You J, Zhang D. A modified matched filter with double-sided thresholding for screening proliferative diabetic retinopathy. IEEE Trans Inf Technol Biomed. 2009;13(4):528–534. doi: 10.1109/TITB.2008.2007201. [DOI] [PubMed] [Google Scholar]
- 12.Agurto C, Murray V, Barriga E, Murillo S, Pattichis M, Davis H, Russell S, Abràmoff M, Soliz P. Multiscale AM-FM methods for diabetic retinopathy lesion detection. IEEE Trans Med Imaging. 2010;29(2):502–512. doi: 10.1109/TMI.2009.2037146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan Silbermankristy Ahlrich, Rob Fergus, Lakshminarayanan Subramanian: Case for automated detection of diabetic retinopathy. Association for the Advancement of Artificial Intelligence, 2010
- 14.Dheeba J, TamilSelvi S: Classification of malignant and benign microcalcification using SVM classifier. IEEE Intl Conf Emerging Trends Electr Comput Technol, (ICETECT’2011), pp. 686–690, 2011
- 15.Kui Wu, Kim Hui Yap: Fuzzy SVM for content-based image retrieval—a pseudo label support vector machine framework. IEEE Comput Intell Mag, may 2006, pp 10–16
- 16.Wang Y, Wang S, Lai KK. A new fuzzy support vector machine to evaluate credit risk. IEEE Trans on fuzzy systems. 2005;13(6):820–831. doi: 10.1109/TFUZZ.2005.859320. [DOI] [Google Scholar]
- 17.Lin CF, Wang SD. Fuzzy support vector machines. IEEE Trans Neural Netw. 2002;18(2):464–471. doi: 10.1109/72.991432. [DOI] [PubMed] [Google Scholar]
- 18.Aquino A, Gegundez-Arias ME, Marin D. Detecting the optical disc boundary in digital fundus images using morphological, edge detection, and feature extraction techniques. IEEE Trans Med Imaging. 2010;29(11):1860–1869. doi: 10.1109/TMI.2010.2053042. [DOI] [PubMed] [Google Scholar]
- 19.Laws KI: Texture energy measures. Proc. Image Understanding Workshop, 1979, pp. 47–51
- 20.Laws KI: Textured image segmentation, Ph.D. dissertation, Univ. Southern California, Los Angeles, CA, USCIPI Rep. 940, 1980
- 21.Laws K: Rapid texture identification. In Image Processing for Missile Guidance, 238, 1980
- 22.Gevers T, Stokman H. Robust histogram construction from color invariants for object recognition. IEEE Trans Pattern Anal Mach Intell. 2003;25(10):1–6. doi: 10.1109/tpami.2004.1261083. [DOI] [PubMed] [Google Scholar]
- 23.Pietikäinen M, Rosenfeld A, Davis LS. Experiments with texture classification using averages of local pattern matches. IEEE Trans Syst Man Cybern. 1983;SMC-13(3):421–426. doi: 10.1109/TSMC.1983.6313175. [DOI] [Google Scholar]
- 24.Guyon I, Matic N, Vapnik VN. Discovering information patterns and data cleaning. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- 25.Pourahmad S, Ayatollahi SMT, Taheri SM. Fuzzy logistic regression: a new possibilistic model and its application in clinical vague status. Iran J Fuzzy Syst. 2011;8(1):1–17. [Google Scholar]
- 26.Zweig MH, Campbell G. Receiver operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]
- 27.Youden WJ. An index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]