Abstract
Background
Resistant hypertension (RH) is a growing health burden in this country affecting as many as one in five adults being treated for hypertension. RH is associated with increased risk of adverse cardiovascular disease (CVD) events and all-cause mortality. Strategies to reduce blood pressure in this high risk population are a national priority.
Methods
TRIUMPH is a single site, prospective, randomized clinical trial (RCT) to evaluate the efficacy of a center-based lifestyle intervention consisting of exercise training, reduced sodium and calorie DASH eating plan, and weight management compared to standardized education and physician advice in treating patients with RH. Patients (N=150) will be randomized in a 2:1 ratio to receive either a 4-month supervised lifestyle intervention delivered in the setting of a cardiac rehabilitation center or to a standardized behavioral counseling session to simulate real-world medical practice. The primary end point is clinic blood pressure; secondary endpoints include ambulatory blood pressure and an array of CVD biomarkers including left ventricular hypertrophy, arterial stiffness, baroreceptor reflex sensitivity, insulin resistance, lipids, sympathetic nervous system activity, and inflammatory markers. Lifestyle habits, blood pressure and CVD risk factors also will be measured at one year follow-up.
Conclusions
The TRIUMPH randomized clinical trial (ClinicalTrials.gov NCT02342808) is designed to test the efficacy of an intensive, center-based lifestyle intervention compared to a standardized education and physician advice counseling session on blood presssure and CVD biomarkers in patients with RH after 4 months of treatment, and will determine whether lifestyle changes can be maintained for a year.
Keywords: Resistant hypertension, DASH diet, exercise, obesity, cardiac rehabilitation
BACKGROUND
The term “resistant hypertension” (RH) is defined as blood pressure (BP) that remains above goal (e.g., systolic blood pressure [SBP]>140 mm Hg and/or diastolic blood pressure [DBP]>90 mm Hg), despite adherence to a regimen of 3 or more optimally-dosed antihypertensive medications of different classes, one of which is a diuretic, or the need for 4 or more antihypertensive agents to achieve goal.1 With the growing prevalence of hypertension in this country, RH is a major public health concern, affecting more than 7.5 million Americans.1-3 Patients with RH are 50% more likely to experience a cardiovascular (CVD) event, including stroke, kidney failure, myocardial infarction, and death, compared to patients with controlled BP.4-10 There is an urgent need for developing RH management strategies to lower BP as well as to reduce the high risk of CVD-related events.11
Lifestyle modifications, including exercise training and dietary modification, are of proven efficacy in lowering BP in unmedicated patients with hypertension and are often recommended as the first step for treating high BP.12 The Dietary Approaches to Stop Hypertension (DASH) diet has been shown to lower BP in hypertensive patients who are not treated with drugs.13-18 Moreover, when the DASH diet is combined with exercise and caloric restriction, even greater, and quite marked, BP reductions can be achieved.19,20 However, the efficacy of these lifestyle modifications in RH patients who are refractory to medical therapy is unknown.
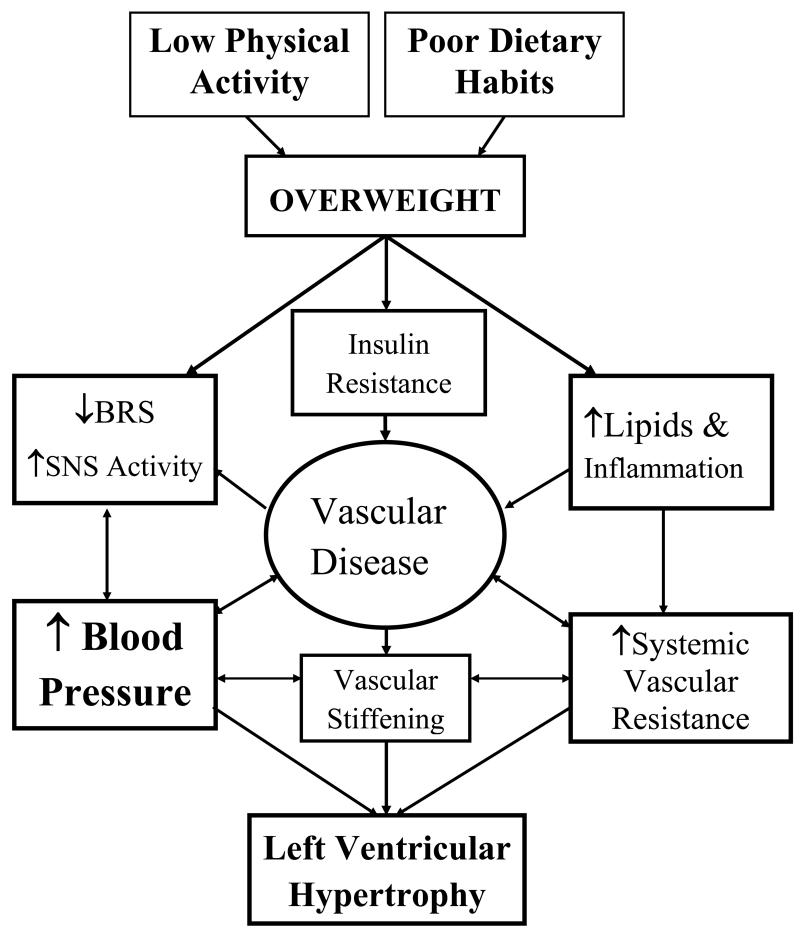
In a recent editorial, Hayward and Krumholz21 provide a compelling argument that treatment decisions should be made on the basis of patients’ overall CVD risk status, with the objective of lowering this risk as much as possible. Effective lifestyle interventions afford the opportunity for not only reduction of BP, but also for modification of multiple risk factors, which can result in significant reduction of overall CVD risk in RH patients. Patient-centered outcomes, including quality of life, may also improve with lifestyle modifications. A premise of the TRIUMPH trial is that unhealthy lifestyles, including poor food choices and sedentary living, play an important role in the development and persistence of RH and that modifying these unhealthy behaviors will arrest and potentially reverse the disease process. A further premise is that despite the apparent motivation of patients to adopt a healthy lifestyle, a structured intervention program is needed for them to achieve and maintain a therapeutic response. Unhealthy behavior patterns are typically well-entrenched and have long-term rather than short-term negative consequences, and lifestyle changes require a significant daily commitment to effortful planning and recalibration of life priorities. Figure 1 depicts a model by which poor nutrition and lack of exercise may adversely impact BP in this population, and how the complex interplay of autonomic, metabolic, inflammatory, cardiac, and vascular function may be both a cause and consequence of RH.
Figure 1. Conceptual Model.
depicts a model by which poor dietary habits and lack of physical activity may adversely impact BP in this population, and how the complex interplay of autonomic, metabolic, inflammatory, cardiac, and vascular function may be both a cause and consequence of RH. The TRIUMPH study is designed primarily to evaluate whether an intensive lifestyle intervention delivered in a CR setting can achieve clinically significant BP lowering in patients whose BP has failed to be adequately controlled by the combined effects of at least 3 antihypertensive medications. It is also designed to provide insight into whether diet and exercise improve comorbid disease and associated biomarkers of CVD risk in RH patients.
Novel Invasive Approaches to the Treatment of Resistant Hypertension
The failure of treatment with antihypertensive drugs to adequately lower BP in RH patients has prompted the development of novel non-pharmacological interventions. One approach is carotid baroreceptor activation to attenuate sympathetic activity and augment cardiac vagal control.22,23 Although this technique has been successful at achieving modest reductions in BP in early clinical trials, it requires permanent implantation of a device and approximately 25% of patients may experience procedure-related adverse outcomes.24,25 Recently, a small, open-label randomized trial of a central iliac arteriovenous anastomosis produced with a coupler device demonstrated significant reductions in both SBP and DBP, but much remains to be learned about the longer term efficacy and safety of this intervention. 26 Another initially promising procedural approach to the treatment of RH is catheter-based renal sympathetic denervation.27 The SYMPLICITY HTN-2 trial28 generated much excitement in the medical community by suggesting that percutaneous renal sympathetic denervation could lower clinic SBP by as much as 30 mm Hg in RH patients.29 However, SYMPLICITY HTN-3,30 a more rigorously designed and pivotal multi-center trial of catheter-based renal denervation performed in the United States, failed to meet its primary efficacy endpoint of a reduction in clinic-based SBP ≥5 mm Hg. This disappointing outcome has tempered the prospects for widespread utilization of renal denervation in the management of RH in the near future, and underscores the need for new avenues of investigation for non-pharmacological approaches to lowering BP and reducing CVD risk in RH patients.
Back to the Future: Potential Benefits of Lifestyle Modification in Patients with Resistant Hypertension
Although the value of lifestyle interventions in patients already taking antihypertensive medications has not been widely studied, the available evidence, acquired primarily in patients treated with 1 or 2 drugs, appears promising. Regular exercise alone lowered DBP and led to regression of left ventricular hypertrophy (LVH) in a small study of medicated African-American men with uncontrolled hypertension,31 and the TONE study demonstrated that in elderly patients receiving antihypertensive monotherapy, sodium restriction and weight loss resulted in improved BP control.32 Surprisingly, there are limited data describing the effects of the DASH diet in medicated hypertensive patients. In a study of 55 hypertensive patients treated with an angiotensin receptor blocker (ARB), the DASH diet was associated with a 5 mm Hg greater reduction in ambulatory SBP compared to patients taking the ARB with their usual diet.33 The ADAPT trial34 was an Australian study of hypertensive patients treated with 1 or 2 drugs in which an intervention designed to promote consumption of a modified DASH diet resulted in a modest (4/2 mm Hg), but statistically significant, reduction in ambulatory BP and reduced dependence on antihypertensive medications. In the DEW-IT study,35 a 9-week ‘feeding’ study of 44 overweight adults on a single BP-lowering agent, the DASH diet coupled with weight loss also resulted in significant BP reductions.
Surprisingly, lifestyle modification has not been rigorously evaluated in patients with RH. Several small studies, however, suggest that changes in diet and physical activity have the potential to lower BP substantially in these individuals. For example, in a study of 12 subjects with RH, 24-hour ambulatory BP was 23/9 mm Hg lower on a 50 mmol/d (1150 mg/d) sodium diet compared to a 250 mmol/d (5750 mg/d) sodium diet.36 In this study, however, the treatment periods were short (7 days) and all food was prepared in a clinical research center; whether similar results could be achieved in the longer-term and in the absence of specially prepared meals is unclear. In another small study, Dimeo et al.37 examined the value of physical activity in 50 patients with RH who were randomized to thrice weekly treadmill exercise or a control condition; exercise decreased ambulatory daytime BP by 6/3 mm Hg. Thus, preliminary evidence suggests that lifestyle modifications may be effective in in reducing BP in RH patients, but such efforts need to be examined in more rigorous RCTs.
METHODS
TRIUMPH is a randomized controlled trial (RCT) designed to evaluate whether an intensive, medically-supervised lifestyle intervention can achieve clinically significant BP lowering in medicated patients with RH. In addition to evaluating the primary endpoint of clinic BP, the study will also examine effects on 24-hour ambulatory blood pressure (ABP), along with a constellation of CVD risk factors that are common in RH patients and that contribute to the increased risk associated with uncontrolled BP.
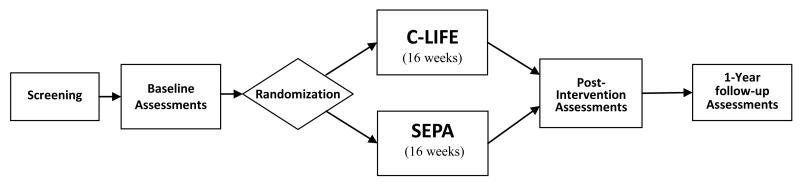
One hundred fifty patients with RH will be evaluated and randomized with 2:1 allocation to one of two treatment groups: (1) Center-based Lifestyle Intervention (C-LIFE) or (2) Standardized Education and Physician Advice (SEPA). Participants will be evaluated at (a) baseline; (b) after a 4-month intervention; and (c) 1 year after randomization. The study design is depicted in Figure 2.
Figure 2. Study Design Overview.
Participants
RH is defined as SBP that is higher than 140 mm Hg, despite treatment with ≥3 optimally-dosed antihypertensive medications of different classes, including a diuretic,12 or the need for 4 or more drugs to achieve a SBP ≤ 140 mmHg.
Inclusion Criteria
Recruitment will target individuals with documented RH within the last 6 months. Individuals treated for two or more weeks with at least 3 antihypertensive medications of different classes (at a stable dosage), including a diuretic, with clinic SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, will be eligible. Individuals being treated with 4 or more antihypertensive medications (including a diuretic) with SBP ≥ 130 or DBP ≥ 85 mm Hg also will be eligible. In addition to hypertension, other inclusion criteria include: overweight or obese (e.g., body mass index [BMI] 25-39.9 kg/m2), sedentary (exercise <30 min/week), age 35-80 years, and willingness to be randomized to either of the 2 treatment groups.
Exclusion criteria
Secondary hypertension, severe chronic kidney disease defined as an estimated glomerular filtration rate [GFR] <45 ml/min/1.73m2, moderate-severe ischemic heart disease (Canadian Cardiovascular Society Class 3 or 4 angina or evidence of ischemia at <85% heart rate reserve on treadmill testing), severe heart failure (New York Heart Association Class 3 or 4), high grade arrhythmias, severe valvular heart disease, severe asthma or chronic obstructive lung disease, diabetes requiring insulin, musculoskeletal or neurologic problems that would preclude participation in aerobic exercise training, current major psychiatric disorder or active drug abuse, current alcohol consumption >14 drinks/week, prior gastric bypass surgery, a life-limiting comorbid medical condition such as cancer, or ‘pseudoresistant’ hypertension due to medication non-adherence or due to the white coat effect.38
Screening Procedures to Determine Eligibility for the TRIUMPH Trial
All eligible patients will undergo clinic blood pressure and medical screening, physical examination, and body weight assessment. Blood pressure will be determined according to JNC 7 guidelines.39 Screening assessments also will include measures of serum creatinine and eGFR, electrolytes, HbA1c, and urinary albumin/creatinine ratio to screen for secondary hypertension and other conditions that would preclude patients from safely participating in the study and to assess comorbidities and target organ manifestations of hypertension. The most common causes of secondary hypertension are CKD and primary hyperaldosteronism. All patients will have baseline measurements of serum creatinine to assess renal function, and renin and aldosterone levels to exclude primary hyperaldosteronism. Thyroid stimulating hormone will be measured to exclude clinically significant thyroid disorders. We will not screen for other secondary causes of hypertension because these conditions tend to be rare (e.g., pheochromocytoma) and/or the impact of current therapies are inconclusive or provides only marginal BP lowering benefit (e.g., renovascular disease and obstructive sleep apnea).
Medication Reconciliation
Treatment regimens will be documented by having participants bring their actual medications to the research clinic and confirmed with referring physicians. To facilitate assessment of medication changes, the intensity of therapy will be quantified as the Daily Defined Dose (DDD) using a method developed by the World Health Organization. The DDD is a system for quantification of drug amount designed to enable comparison across drug classes (e.g., 1×DDD=150 mg irbesartan or 5 mg amlodipine)40 and was previously used in the BP Guide and TASMIN-SR studies.41,42
Medication Adherence Screening
Because patients who are non-adherent to their antihypertensive medications could be erroneously classified as RH, we will perform an initial screen for medication adherence using the Morisky Medication Adherence Measure.43 This 8-item scale was developed from the well-validated and widely-used 4-item version44 with the objective of specifically evaluating medication adherence in patients with hypertension. The scale has established reliability and validity45 and is related to BP control, with a score ≥ 3 identifying patients with poor BP control due to non-adherence with a sensitivity of 93%.43
Treatment Conditions
Participants will be randomized to one of two treatment arms and encouraged to maintain their prescribed antihypertensive medications at the discretion of their personal physicians, unless contraindicated by safety issues.
- (1) Center-based Lifestyle Intervention (C-LIFE): The 4-month C-LIFE intervention will consist of 4 components delivered by our research staff at participating community CR centers:
- (i) Diet: Participants will receive instruction on the DASH diet with caloric and sodium restriction (2300 mg/day) and feedback on their adherence to the diet in 16 half-hour weekly sessions. The goal of the weekly sessions is to assist participants in knowing how to buy and prepare the appropriate foods, to enhance their motivation to choose to eat those foods, and to overcome obstacles to following the diet. The nutritionist will utilize motivational interviewing strategies as a way to encourage participants to make the needed changes. A key component, used in the DASH, PREMIER, and ENCORE studies and incorporated into the proposed intervention, is motivational enhancement to reinforce commitment and confidence for behavior change and to help participants explore and resolve ambivalence.46,47 Specific efforts to provide culturally-sensitive adaptations of the diet will be provided as our prior work indicated that African-American participants may have difficulty adopting the DASH diet.48 Greater emphasis will be placed on the personal, financial, and cultural needs of the participants while they attempt to adopt the DASH diet and reduce dietary sodium (2300 mg/day), and thus provide an individually tailored intervention.
-
(ii) Behavioral Weight Management: The Weight Management program will consist of 16 half-hour weekly sessions that emphasize the initiation of eating behavior changes, individualized problem-solving, and strategies to facilitate the transition to a long-term self-care plan that is designed to prepare participants to maintain treatment gains on their own following termination of treatment. The weight management employed in TRIUMPH will include focus on the attitudes and skills needed to develop and implement a long-term self-care health plan.The weight management intervention includes standard cognitive and behavioral strategies with a particular emphasis on the importance of self-monitoring as a strategy to initiate behavior change and an understanding of the roles of motivation for change and automaticity of behavioral patterns in maintaining behavioral changes. Self-monitoring of DASH food goals is used to enhance compliance with the recommended dietary content, and appetite monitoring is used to regulate the amount of food eaten. Appetite Awareness Training (AAT) serves to enhance portion control, facilitate reductions in caloric consumption, and reduce perceptions of deprivation by raising awareness of internal fullness cues. Weekly weight monitoring is to be continued indefinitely as small increases (which are more easily reversed) are not always noticeable. The weight management training will emphasize individualized problem-solving to remove barriers to adherence. The syllabus for the program is provided in an Appendix (Table S1).
- (iii) Supervised Exercise: Participants initially will exercise at one of the designated CR facilities 3 times per week at a level of 70-85% of their initial heart rate reserve, determined at the time of their baseline treadmill test.49 The exercise routine will consist of 10 minutes of warm up exercises, 30-45 minutes of aerobic exercise, and 10 minutes of cool down exercises. Patients with pre-exercise SBP levels >200 mm Hg or DBP >110 mm Hg will not be permitted to exercise; exercise will be stopped immediately if patients’ SBP values exceed 250 mm Hg or DBP levels exceed 115 mm Hg. While exercise alone has been shown to be of limited value in reducing BP50 and in inducing weight loss,51 continued exercise and physical activity are widely recognized as being critically important for successful weight maintenance.52 Therefore, participants will be counseled to develop a plan to remain active and to exercise on their own, using strategies previously employed to help patients transition from supervised to home-based exercise protocols.53,54
- (iv) Maintenance: Participants will be prepared to maintain all components that constitute the lifestyle intervention (i.e., DASH eating plan, limited sodium consumption, weight management, and exercise). The C-LIFE intervention will emphasize the transition to self-care by carefully preparing participants to meet the challenges of maintaining lifestyle changes on their own. We hope to maximize the likelihood that those participants who show improvements with treatment will be able to maintain those improvements over time, especially since CR programs do not offer provision for long-term support for behavior change. Because patients with RH currently have few options to lower their BP and hypertension is a chronic disease, it is particularly critical to teach patients to establish a life-long self-care plan.
(2) Medical Management with Standardized Education and Physician Advice (SEPA): Although patients will be encouraged to achieve an ideal body weight and engage in exercise as part of routine counseling in primary care, no special programs will be provided that enhance patients’ ability to comply with these recommendations. The SEPA arm will consist of routine medical care provided by patients’ primary care physicians supplemented by an educational session on hypertension management. Patients will receive a dietary consultation and an individualized exercise prescription delivered by a health educator. In order to compare the intensive, structured C-LIFE program with patients’ own efforts at adhering to physician advice, patients will be free to engage in their own diet and exercise programs, which we will document over the course of the trial. Referral for an individual consultation with a nutritionist and exercise specialist is an option that is available to physicians in the care for patients with hypertension, and will be standardized at its optimal level of implementation for this research protocol.
Assessments
Blood pressure will be obtained in the clinic and during daily life using 24-hr ambulatory blood pressure monitoring (see Table 1 for assessment methods and testing schedule). In addition to measuring BP, we will assess an array of CVD biomarkers including left ventricular hypertrophy (LVH), cardiovascular hemodynamics (including cardiac output and systemic vascular resistance), arterial stiffness, endothelial function, baroreflex sensitivity, metabolic factors such as glucose and insulin resistance, inflammatory markers, and aerobic capacity. We also will document the effects of the interventions on relevant lifestyle habits including physical activity, body weight, and diet.
Table 1.
Study Outcomes, Assessment Methods and Schedule
| Study Outcomes/ Endpoints | Assessment Methods | Timepoint |
|---|---|---|
|
| ||
| Clinical Outcomes | ||
| Blood pressure | Automated BP monitor | Screening, baseline, 4M, 12M |
| 24-hour ambulatory BP monitor | Baseline, 4M, 12M | |
| Body weight | Calibrated digital scale | Screening, baseline, 4M, 12M |
| Waist circumference | Tape measure | Baseline, 4M, 12M |
|
| ||
| Metabolic profile | Fasting serum glucose, insulin and lipids | Baseline, 4M, 12M |
|
| ||
| Neuroendocrine profile | Serum aldosterone, plasma renin activity, 24- hour urine catecholamines |
Baseline, 4M |
|
| ||
| Kidney function | Serum creatinine | Screening, baseline, 4M, 12M |
| Urine albumin and creatinine | Baseline, 4M, 12M | |
|
| ||
| Systemic inflammation markers | High-sensitivity C-reactive protein | Baseline, 4M |
|
| ||
| Cardiovascular Outcomes | ||
| Left ventricular mass/geometry |
Echocardiography | Baseline, 4M, 12M |
| Arterial Stiffness | Pulse wave velocity | Baseline, 4M, 12M |
| Baroreflex sensitivity | Finometer instrument | Baseline, 4M |
| Estimated CVD Risk | Framingham Risk Score | Baseline, 4M, 12M |
|
| ||
| Aerobic Capacity | Modified Balke exercise treadmill test | Baseline, 4M, 12M |
|
| ||
| Physical Activity | 7-day accelerometer monitoring, Community Healthy Activities Model Program for Seniors Activity Questionnaire |
Baseline, 4M, 12M |
|
| ||
| Dietary Intake | Automated Self-administered 24-hour recall, 24- hour urine sodium, potassium, and creatinine |
Baseline, 4M, 12M |
|
| ||
| Psychosocial Outcomes | ||
| Quality of Life | Short-form-36 | Baseline, 4M, 12M |
| Depression | Beck Depression Inventory | Baseline, 4M, 12M |
| Anxiety | State-Trait Anxiety Inventory | Baseline, 4M, 12M |
| Self-esteem | Rosenberg Self-Esteem Scale | Baseline, 4M, 12M |
| Health Locus of Control | Health Locus of Control Scale | Baseline, 4M, 12M |
|
| ||
| Medication Adherence | Morisky Adherence Scale, Medication Event Monitoring System |
Screening, baseline, 4M, 12M |
|
| ||
| Clinical Events | Interview, medical record adjudication | 4M, 12M and annually up to 5 yrs |
| All-cause mortality, non-fatal myocardial infarction, stroke, CV-related hospitalization, hypertensive emergency, CV procedure, doubling of serum creatinine or end-stage renal disease | ||
Note: BP= blood pressure; CV = cardiovascular; 4M= 4-months; 12M= 12-months.
In addition to better BP control, several patient-centered outcomes that could represent important benefits of a lifestyle intervention program will be assessed. These include improvements in other risk factors (e.g., hypercholesterolemia and diabetes) and overall CVD risk55 and improved quality of life. A detailed description of the measures obtained in the TRIUMPH study is available in the Appendix (Table S2).
Follow-up
Participants will be followed at 1-year post-randomization (8 months after completion of the 4-month intervention program). All participants will be asked to report any changes in BP medications or other interventions to reduce BP that they may have initiated (under the care of their own physicians). Medication changes will be incorporated into our data analytic plan described below. At 1 year following randomization, measures of clinic BP, ABP, CVD biomarkers, body weight, dietary and exercise habits, and QoL will be reassessed. At the follow-up visit, patients will be asked to bring with them all medications that they are currently taking, so that any adjustments to their antihypertensive therapy can be documented. Patients will also be queried as to whether they participated in any supplemental programs to lose weight (including bariatric surgery), diet, or engage in exercise. This naturalistic follow-up has been used in prior work56-58 and yields important information regarding maintenance of lifestyle habits over time and will used to help interpret any group differences in BP and CVD biomarkers after 1 year. We also will document a number of clinical endpoints including cardiovascular death, non-fatal MI, non-fatal stroke or transient ischemic attack, coronary revascularization, hospitalization for hypertension, angina, or heart failure, and progression to end-stage renal disease during a follow-up period of up to 5 years.
DATA ANALYSIS
We will evaluate the primary outcome, clinic SBP, at the immediate post-treatment measurement occasion (4 months), with treatment assignment (C-LIFE vs. SEPA) as a between subjects factor, and ethnicity, gender, age, diabetes/chronic kidney disease, baseline medication adherence, and pre-treatment clinic SBP as covariates, selected a priori.59 We will conduct an ancillary analysis in order to evaluate the possible role of change in BP medication on the treatment effect by adding the DDD measure as a covariate in the models testing treatment effects. Ambulatory daytime SBP will be evaluated as a supportive analysis, using the same modeling approach as above. Analysis of additional outcomes, including ambulatory and clinic DBP, body weight, aerobic capacity, dietary intake, LV mass and relative wall thickness, CVD biomarkers, and patient-centered outcomes will be conducted using the same modeling approach. For secondary outcomes, we again will use separate models for each outcome, using Benjamini and Hochberg’s60 False Discovery Rate correction for multiple testing. All analyses of primary and secondary outcomes will adhere to the intent-to-treat principle, using multiple imputation for missing data with Harrell’s aregImpute algorithm in R. Mediational analyses will be carried out following the bootstrap procedures described by MacKinnon61 using the mediation package available in R.62 Specifically, we will use this approach to explore the potential role of changes in aerobic capacity, adherence to the DASH diet, and body weight as mediators that might explain a treatment effect. We also will examine potential moderators of treatment by testing interaction terms between treatment group and background characteristics. The moderators of primary interest are race, age, gender, presence of kidney disease or diabetes, BRS, and baseline levels of BMI and physical activity. In order to reduce the chance of a Type I Error, we will evaluate these interactions using a pooled 2 degree of freedom test using p = .05 as the criterion for statistical significance.63 For the 1-year BP outcomes, we will use mixed models, incorporating the same covariates as in the primary analyses, but also including the immediate post-treatment and 1-year BP as repeated measures. All analyses will be carried out using SAS (Cary, NC) or R (http://cran.r-project.org/) software.
Power Analysis
The primary effect of interest is the treatment group difference in clinic SBP at 4 months. Power estimates were made based on the following specifications, based in part on our prior work with hypertensive adults13: 1) a general linear model for clinic SBP; 2) an initial sample size of 150 patients with 2:1 group allocation and 15% attrition at 16 weeks; 3) alpha of 0.05; 4) a standard deviation of 10.2 mm Hg for clinic SBP, and 13 mm Hg for the supportive ambulatory SBP outcome. For clinic SBP, we will have 80% power to detect about a 5.4 mm Hg group difference, and 90% power to detect about a 6.3 mm Hg difference. For ambulatory SBP, we will have 80% power to detect a group difference of about 6.9 mm Hg; at 90% power, we will be able to detect about an 8.1 mm Hg treatment difference. With respect to secondary biomarker endpoints (e.g., BRS, arterial stiffness) we will have 80% power to detect at least a 0.53 standard deviation difference between treatment groups on a given endpoint.
DISCUSSION
In order to make informed choices, patients and their providers need valid empirical evidence about the merits (or ‘demerits’) of structured lifestyle modification for patients with RH. Because the existing research is relatively sparse and was conducted primarily in unmedicated hypertension patients, it remains to be seen if patients with RH will be able to achieve significant lifestyle changes, and whether such changes will produce clinically significant improvements in BP control. It cannot be assumed that RH patients, who have complied with drug therapy yet failed to achieve target BP levels, will respond in the same way to lifestyle modifications as those who are not on medication. Patients who are refractory to medications may not achieve significant BP reductions from lifestyle changes, either. The value of lifestyle modification for RH patients remains an important and unanswered empirical question that needs to be examined in order to ascertain whether intensive, structured lifestyle interventions may be an effective treatment option for these patients. Indeed, the recently published American Heart Association and American College of Cardiology joint Guidelines on Lifestyle Management to Reduce Cardiovascular Risk noted that there is a lack of data on the effects of lifestyle modification in treated hypertension patients and identified this as being a priority area for future research.64 If the TRIUMPH lifestyle intervention proves successful in lowering BP and if the results are confirmed in subsequent studies, it could be argued that third-party payers should support the participation of patients with RH in CR programs.
Patients with RH are typically sedentary and obese,1 so a lifestyle intervention including exercise, weight loss, and optimal nutrition has the potential to lower BP substantially. There is now good evidence that for behavior change to be successful, interventions should be linked to structured programs designed to facilitate behavior change.65 To date, no RCT has tested an intensive, structured lifestyle intervention in patients who have uncontrolled BP despite being on 3 or more antihypertensive medications. Also missing from the research literature is an examination of “moderators” of lifestyle interventions. It is important to determine characteristics of patients who respond to lifestyle modifications so that interventions can be delivered to those most likely to benefit, and so that alternative strategies can be developed for those unlikely to respond. TRIUMPH will examine a variety of possible moderators as potential individual difference measures that could affect BP response to lifestyle modification.
In summary, TRIUMPH is a randomized clinical trial that will test the hypothesis that an intensive, 4-month, cardiac rehabilitation-based lifestyle modification program to promote exercise, the DASH eating plan, and weight loss will result in a meaningful decrease in BP, improvements in other biomarkers of CVD risk, and enhanced quality of life in patients with RH. Moreover, we will determine if lifestyle changes can be maintained for a year. The results of this study will inform patients, providers, and policy makers in judging whether structured lifestyle interventions may be an effective treatment option for individuals with hypertension that is refractory to medical therapy.
Supplementary Material
Acknowledgments
FUNDING SOURCE: Supported by a grant (HL122836) from the National Heart, Lung, and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
ABBREVIATIONS
- ABP
Ambulatory blood pressure
- ARB
Angiotensin receptor blocker
- BMI
Body Mass Index
- BDI
Beck Depression Inventory
- BP
Blood pressure
- BRS
Baroreflex sensitivity
- C-LIFE
Center-based lifestyle intervention
- CO
Cardiac output
- CVD
Cardiovascular disease
- CR
Cardiac rehabilitation
- DASH
Dietary Approaches to Stop Hypertension
- DBP
Diastolic blood pressure
- DDD
Daily Defined Dose
- EEG
Electrocardiogram
- GFR
Glomerular filtration rate
- hsCRP
High-sensitivity C-reactive protein
- HR
Hazard Ratio
- HRV
Heart rate variability
- IVST
Interventricular septal thickness
- ITT
Intent to Treat
- LVEDD
left ventricular end-diastolic diameter
- LVH
Left ventricular hypertrophy
- MAP
Mean arterial pressure
- PWT
Posterior wall thickness
- PWV
Pulse wave velocity
- QoL
Quality of life
- RCT
Randomized clinical trial
- RH
Resistant hypertension
- SBP
Systolic blood pressure
- SEPA
Standardized Education and Physician Advice
- STAI
State-Trait Anxiety Inventory
- SVR
Systemic vascular resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Clinical Trial Registry: NCT02342808
REFERENCES
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008 Jun;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011 Aug 30;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. Journal of the American College of Cardiology. 2008 Nov 25;52(22):1749–1757. doi: 10.1016/j.jacc.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012 Apr 3;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. American journal of hypertension. 2005;18(11):1422–1428. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 6.De Nicola L, Gabbai FB, Agarwal R, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. Journal of the American College of Cardiology. 2013;61(24):2461–2467. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 7.Bangalore S, Fayyad R, Laskey R, et al. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. The American journal of medicine. 2014;127(1):71–81. e71. doi: 10.1016/j.amjmed.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Irvin MR, Booth JN, 3rd, Shimbo D, et al. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. Journal of the American Society of Hypertension: JASH. 2014;8(6):405–413. doi: 10.1016/j.jash.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SM, Gong Y, Handberg E, et al. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. Journal of hypertension. 2014;32(3):635–643. doi: 10.1097/HJH.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. European heart journal. 2013;34(16):1204–1214. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 11.Vemulapalli S, Ard J, Bakris GL, et al. Proceedings from Duke resistant hypertension think tank. American heart journal. 2014;167(6):775–788. e771. doi: 10.1016/j.ahj.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA: the journal of the American Medical Association. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 13.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New England Journal of Medicine. 1997 Apr 17;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA: the journal of the American Medical Association. 2003 Apr 23-30;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 15.Harsha DW, Lin P-H, Obarzanek EVA, Karanja NM, Moore TJ, Caballero B. Dietary Approaches to Stop Hypertension: A Summary of Study Results. Journal of the American Dietetic Association. 1999;99(8, Supplement 1):S35–S39. doi: 10.1016/s0002-8223(99)00414-9. [DOI] [PubMed] [Google Scholar]
- 16.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. New England Journal of Medicine. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 17.Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. The American journal of cardiology. 2004 Jul 15;94(2):222–227. doi: 10.1016/j.amjcard.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 18.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA: the journal of the American Medical Association. 2003 Apr 23-30;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Archives of internal medicine. 2010 Jan 25;170(2):126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenthal JA, Babyak MA, Sherwood A, et al. Effects of the dietary approaches to stop hypertension diet alone and in combination with exercise and caloric restriction on insulin sensitivity and lipids. Hypertension. 2010 May;55(5):1199–1205. doi: 10.1161/HYPERTENSIONAHA.109.149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayward RA, Krumholz HM. Three Reasons to Abandon Low-Density Lipoprotein Targets: An Open Letter to the Adult Treatment Panel IV of the National Institutes of Health. Circulation. Cardiovascular quality and outcomes. 2012 Jan 1;5(1):2–5. doi: 10.1161/CIRCOUTCOMES.111.964676. [DOI] [PubMed] [Google Scholar]
- 22.Scheffers IJM, Kroon AA, Schmidli J, et al. Novel baroreflex activation therapy in resistant hypertension: Results of a European multi-center feasibility study. Journal of the American College of Cardiology. 2010 Oct 5;56(15):1254–1258. doi: 10.1016/j.jacc.2010.03.089. 2010. [DOI] [PubMed] [Google Scholar]
- 23.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009 Apr 11;373(9671):1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 24.Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. Journal of the American College of Cardiology. 2011 Aug 9;58(7):765–773. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Scheffers IJ, Kroon AA, Schmidli J, et al. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. Journal of the American College of Cardiology. 2010 Oct 5;56(15):1254–1258. doi: 10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 26.Schlaich M, Hering D. Central arteriovenous anastomosis in resistant hypertension? The Lancet. 2015 doi: 10.1016/S0140-6736(14)62290-X. [DOI] [PubMed] [Google Scholar]
- 27.Davis MI, Filion KB, Zhang D, et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2013;62(3):231–241. doi: 10.1016/j.jacc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Investigators SHTN Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010 Dec 4;376(9756):1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 29.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010 Dec 4;376(9756):1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 30.Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clinical cardiology. 2012 Sep;35(9):528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokkinos PF, Narayan P, Colleran JA, et al. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. New England Journal of Medicine. 1995 Nov 30;333(22):1462–1467. doi: 10.1056/NEJM199511303332204. [DOI] [PubMed] [Google Scholar]
- 32.Whelton PK, Appel LJ, Espeland MA, et al. Sodium Reduction and Weight Loss in the Treatment of Hypertension in Older Persons: A Randomized Controlled Trial of Nonpharmacologic Interventions in the Elderly (TONE) JAMA: the journal of the American Medical Association. 1998 Mar 18;279(11):839–846. doi: 10.1001/jama.279.11.839. 1998. [DOI] [PubMed] [Google Scholar]
- 33.Conlin PR, Erlinger TP, Bohannon A, et al. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. American journal of hypertension. 2003 May;16(5 Pt 1):337–342. doi: 10.1016/s0895-7061(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 34.Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. Journal of hypertension. 2005 Jun;23(6):1241–1249. doi: 10.1097/01.hjh.0000170388.61579.4f. [DOI] [PubMed] [Google Scholar]
- 35.Miller ER, 3rd, Erlinger TP, Young DR, et al. Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT) Hypertension. 2002 Nov;40(5):612–618. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- 36.Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012 Sep;60(3):653–658. doi: 10.1161/HYPERTENSIONAHA.112.197780. [DOI] [PubMed] [Google Scholar]
- 38.de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011 May;57(5):898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 39.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 40.Methodology. WCCfDS. Daily defined dose: definition and general considerations. 2012. [Google Scholar]
- 41.Sharman JE, Marwick TH, Gilroy D, et al. Randomized Trial of Guiding Hypertension Management Using Central Aortic Blood Pressure Compared With Best-Practice Care Principal Findings of the BP GUIDE Study. Hypertension. 2013 Dec;62(6):1138–1145. doi: 10.1161/HYPERTENSIONAHA.113.02001. [DOI] [PubMed] [Google Scholar]
- 42.McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA: the journal of the American Medical Association. 2014 Aug 27;312(8):799–808. doi: 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- 43.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008 May;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical care. 1986 Jan;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. The American journal of managed care. 2009 Jan;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 46.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Guilford Press; New York, NY: 1991. [Google Scholar]
- 47.Rollnick S, Mason P, Butler C. Health behavior change: A guide for practitioners. Churchhill Livingstone; New York NY: 1999. [Google Scholar]
- 48.Epstein DE, S A, Smith PJ, Craighead L, et al. Determinants and consequences of adherence to the Dietary Approaches to Stop Hypertension Diet in African-American and Whtie Adults with High Blood Pressure: Results from the ENCORE Trial. Journal of the Acadmey of Nutrition and Dietetics. 2012 doi: 10.1016/j.jand.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate. Annales Medicinae Experimentalis Et Biologiae Fenniae. 1957;35:307–315. [PubMed] [Google Scholar]
- 50.Blumenthal JA, Siegel WC, Appelbaum M. Failure of Exercise to Reduce Blood-Pressure in Patients with Mild Hypertension - Results of a Randomized Controlled Trial. Jama-J Am Med Assoc. 1991 Oct 16;266(15):2098–2104. [PubMed] [Google Scholar]
- 51.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obesity research. 2004 Dec;12(Suppl):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 52.Epstein LH, Wing RR, Koeske R, Valoski A. A Comparison of Lifestyle Exercise, Aerobic Exercise, and Calisthenics on Weight-Loss in Obese Children. Behav Ther. 1985;16(4):345–356. [Google Scholar]
- 53.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA: the journal of the American Medical Association. 2012 Aug 1;308(5):465–474. doi: 10.1001/jama.2012.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA: the journal of the American Medical Association. 2009 Apr 8;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardiosource . 2013 Prevention Guidelines ASCVD Risk Estimator. [Accessed February 20, 2014]. 2013. [Google Scholar]
- 56.Hinderliter AL, Sherwood A, Craighead LW, et al. The Long-Term Effects of Lifestyle Change on Blood Pressure: One-Year Follow-Up of the ENCORE Study. American journal of hypertension. 2013 Oct 1; doi: 10.1093/ajh/hpt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffman BM, Babyak MA, Craighead WE, et al. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosomatic medicine. 2011 Feb-Mar;73(2):127–133. doi: 10.1097/PSY.0b013e31820433a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babyak MA, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosomatic medicine. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 59.International Conference on Harmonisation: Statistical Principles in Clinical Trials (E9); 1997; http://www.ich.org/LOB/media/MEDIA485.pdf. [Google Scholar]
- 60.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B, Statistical Methodology. 1995(57):289–300. [Google Scholar]
- 61.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002 Mar;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tingley D, Yamamoto T, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. 2013 R package version 4.4. [Google Scholar]
- 63.Harrell FE. Regression Modeling Strategies: With applications to linear modeling, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 64.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12; [Google Scholar]
- 65.Wadden TA, Osei S. The treatment of obesity: An overview. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York,: Guilford Publications; New York: 2002. pp. 229–248. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.