Abstract
Increasing motivation can positively impact cognitive performance. Here we employed a cognitive timing task that allows us to detect changes in cognitive performance that are not influenced by general activity or arousal factors such as the speed or persistence of responding. This approach allowed us to manipulate motivation using three different methods; molecular/genetic, behavioral and pharmacological. Increased striatal D2Rs resulted in deficits in temporal discrimination. Switching off the transgene improved motivation in earlier studies, and here partially rescued the temporal discrimination deficit. To manipulate motivation behaviorally, we altered reward magnitude and found that increasing reward magnitude improved timing in control mice and partially rescued timing in the transgenic mice. Lastly, we manipulated motivation pharmacologically using a functionally selective 5-HT2C receptor ligand, SB242084, which we previously found to increase incentive motivation. SB242084 improved temporal discrimination in both control and transgenic mice. Thus, while there is a general intuitive belief that motivation can affect cognition, we here provide a direct demonstration that enhancing motivation, in a variety of ways, can be an effective strategy for enhancing temporal cognition. Understanding the interaction of motivation and cognition is of clinical significance since many psychiatric disorders are characterized by deficits in both domains.
Keywords: Time perception, 5-HT2C receptor, reward, Dopamine D2 receptor, Striatum
Introduction
Deficits in incentive motivation, the energizing of behavior in pursuit of a goal, occur in several psychiatric disorders including schizophrenia and some affective disorders. Such deficits in motivation significantly impact functional outcome (Barch, Treadway, & Schoen, 2014; Green, Hellemann, Horan, Lee, & Wynn, 2012) and in the case of schizophrenia, interact with cognitive deficits (Fervaha et al., 2014; Mann, Footer, Chung, Driscoll, & Barch, 2013). Therefore, developing methods to enhance motivation (Randall et al., 2015; Thomsen, 2015) could result in improvements in cognitive function and quality of life for psychiatric patients.
Here we examine the effect of motivation on temporal information processing. We chose this domain both because of its relevance to psychiatric disorders (Droit-Volet, 2013; Ward, Kellendonk, Kandel, & Balsam, 2012) as well as for what is known about the underlying neurobiology of temporal information processing and motivation. There is overlap between neural circuits involved in timing and those regulating motivation. Cortical-striatal circuits that are directly involved in encoding and use of temporal information (Buhusi & Meck, 2005; Rao, Mayer, & Harrington, 2001) overlap with those regulating motivation (Berridge & Kringelbach, 2013; Hart, Leung, & Balleine, 2014; Salamone & Correa, 2012). Dopaminergic modulation of these circuits affects both timing (Coull, Hwang, Leyton, & Dagher, 2012; Maricq, Roberts, & Church, 1981) and motivation (Berridge & Kringelbach, 2013; Salamone & Correa, 2012; Simpson, Waltz, Kellendonk, & Balsam, 2012). More specifically, dopamine D2 receptors (D2Rs) are important mediators of the dopaminergic modulation of timing (Drew, Fairhurst, Malapani, Horvitz, & Balsam, 2003; Maricq & Church, 1983; Meck, 1986) and it is well known that D2R also play an important role in motivation (Drew et al., 2007; Nowend, Arizzi, Carlson, & Salamone, 2001; Salamone & Correa, 2012; Simpson et al., 2011; Ward, Simpson, et al., 2012). There are also a few studies indicating that manipulation of reward can affect temporal information processing (Balcı, 2014; Galtress & Kirkpatrick, 2010; Ward, Avlar, & Balsam, 2015; Ward et al., 2009)
It is within this context that we explored the interaction of motivation and cognition. We manipulated motivation using three different methods; molecular/genetic, behavioral and pharmacological. We found that altering dopamine D2R expression, increasing the reward magnitude, and administering a motivationally enhancing drug all improved timing. Therefore, we here provide a concrete demonstration that enhancing motivation is an effective strategy for enhancing temporal cognition and might well be an effective strategy for enhancing other cognitive domains.
Experiment 1: D2R expression and timing
For the molecular manipulation we used a transgenic line of mice that we previously generated to model the increase in occupancy of D2Rs observed in patients with schizophrenia (Kellendonk et al., 2006). These mice selectively and reversibly overexpress D2Rs in post-synaptic medium spiny neurons in the striatum (D2R-OE mice). D2R-OE mice display cognitive motivational phenotypes similar to those observed in patients including deficits in timing (Drew et al., 2007; Ward et al., 2009) and motivation (Drew et al., 2007; Simpson et al., 2011). In D2R-OE mice this motivational deficit is rescued in adulthood by switching the transgene off (Drew et al., 2007; Ward, Simpson, et al., 2012). We previously reported an interval timing deficit in the D2R-OE mice using a peak interval procedure in which animals are reinforced for pressing after a specific target time has elapsed since the presentation of a cue (Drew et al., 2007) and that turning off the transgene resulted in a partial rescue of timing deficits. In a peak interval procedure, accuracy and precision of timing is evidenced by increased rate of responding close to the target duration and followed by a decline in rate after the expected time of reinforcement on long duration test trials in which no reward is presented (called peak trials). While D2R-OE mice displayed a reduced rate of responding and relatively flat response gradients, D2R-OE mice in which the transgene was switched off by treatment with doxycycline (D2R-OE-Dox mice) had higher response rates and timing accuracy, but only partially improved in timing precision (Drew et al., 2007). A limitation of the peak interval procedure, however is that the measure of timing depends on the animal's response rate. Consequently it is difficult to separate timing deficits from motivational factors. To overcome this problem in the current experiments, we used a temporal discrimination task, which only requires a single response on each trial and is thus independent of the subject's response vigor. In this task, referred to as a temporal bisection task, a subject is reinforced for successfully discriminating between two sample stimuli, which differ only in duration. In our experiments mice were presented with either a long (24s) or short (6s) tone, followed by the presentation of two choice levers. A single response on one of the two levers was rewarded conditional on the duration of the tone presented (e.g. after a short tone a left lever press was reinforced and after a long tone a right lever press was reinforced). Following this discrimination training, 5 logarithmically spaced intermediate sample durations (7.6-s, 9.5-s, 12-s, 15.1-s, and 19-s) were presented on half of the trials. As detailed in the methods section, psychometric functions of the animals' responses to these intermediate durations was used to determine accuracy (how close the animal is to normatively perceiving and responding to the exact duration of the cues) as well as precision (each subject's intrinsic variability in making temporal judgments). We tested D2R-OE and D2R-OE mice that were fed with doxycycline as well as control mice (half fed doxycycline, half not).
Methods and Materials
Subjects
A detailed description of the generation of the transgenic model can be found in previous publications (Kellendonk et al., 2006). Mice expressing the human D2 receptor under control of the tet operator (tetO_hD2R mice) were maintained on a congenic C57BL/6(J) background and mice expressing the tetracycline transactivator transgene under the calcium/calmodulin- dependent kinase IIα promoter (tTA-CaMKIIα mice) (Mayford et al., 1996) were maintained on 129SveV(Tac) congenic background. F1 animals obtained from intercrossing these two lines were used for all experiments. To specifically test the effect of transgenic D2R overexpression, double transgenic mice (D2R-OE) were compared to control mice that included single-transgenic and wild type littermates
Mice were genotyped at weaning by triplex polymerase chain reaction (PCR) using primers specific for tTA, tet-O and a fragment of an unrelated endogenous gene (Sim1), to provide a positive control for the PCR. All genotypes were re-confirmed using the same method after the termination of all experiments.
To regulate tet-O-driven gene expression, mice were fed doxycycline-supplemented chow (40 mg/kg; Mutual Pharmaceutical, Philadelphia, PA) beginning approximately 16-20 weeks of age. Two weeks after commencing doxycycline feeding, behavioral testing was started. Experimental protocols were approved by the Institutional Animal Care and Use Committees of both the New York State Psychiatric Institute and Columbia University. Mice were maintained and bred under standard conditions, consistent with NIH guidelines.
The following groups of female mice were used in the first experiment described: Double transgenic mice fed a regular diet (Isopro RMH 3000 complete mouse diet) that overexpress the D2R transgene (D2R-OE) (n = 12), double transgenic mice fed a doxycycline supplemented diet (doxycycline-supplemented chow), to switch off the transgene in adulthood, (D2R-OE-Dox) (n=13), control mice fed a regular diet (Control) (n=13) and control mice fed a doxycycline supplemented diet (Control-Dox) (n =13). Mice used in this study had restricted daily access to food (1 hour and 30 minutes) in order to motivate them to earn rewards during behavioral testing. Water was available ad libitum.
Apparatus
In the present experiments, eight matching experimental chambers (model env-307w; Med- Associates, St. Albans, VT) equipped with liquid dippers were used. Each chamber was located in a light- and sound-attenuating cabinet equipped with an exhaust fan. The internal dimensions of the experimental chamber were 22×18×13 cm, and the floor consisted of metal rods placed 0.87cm apart. A feeder trough was centered on one wall of the chamber. An infrared photocell detector 4 mm from the trough opening was used to record head entries. Raising the dipper located inside the feeder trough provided 0.01 cc of evaporated milk as a reward. Two retractable levers located 5 cm on either side of the feeder trough could be inserted into the chamber. A house light (model 1820; Med Associates) located at the top of the chamber provided illumination throughout all sessions. An audio speaker was positioned 8.5 cm above the floor on the wall opposite the feeder trough. This speaker delivered a brief tone (90 db, 2500 Hz, 250 ms) to signal that the liquid dipper was raised.
Operant lever press training
During dipper training, mice were trained to consume the liquid reward from the dipper. Initially, mice were placed inside the experimental chamber while the dipper was in the raised position. The dipper was retracted 10 s after a head entry into the feeder trough was detected. Subsequent dipper presentations were separated by variable duration intertrial intervals which averaged 45 s. The session ended after 30 min or 20 dipper presentations. On the following day, mice received another session similar to the first, except that the dipper remained up for 8 s and then lowered whether or not mice had made a head entry. Dipper training continued until a mouse made head entries at least 20 of 30 dipper presentations in a session. During all behavioral testing of this experiment, sessions occurred once per day, 7 d per week.
During bar press training, mice were required to press a lever to earn the liquid reward. For the first lever press training session, mice were put in the experimental chamber for 8 h. At the beginning of the session, both levers were extended into the chamber, and lever presses were reinforced on a continuous reinforcement schedule. In this and all subsequent sessions (except the reward magnitude manipulation phase), the reward consisted of raising the dipper for 5 s. After 20 reinforcements, the lever was retracted to familiarize mice with the retraction and extension of the lever. The lever was extended again following a variable delay averaging 45s and the cycle was repeated. Mice had to earn 100 reinforcements in a session. If they did not, the procedure was repeated the next day. Two days after the first successful lever press training session, mice received a session which began with the lever extended. The lever was retracted after every two reinforcements and then re-extended after an intertrial interval that averaged 45 s. The session ended when the mouse earned 40 reinforcements or 1 h elapsed. Mice continued receiving sessions like this until they earned 40 rewards in one session.
Temporal Discrimination Training
A detailed explanation of temporal discrimination training can be found in (Ward et al., 2009) (see temporal bisection procedure). Briefly, a tone was presented either for a short or long duration. Once the sample terminated, two levers were inserted into the chamber. A single response to one of the two levers was rewarded conditional on the duration of the preceding sample. For half of the mice, the left lever was designated as the correct following a 6-s (short) duration tone and the right lever was designated as the correct following a 24-s (long) duration tone. This rule was reversed for the remaining mice. Following lever training, 5 logarithmically spaced (7.6-s, 9.5-s, 12-s, 15.1-s, 19-s) intermediate sample durations were presented on half of the trials. Correct responses to the anchor durations were continued to be reinforced but responses on the trials of intermediate duration were never reinforced. Each daily session was consisted of 60 trials separated by the 45 s variable ITI. Mice earned a single reward for correct responses on both short (6 s) and long (24 s) sample trials. Each subject received 12 days of test sessions and the final 5 days were used in the data analysis for this part of the study.
Data Analysis
The basic datum of these experiments is the psychometric functions obtained by plotting the proportion of long lever choices as a function of sample duration. Multilevel binomial logistic regressions were conducted using the R (R Development Core Team) statistical software with the lme4 mixed model package to obtain fits of the psychometric data.
The point of subjective equality (PSE) is the duration that subjects are equally likely to classify as long or short. Past research has shown that the PSE for time is usually at the geometric mean of the anchor durations (Church & Deluty, 1977; Wearden & Ferrara, 1995). Consequently, for all experiments, the regressions were conducted on the logarithms of sample durations centered with respect to the logarithm of the geometric mean of the anchor durations. The goodness of fit was determined by comparing the change in the deviance from the null model (intercept only) to the full model (with all fixed effects) (Nelder & Wedderburn, 1972). The Bayesian Information Criterion was used to take model complexity into account when comparing models (Schwartz, 1978). The Bayesian Information Criterion values and residual deviance were significantly lower in the full model than in the null model for all of the experiments (p<.000) in the present study, therefore the full model was utilized.
The multilevel logistic model for estimating the probability of choosing the long lever, P(long) is based on the standard logistic function that relates a sample duration X to P(long) and is given by
| (1) |
where the beta coefficients, β0 and β1 are the constant and slope parameters of the model, and here represent indexes of the accuracy and the precision of timing, respectively. The point of subjective equality (PSE) can be obtained from these two parameters (-β0 / β1).
These coefficients derive from model estimates at each level of the multilevel analysis. In different experiments the model estimates represent the fixed effects of different experimental groups, reward magnitudes, and drug manipulations. We specified the model to allow slope and intercept to vary randomly by individual mouse (Bolger & Laurenceau, 2013). For each experiment, fixed effects estimates are calculated by
| (2) |
| (3) |
| (4) |
| (5) |
Where F denotes each of the fixed effects used in the three experiments, the index j represents an individual mouse, the index i stands for trials, and Xij represents log durations for each trial and individual mouse. Finally, u0j and u1j represent the error terms for the constant and the slope parameters in the model.
Estimates of γ0 and γ1 in the model correspond to the unit change (in log-odds units) for different fixed effects on the constant and the slope, respectively. β0 and β1 were estimated by summing the contribution of each fixed effect to produce the composite constant (β0) and slope parameters (β1) for each group. Negative and positive values of the β0 indicate response bias for short and long lever choices at the geometric mean of the sample durations. Because previous research has shown that normal timing accuracy is characterized by PSE's at the geometric mean (Church & Deluty, 1977; Wearden & Ferrara, 1995), a β0 value that is closer to zero indicates better timing accuracy. A flatter psychometric function is indicative of a lack of discrimination between different durations. Consequently, higher values of the β1 (slope) corresponds to more precise temporal discriminations, referred to in this study as greater precision.
In the first experiment normalizing D2R expression in D2R-OE mice, relationships of the predictor variables; group (Control, Control-Dox, D2R-OE, and D2R-OE-Dox) and sample duration, to the probability of choosing the long lever were assessed. We analyzed trial-by-trial choices for each animal over the last five days of training. Control and Control-Dox groups were not different in any of the dependent measures (p>.05), so they were pooled into a single control group.
For the other manipulations, predictor variables of reward magnitude, drug and sample duration were analyzed to model the change in the probability of choosing the long lever. In both these experiments, we centered the genotype variable to zero to be able to observe the main effects of the manipulations. If an interaction with genotype was found in this initial analysis, we performed separate post-hoc analyses for each genotype.
Results and Discussion
Normalizing D2 Receptor Overexpression Improves Temporal Discrimination
D2R-OE mice had flatter timing functions than control mice, generally performing at chance when cue durations were longer than the geometric mean of the anchor durations (Figure 1A). D2R-OE-Dox displayed a sharper temporal discrimination compared to D2R-OE mice but did not completely recover the level of discrimination observed in the control mice (Figure 1A). To characterize the timing performance of individual mice in more detail, we fit a multilevel binomial logistic function to the data. Unit changes (Δ) in estimates of slope (γ1) and constant (γ0) coefficients from Control (baseline) values to D2R-OE and D2R-OE-Dox values (in log-odds) are given in Table 1 and model fits for each group are presented in Figure 1B. There was no significant difference between groups in timing accuracy (γ0 estimates) but the timing precision was significantly altered: the slope parameter (γ1) of the logistic function was -0.76 log-odds units (p<.01) lower in D2R-OE group in comparison to Control group. There was no significant difference between the Control and the D2R-OE-Dox mice (-0.40, p>.05), suggesting that switching off the transgene with doxycycline reduced or eliminated the source of impairment.
Figure 1.
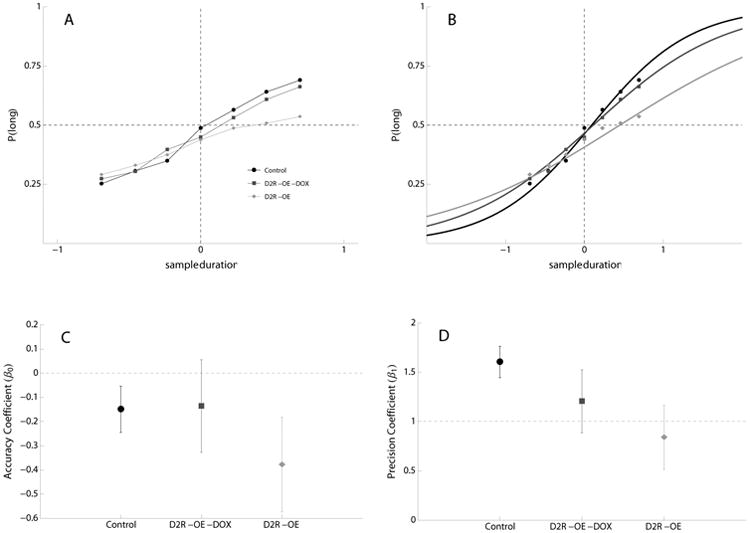
Performance of Control, D2R-OE and D2R-OE-Dox Mice in the Temporal Bisection Task.
A) Mean proportion of responses to the lever associated with the ‘long’ sample duration as a function of sample duration (seconds) in control mice (black circles and lines), D2R-OE mice (light gray diamonds and lines) and D2R-OE mice fed with doxycycline, D2R-OE-DOX (dark gray squares lines). B) Data fit by binomial logistic regression is superimposed with the mean proportion of long responses per sample duration for Control (black circles and lines), D2R-OE (light gray diamonds and lines) and D2R-OE-DOX (dark gray squares and lines) mice. C and D) Model coefficients for each group after addition of fixed effect estimates: C) the accuracy coefficient (β0±SE), D) the slope coefficient (β1±SE).
Table 1. Characterization of Performance on the Temporal Bisection Task using Multilevel Logistic Regression.
Δγ-estimates, standard error (S.E.), z-values and p-values for fixed effects of groups. Δγ-estimate indicates the unit change for either the constant coefficient (γ0) or the slope coefficient (γ1) in log-odds units. The model specifies the control group as the baseline condition and values for D2R-OE and D2R-OE-Dox correspond to the contribution of those group variables to the baseline γ-coefficients. For details of the model see the data analysis section in the Methods. n.s. = not significant.
| Coefficient | Group | Δ γ-estimate | S.E. | Z-value | p-value |
|---|---|---|---|---|---|
| Constant (γ0) (Timing Accuracy) | D2R-OE | -0.23 | 0.17 | -1.34 | ns. |
| D2R-OE -Dox | 0.01 | 0.16 | -0.08 | ns. | |
|
| |||||
| Slope (γ1) (Timing Precision) | D2R-OE | -0.76 | 0.28 | -2.7 | < 0.01 |
| D2R-OE -Dox | -0.40 | 0.27 | -1.45 | n.s. | |
In a separate analysis comparing D2R-OE and D2R-OE-Dox groups, the timing precision was not significantly different between these two groups either (γ1 = 0.37, SE=0.32, z-value=1.13, p>.05). Figure 1C shows the composite accuracy and precision coefficients for all groups.
Overall, the logistic regression indicates that normalizing D2R expression levels rescues timing accuracy and rescues precision at least partially, results similar to those we obtained in the peak interval procedure (Drew et al., 2007). However here, because of the alternate testing method, we are confident that the rescue in cognitive performance was not simply due to an increase in the animal's speed or vigor of responding.
Experiment 2: Reward magnitude and timing
One factor that might have mediated the improvement in performance in D2R-OE-Dox mice in experiment 1 is the increase in motivation that accompanies the normalization of striatal D2Rs when transgenic mice are treated with doxycycline (Simpson et al., 2011). Though motivation and timing were initially considered independent of one another (Roberts, 1981) more recent studies have found that motivational factors may alter timing precision and/or accuracy (Galtress & Kirkpatrick, 2010; Ward et al., 2009; Ward, Simpson, et al., 2012). For example, in a temporal bisection task similar to the one we employ here, Galtress & Kirkpatrick (Galtress & Kirkpatrick, 2010) increased the magnitude of reward either for short or long durations and found that this manipulation changed the slope of the psychophysical function and PSE values. In experiment 1, we found that the D2R-OE mice had a specific deficit in accurately classifying long durations. To see whether an explicit manipulation of motivation would also sharpen temporal control of behavior in D2R-OE mice, we increased the reward magnitude associated with the long response. In the first phase of training, D2R-OE and Control mice earned a single reward for all correct responses. In phase 2, we doubled the reward magnitude for correct long responses and in the final phase of the experiment all subjects received a single reward for correct responses to both durations.
Method
Subjects
10 D2R-OE and 11 Control subjects from experiment 1 were subjects in experiment 2.
Procedure
In the first phase (low reward) of the reward magnitude manipulation, subjects earned a single reward for correct responses on both short (6 s) and long (24 s) sample trials for 12 daily test sessions. In phase 2 (high reward) we doubled the reward magnitude on long trials and tested mice for an additional 12 sessions. During these sessions when a reward was earned the dipper was raised for 5s and then lowered back into the liquid well and an additional milk reward was immediately presented by raising the dipper for another 5s. In the third phase (low reward), which lasted for 9 sessions, mice again received a single dipper presentation for correct responses to both the 6 and 24-s samples. All other aspects of the procedure were the same as in experiment 1. Again, data from the final five sessions of each phase were analyzed.
Results and Discussion
Increasing Reward Magnitude Improved Timing Precision in both Groups and Accuracy in Control Mice
In phase 2, we doubled the reward magnitude for correct long responses and the temporal discrimination sharpened in both groups (Figure 2A) relative to performance in the first phase when only one pellet could be earned for correct responses. The precision of timing was improved in both D2R-OE and Control subjects. Table 2 shows the unit changes (Δ) in estimates of slope (γ1) and constant (γ0) coefficients from low reward to high reward magnitude (in logodds) and model fits for each group are presented in Figure 2B. There was a significant main effect of increasing the reward magnitude on the slope of the logistic curve (p<.05). There was no main effect of increasing the reward magnitude on the timing accuracy; however, the interaction between reward magnitude and genotype for this parameter of the logistic regression was significant (p<.01; Table 2 and Figure 2B). In two separate, within subject, post-hoc analyses, increasing reward magnitude was found to improve timing accuracy in Control mice, albeit a modest but consistent shift in accuracy (γ0 = 0.20, SE=0.05, z-value= 3.6, p<.001); accuracy of D2R-OE was not improved (γ0 = -0.06, SE=0.06, z-value= -1.05, p>.05; Figure 2C). In summary, increasing the reward magnitude improved the timing precision in both Control and D2R-OE mice, but accuracy of timing was only improved in the Control mice.
Figure 2.
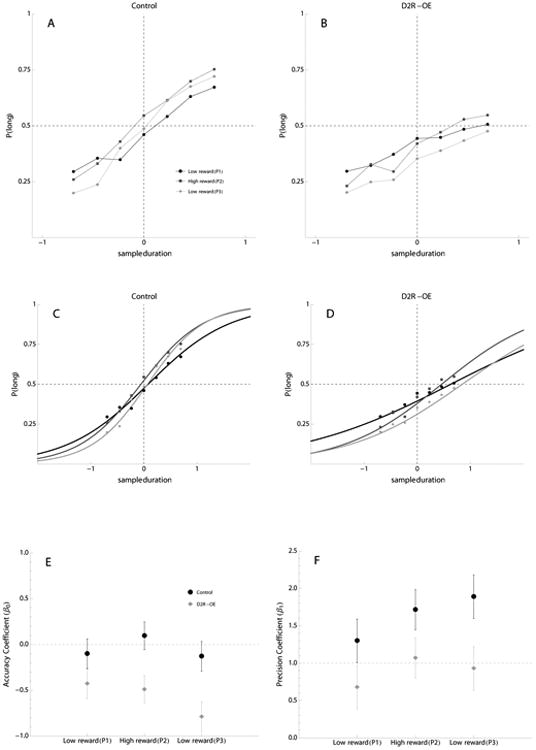
Performance of Control and D2R-OE Mice in the Temporal Bisection Task with Changes in Reward Magnitude.
The top row of graphs show mean proportion of responses to the lever associated with the ‘long’ sample duration as a function of sample duration (seconds) in 3 phases (phase1-low reward = black circles and lines, phase2-high reward = dark gray squares and lines, and phase3-low reward = light gray diamonds and lines). A) Control mice, B) D2R-OE mice. The middle row of graphs show data fit by binomial logistic regression superimposed with the mean proportion of long responses per sample duration for C) Control mice and D) D2R-OE mice in each phase (Same phase symbols as A and B). The lower row of graphs show model coefficients for each group after addition of fixed effect estimates: E) the accuracy coefficient (β0±SE), F) the slope coefficient (β1±SE). Control = black circles, D2R-OE= gray diamonds. Note that SE is based on the between subject variability, the statistical results in the text are based on a within subject analysis.
Table 2. Characterization of the Effect of Changing Reward Magnitude in the Temporal Bisection Task using Multilevel Logistic Regression.
Δγ-estimates, standard error (S.E.), z-values and p-values for fixed effects of reward magnitude and the interaction between genotype and reward magnitude. Low Reward → High Reward indicates the shift from phase 1 to phase 2, High Reward → Low Reward indicates the shift from phase 2 to phase 3. The genotype variable is centered at zero. Δγ-estimate indicates the unit change for either the constant coefficient (γ0) or the slope coefficient (γ1) in log-odds units. n.s. = not significant.
| Coefficient | Reward Change | Δ γ-estimate | S.E. | Z-value | p-value |
|---|---|---|---|---|---|
| Constant (γ0) (Timing Accuracy) | Low Reward→ High Reward | 0.06 | 0.04 | 1.64 | n.s. |
| Genotype × Low → High | -0.26 | 0.08 | -3.2 | <0.01 | |
|
| |||||
| High Reward→ Low Reward | -0.26 | 0.04 | -6.30 | <0.001 | |
| Genotype × High → Low | -0.07 | 0.08 | -0.89 | n.s. | |
|
| |||||
| Slope (γ1) (Timing Precision) | Low Reward→ High Reward | 0.40 | 0.08 | 5.24 | <0.001 |
| Genotype × Low → High | 0.02 | 0.16 | 0.15 | n.s. | |
|
| |||||
| High Reward→ Low Reward | 0.02 | 0.08 | 0.21 | n.s. | |
| Genotype × High → Low | -0.31 | 0.16 | -1.98 | <0.05 | |
A Downshift in Reward Magnitude Reduced Timing Accuracy in both Groups and Reduced Timing Precision in D2R-OE mice
To further understand the effect of altering the reward value on timing performance, mice were returned to the lower and equal payoffs for each anchor cue in the final phase of this experiment. Multilevel logistic regression contrasting phase 2 and phase 3 (see table 2) indicated a significant main effect of returning to the lower reward condition on timing accuracy (p<.01), without any significant interaction between genotype and phase on the accuracy parameter (p>.05) (Figure 2A). In the analysis of timing precision, there was no main effect of repeating the lower reward condition on the slope parameter (p>.05) but, there was a significant genotype × phase interaction (p<.05). Post-hoc analyses indicated that the source of the interaction was that decreasing reward magnitude produced a trend toward decreasing timing precision in D2R-OE (γ0 = -0.14, SE=0.12, z-value= -1.20, p>.05; Figure 2C) and an increasing trend in timing precision for Control mice (γ0 = 0.17, SE=0.05, z-value= 1.62, p>.05). The slope of the D2R-OE group was significantly lower than the Control group (γ1 =-.31, SE=0.16, z-value=-1.98, p<.05) following the reward downshift. The results show that the downshift in reward magnitude during phase 3 lowered the timing accuracy and that the downshift resulted in a significantly lower timing precision in the D2R-OE group relative to the controls.
In summary, in Control mice increasing reward magnitude for the long duration anchor stimulus increased accuracy, and decreasing reward magnitude (returning to equal payoffs) decreased accuracy, demonstrating that cognitive performance can be bi-directionally affected by the value of the rewards available. Furthermore, in control mice precision of timing increased with each phase. We cannot rule out the possibility that the latter result is produced by the continued training of the mice. However, in other studies (Ward et al., 2009) and in experiment 1 of this paper we did not observe continued increases in precision beyond about a week of training on this task. In contrast, the timing accuracy of D2R-OE mice was not improved when the reward magnitude was increased, yet it still worsened after the reward magnitude downshift in phase 3. This is suggestive that overexpression of the D2R may create an asymmetry in motivation: lower sensitivity to positive changes in reward value than to negative shifts in reward value.
Experiment 3: Impact of a selective serotonin 2C receptor ligand on timing
Increasing the payoff selectively for correct responses to the long anchor duration generally improved timing in control mice but in D2R-OE mice timing accuracy did not improve. In the current experiment we used a pharmacological manipulation to produce a more global change in motivation. Previously, Simpson et al. (2011) found that administration of SB242084, a functionally selective ligand at the 5-HT2C receptor, increased the motivation to work for rewards. Additionally, the drug alleviated the motivational deficits of D2R-OE mice. Specifically, a dose of 0.75 mg/kg was sufficient to restore the D2R-OE mice's willingness to work to earn rewards to the level of control subjects when tested on a progressive ratio schedule in which the number of responses required to earn each successive was doubled. The purpose of experiment 3 was to determine whether SB242084 treatment would also alleviate the timing deficits observed in D2R-OE mice.
Method
Subjects
All mice from experiment 2 were used as subjects in experiment 3.
Procedure
All features of the procedure were identical to experiment 1 except that, four days of saline were followed by four days of drug injections. The 5-HT2C selective ligand SB24280 (Sigma Aldrich) was dissolved in 0.9% saline and injected, intraperitoneally 20 minutes before behavioral testing, as our previous studies demonstrated that this dose and timing was effective in alleviating the motivational deficit (Simpson et al., 2011). Data from all days of drug and saline injections were included in the analysis.
Results and Discussion
Interval Timing Performance is Improved by Modulation of the 5-HT2C Receptor in both Groups
Administration of SB 242084 resulted in improvement of temporal discrimination performance in both Control and D2R-OE mice. (See Table 3; Figure 3A). There was a main effect of the drug on both timing accuracy and precision. For accuracy, there was also a significant interaction between drug and genotype. Separate post-hoc analyses showed that the drug improved accuracy in both Control (γ0 =0.53, SE=0.06, z-value=8.14, p<.001) and D2R-OE groups (γ0 =0.23, SE=0.07, z-value=3.25, p<.01). Composite accuracy and precision coefficients for all groups in saline and drug phases are shown in Figure 3C. Overall, SB242084 improved the timing accuracy and precision in both groups showing that a global increase in motivation can produce quite general improvements in cognition.
Table 3. Characterization of the Effect of SB242084 on Performance in the Temporal Bisection Task using Multilevel Logistic Regression.
Δγ-estimates, standard error (S.E.), z values and p-values for fixed effects of drug condition and the interaction between genotype and drug condition. Δγ-estimates indicate unit change for either the constant coefficient (γ0) or the slope coefficient (γ1) in log-odds units. n.s. = not significant.
| Coefficient | Treatment | Δ γ-estimate | S.E. | Z-value | p-value |
|---|---|---|---|---|---|
| Constant (γ0) (Timing Accuracy) | Saline - Drug | 0.38 | 0.05 | 7.89 | <0.001 |
| Genotype × Saline-Drug | -0.30 | 0.10 | -3.1 | <0.01 | |
|
| |||||
| Slope (γ1) (Timing Precision) | Saline - Drug | 0.22 | 0.09 | 2.43 | <0.05 |
| Genotype × Saline-Drug | 0.27 | 0.18 | 1.50 | n.s. | |
Figure 3.
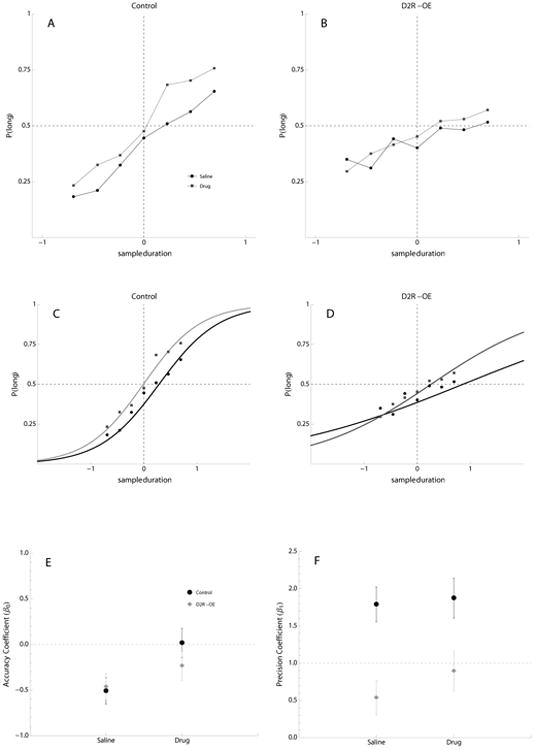
The Effect of SB242084 on the Performance of Control and D2R-OE Mice in the Temporal Bisection Task.
The top row of graphs show mean proportion of responses to the lever associated with the ‘long’ sample duration as a function of sample duration (seconds) in saline (black circles and lines) and drug (gray squares and lines) phases for A) control mice and B) D2R-OE mice. The middle row of graphs show data fit by binomial logistic regression superimposed with the mean proportion of long responses per log sample duration for C) Control mice and D) D2R-OE mice in each phase (saline = black circles and lines, drug = gray squares and lines). The lower row of graphs show model coefficients for each group after addition of fixed effect estimates: E) the accuracy coefficient (β0±SE), F) the slope coefficient (β1±SE). Control = black circles, D2R-OE = gray diamonds, Note that SE is based on the between subject variability.
General Discussion
We demonstrated that increasing motivation can enhance cognitive performance using several different methods of altering motivation. We show that in Control mice, accuracy and precision of timing were modulated by reward magnitude as well as by a global increase in motivation produced by a 5-HT2C selective ligand (SB242084) previously shown to increase motivation (Simpson et al., 2011). Additionally, we showed that mice that overexpress D2 receptors in the striatum have a deficit in temporal discrimination in addition to a deficit in incentive motivation (Simpson et al., 2011). In these mice, we found that cognitive performance could be improved by using three methods to increase motivation. First, we normalized receptor levels by switching off the transgene with doxycycline and produced a partial rescue of the timing deficit. As in our previous study that employed a different assay of timing behavior (Drew et al., 2007), normalizing the D2R overexpression, resulted in a recovery of accurapcy and partial rescue of precision of timing. To explore the effect of reward magnitude, we altered the levels of motivation by first increasing the reward magnitude selectively for the longer anchor cue duration. This improved timing precision but not accuracy in D2R-OE mice. Finally, we used the 5-HT2C selective ligand to increase motivation and found that this drug improved both the precision and the accuracy of timing. Taken together, these results demonstrate that increasing motivation can improve the discrimination and use of time to guide actions.
Because our study involved modulation of motivation by 3 different methods, and our temporal cognition assay allowed us to quantify two different performance metrics (precision and accuracy), our results allow us investigate how different components of motivation may impact specific aspects of timing performance.
Good temporal discrimination depends on both attention to and perception of time, updating of working memory as time elapses, long-term recall of which particular actions are rewarded after different amounts of time and the proper selection of the rewarded actions (Ward et al., 2015; Ward, Kellendonk, et al., 2012). Consideration of what we have previously learned about D2R-OE mice gives us some clues as to which processes might have been modulated by motivation in the current studies. We previously found no deficit in sustained attention in D2R-OE mice, as well as intact maintenance of working memory stimuli (Ward, Winiger, Higa, Kahn, Kandel, Balsam, and Simpson, in press). D2R-OE mice also show normal accuracy as well as normal precision in timing on a temporal bisection task with shorter anchor durations (2s and 8s) than the ones we used here (6s and 24s) (Ward et al., 2009). Thus, the deficit in D2R-OE mice does not appear to be the result of impaired attention, maintenance of working memory or timing perception, per se. Good performance with short duration cues also indicates that the D2R-OE mice have intact long-term memory about which actions are rewarded after different amounts of time. Thus we are left with the hypothesis that the deficit involves the updating of working memory with new information. Either a compromised facility with updating memories or a lowered working memory capacity might limit the ability of D2R-OE mice to accurately accumulate information about elapsing time during longer intervals. Patients with schizophrenia also show intact maintenance of working memory stimuli (Gold et al., 2010) and instead have deficits in the aspects of working memory that involve the updating and manipulation of information (Kim, Glahn, Nuechterlein, & Cannon, 2004) as well as in the capacity of working memory (Gold et al., 2010). Consequently, our results suggest that these alterations in cognitive function may arise from altered D2R signaling found in many patients (Kuepper, Skinbjerg, & Abi-Dargham, 2012) and may, at least in part, be caused by the impact of altered D2R signaling on motivation. Consequently, though we have only demonstrated an effect of motivation on temporal information processing here, our results suggest that motivation may more generally modulate the updating and/or capacity of working memory and imply that enhancing motivation to use this specific aspect of cognitive function might be of considerable value to patients.
Just as temporal discrimination involves multiple component processes (attention, perception, working memory etc.); motivation is a similarly complex construct involving several different elements. For a subject to be motivated toward a particular goal, they must like the goal (it must elicit a positive hedonic reaction), want the goal (be willing to work for it), be able to represent the hedonic value of future rewards and they must also understand the relationship between the effort required and the value of the possible outcome. Disruption to any of these processes may result in a deficit in motivation. In the specific case of the D2R-OE mice, our previous work indicates that overexpression of D2Rs in the striatum results in a deficit in representing the value of positive outcomes as well as a deficit in assessing the tradeoff between the costs and benefits of their actions (Simpson et al., 2011; Ward, Simpson, et al., 2012). When we increased reward magnitude in the current study, Control mice demonstrated an improvement in both accuracy and precision in the temporal discrimination assay, which may reflect an increase in both attention as well as working memory. In contrast, D2R-OE mice showed no improvement in accuracy when the reward magnitude was increased, which may reveal another interesting aspect of how reward processing may be changed by D2R overexpression. As in the previous study (Ward, Simpson, et al., 2012), the D2R-OE mice were less sensitive to an increase in reward value. However, D2R-OE mice were not less sensitive to a decrease in reward value because when the magnitude of reward was shifted back to the smaller reward, the accuracy of timing in D2R-OE mice declined, as it did for controls.
The pattern of a greater sensitivity to a reduction or loss in reward value and a lowered sensitivity to increases in reward value of the D2R-OE mice is similar to the deficits in reward processing that has been described in patients (Gold et al., 2012; Waltz, Frank, Robinson, & Gold, 2007; Waltz, Frank, Wiecki, & Gold, 2011). As previously suggested (Simpson et al., 2012), this asymmetry may arise from a general deficit in the capacity to represent the value of future positive rewards in D2R-OE mice (Ward, Simpson, et al., 2012) and in patients with schizophrenia (Barch & Dowd, 2010; Strauss, Wilbur, Warren, August, & Gold, 2011; Ziauddeen & Murray, 2010). The results of the current study along with the similar characterization of reward processing deficits in patients suggests that the 5-HT2C receptor might be a novel therapeutic target to consider for meliorating specific deficits in processing positive rewards.
Moreover, our study suggests that modulation of the 5-HT2C receptor may be a useful therapeutic target for generally improving both motivation and cognition. Our earlier work demonstrates that SB242084 increases motivation as reflected in the willingness to expend effort to obtain rewards (Simpson et al., 2011). Our current results provide the first demonstration that 5-HT2C receptor modulation increases both timing precision and accuracy. Previously, modulation of the 5-HT2C receptor with SB242084 has been found to improve cognitive function in a rodent assay of cognitive flexibility when delivered systemically, (Boulougouris, Glennon, & Robbins, 2008) or selectively to the orbitofrontal cortex (Boulougouris & Robbins, 2010). SB242084 has also been found to have antidepressant related effects in rodent models with a faster onset than traditional serotonergic antidepressant drugs (Opal et al., 2013). These studies suggest that targeting the 5-HT2C receptor may have very general effects on motivation and cognition and thus might be efficacious for a number of psychiatric disorders. It is therefore important to consider the mechanisms by which SB242084 enhances motivated behavior and cognition.
SB242084 is one of several compounds known to display functional selectivity at the 5-HT2C receptor, having a mixed effect of signal transduction pathways downstream of the receptor. Specifically, in vitro studies have determined that SB242084 acts as an inverse agonist for PLA2 and Gαi, and as an agonist for PLC (De Deurwaerdere, Navailles, Berg, Clarke, & Spampinato, 2004). It is unclear how the drug impacts these signaling pathways in vivo. However, several studies suggest that this compound exerts its behavioral effect through an interaction with the mesolimbic dopamine system. In the anesthetized rat, systemic injection of SB242084 at 0.64 - 1.0mg/kg (i.e. similar to the dose used in our studies) increases the rate of firing of VTA DA neurons (Di Matteo, Di Giovanni, Di Mascio, & Esposito, 1999) and also augments pharmacologically stimulated dopamine release (Hutson et al., 2000; Navailles, De Deurwaerdere, Porras, & Spampinato, 2004). The enhanced increase in extracellular dopamine (DAex) was observed in the nucleus accumbens (NAc), an area of the mesolimbic DA projection from the VTA that is involved in the dopaminergic regulation of motivated behaviors. Thus at least some of the behavioral impact of 5-HT2C receptor modulation may be mediated by DA in the NAc.
It seems plausible that altered dopamine signaling would be central to the general interaction of motivation and cognition. First, dopamine plays a pivotal role in reward processing (Liu, Hairston, Schrier, & Fan, 2011; Markou et al., 2013; Salamone & Correa, 2012) through a network that computes a cost and benefit calculations when expected reward magnitude and effort are manipulated (Croxson, Walton, O'Reilly, Behrens, & Rushworth, 2009). Second, altering dopamine related circuitry and baseline DA levels in frontal cortices and striatum is a determinant of cognitive processes such as working memory (Abi-Dargham et al., 2002), attention (Boulougouris & Tsaltas, 2008; Nieoullon, 2002) and timing (Coull, Cheng, & Meck, 2011). Interestingly this relation is bidirectional. Not only does cognition depend on dopamine signaling but cognitive training also alters dopamine function. In fact, working memory training for 5 weeks altered the D1R binding potential in prefrontal and parietal cortices (McNab et al., 2009) and D2R binding potential in the striatum (Backman et al., 2011).
One limitation of the current work arises from our sole use of female subjects. We did this because our prior work on motivation was also done with females (Drew et al, 2007; Simpson et al., 2011). It is well known that there are many differences in both serotonin (Rubinow, Schmidt, & Roca, 1998) and dopamine (Becker, 1999; Munro et al., 2006) systems in males and females. Consequently, it will be important to test the generality of our findings in males.
In conclusion, cognition and motivation are inseparable mental operations that are required for everyday functioning. We demonstrate here that the accuracy and precision of timing is altered by changes in motivation. These alterations in timing likely depend on the impact of motivation on working memory updating and/or capacity. Since timing and working memory are such an integral part of so many functional behaviors, an altered interaction between motivation and these processes in patients may contribute significantly to functional deficits in a very broad range of activities including academic pursuits, maintaining a job and relationship, and even in the basic activities of daily living. More generally, our results suggest that patients have two difficulties to overcome to improve their daily functioning; motivational and cognitive impairments. Whatever deficits exist in cognition may be exacerbated by a low level of motivation and conversely, whatever deficits exist in motivation may be aggravated by cognitive deficits. Our experiments demonstrate that altering motivation can improve temporal cognition. Similarly, it has been found that increasing intrinsic motivation in patients enhances arithmetic skills (Choi & Medalia, 2010). Therefore, using behavioral and pharmacological strategies to increase motivation in patients may not only improve motivationally relevant symptoms but may also significantly improve cognitive functions.
Acknowledgments
This work was supported by NIH grants R01MH068073 (PDB) and P50MH086404 (EHS and ERK), the Lieber Institute for Brain Development (EHS and ERK), and the Howard Hughes Medical Institute (ERK).
We are grateful to Iram Haq for maintaining and genotyping the transgenic mouse colony.
Abbreviations
- D2R Dopamine
D2 receptor
- D2R-OE mice
Dopamine D2 receptor over-expressing mice
- PSE
Point of Subjective Equality
- SB242084 (Chemical Name)
6-Chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride
Footnotes
Conflict of interests: The authors declare no competing financial interests.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. Journal of Neuroscience. 2002;22(9):3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, et al. Rinne JO. Effects of working-memory training on striatal dopamine release. Science. 2011;333(6043):718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- Balcı F. Interval Timing, Dopamine, and Motivation. Timing & Time Perception. 2014;2(3):379–410. doi: 10.1163/22134468-00002035. [DOI] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123(2):387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.Review. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23(3):294–303. doi: 10.1016/j.conb.2013.01.017. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Laurenceau JP. Modelleing categorical outcomes. In: Bolger N, Laurenceau JP, editors. Intensive Longitudinal Methods: An Introduction to Diary and Experience Sampling Research. New York: Guilford Press; 2013. pp. 105–1025. [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33(8):2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30(3):930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Choi J, Medalia A. Intrinsic motivation and learning in a schizophrenia spectrum sample. Schizophr Res. 2010;118(1-3):12–19. doi: 10.1016/j.schres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. J Exp Psychol Anim Behav Process. 1977;3(3):216–228. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36(1):3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Hwang HJ, Leyton M, Dagher A. Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J Neurosci. 2012;32(47):16704–16715. doi: 10.1523/JNEUROSCI.1258-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain. J Neurosci. 2009;29(14):4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24(13):3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38(8):1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav. 2003;75(1):9–15. doi: 10.1016/s0091-3057(03)00036-4. Research Support, U.S. Gov't, P.H.S. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S. Time perception, emotions and mood disorders. Journal of Physiology-Paris. 2013;107(4):255–264. doi: 10.1016/j.jphysparis.2013.03.005. http://dx.doi.org/10.1016/j.jphysparis.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014;71(9):1058–1065. doi: 10.1001/jamapsychiatry.2014.1105. [DOI] [PubMed] [Google Scholar]
- Galtress T, Kirkpatrick K. The role of the nucleus accumbens core in impulsive choice, timing, and reward processing. Behav Neurosci. 2010;124(1):26–43. doi: 10.1037/a0018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67(6):570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, et al. Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69(12):1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: The distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol Learn Mem. 2014;108(0):104–118. doi: 10.1016/j.nlm.2013.11.003. http://dx.doi.org/10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson PH, Barton CL, Jay M, Blurton P, Burkamp F, Clarkson R, Bristow LJ. Activation of mesolimbic dopamine function by phencyclidine is enhanced by 5-HT(2C/2B) receptor antagonists: neurochemical and behavioural studies. Neuropharmacology. 2000;39(12):2318–2328. doi: 10.1016/s0028-3908(00)00089-7. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49(4):603–615. doi: 10.1016/j.neuron.2006.01.023. see comment. [DOI] [PubMed] [Google Scholar]
- Kim J, Glahn DC, Nuechterlein KH, Cannon TD. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res. 2004;68(2-3)(03):173–187. 00150–6. doi: 10.1016/S0920-9964. [DOI] [PubMed] [Google Scholar]
- Kuepper R, Skinbjerg M, Abi-Dargham A. The dopamine dysfunction in schizophrenia revisited: new insights into topography and course. Handb Exp Pharmacol. 2012;(212):1–26. doi: 10.1007/978-3-642-25761-2_1. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CL, Footer O, Chung YS, Driscoll LL, Barch DM. Spared and impaired aspects of motivated cognitive control in schizophrenia. J Abnorm Psychol. 2013;122(3):745–755. doi: 10.1037/a0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology (Berl) 1983;79(1):10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Roberts S, Church RM. Methamphetamine and time estimation. J Exp Psychol Anim Behav Process. 1981;7(1):18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- Markou A, Salamone JD, Bussey TJ, Mar AC, Brunner D, Gilmour G, Balsam P. Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci Biobehav Rev. 2013;37(9 Pt B):2149–2165. doi: 10.1016/j.neubiorev.2013.08.007. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol Biochem Behav. 1986;25(6):1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–974. doi: 10.1016/j.biopsych.2006.01.008. Controlled Clinical Trial Research Support, N.I.H., Extramural. [DOI] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdere P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29(2):319–326. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- Nelder JA, Wedderburn RWM. Generalized Linear Models. Journal of the Royal Statistical Society. 1972;135(3):370–384. [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69(3-4):373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Opal MD, Klenotich SC, Morais M, Bessa J, Winkle J, Doukas D, et al. Dulawa SM. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.144. [DOI] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M, et al. Salamone JD. Bupropion Increases Selection of High Effort Activity in Rats Tested on a Progressive Ratio/Chow Feeding Choice Procedure: Implications for Treatment of Effort-Related Motivational Symptoms. International Journal of Neuropsychopharmacology. 2015;18(2) doi: 10.1093/ijnp/pyu017. 10.1093/ijnp/pyu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4(3):317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. J Exp Psychol Anim Behav Process. 1981;7(3):242–268. [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–850. doi: 10.1016/s0006-3223(98)00162-0. Research Support, Non-U.S. Gov't Review. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, et al. Balsam PD. Pharmacologic Rescue of Motivational Deficit in an Animal Model of the Negative Symptoms of Schizophrenia. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Waltz JA, Kellendonk C, Balsam PD. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr Bull. 2012;38(6):1111–1117. doi: 10.1093/schbul/sbs114. Research Support, N.I.H., Extramural Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187(1-2):36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen KR. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Frontiers in behavioral neuroscience. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25(1):86–97. doi: 10.1037/a0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Avlar B, Balsam PD. Interactions of timing and motivational impairments in schizophrenia. In: Vatakis A, Allman MJ, editors. Time distortions in mind: Temporal processing in clinical populations. Boston, MA: Brill Academic Publishers; 2015. [Google Scholar]
- Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62(3):1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, et al. Balsam PD. Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behav Neurosci. 2009;123(4):720–730. doi: 10.1037/a0016503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, et al. Balsam PD. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37(7):1699–1707. doi: 10.1038/npp.2012.15. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearden JH, Ferrara A. Stimulus spacing effects in temporal bisection by humans. Q J Exp Psychol B. 1995;48(4):289–310. [PubMed] [Google Scholar]
- Ziauddeen H, Murray GK. The relevance of reward pathways for schizophrenia. Curr Opin Psychiatry. 2010;23(2):91–96. doi: 10.1097/YCO.0b013e328336661b. [DOI] [PubMed] [Google Scholar]


