Summary
Noroviruses (NoVs) are the most common cause of sporadic and epidemic gastroenteritis in the United States and Europe and are responsible for 20% of acute gastroenteritis worldwide. Over the past decade, the understanding of NoV immunology has grown immensely. Studies of the natural immune response to NoV in humans and animal models have laid the foundation for innovations in cell culture systems for NoV and development of new therapeutics. Evidence from animal models, NoV surrogates, observational human research, and human challenge studies suggest that the innate immune response is critical for limiting NoV infection but is insufficient for viral clearance. NoV may antagonize the innate immune response to establish or prolong infection. However, once a robust adaptive immune response is initiated, the immune system clears the infection through the action of T cells and B cells, simultaneously generating highly specific protective immunologic memory. We review here both the current knowledge on norovirus immunity and exciting new developments, with a focus on ongoing vaccine development work, novel cell culture systems, and advances in understanding the role of the gut microbiome. These changes reinforce the need for a better understanding of the human immune response to NoV and suggest novel hypotheses.
Keywords: Norovirus, animal models, human, vaccine, microbiome
1) Introduction
Noroviruses (NoV) are the most common cause of sporadic and epidemic gastroenteritis in the United States and Europe across all age groups, and are estimated to be responsible for as much as 20% of all acute gastroenteritis worldwide [1–3]. Infection with this food-borne pathogen causes profuse vomiting and diarrhea, which is typically self-resolving, though among young children, the elderly, and the immunosuppressed, it may lead to more severe or protracted illness [4–7]. In low resource settings, such as many countries in Africa and southeast Asia, NoV and other causes of acute gastroenteritis (e.g. rotavirus, enteropathogenic E. coli) remain a major cause of morbidity and mortality, especially among children [8–12]. There is also growing recognition that NoV infection may be associated with some long-term sequelae, such as post-infection irritable bowel syndrome [13] and exacerbation of inflammatory bowel disease [14].
NoV infections are also a major economic concern. In the United States, food-borne NoV infection is estimated to cost over $2 billion annually in direct and indirect costs [15]. A similar study estimated that in the Netherlands NoV has the highest estimated annual costs of any food-borne pathogen [16]. The collective health and economic burden of NoV infection has led to increasing awareness of the need for better funding of NoV research [17].
In the past decade, there has been a dramatic expansion of NoV research and a corresponding increase in the understanding of NoV pathogenesis and immunology. In particular, there have been exciting recent advances in models of NoV infection and understanding the cellular immune response to NoV. However until recently, the field has been limited by the lack of cell culture systems and small animal models of human NoV infection. Further, the broad diversity of NoV strains (currently over 30 genotypes of GI and GII NoVs [18]) continues to challenge researchers. Recent reviews have addressed advances in NoV detection [18], molecular virology [19, 20], animal models for NoV research [19], the role of histo blood group antigens (HBGA) in NoV infection [21], and vaccine and antiviral medication development [22–24]. Though some of these topics will be touched upon briefly, the focus of this review is to provide a detailed overview of NoV immunology.
2) NoV structure and models
The unenveloped positive sense RNA virus family of Caliciviridae is divided into five genera: Vesivirus, Lagovirus, Sapovirus, Nebovirus, and Norovirus [25, 26].
The genus Norovirus is divided into six genogroups (GI-VI; though a seventh has been proposed [18]). Each genogroup contains many numbered genotypes, which are used in conjunction with genogroups, to designate strain types (e.g. GI.1 viruses belong to genogroup I, genotype 1). Humans can be infected by NoVs from genogroups I, II, and IV [18]. The other genogroups generally infect cattle (GIII), mice and rats (GV), and dogs (GVI) [18]. Though some humans have been found to carry antibodies to genogroup VI NoV [27], it is unknown whether this genogroup can cause symptomatic infection.
The NoV genome has three to four open reading frames (ORFs) and is capped on the 5’ end by the virally encoded protein VPg. ORF1 codes for non-structural proteins (including Vpg), ORF2 codes for the NoV capsid protein (VP1), and ORF3 codes for a minor structural protein (VP2). Murine NoVs (MNV) have a fourth ORF (ORF4), which codes for virulence factor 1 [28]. Traditionally, NoVs have been classified using pairwise distances based on complete VP1 sequences [29], but, due to the diversity of novel NoV strains, ORF1 sequences should be included in order to better identify related strains [30].
Most NoV strains generally infect hosts in a species-dependent manner, which has hindered research on human NoV because of a lack of small animal models. However, many surrogate Caliciviridae have been used in lieu of human NoV (Table 1). Though these surrogate viruses are diverse, much like human NoV, the majority of research has been with specific canonical strains, such as Tulane virus and MNV-1.CW3 (Table 1).
Table 1.
Surrogate viruses and model systems used to study human NoV.a
| Virus | Common lab strains |
Family/ genus |
Enteric virus |
Host receptors co-receptors |
Hosts | Symptomsb | Infection duration in host |
Cell tropism | Cell culture system |
Animal models |
|---|---|---|---|---|---|---|---|---|---|---|
| Human NoV | GI.1 NW, GII.1 HI, GII.2 SMV, GII.4 Sydney |
Caliciviridae/ Norovirus |
Yes | HBGA [34], heparin sulfate [134] |
Human | Vomiting, diarrhea |
1–4 weeks | Unknown, possibly gut epithelial [55, 56, 135] and B cells [31] |
Human B cell line BJAB co-cultured with killed HBGA- like antigen expressing bacteria [31] |
Chimpanzee [36], Gn pig [43], Gn calf [39], mouse [44] |
| Feline calicivirus (FCV) |
F9, Urbana strain, 255 |
Caliciviridae/ Vesivirus |
No | JAM-1 [136], sialic acid [137] |
Cat | Pneumonia, stomatitis, gingivitis, lameness |
Acute-chronicc | Mucosal epithelial cells, macrophage s [138] |
Crandell-Reese feline kidney cells [139] |
Cat |
| Murine NoV (MNV) |
MNV-1.CW1, MNV-1.CW3, MNV-3, MNV.CR6, MNV-4 |
Caliciviridae/ orovirus |
Yes | Sialic acid [140], glycoproteins [141] |
Mouse | None | Acute-chronicc | Dendritic cells, macrophages [77] |
Murine macrophage cell line RAW264.7 [77] |
Mouse |
| Bovine NoV | GIII.1, GIII.2 | Caliciviridae/ Norovirus |
Yes | αGalactose epitope [142] |
Cow | Diarrhea | 1–3+ days (GIII.1) and 1– 3 weeks (GIII.2) [60, 143] |
Gut epithelial cells and lamina propria (GIII.1) [60], unknown for GIII.2 [143] |
None | Calf, Gn calf [60, 143] |
| Porcine sapovirus (Porcine enteric calicivirus) |
Cowden strain |
Caliciviridae/ Sapovirus |
Yes | α2,3- and α 2,6-linked sialic acids on o-linked glycoproteins [144] |
Pig | Diarrhea | 1–7+ days | Gut epithelial cells [59] |
Pig kidney cell line LLC-PK with added bile acids [145, 146] |
Piglet, Gn pig [59] |
| Recoviruses (Rhesus Enteric Caliciviruses) |
Tulane Virus | Caliciviridae/ Recovirus |
Yes | HBGA [147] | Rhesus macaque |
Diarrhea, fever |
8–10 days | B cells [61] | Monkey kidney cell line LLC-MK2 [148] |
Rhesus macaque |
| Bovine nebovirus (Bovine enteric caliciviruses) |
Nebraska, Newbury1 |
Calicivirus/ Nebovirus |
Yes | Unknown | Cow | Diarrhea, anorexia |
3–7 days | Unknown | None | Calf, Gn calf [149] |
Abbreviations: NoV, norovirus; NW, Norwalk virus; HI, Hawaii virus; SMV, Snow Mountain virus; HBGA, histo blood group antigens; Gn, gnotobiotic
Symptoms in immunocompetent host.
Short (acute) or long (chronic) duration of infection varies based on virus strain.
Limitations of the existing in vivo models of human NoV infection include differences in cellular tropism, host-receptor targets, enteric pathology, and mechanism of infection. Until recently [31] in vitro models have faced similar challenges, including the absence of replicable cell culture systems for human NoV [32].
Although it has not yet been replicated or used in other studies, a recent study reported modest replication of human NoV in the human BJAB B-cell line in vitro [31]. The cell culture system built on an earlier discovery that some bacteria produce histo-blood group antigen (HBGA)-like substances on their surfaces, which can bind human NoVs [33]. HBGAs are present on the surface of erythrocytes and intestinal epithelial cells. NoVs bind HBGAs in a strain-dependent manner, with certain strains only binding to specific HBGAs [34]. HBGA-blocking antibodies have been shown to prevent NoVs from binding to HBGAs, which may reduce the ability of NoV to enter host cells (reviewed in [21, 35]). Jones et al. [31] co-cultured BJAB cells with killed Enterobacter cloacae, a bacterium that expresses H-, A-, and B-type HBGAs on its surface [33], to grow GII.4-Sydney NoV. When NoVs bound HBGAs, it allowed for attachment to the surface of B cells, leading to infection. The BJAB model also sustained NoV replication when the cells were cultured with synthetic H antigen [31], suggesting that it may be possible to allow replication of other NoV strains through the addition of different HBGA to the culture. The researchers also found that NoV in unfiltered stool containing HBGA were able to pass through a polarized epithelial cell barrier to infect B cells in a separate compartment [31], which may be a potential mechanism of in vivo infection (Fig. 1).
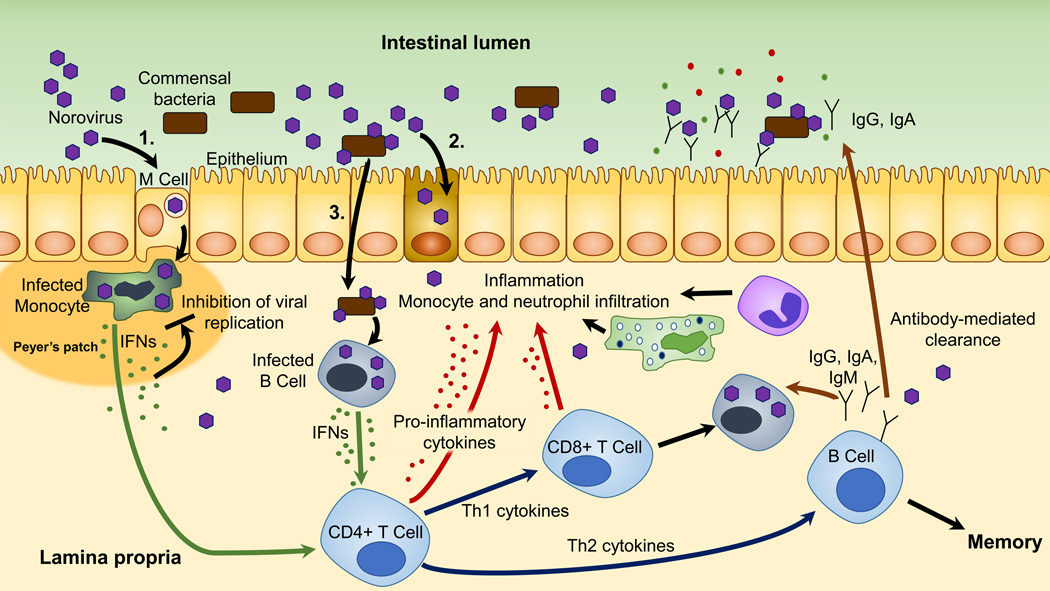
Figure 1.
Proposed schematic of norovirus infection in the gut and immune response. Noroviruses (NoV) may infect multiple cell types in the gut, and this may vary between humans and animal models. Three major hypothetical infection pathways are shown. (1) NoV may infect monocytes and other cells associated with Peyer’s patches in a microfold (M) cell-dependent manner, (2) NoV may infect epithelial cells directly, or (3) NoV may be transported across the epithelium, perhaps through interactions with commensal gut bacteria, where they may infect lymphoid cells. Infected cells produce IFNs. IFNs inhibit viral replication in infected cells and may stimulate CD4+ T-cell differentiation and activation, which may lead to CD8+ T-cell activation and B-cell maturation. This schematic is not intended to make suggestions on the host location of CD4+ and CD8+ differentiation and activation following NoV infection because these data are unknown. Cytokines may lead to inflammation of the gut epithelium and recruitment of neutrophils and monocytes. B cells produce NoV-specific antibodies that are critical for clearing the infection. NoV infection also generates memory B cells, which may protect against future infection by the same strain.
There are multiple in vivo models of human NoV infection, but each has substantial limitations. For example, chimpanzees have been shown to be susceptible to GI.1 human NoV, have a similar immune system to humans, and shed virus for a similar duration (duration in GI.1-infected chimpanzees: 23 days [36]; in GI.1-infected humans: 17–28 days [37, 38]), but their infection is asymptomatic and regulatory challenges make widespread use prohibitive [36]. Gnotobiotic pigs and calves can be infected with human GII.4 NoV, and they develop gastrointestinal symptoms, but they shed virus for a shorter period of time (mean duration in GII.4-infected pigs and calves: 4 days [39, 40]; mean duration in GII.4-infected humans: 6+ days [41, 42]) and the financial and technical difficulties of working with these animals limits their availability [39, 43]. BALB/c mice deficient in Rag-γc also are susceptible to human NoV and are more widely available, but they require intraperitoneal inoculation and experience short and asymptomatic infection [44]. These difficulties have led to the use of human challenge studies as a major means of understanding NoV immunology (Table 2) [42, 45–49].
Table 2.
Summary of published human NoV challenge study data collection and results.a
| Ref. | Year | Strain | Intervention | n | Infected | Symptomatic | Rechallenge/ reinfection |
Histology | Serology | Viral shedding |
|---|---|---|---|---|---|---|---|---|---|---|
| [150] | 1971 | NV | None | 16 | 10b | 10 | No/NA | No | No | No |
| [151] | 1972 | NV | None | 7 | 6b | 6 | No/NA | No | Yes | No |
| [152] | 1972 | NV | Heat, ether, acid, filtration | 103 | 35b | 35 | Yes/No | No | No | No |
| [55] | 1973 | NV | None | 15 | 12b | 12 | No/NA | Yes | No | No |
| [153] | 1973 | NV | None | 7 | 4b | 4 | No/NA | Yes | No | No |
| [117] | 1974 | NV, HI, MC | None | 52 (NV), 23 (HI), 18 (MC) |
31 (NV), 12 (HI), 10 (MC)b |
31 (NV), 12 (HI), 10 (MC)* |
Yes/Yes | No | No | No |
| [92] | 1975 | HI | None | 7 | 4b | 4 | No/NA | Yes | Yes | No |
| [154] | 1975 | NV | None | 15 | 9b | 9 | No/NA | Yes | No | No |
| [121] | 1977 | NV | None | 12 | 6b | 6 | Yes/Yes | Yes | Yes | No |
| [68] | 1979 | NV | None | 38 | 18b | 18 | No/NA | No | Yes | No |
| [155] | 1982 | SMV | None | 12 | 9b | 9 | No/NA | No | Yes | No |
| [156] | 1985 | NV | Chlorinated water | 32 | 17b | 17 | No/NA | No | Yes | No |
| [157] | 1988 | HI | None | 10 | 8b | 8 | No/NA | No | Yes | No |
| [69] | 1990 | NV | None | 42 | 27b | 25 | Yes/Yes | No | Yes | No |
| [158] | 1994 | NV | None | 50 | 41 | 28 | No/NA | No | Yes | Yes |
| [47] | 2003 | NV | None | 55 Se+, 22 Se− | 34 (all Se+) | 22 (all Se+) | No/NA | No | Yes | No |
| [46] | 2005 | SMV | None | 15 | 9 | 7 | No/NA | No | Yes | No |
| [37] | 2008 | NV | None | not reported | 16 | 11 | No/NA | No | Limited | Yes |
| [159] | 2008 | NV | None | 80 Se+, 28 Se− | 40 (all Se+) | 24 (all Se+) | No/NA | No | No | No |
| [122] | 2011 | NV | Monovalent VLP vaccine | 43 vaccinated, 41 placebo |
23 vaccinated, 32 placebo |
14 vaccinated, 27 placebo |
No/NA | No | Yes | No |
| [48] | 2011 | NV | Hydrostatic pressure processingc | 44 in four arms | 13 | 9 | No/NA | No | Yes | Yes |
| [49] | 2011 | NV | Persistence in water | 13 | 10 | 10 | No/NA | No | Yes | Yes |
| [42] | 2012 | GII.4 | None | 23 Se+, 17 Se− | 16 Se+, 1 Se− | 12 Se+, 1 Se− | No/NA | No | Yes | Yes |
| [41] | 2014 | GII.4 | Bivalent VLP vaccine | 50 vaccinated, 48 placebo |
27 vaccinated, 30 placebo |
13 vaccinated, 16 placebo |
No/NA | No | Yes | Yes |
Abbreviations: NV, Norwalk virus (GI.1); HI, Hawaii virus (GII.1); MC, Montgomery County virus (GI.5); SMV, Snow Mountain virus (GII.2); VLP, virus-like particle; Se+, secretor positive; Se−, secretor negative; NA, not applicable; Ref., reference.
Study did not use genomic testing for infection status.
Study included four arms—placebo and three processing conditions.
3) NoV infection
Human NoV infection can be symptomatic or asymptomatic. Based on human challenge studies and outbreak data, 15%-35% of all infected individuals are asymptomatic [37, 38, 42, 45, 50]. Symptom onset occurs 24–48 hours after exposure. Individuals are typically afebrile or have a low fever with vomiting and diarrhea [51]. In immunocompetent individuals, diarrhea is non-bloody, has low levels of lactoferrin (a marker of intestinal polymorphonuclear leukocyte inflammation) and few fecal leukocytes relative to bacterial diarrheas [52]. Diarrhea may be caused by epithelial barrier dysfunction and increased anion transport [53]. In immunocompetent individuals, symptoms generally resolve within 24–48 hours but intensity of symptoms may depend on NoV strains. Immunosuppressed individuals and neonates can have more severe and protracted symptoms, including fatal complications [5, 7].
Symptomatic and asymptomatic individuals both shed virus in stool at high levels for extended periods of time following infection [37, 38, 50]. Peak viral RNA titers reach 109-1012 genomic equivalent copies (GEC)/g stool in symptomatic individuals and may be 1–2 logs lower in asymptomatic individuals [37, 38]. A heterogeneity in the duration of shedding has been shown, with individuals shedding detectable levels of virus for a median of approximately 30 days, though durations as short as 5 or as long as 60 days have been reported in healthy adults [37, 38, 45, 50]. Immunocompromised individuals may be chronically infected and have been documented to shed NoV for years [54].
Histologically, human NoV infection causes alterations of the gut mucosa. Infected individuals may have small lesions of the duodenum with increased enterocyte apoptosis, flattened villi, crypt hypertrophy, mucosal inflammation, and disruption of the epithelial barrier function [53, 55, 56]. This is accompanied by neutrophil and mononuclear cell infiltration of the lamina propria and disruption of absorptive cells [55]. In the epithelium, there is a significant increase in perforin-producing CD8+ intraepithelial lymphocytes (IELs) and a slight increase in CD4+ IELs [53]. Histologic changes have been reported in both symptomatic and asymptomatic individuals, suggesting that asymptomatic individuals may have sub-clinical symptoms [55, 56]. Though the cellular tropism of human NoV remains unknown, the observed changes to epithelial cells and cells in the lamina propria following infection suggest that they may be possible sites of replication [55, 56].
Animal models of NoV infection display a broad range of gut histology, which partially mimic human infection (Table 1). For example, symptomatic AG129 mice infected with MNV-1.CW3 exhibit epithelial necrosis and mild lymphocytic infiltrate [57]. Some gnotobiotic calves and pigs infected with GII.4 NoV develop villous blunting in the duodenum, which appears similar to the pathological changes observed in humans [39, 43, 58]. In these animals, gut epithelial cells and cells in the lamina propria serve as targets for NoV infection [59, 60]. Rhesus macaques inoculated with Tulane Virus have lymphocytic infiltration of the lamina propria and moderate villous blunting [61]. Chimpanzees do not have histopathologic changes to the duodenum or jejunum following infection with GI.1 NoV [36].
4) Norovirus Immunity
Protection from NoV infection has both genetic and immunologic components. The genetic factors associated with protection from NoV infection are well reviewed [21, 62]. Briefly, secretor status is defined by the presence of functional fucosyltransferase 2 (FUT2) alleles, which express α1,2 fucosyltransferase 2, an enzyme that allows individuals with at least one functional FUT2 allele (i.e. secretors or secretor-positive) to express A, B, H-type 1, and Lewis b HBGAs antigens on their mucosal epithelial cells and in secretions [62]. Lack of functional α1,2 fucosyltransferase 2 (i.e. non-secretors or secretor-negative) has been associated with resistance to infection by certain strains of NoV [47, 63, 64]. Individuals who are homozygous recessive for inactivating mutations in the FUT2 gene (i.e. secretor negative) are largely resistant to infection by GI.1 NoV [47] and may also have decreased susceptibility to infection by other genogroups of NoV [42]. Overall, an estimated 70–80% of the population are secretors [65], though secretor genotype varies by ancestry [66].
Immunologic memory also factors into protection from NoV infection. Historically, broadly reactive NoV-specific IgG antibodies were not consistently associated with protection from subsequent NoV infection [67–69]. This may have been because in vitro testing by ELISA for NoV-specific antibodies did not correlate with in vivo ability to block interaction between NoVs and host cells [67] or because of confounding by genetic susceptibility [70]. Confounding by genetic susceptibility may occur because individuals who are genetically susceptible to NoV (e.g. secretor positive), compared to individuals who are genetically resistant, may be more likely to have high antibody titers. Specifically, individuals who are genetically susceptible to NoV, compared to those who are resistant, likely have been repeatedly infected and therefore developed an adaptive immune response to NoV (i.e. higher titers of NoV-specific antibodies). Some studies have found that strain-specific NoV-blocking antibodies were associated with protection from NoV infection in a strain-dependent manner in humans [71–73] and in non-human primates [36]. For example, a study in chimpanzees suggests that strain-specific IgG may provide long-term resistance to infection by homologous NoV strains [36]. In addition to IgG antibodies, IgA antibodies may also help protect against NoV infection. An early salivary IgA antibody response to NoV challenge has been associated with protection from infection by GI.1 NoV [47]. This was further supported by a recent study by Ramani, et al. comprising a NoV challenge of human volunteers, which found that pre-challenge IgA-producing memory B cells significantly correlated with protection from infection by GII.4 NoV [74].
Researchers have also identified immunologic correlates of protection from symptomatic infection. One of the first described immunologic correlates of protection from symptomatic infection was HBGA-blocking antibodies [67]. HBGA are present on the surface of enterocytes in the mucosal epithelium of the gut. Though the mechanism of NoV entry into host cells is still unknown, HBGA-blocking antibodies prevent NoV from binding to HBGA, which may reduce NoV’s ability to enter host cells (reviewed in [21, 35]). Another correlate of protection from symptomatic infection are hemagglutination inhibition (HAI) antibodies, a closely associated group of antibodies [75]. In addition, both HBGA-blocking and HAI antibodies have been proposed as surrogates for measuring virus-neutralizing antibodies, which has been supported by human [67, 75, 76] and non-human primate data [36]. Additional surrogates of protection from illness have also been proposed. For example, Ramani, et al. found that pre-challenge salivary IgA antibodies and NoV-specific IgG memory B cells were significantly associated with protection from symptomatic NoV infection [74]. As vaccine development continues, the need for reliable correlates of immunity and symptomatic protection remain important areas of future research.
5) Innate immunity to NoV
Over the past decade, the understanding of NoV immunology has grown immensely. Studies of the natural immune response to NoV have led to observations that were important for growing NoV in cell culture and to developing therapeutics. Although some work has been conducted in humans, most of the evidence comes from animal models and human NoV surrogates. Based on this work, the innate immune response appears to play a critical role in limiting viral replication and initiating a memory-generating adaptive immune response to NoV (Fig. 1).
5.1 Mouse models
In mouse models of NoV, the innate immune response limits viral replication largely through interferon (IFN)-dependent pathways. MNV infects macrophages and dendritic cells [77], possibly in a manner dependent on microfold (M) cells (Fig. 1), an epithelial cell associated with Peyer’s patches and involved in antigen sampling from the gut lumen [78]. In in vivo MNV infection, viruses are recognized by MDA-5, a Rig-I-like helicase, which initiates the innate immune response [79] (Fig. 2). This innate sensing leads to the production of type I and type II IFN by antigen-presenting cells, which leads to the production of pro-inflammatory cytokines through the STAT-1 pathway [80, 81] (Fig. 2). In primary macrophages isolated from wild-type mice, infection induces transcription factors interferon regulatory factor 3 (IRF-3) and IRF-7, which stimulate type I IFN production (IFN-α and IFN-β), though they are not necessary for governing their downstream antiviral effects [82].
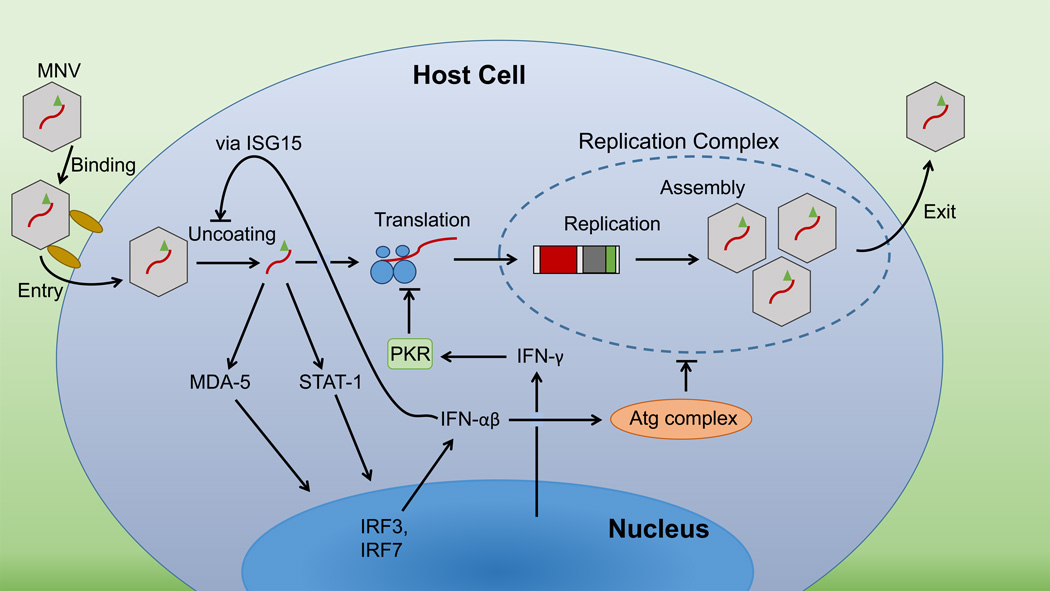
Figure 2.
Proposed schematic of intracellular immune response to murine norovirus (MNV) infection. MNV may infect macrophages or dendritic cells in the gut. Infected cells sense MNV via MDA-5 and produce IFNs through this or the STAT-1 pathway. Type I IFNs inhibit MNV uncoating via ISG15 and may inhibit replication complex formation via an Atg complex. Type II IFNs block MNV translation via RNA-activated protein kinase (PKR). VF1-related immune suppression by MNV is not included in this schematic.
This innate response leads to inhibition of viral replication. Type I and type II IFN have been shown to inhibit MNV translation in macrophages and dendritic cells, with type I IFN blocking viral replication in a double-stranded RNA-activated protein kinase (PKR)-independent manner and type II IFN (IFN-γ) arresting it in a PKR-dependent manner [83] (Fig. 2). In the absence of type I interferons, IFN-γ mediates antiviral activity against MNV by activating the autophagy protein (Atg) complex Atg5-Atg12/Atg16L1 (Fig. 2), which inhibits the MNV replication complex in the cytoplasm of infected macrophages but does not act through autophagy-associated degradation [84]. In the absence of IFN-y, type I IFNs may limit MNV replication through the activation of interferon-stimulated gene 15 (ISG15). ISG15 may act to impede an early stage in the MNV life-cycle, such as entry or uncoating [85] (Fig. 2), but IFNs alone are not enough to clear the infection [86]. However the innate response may not occur unimpeded. MNV carries an additional ORF4, encoding virulence factor 1 (VF1), which antagonizes the innate immune response [28]. VF1 delays up-regulation of CXCL10, ISG54, and IFN-β, and therefore enhances viral replication [28]. Interestingly, other NoVs (including human NoVs) do not share this gene, suggesting that human NoV may regulate the immune response through other mechanisms.
In some cases, the innate immune response may help with viral clearance or be exogenously stimulated to do so. Recent work has examined the role of IFN-λ, a type III IFN, in MNV infection. IFN-λ has been shown to be produced by cells in the mesenteric lymph nodes and Peyer’s patches of healthy C57BL/6 control mice in response to infection by MNV-1.CW3 [86]. In contrast, MNV.CR6 infection of mice led to persistent infection and there was no initial induction of type III IFN production. However, treating the persistently MNV.CR6-infected mice with exogenous IFN-λ reduced viral persistence in the gut, eventually leading to viral clearance, without initiating an adaptive immune response [86]. Interestingly, antibiotic treatment of mice increased the antiviral effect of IFN-λ, suggesting that the bacterial microbiota may alter the innate immune response to viruses or its efficacy [87].
5.2) Gnotobiotic pigs
Gnotobiotic pigs are one of the few models with available data regarding their innate immune response to human NoV. Their response to NoV is similar to the response seen in mouse models of MNV. Most animals used are relatively young and may have less developed innate immune systems, rendering them more sensitive to low doses of NoV [88]. Gnotobiotic pigs have less robust neutrophil responses [89], fewer intraepithelial lymphocytes [90], and less developed Peyer’s patches [91] with a preponderance of T cells rather than B cells [90]. Nevertheless, they still mount an innate immune response to human NoV, in the form of elevated serum IFN-α, intestinal IFN-α, and serum IFN-γ, though IFN-α does not appear until later in infection [40]. As in wild-type mice infected with MNV, this innate response is important for limiting viral replication in an IFN-dependent manner. Studies have also assessed gnotobiotic pigs treated with simvastatin, a cholesterol-lowering medication that also inhibits IFN-α production and major histocompatibility complex (MHC) class II-dependent T-cell activation [58, 88]. These animals were more susceptible to NoV infection and shed virus for a longer duration and at higher levels [58, 88]. When gnotobiotic pigs which had not been administered simvastatin were treated with human IFN-α, they shed virus for a shorter duration and at lower levels than gnotobiotic pigs which were not treated with IFN-α or statins did. This result supports the role of IFN-α in controlling viral replication during NoV infection [58].
5.3) Humans
There are limited human data regarding the innate immune response to NoV. Past studies of humans challenged with Norwalk virus (NV—GI.1) and Hawaii virus (GII.1) did not show induction of detectable levels of IFN in sera, jejunal aspirates, or jejunal biopsy specimens taken 48 to 96 hours post-challenge [92]. However, a Snow Mountain virus (SMV—GII.2) challenge study reported a significant rise in serum IFN-γ and IL-2 levels 48 hours post-challenge in infected individuals as compared with that of uninfected controls [46]. This result was supported by a study of fecal cytokines in NoV-infected travelers; they exhibited significantly increased fecal IL-2 and IFN-γ levels in diarrhea specimens [52]. These conflicting data suggest that more research is needed to clarify the nature of the human innate immune response to NoV and whether it differs by strain.
6) Adaptive immunity to NoV
Though the innate immune response is important for blocking viral replication through early IFN production, its other key role is in initiating the adaptive immune response. Based on animal models, the adaptive immune response helps clear NoV infection and may generate immunologic memory to prevent reinfection (Fig. 1).
6.1) Mice
While the action of IFNs and the innate immune response inhibits viral replication, adaptive immunity seems critical for viral clearance. B cells and T cells seem necessary to clear NoV infection. B- and T-cell deficient RAG1 and RAG2 knockout mice have been shown to develop persistent or extended MNV infections with high viral RNA titers [80, 93]. B cells are hypothesized to help clear MNV infection through antibody production. In support of this hypothesis, B-cell deficient RAG1 knockout mice that received a transfer of B cells, incapable of producing NoV antibodies, did not clear MNV and were statistically no different from B-cell deficient RAG1 knockout mice [93]. However, RAG1 knockout mice that received infusions of MNV antibodies showed reduced MNV RNA titers [93]. Further, in a study of MNV capsid protein-vaccinated mice, broad T- and B-cell activation, including CD4+ and CD8+ T cells, was necessary for NoV resistance [94], indicating that T cells may also be necessary, alongside antibody-producing B cells, for clearing NoV infections. This multifaceted response is reflected in the serum cytokine response of wild-type mice orally administered MNV [95]. Ten days post-challenge, these mice were shown to have significantly elevated serum levels of IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, IL-17-A, IFN-γ, TNF-α, G-CSF, and GM-CSF compared to unchallenged mice [95]. This systemic cytokine response occurs in the absence of visible histopathologic changes in the gut [95], indicating that an immune response to NoV is not necessarily associated with NoV-induced pathology.
Recent work indicates that a suboptimal NoV-specific CD8+ T-cell response results in persistent MNV infection [96]. Mice with lower levels of activated MNV-specific CD8+ T cells were shown to have long-term MNV infections that they were unable to clear, whereas mice with higher levels of CD8+ T-cell activation were able to clear the MNV infection [96]. Furthermore, RAG1 knockout mice that received a transfer of activated MNV-specific CD8+ T cells had significantly reduced viral loads, indicating that CD8+ T cells are another key component of the adaptive immune response that clears NoV infection [96]. However, to prevent successive NoV infection, antibodies and CD4+ T cells are more important [97]. The presence of MNV-3-specific antibodies or CD4+ T cells were each sufficient for partial protection from reinfection with MNV-3, but CD8+ T cells alone did not provide protective immunity from infection with MNV-3. These situations may be similar to the chronic NoV infection observed in immunocompromised patients with impaired B- and T-cell responses, who are unable to clear the virus [54, 98–100]. Indeed, many transplant recipients are only cured of their NoV infections when their immunosuppressive medication dosing is relaxed [101].
In vitro work supports the importance of the Th1-type response in MNV infection and the production of IFNs in the immune response to NoV. MNV-1 infected murine RAW264.7 macrophages were shown to have a Th1-skewed response, with upregulation of CCL2, CCL3, CCL4, CCL5, CXCL2, CXCL10, and CXCL11 genes [102]. In vivo, some of these chemokines (e.g. CXCL10, CXCL11) can be induced by type I or type II IFNs and are important for Th1-cell trafficking [103]. Thus, the early innate response may be critical for laying the foundation for later viral clearance.
Interestingly, different MNV strains induce different levels of protective immunity. In wild-type mice, MNV-3 has been shown to be associated with strong homotypic and heterotypic protection against re-challenge by MNV-3 or MNV-1 in a type I IFN-independent and B- and CD4+ T-cell dependent manner [97]. MNV-3 induced significant serum IgG and mucosal IgA antibody responses that were reactive against antigens from both MNV-3 and MNV-1 [97]. However, MNV-1, a more virulent strain, did not induce heterotypic antibody production and led to lower levels of IgG and IgA antibody production compared with that following MNV-3 infection. One hypothesis for this strain-specific difference may be antagonism of the innate immune response. MNV-1 infected RAW264.7 cells exhibited lower levels of IFN-β, TNF-α, and MCP-1 transcripts [28, 97]. MNV-1 may also interfere with maturation of antigen presenting cells, leading to decreased T-cell activation [97]. Some of these effects may be related to MNV strain-specific differences in a protruding region of VP1, a capsid protein, as alterations in the sequences of the genes coding for the P2 region of VP1 have been found to be associated with differences in MNV strain virulence in STAT1 knockout mice [104], however others may be related to strain-specific differences in VF1 [97]. The presence of strain-related differences in MNV virulence and its impact on the immune response suggest that future work should consider whether strain-specific differences are present in human NoVs and impact the human immune response to NoV.
6.2) Gnotobiotic pigs and calves
NoV-infected gnotobiotic pigs and calves exhibit similar innate and adaptive cytokine and antibody responses to that in mice, and may serve as better models of primary NoV infection in humans because of immunologic and physiologic similarities to humans [89] and the ability to be infected with human NoVs (Table 1). NoV-infected gnotobiotic pigs show an early increase in IFN-γ [40], suggestive of an IFN-dependent innate anti-viral response. This is then followed by Th1 and Th2 activation. Specifically, infected animals develop significant elevations in serum IL-4, IL-6, and IL-10 between 2 and 8 days post-challenge [40]. They also exhibit persistently elevated serum IL-12 and intestinal IL-12 post-challenge [40]. Viral clearance in gnotobiotic pigs is associated with antibody responses in the form of low titers of NoV-specific serum IgG antibody [43] and detectable IgM, IgA, and IgG andtibodies in their intestines [40]. These levels of antibodies are positively correlated with the severity of diarrhea [40].
Gnotobiotic calves inoculated with human GII.4 NoV were shown to initially respond similarly to gnotobiotic pigs administered the same inoculum. Namely, the initial innate response appeared to be IFN-mediated, and was followed by serum and fecal cytokine elevations that suggested Th1 and Th2 activation [39]. NoV-infected gnotobiotic calves have an early increase in serum IFN-γ compared to uninfected animals [39]. Moreover, over the course of infection, infected gnotobiotic calves show increases in serum TNF-α, IL-4, IL-10, and IL-12 cytokine levels [39]. Fecal TNF-α, IFN-γ, IL-4, IL-10 and IL-12 were also elevated in infected calves as compared to controls. Adaptive immunity, through antibody responses following challenge, includes low titers of IgA, IgM, and IgG antibodies [39] and IgA- and IgG- secreting cells in the intestine and serum 28 days following challenge, with the highest concentration found in the intestine.
6.3) Humans
In humans, NoV infection leads to Th1-skewed T-cell activation in addition to a B-cell response. A study of five healthy volunteers with evidence of prior GII.4 infection (or infection by a strain that generated cross-reactive antibodies) showed that their peripheral blood mononuclear cells (PBMCs) expressed CD80, CD86, CD40, and HLADR activation markers, following GII.4 NoV virus-like particle (VLP) stimulation, indicating T-cell maturation [105]. Stimulation with GII.4 NoV VLPs also led to secretion of IFN-γ, IL-6, and TNF-α, suggesting Th1 and some possible Th2 activation [105]. NoV challenge study results have also shown in vitro cytokine responses by PBMCs collected 4 to 21 days post-challenge to be consistent with Th1 and some possible Th2 activation following stimulation with challenge study virus VLPs with significant elevations in IFN-γ, IL-2, and IL-5 and non-significant elevations in TNF-α, IL-4, and IL-10 [46, 106]. Depletion of CD4+ and CD8+ T cells in vitro has shown that CD4+ T cells are largely responsible for IFN-γ production following in vitro VLP stimulation [46].These results are consistent with serum cytokine changes observed during challenge studies and observational research. Specifically, SMV-infected challenge study participants showed significant elevations in serum IFN-γ and IL-2 concentrations 2 days post-challenge [46]. Some other studies have also identified elevated serum IL-5, IL-6, IL-8, and MCP-1 in response to natural NoV infection [46, 107, 108]. These results indicate that human NoV infection leads to a Th1-driven response with possible Th2 involvement. This is further supported by challenge study and population studies indicating that, following NoV infection, IgG1 antibody predominates among IgG subclasses [46, 109].
As previously noted, immunocompromised patients can develop chronic NoV infections [54, 98–100]. Anecdotal evidence from these case reports suggest that T cells, in particular CD4+ cells, and B cells may be important for NoV clearance [100, 110]. In one case report, an HIV-positive individual with AIDS and a chronic NoV infection showed a decrease in NoV RNA titer after successful initiation of an antiretroviral regimen and subsequent increase in CD4+ cell count [110]. In a case study, T-cell recovery following pediatric hematopoietic stem cell transplant was correlated with clearance of chronic NoV infection [100]. Other reports of immunocompromised patients have suggested that intravenous or enteral immunoglobulin may be a potential therapy for chronic NoV infection, though its efficacy is unproven [98, 99]. However, B cells alone do not appear sufficient in immunocompromised patients to prevent chronic NoV infection, as evidenced by a case of an individual with normal serum concentrations of IgG, IgA, and IgM antibodies but with severely compromised T-cell function who developed a chronic NoV infection lasting over two years [111].
In humans, NoV infection has been shown to induce increases in IgA, IgG, and IgM antibodies [74, 106, 112–114], the kinetics of which have been summarized in greater detail elsewhere [115]. IgA is one of the earliest antibodies produced at detectable levels and begins to rise around day 5 post-challenge in serum and as early as day 2 post-challenge in saliva [45, 47]. Strain-specific fecal IgA antibody levels are associated with lower viral RNA titer in stool and less severe illness [74]. NoV infection also results in IgM antibody production [114]. IgG antibodies begin to develop around day 7 post-challenge, with 100% of infected individuals exhibiting IgG sero-response 14 days post-challenge [45]. Some of these antibodies may prevent reinfection with the same or related strain, but given the patterns of cross-reactivity and broad within-genogroup diversity, antibody-mediated protection derived from a single NoV infection may not extend to the entire genogroup of related NoVs, particularly for GII NoVs [46, 106]. For example, GII.1 and and GII.2 infections sometimes generate serum IgG antibodies that are cross-reactive in vitro against GI.1 capsid antigens, but the antibody reactivity is lower than that in individuals who are infected with a GI.1 NoV, and they do not generally produce cross-reactive serum IgA or IgM antibodies [116]. To date, challenge studies and epidemiologic research provide evidence both for and against the hypothesis that prior infection protects from subsequent infection with a heterologous strain (across genotype or genogroup) [117–119].
Interestingly, there is conflicting evidence regarding the duration of protection from symptomatic infection by homologous strains. Epidemiologic modeling suggests that duration of immunity may range from 4 to 8 years [120]. One challenge study found complete protection against symptomatic infection by a homologous strain when rechallenge took place 6 to 14 weeks later [117], though in a different study, homologous rechallenge 8 weeks after an earlier symptomatic infection led to a second symptomatic infection [121]. The only studies to consider longer timeframes have found some individuals who initially became ill were not protected from symptomatic infection when rechallenged with a homologous strain 6 months later [69] and no individuals who became ill in an earlier challenge were protected from infection when rechallenged 27 to 42 months later [69, 121].
Overall, the evidence from animal models, NoV surrogates, observational human research, and human challenge studies suggest that while the innate immune response is critical for limiting the severity of NoV infection, it is insufficient for viral clearance. NoV may antagonize the innate immune response in order to establish or prolong infection, but once a robust adaptive immune response is initiated, the immune system clears the infection through the action of both T cells and B cells, simultaneously generating immunologic memory, which is highly specific and may be short-lived.
7) Frontiers in NoV immunology
NoV immunology is in the midst of exciting transformations, thanks to vaccine development work, novel cell culture systems, and advances in understanding the role of the gut microbiome. These changes reinforce the need for better understanding of the human immune response to NoV and suggest novel hypotheses regarding pathogenesis and protection.
7.1) Vaccine development
One of the most significant public health-related developments in NoV immunology is the clinical testing of VLP-based vaccines [41, 122]. A NoV vaccine is most needed in developing parts of the world, such as in many African and southeast Asian countries, where the burden of diarrheal disease remains highest [8–12]. The most recent vaccine iteration is a bivalent vaccine GI.1 and GII.4 NoV VLPs expressing VP1. This bivalent vaccine has proven effective in reducing the severity of illness. However, it has not been associated with a significant reduction in infection rate in healthy adults challenged with a GII.4 strain following vaccination with VLP-based vaccine or placebo [41]. An earlier monovalent formulation of the vaccine with GI.1 NoV VLPs showed a reduction in the rates of infection and illness among healthy adults challenged with GI.1 NoV following vaccination [122]. VLP vaccines for NoV have been shown to be immunogenic when administered through a variety of different methods [123–126], generating NoV VLP-specific serum IgG and IgA antibodies [125, 126], antibodies in serum blocking the interaction between vaccine-strain NoV VLPs and HBGAs [126], and an expansion in the number of antibody-secreting cells as early as 7 days post-vaccination [124, 125].
In addition to the difficulty in establishing protective immunity from infection, there are some additional questions regarding the vaccine’s long-term prospects (reviewed in [127]). Some key issues are that NoVs are highly diverse, and the lack of strong host heterotypic protection from prior NoV infection [117] may foretell difficulties in creating any single vaccine with broad protection. Total NoV-specific IgG antibody concentration is not consistently associated with protection from infection [67], suggesting that cross-reactive antibodies may not confer complete protection. Because these vaccine studies challenged individuals with NoV relatively soon after vaccination [41, 122], it was not possible to measure the duration of protection to these vaccines. Lastly, in areas that would benefit most from a vaccine, many individuals may be malnourished. Malnourished mice have been shown to develop less protective immunity following NoV infection, including weaker antibody responses and higher viral RNA titers, [128], suggesting that malnourished, NoV-infected humans may form a similar response. Therefore, it will also be critical to address the immunogenicity of a NoV vaccine under similar circumstances. Nevertheless, there are plans to complete field trials in adults, and safety trials in children, with VLP-based vaccines in the near future (ClinicalTrials.gov NCT02142504 and NCT02153112).
7.2) NoV and the microbiome
In addition to vaccine development work, researchers have begun to investigate the interplay between NoVs and the microbiome in vivo. There is evidence that NoV infection might alter the composition of the gut microbiome [128–130]. A subset of travelers infected with NoV were shown to have significantly reduced numbers of Bacteriodetes and increases in Proteobacteria compared to uninfected controls [130]. The increase in intestinal Proteobacteria was driven by increases in non-enteropathogenic Escherichia coli during NoV infection. Studies in mice have been equivocal with regard to the response of the gut microbiota to MNV infection. One study reported that C57BL/6 mice did not have disruption of the gut microbiota following MNV-1 or MNV-4 infection [129]. A later study found that healthy C57BL/6 mice did show alterations in the composition of their gut microbiota following MNV-1 infection but malnourished mice did not [128]. Non-malnourished mice infected with MNV-1 showed a significant reduction in the proportion of Bacteriodetes, as was seen in humans [130], and an increase in Firmicutes.
Beyond descriptive findings, there is a growing body of knowledge related to the complex interplay between NoV and the microbiome. Research in mice indicates that gut bacteria may play a critical role in NoV infection [87]. Mice treated with broad-spectrum antibiotics were largely resistant to persistent infection by MNV CR6. No single antibiotic was solely responsible for resistance, and resistance depended on intact innate immune responses, particularly IFN-λ, STAT1 and IRF3 [87]. The work suggests that commensal bacteria, which had been largely reduced by treatment with antibiotics, may counteract the innate immune response to NoV, limiting its efficacy in preventing infection. A second hypothesis is that commensal bacteria may even help NoV enter target cells, a hypothesis supported by the BJAB culture system for human NoV, which relies on the presence of an enteric bacterium for infection to occur [31] (Fig. 1). There is also a growing appreciation for the impact of NoV infection on the immune response to other microbes. MNV has been shown to suppress the human immune response rather than simply evade it [28, 97], and in the presence of underlying genetic and microbiome factors, may lead to inflammatory illness [131, 132]. Mice deficient in Atg16L1, an autophagy gene that is associated in humans with Crohn’s disease, when infected with MNV CR6, were shown to develop a Crohn’s-like illness mediated by TNF-α and IFN-γ, but they did so only when commensal bacteria were present [131]. When treated with broad-spectrum antibiotics, the mice were protected from developing a Crohn’s-like illness, suggesting that normal gut microbiomes may become a nidus for inflammatory disease following an infectious trigger in individuals who are genetically more susceptible to inflammatory conditions [131]. NoVs may be one such trigger. In a similar set of experiments, Basic et al. found that IL-10 knockout mice with normal gut microbiota developed mucosal inflammation following MNV infection, but germ-free knockout mice did not develop illness following MNV infection [132], suggesting that NoV infection may be an inciting event for inflammatory gastrointestinal illness among susceptible individuals.
The effect of NoV infection on gut homeostasis may be even more complicated. NoV infection in the presence of specific genetic and environmental factors may lead to illness, but in the absence of an intact bacterial microbiome, MNV has been shown to promote the healthy development of mucosal immunity [133]. Specifically, germ-free mice experimentally infected with MNV CR6 developed apparently healthy small intestines with more normal levels of CD4+ and CD8+ T cells compared with those of uninfected germ-free mice and more comparable serum antibody levels and markers of cellular function, such as Paneth cell granules, lysozyme expression, and IFN-γ expression [133]. In antibiotic-treated mice, MNV infection also prevented intestinal injury by Citrobacter rodentium and enhanced the immune response [133]. Based on this work, it appears that NoV may play a commensal role under certain conditions.
8) Conclusions
Despite huge advances since the identification of NoV over 40 years ago, major questions remain unanswered. The cellular tropism of NoV remains unknown. There are no small animal models that mimic human NoV pathogenesis and there is still no in vitro infectivity assay, though the novel cell culture system may be one possible option. The role of antibodies in resistance to NoV infection remains an open question. The pathogenesis of human NoV infection and causes of symptoms are ill-understood. As vaccine studies advance, researchers continue searching for optimal surrogates and correlates of protection. Studies of interactions between NoV and the human microbiota are still in their infancy. The field of NoV immunology remains open to innovation and discovery to prevent and treat this major cause of illness worldwide.
Acknowledgements
This work was supported by the F30 grant (K.L.N., grant 1F30DK100097), the ARCS Foundation (K.L.N), the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (J.S.L., grant 1K01AI087724-01), and U.S. Department of Agriculture, National Institute of Food and Agriculture (J.S.L. grant 2015-67017-23080). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- NoV
Norovirus
- HBGA
histo blood group antigen
- ORF
open reading frame
- IEL
intraepithelial lymphocyte
- MNV
murine norovirus
- FUT2
fucosyltransferase 2
- PKR
RNA-activated protein kinase
- Atg
autophagy protein
- VF1
virulence factor 1
- MHC
major histocompatibility complex
- NV
Norwalk virus
- SMV
Snow Mountain virus
- VLP
virus-like particle
- HI
Hawaii virus
- MC
Montgomery Country virus
- Se+
secretor positive
- Se−
secretor negative
Footnotes
Conflicts of Interest
The authors declare no commercial or financial conflict of interest
References
- 1.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhoef L, Koopmans M, W VANP, Duizer E, Haagsma J, Werber D, L VANA, Havelaar A. The estimated disease burden of norovirus in The Netherlands. Epidemiol Infect. 2013;141:496–506. doi: 10.1017/S0950268812000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivedi TK, DeSalvo T, Lee L, Palumbo A, Moll M, Curns A, Hall AJ, Patel M, Parashar UD, Lopman BA. Hospitalizations and mortality associated with norovirus outbreaks in nursing homes, 2009–2010. JAMA. 2012;308:1668–1675. doi: 10.1001/jama.2012.14023. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, Flegel WA, Thiel E, Schneider T. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117:5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebenga JJ, Beersma MF, Vennema H, van Biezen P, Hartwig NJ, Koopmans M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J Infect Dis. 2008;198:994–1001. doi: 10.1086/591627. [DOI] [PubMed] [Google Scholar]
- 7.Bagci S, Eis-Hubinger AM, Yassin AF, Simon A, Bartmann P, Franz AR, Mueller A. Clinical characteristics of viral intestinal infection in preterm and term neonates. Eur J Clin Microbiol Infect Dis. 2010;29:1079–1084. doi: 10.1007/s10096-010-0965-4. [DOI] [PubMed] [Google Scholar]
- 8.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 9.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 12.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Child Health Epidemiology Reference Group of the World Health, O. and Unicef, Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, Lanzini A, San Felice del Benaco Study I. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 14.Khan RR, Lawson AD, Minnich LL, Martin K, Nasir A, Emmett MK, Welch CA, Udall JN., Jr Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:328–333. doi: 10.1097/mpg.0b013e31818255cc. [DOI] [PubMed] [Google Scholar]
- 15.Gastanaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting--United States, 2001–2009. J Infect Dis. 2013;207:1058–1065. doi: 10.1093/infdis/jis942. [DOI] [PubMed] [Google Scholar]
- 16.Belliot G, Lopman BA, Ambert-Balay K, Pothier P. The Burden of Norovirus gastroenteritis: an important foodborne and healthcare-related infection. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head MG, Fitchett JR, Atun R. Systematic analysis of funding awarded for norovirus research to institutions in the United Kingdom, 1997–2010. J R Soc Med. 2014;107:110–115. doi: 10.1177/0141076813511450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinje J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J Clin Microbiol. 2014 doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in norovirus biology. Cell Host Microbe. 2014;15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karst SM, Zhu S, Goodfellow IG. The molecular pathology of noroviruses. J Pathol. 2015;235:206–216. doi: 10.1002/path.4463. [DOI] [PubMed] [Google Scholar]
- 21.Tan M, Jiang X. Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev Mol Med. 2014;16:e5. doi: 10.1017/erm.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias A, Emmott E, Vashist S, Goodfellow I. Progress towards the prevention and treatment of norovirus infections. Future Microbiol. 2013;8:1475–1487. doi: 10.2217/fmb.13.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramani S, Atmar RL, Estes MK. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol. 2014;30:25–33. doi: 10.1097/MOG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha-Pereira J, Neyts J, Jochmans D. Norovirus: Targets and tools in antiviral drug discovery. Biochem Pharmacol. 2014 doi: 10.1016/j.bcp.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke INEMK, Green KY, Hansman GS, Knowles NJ, Koopmans MK, Matson DO, Meyers G, Neill JD, Radford A, Smith AW, Studdert MJ, Thiel H-J, Vinjé J. Caliciviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012. pp. 977–986. [Google Scholar]
- 26.Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009) Arch Virol. 2010;155:133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesquita JR, Costantini VP, Cannon JL, Lin SC, Nascimento MS, Vinje J. Presence of antibodies against genogroup VI norovirus in humans. Virol J. 2013;10:176. doi: 10.1186/1743-422X-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFadden N, Bailey D, Carrara G, Benson A, Chaudhry Y, Shortland A, Heeney J, Yarovinsky F, Simmonds P, Macdonald A, Goodfellow I. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog. 2011;7:e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Kroneman A, Vega E, Vennema H, Vinje J, White PA, Hansman G, Green K, Martella V, Katayama K, Koopmans M. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol. 2013 doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, Tibbetts SA, Wallet SM, Karst SM. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papafragkou E, Hewitt J, Park GW, Greening G, Vinje J. Challenges of culturing human norovirus in three-dimensional organoid intestinal cell culture models. PLoS One. 2013;8:e63485. doi: 10.1371/journal.pone.0063485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura T, Sano D, Suenaga A, Yoshimura T, Fuzawa M, Nakagomi T, Nakagomi O, Okabe S. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol. 2013;87:9441–9451. doi: 10.1128/JVI.01060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, Altaye M, Pickering LK, Newburg DS, LePendu J, Jiang X. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 35.Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, Engle R, Yu C, Kapikian AZ, Sosnovtsev SV, Purcell RH, Green KY. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A. 2011;108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirby AE, Shi J, Montes J, Lichtenstein M, Moe CL. Disease course and viral shedding in experimental Norwalk virus and Snow Mountain virus infection. J Med Virol. 2014 doi: 10.1002/jmv.23905. [DOI] [PubMed] [Google Scholar]
- 39.Souza M, Azevedo MS, Jung K, Cheetham S, Saif LJ. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus. J Virol. 2008;82:1777–1786. doi: 10.1128/JVI.01347-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain) J Virol. 2007;81:9183–9192. doi: 10.1128/JVI.00558-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinje J, Gregoricus N, Frenck RW, Jr, Moe CL, Al-Ibrahim MS, Barrett J, Ferreira J, Estes MK, Graham DY, Goodwin R, Borkowski A, Clemens R, Mendelman PM. Norovirus Vaccine Against Experimental Human GII.4 Virus Illness: A Challenge Study in Healthy Adults. J Infect Dis. 2014 doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frenck R, Bernstein DI, Xia M, Huang P, Zhong W, Parker S, Dickey M, McNeal M, Jiang X. Predicting susceptibility to norovirus GII.4 by use of a challenge model involving humans. J Infect Dis. 2012;206:1386–1393. doi: 10.1093/infdis/jis514. [DOI] [PubMed] [Google Scholar]
- 43.Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J Virol. 2006;80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taube S, Kolawole AO, Hohne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. A mouse model for human norovirus. MBio. 2013;4 doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis. 2014;209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol. 2005;79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 48.Leon JS, Kingsley DH, Montes JS, Richards GP, Lyon GM, Abdulhafid GM, Seitz SR, Fernandez ML, Teunis PF, Flick GJ, Moe CL. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl Environ Microbiol. 2011;77:5476–5482. doi: 10.1128/AEM.02801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seitz SR, Leon JS, Schwab KJ, Lyon GM, Dowd M, McDaniels M, Abdulhafid G, Fernandez ML, Lindesmith LC, Baric RS, Moe CL. Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol. 2011;77:6884–6888. doi: 10.1128/AEM.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teunis PF, Sukhrie FH, Vennema H, Bogerman J, Beersma MF, Koopmans MP. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol Infect. 2014:1–8. doi: 10.1017/S095026881400274X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko G, Jiang ZD, Okhuysen PC, DuPont HL. Fecal cytokines and markers of intestinal inflammation in international travelers with diarrhea due to Noroviruses. J Med Virol. 2006;78:825–828. doi: 10.1002/jmv.20630. [DOI] [PubMed] [Google Scholar]
- 53.Troeger H, Loddenkemper C, Schneider T, Schreier E, Epple HJ, Zeitz M, Fromm M, Schulzke JD. Structural and functional changes of the duodenum in human norovirus infection. Gut. 2009;58:1070–1077. doi: 10.1136/gut.2008.160150. [DOI] [PubMed] [Google Scholar]
- 54.Schorn R, Hohne M, Meerbach A, Bossart W, Wuthrich RP, Schreier E, Muller NJ, Fehr T. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin Infect Dis. 2010;51:307–314. doi: 10.1086/653939. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber DS, Blacklow NR, Trier JS. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N Engl J Med. 1973;288:1318–1323. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber DS, Blacklow NR, Trier JS. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J Infect Dis. 1974;129:705–708. doi: 10.1093/infdis/129.6.705. [DOI] [PubMed] [Google Scholar]
- 57.Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MS, Neyts J. The viral polymerase inhibitor 2'-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J Virol. 2013;87:11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung K, Wang Q, Kim Y, Scheuer K, Zhang Z, Shen Q, Chang KO, Saif LJ. The effects of simvastatin or interferon-alpha on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PLoS One. 2012;7:e41619. doi: 10.1371/journal.pone.0041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flynn WT, Saif LJ, Moorhead PD. Pathogenesis of porcine enteric calicivirus-like virus in four-day-old gnotobiotic pigs. Am J Vet Res. 1988;49:819–825. [PubMed] [Google Scholar]
- 60.Otto PH, Clarke IN, Lambden PR, Salim O, Reetz J, Liebler-Tenorio EM. Infection of calves with bovine norovirus GIII.1 strain Jena virus: an experimental model to study the pathogenesis of norovirus infection. J Virol. 2011;85:12013–12021. doi: 10.1128/JVI.05342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sestak K, Feely S, Fey B, Dufour J, Hargitt E, Alvarez X, Pahar B, Gregoricus N, Vinje J, Farkas T. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS One. 2012;7:e37973. doi: 10.1371/journal.pone.0037973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rydell GE, Kindberg E, Larson G, Svensson L. Susceptibility to winter vomiting disease: a sweet matter. Rev Med Virol. 2011;21:370–382. doi: 10.1002/rmv.704. [DOI] [PubMed] [Google Scholar]
- 63.Thorven M, Grahn A, Hedlund KO, Johansson H, Wahlfrid C, Larson G, Svensson L. A homozygous nonsense mutation (428G-->A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J Virol. 2005;79:15351–15355. doi: 10.1128/JVI.79.24.15351-15355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutson AM, Airaud F, LePendu J, Estes MK, Atmar RL. Norwalk virus infection associates with secretor status genotyped from sera. J Med Virol. 2005;77:116–120. doi: 10.1002/jmv.20423. [DOI] [PubMed] [Google Scholar]
- 65.Ferrer-Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, Blancher A, Calafell F, Bertranpetit J, Casals F. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26:1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 66.Currier RL, Payne DC, Staat MA, Selvarangan R, Shirley SH, Halasa N, Boom JA, Englund JA, Szilagyi PG, Harrison CJ, Klein EJ, Weinberg GA, Wikswo ME, Parashar U, Vinje J, Morrow AL. Innate Susceptibility to Norovirus Infections Influenced by FUT2 Genotype in a United States Pediatric Population. Clin Infect Dis. 2015;60:1631–1638. doi: 10.1093/cid/civ165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blacklow NR, Cukor G, Bedigian MK, Echeverria P, Greenberg HB, Schreiber DS, Trier JS. Immune response and prevalence of antibody to Norwalk enteritis virus as determined by radioimmunoassay. J Clin Microbiol. 1979;10:903–909. doi: 10.1128/jcm.10.6.903-909.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis. 1990;161:18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- 70.Larsson MM, Rydell GE, Grahn A, Rodriguez-Diaz J, Akerlind B, Hutson AM, Estes MK, Larson G, Svensson L. Antibody prevalence and titer to norovirus (genogroup II) correlate with secretor (FUT2) but not with ABO phenotype or Lewis (FUT3) genotype. J Infect Dis. 2006;194:1422–1427. doi: 10.1086/508430. [DOI] [PubMed] [Google Scholar]
- 71.Malm M, Uusi-Kerttula H, Vesikari T, Blazevic V. High Serum Levels of Norovirus Genotype-Specific Blocking Antibodies Correlate with Protection from Infection in Children. J Infect Dis. 2014 doi: 10.1093/infdis/jiu361. [DOI] [PubMed] [Google Scholar]
- 72.Nurminen K, Blazevic V, Huhti L, Rasanen S, Koho T, Hytonen VP, Vesikari T. Prevalence of norovirus GII-4 antibodies in Finnish children. J Med Virol. 2011;83:525–531. doi: 10.1002/jmv.21990. [DOI] [PubMed] [Google Scholar]
- 73.Ryder RW, Singh N, Reeves WC, Kapikian AZ, Greenberg HB, Sack RB. Evidence of immunity induced by naturally acquired rotavirus and Norwalk virus infection on two remote Panamanian islands. J Infect Dis. 1985;151:99–105. doi: 10.1093/infdis/151.1.99. [DOI] [PubMed] [Google Scholar]
- 74.Ramani S, Neill FH, Opekun AR, Gilger MA, Graham DY, Estes MK, Atmar RL. Mucosal and Cellular Immune Responses to Norwalk Virus. J Infect Dis. 2015 doi: 10.1093/infdis/jiv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czako R, Atmar RL, Opekun AR, Gilger MA, Graham DY, Estes MK. Serum hemagglutination inhibition activity correlates with protection from gastroenteritis in persons infected with Norwalk virus. Clin Vaccine Immunol. 2012;19:284–287. doi: 10.1128/CVI.05592-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol. 2002;76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Hernandez MB, Liu T, Payne HC, Stencel-Baerenwald JE, Ikizler M, Yagita H, Dermody TS, Williams IR, Wobus CE. Efficient Norovirus and Reovirus Replication in the Mouse Intestine Requires Microfold (M) Cells. J Virol. 2014;88:6934–6943. doi: 10.1128/JVI.00204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, Colonna M. MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HWt. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 81.Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol. 2007;81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thackray LB, Duan E, Lazear HM, Kambal A, Schreiber RD, Diamond MS, Virgin HW. Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication. J Virol. 2012;86:13515–13523. doi: 10.1128/JVI.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Changotra H, Jia Y, Moore TN, Liu G, Kahan SM, Sosnovtsev SV, Karst SM. Type I and type II interferons inhibit the translation of murine norovirus proteins. J Virol. 2009;83:5683–5692. doi: 10.1128/JVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, Green KY, Lopez-Otin C, Xavier RJ, Thackray LB, Virgin HW. Nondegradative role of Atg5-Atg12/ Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe. 2012;11:397–409. doi: 10.1016/j.chom.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez MR, Monte K, Thackray LB, Lenschow DJ. ISG15 functions as an interferon-mediated antiviral effector early in the murine norovirus life cycle. J Virol. 2014;88:9277–9286. doi: 10.1128/JVI.01422-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, Diamond MS, Virgin HW. Interferon lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science. 2014 doi: 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science. 2015;347:266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bui T, Kocher J, Li Y, Wen K, Li G, Liu F, Yang X, Leroith T, Tan M, Xia M, Zhong W, Jiang X, Yuan L. Median infectious dose of human norovirus GII.4 in gnotobiotic pigs is decreased by simvastatin treatment and increased by age. J Gen Virol. 2013 doi: 10.1099/vir.0.054080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pabst R, Rothkotter HJ. Postnatal development of lymphocyte subsets in different compartments of the small intestine of piglets. Vet Immunol Immunopathol. 1999;72:167–173. doi: 10.1016/s0165-2427(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 91.Pabst R, Geist M, Rothkotter HJ, Fritz FJ. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988;64:539–544. [PMC free article] [PubMed] [Google Scholar]
- 92.Dolin R, Levy AG, Wyatt RG, Thornhill TS, Gardner JD. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am J Med. 1975;59:761–768. doi: 10.1016/0002-9343(75)90461-1. [DOI] [PubMed] [Google Scholar]
- 93.Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HWt. Antibody is critical for the clearance of murine norovirus infection. J Virol. 2008;82:6610–6617. doi: 10.1128/JVI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 2008;4:e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park K, Cha KE, Myung H. Observation of inflammatory responses in mice orally fed with bacteriophage T7. J Appl Microbiol. 2014 doi: 10.1111/jam.12565. [DOI] [PubMed] [Google Scholar]
- 96.Tomov VT, Osborne LC, Dolfi DV, Sonnenberg GF, Monticelli LA, Mansfield K, Virgin HW, Artis D, Wherry EJ. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J Virol. 2013;87:7015–7031. doi: 10.1128/JVI.03389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN, 3rd, Devabhaktuni D, Jones MK, Karst SM. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog. 2013;9:e1003592. doi: 10.1371/journal.ppat.1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chagla Z, Quirt J, Woodward K, Neary J, Rutherford C. Chronic norovirus infection in a transplant patient successfully treated with enterally administered immune globulin. J Clin Virol. 2013;58:306–308. doi: 10.1016/j.jcv.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 99.Ronchetti AM, Henry B, Ambert-Balay K, Pothier P, Decroocq J, Leblond V, Roos-Weil D. Norovirus-related chronic diarrhea in a patient treated with alemtuzumab for chronic lymphocytic leukemia. BMC Infect Dis. 2014;14:239. doi: 10.1186/1471-2334-14-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, Babiker ZO, Guiver M, Turner AJ, Hughes S, Wynn RF. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant. 2011;15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 101.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waugh E, Chen A, Baird MA, Brown CM, Ward VK. Characterization of the chemokine response of RAW264.7 cells to infection by murine norovirus. Virus Res. 2014;181:27–34. doi: 10.1016/j.virusres.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 103.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strong DW, Thackray LB, Smith TJ, Virgin HW. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol. 2012;86:2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ponterio E, Petrizzo A, Di Bartolo I, Buonaguro FM, Buonaguro L, Ruggeri FM. Pattern of activation of human antigen presenting cells by genotype GII.4 norovirus virus-like particles. J Transl Med. 2013;11:127. doi: 10.1186/1479-5876-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol. 2010;84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen SM, Lin CP, Tsai JD, Chao YH, Sheu JN. The significance of serum and fecal levels of interleukin-6 and interleukin-8 in hospitalized children with acute rotavirus and norovirus gastroenteritis. Pediatr Neonatol. 2014;55:120–126. doi: 10.1016/j.pedneo.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Long KZ, Garcia C, Ko G, Santos JI, Al Mamun A, Rosado JL, DuPont HL, Nathakumar N. Vitamin A modifies the intestinal chemokine and cytokine responses to norovirus infection in Mexican children. J Nutr. 2011;141:957–963. doi: 10.3945/jn.110.132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hinkula J, Ball JM, Lofgren S, Estes MK, Svensson L. Antibody prevalence and immunoglobulin IgG subclass pattern to Norwalk virus in Sweden. J Med Virol. 1995;47:52–57. doi: 10.1002/jmv.1890470111. [DOI] [PubMed] [Google Scholar]
- 110.Wingfield T, Gallimore CI, Xerry J, Gray JJ, Klapper P, Guiver M, Blanchard TJ. Chronic norovirus infection in an HIV-positive patient with persistent diarrhoea: a novel cause. J Clin Virol. 2010;49:219–222. doi: 10.1016/j.jcv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 111.Nilsson M, Hedlund KO, Thorhagen M, Larson G, Johansen K, Ekspong A, Svensson L. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J Virol. 2003;77:13117–13124. doi: 10.1128/JVI.77.24.13117-13124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iritani N, Seto T, Hattori H, Natori K, Takeda N, Kubo H, Yamano T, Ayata M, Ogura H, Seto Y. Humoral immune responses against norovirus infections of children. J Med Virol. 2007;79:1187–1193. doi: 10.1002/jmv.20897. [DOI] [PubMed] [Google Scholar]
- 113.Rockx B, Baric RS, de Grijs I, Duizer E, Koopmans MP. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J Med Virol. 2005;77:439–446. doi: 10.1002/jmv.20473. [DOI] [PubMed] [Google Scholar]
- 114.Cukor G, Nowak NA, Blacklow NR. Immunoglobulin M responses to the Norwalk virus of gastroenteritis. Infect Immun. 1982;37:463–468. doi: 10.1128/iai.37.2.463-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leon J, Souza M, Qiuhong W, Smith E, Saif L, Moe C. Immunology of Norovirus Infection. In: M V, editor. Immunity Against Mucosal Pathogens. New York: Springer; 2008. pp. 219–262. [Google Scholar]
- 116.Treanor JJ, Jiang X, Madore HP, Estes MK. Subclass-specific serum antibody responses to recombinant Norwalk virus capsid antigen (rNV) in adults infected with Norwalk, Snow Mountain, or Hawaii virus. J Clin Microbiol. 1993;31:1630–1634. doi: 10.1128/jcm.31.6.1630-1634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, Kapikian AZ, Chanock RM. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis. 1974;129:709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]
- 118.Parra GI, Green KY. Sequential gastroenteritis episodes caused by 2 norovirus genotypes. Emerg Infect Dis. 2014;20:1016–1018. doi: 10.3201/eid2006.131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saito M, Goel-Apaza S, Espetia S, Velasquez D, Cabrera L, Loli S, Crabtree JE, Black RE, Kosek M, Checkley W, Zimic M, Bern C, Cama V, Gilman RH. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin Infect Dis. 2014;58:483–491. doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simmons K, Gambhir M, Leon J, Lopman B. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis. 2013;19:1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med. 1977;297:86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- 122.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology. 1999;117:40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 124.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, Bargatze RF, Sztein MB, Tacket CO. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 126.Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, Ferreira J, Goodwin R, Borkowski A, Clemens R, Mendelman PM. A Novel Intramuscular Bivalent Norovirus Virus-Like Particle Vaccine Candidate-Reactogenicity, Safety, and Immunogenicity in a Phase 1 Trial in Healthy Adults. J Infect Dis. 2014 doi: 10.1093/infdis/jiu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Atmar RL, Estes MK. Norovirus vaccine development: next steps. Expert Rev Vaccines. 2012;11:1023–1025. doi: 10.1586/erv.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hickman D, Jones MK, Zhu S, Kirkpatrick E, Ostrov DA, Wang X, Ukhanova M, Sun Y, Mai V, Salemi M, Karst SM. The effect of malnutrition on norovirus infection. MBio. 2014;5:e01032–01013. doi: 10.1128/mBio.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nelson AM, Elftman MD, Pinto AK, Baldridge M, Hooper P, Kuczynski J, Petrosino JF, Young VB, Wobus CE. Murine norovirus infection does not cause major disruptions in the murine intestinal microbiota. Microbiome. 2013;1:7. doi: 10.1186/2049-2618-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nelson AM, Walk ST, Taube S, Taniuchi M, Houpt ER, Wobus CE, Young VB. Disruption of the human gut microbiota following Norovirus infection. PLoS One. 2012;7:e48224. doi: 10.1371/journal.pone.0048224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Basic M, Keubler LM, Buettner M, Achard M, Breves G, Schroder B, Smoczek A, Jorns A, Wedekind D, Zschemisch NH, Gunther C, Neumann D, Lienenklaus S, Weiss S, Hornef MW, Mahler M, Bleich A. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm Bowel Dis. 2014;20:431–443. doi: 10.1097/01.MIB.0000441346.86827.ed. [DOI] [PubMed] [Google Scholar]
- 133.Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516:94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tamura M, Natori K, Kobayashi M, Miyamura T, Takeda N. Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J Virol. 2004;78:3817–3826. doi: 10.1128/JVI.78.8.3817-3826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murakami K, Kurihara C, Oka T, Shimoike T, Fujii Y, Takai-Todaka R, Park Y, Wakita T, Matsuda T, Hokari R, Miura S, Katayama K. Norovirus binding to intestinal epithelial cells is independent of histo-blood group antigens. PLoS One. 2013;8:e66534. doi: 10.1371/journal.pone.0066534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Makino A, Shimojima M, Miyazawa T, Kato K, Tohya Y, Akashi H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J Virol. 2006;80:4482–4490. doi: 10.1128/JVI.80.9.4482-4490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stuart AD, Brown TD. Alpha2,6-linked sialic acid acts as a receptor for Feline calicivirus. J Gen Virol. 2007;88:177–186. doi: 10.1099/vir.0.82158-0. [DOI] [PubMed] [Google Scholar]
- 138.Monne Rodriguez JM, Soare T, Malbon A, Blundell R, Papoula-Pereira R, Leeming G, Kohler K, Kipar A. Alveolar macrophages are the main target cells in feline calicivirus-associated pneumonia. Vet J. 2014;201:156–165. doi: 10.1016/j.tvjl.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 139.Kreutz LC, Seal BS, Mengeling WL. Early interaction of feline calicivirus with cells in culture. Arch Virol. 1994;136:19–34. doi: 10.1007/BF01538814. [DOI] [PubMed] [Google Scholar]
- 140.Taube S, Perry JW, Yetming K, Patel SP, Auble H, Shu L, Nawar HF, Lee CH, Connell TD, Shayman JA, Wobus CE. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J Virol. 2009;83:4092–4101. doi: 10.1128/JVI.02245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Taube S, Perry JW, McGreevy E, Yetming K, Perkins C, Henderson K, Wobus CE. Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner. J Virol. 2012;86:5584–5593. doi: 10.1128/JVI.06854-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zakhour M, Ruvoen-Clouet N, Charpilienne A, Langpap B, Poncet D, Peters T, Bovin N, Le Pendu J. The alphaGal epitope of the histo-blood group antigen family is a ligand for bovine norovirus Newbury2 expected to prevent cross-species transmission. PLoS Pathog. 2009;5:e1000504. doi: 10.1371/journal.ppat.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jung K, Scheuer KA, Zhang Z, Wang Q, Saif LJ. Pathogenesis of GIII.2 bovine norovirus, CV186-OH/00/US strain in gnotobiotic calves. Vet Microbiol. 2014;168:202–207. doi: 10.1016/j.vetmic.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim DS, Hosmillo M, Alfajaro MM, Kim JY, Park JG, Son KY, Ryu EH, Sorgeloos F, Kwon HJ, Park SJ, Lee WS, Cho D, Kwon J, Choi JS, Kang MI, Goodfellow I, Cho KO. Both alpha2,3- and alpha2,6-linked sialic acids on o-linked glycoproteins act as functional receptors for porcine sapovirus. PLoS Pathog. 2014;10:e1004172. doi: 10.1371/journal.ppat.1004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang KO, Sosnovtsev SV, Belliot G, Kim Y, Saif LJ, Green KY. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A. 2004;101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parwani AV, Flynn WT, Gadfield KL, Saif LJ. Serial propagation of porcine enteric calicivirus in a continuous cell line. Effect of medium supplementation with intestinal contents or enzymes. Arch Virol. 1991;120:115–122. doi: 10.1007/BF01310954. [DOI] [PubMed] [Google Scholar]
- 147.Farkas T, Cross RW, Hargitt E, 3rd, Lerche NW, Morrow AL, Sestak K. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol. 2010;84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Farkas T, Sestak K, Wei C, Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smiley JR, Chang KO, Hayes J, Vinje J, Saif LJ. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J Virol. 2002;76:10089–10098. doi: 10.1128/JVI.76.20.10089-10098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dolin R, Blacklow NR, DuPont H, Formal S, Buscho RF, Kasel JA, Chames RP, Hornick R, Chanock RM. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. J Infect Dis. 1971;123:307–312. doi: 10.1093/infdis/123.3.307. [DOI] [PubMed] [Google Scholar]
- 151.Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dolin R, Blacklow NR, DuPont H, Buscho RF, Wyatt RG, Kasel JA, Hornick R, Chanock RM. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc Soc Exp Biol Med. 1972;140:578–583. doi: 10.3181/00379727-140-36508. [DOI] [PubMed] [Google Scholar]
- 153.Agus SG, Dolin R, Wyatt RG, Tousimis AJ, Northrup RS. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Histologic and enzymatic alterations during illness produced by the Norwalk agent in man. Ann Intern Med. 1973;79:18–25. doi: 10.7326/0003-4819-79-1-18. [DOI] [PubMed] [Google Scholar]
- 154.Widerlite L, Trier JS, Blacklow NR, Schreiber DS. Structure of the gastric mucosa in acute infectious bacterial gastroenteritis. Gastroenterology. 1975;68:425–430. [PubMed] [Google Scholar]
- 155.Dolin R, Reichman RC, Roessner KD, Tralka TS, Schooley RT, Gary W, Morens D. Detection by immune electron microscopy of the Snow Mountain agent of acute viral gastroenteritis. J Infect Dis. 1982;146:184–189. doi: 10.1093/infdis/146.2.184. [DOI] [PubMed] [Google Scholar]
- 156.Keswick BH, Satterwhite TK, Johnson PC, DuPont HL, Secor SL, Bitsura JA, Gary GW, Hoff JC. Inactivation of Norwalk virus in drinking water by chlorine. Appl Environ Microbiol. 1985;50:261–264. doi: 10.1128/aem.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Treanor JJ, Madore HP, Dolin R. Development of an enzyme immunoassay for the Hawaii agent of viral gastroenteritis. J Virol Methods. 1988;22:207–214. doi: 10.1016/0166-0934(88)90103-6. [DOI] [PubMed] [Google Scholar]
- 158.Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 159.Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. Norwalk virus: how infectious is it? J Med Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]