Abstract
Background:
Oocyte cryopreservation is an important part of modern fertility treatment. The effect of vitrification on the fertilization and developmental rates of embryo is still a matter of debate.
Objectives:
This study aimed to investigate the effect of vitrification on the success of mouse oocyte maturation, fertilization, and preimplantation development in vitro.
Materials and Methods:
In this experimental study, a total of 200 germinal vesicle (GV) and 200 metaphase II (MII) oocytes were obtained from ovaries and fallopian tubes of NMRI mice, respectively and divided into two control and experimental (vitrified) groups. Oocytes in the experimental group were vitrified by Cryotop using vitrification medium (Origio, Denmark) and kept in liquid nitrogen for one month. Then, they were cultured in maturation medium for 24 hours. In vitro maturated metaphase 2 (IVM-MII) and ovulated metaphase 2 (OV-MII) oocytes were inseminated and the fertilized embryos assessed until the hatching blastocyst stage. Outcomes were assessed for statistical significance by Chi-square test using SPSS software.
Results:
Vitrification caused a significant reduction in the maturation rate of oocytes. Of those that matured, the fertilization rate of vitrified IVM-MII (44.1%) and OV-MII oocytes (50%) was not significantly different from each other but both were significantly lower than the control group (P < 0.05). There was no significant difference in developmental rates of both vitrified groups and the control group.
Conclusions:
The present study showed that vitrification using Cryotop and freezing medium can damage oocytes by reducing the maturation and fertilization rates in both developmental stages.
Keywords: Vitrification, Fertilization, Maturation, Cryotop, Developmental Rate, Oocyte
1. Background
There are few effective clinical alternatives for fertility preservation in women. In recent years, fertility preservation in women, especially in cases where the reproductive organs and fertility are at risk, has become an important component of assisted reproductive technologies (ART). Embryo freezing is a common and efficient method, however, most young women, suffering from cancer in reproductive age, cannot freeze embryos for their fertility preservation due to lack of partner, reproductive age restrictions as well as legal and ethical problems. Oocyte freezing is an alternative method to preserve fertility in women (1). The ability to mature oocyte in vitro (in vitro maturation, IVM) also provides greater flexibility for ART treatments and the capacity to both undertake IVM and oocyte maturation provides a major advance in fertility preservation. In some cases, such as immature girls (2) and some chromosomal disorders like mosaic Turner syndrome, cryopreservation of immature oocyte is the preferred strategy (3).
Over the last decade, cryopreservation by vitrification technique has begun to dominate in ART treatments. Vitrification is a physical process through which the concentrated cryoprotectant solution solidifies without ice crystal formation. Therefore, the formation of ionic concentration gradients is avoided. In general, transition to glass formation is more a kinetic phenomenon than a thermodynamic one. Ice crystallization can cause intra-cellular damage and (4) and harm zona pellucida (5-10). In vitrification, the oocytes may be prone to damage by a variety of factors, including the toxicity of cryoprotectant, cold shock, incomplete vitrification (allowing some ice crystal formation), and resulting osmotic stress (11).
2. Objectives
This study aimed to investigate the effect of oocyte freezing on maturation, fertilization, and normal development of the embryos to assess whether this process is suitable for use on immature oocytes. The results showed that there are no acute adverse effects of using immature oocytes compared to ovulated oocytes, however, both suffered some reduced function after vitrification compared to controls.
3. Materials and Methods
3.1. Preparation of Immature and Mature Oocytes
All culture media for flushing, in vitro maturation (IVM), in vitro fertilization, vitrification, and thawing in this experimental study were purchased from Origio, Denmark. Oocytes were obtained from adult female (6 - 8 weeks old) and spermatozoa from male (8 - 12 weeks old) NMRI mice obtained from Razi Institute (Karaj, Iran). All animal experiments were carried out in accordance with the European Communities Council Directive on 24 November, 1986 (86/609/EEC). Animals were kept in controlled condition under a 12:12 hour light/dark cycle in a room at 22 ± 2°C and humidity of 40% - 50%. Induction of ovulation was performed by intraperitoneal injection of 10 units equine chorionic gonadotropin (Sigma, UK) followed by 10 IU hCG (human chorionic gonadotropin) 48 hours later. About 13 hour after hCG administration, mice were sacrificed by cervical dislocation. Germinal vesicle (GV) oocytes were collected from ovarian follicles by dissection method and MII oocytes obtained from fallopian tube by flushing method. To eliminate sampling biases, the oocytes with clear cytoplasm and uniform zona pellucida were selected for each experiment group.
The GV oocytes without cumulus cells were obtained by removal of the surrounding cumulus cells using a Pasteur pipette through frequent pipetting and the MII stage oocytes separated from the cumulus-oocyte complex (COC) by the enzymatic action of intracytoplasmic sperm injection (ICSI) cumulase. Oocytes without cumulus cells were transferred to fresh culture medium. A total of 400 oocytes (200 GV stage oocytes and 200 MII stage oocytes) were divided into two control and experimental (vitrified) groups and used for IVM and IVF assessments.
3.2. Vitrification and Thawing of Oocytes
The GV and MII oocytes were vitrified using a two-step exposure to equilibrium and vitrification solutions. In the first step (equilibrium/dehydration), the GV and MII oocytes of both groups were exposed to the equilibrium solution for 10 minutes (12). Then, the oocytes were exposed to vitrification solution followed by loading 1 - 3 oocytes onto the propylene strip of Cryotop (Kitazato, Japan) within less than 60 seconds (13). During thawing, the protective coating of Cryotops was initially removed while the propylene strips were still in liquid nitrogen followed by immediate and rapid transfer of Cryotops from liquid nitrogen (LN2) into the thawing medium. Later, the oocytes were transferred to culture medium and the survival rate of oocytes was evaluated using an inverted microscope after 2 hours incubation. Oocytes with spherical shape, intact membranes, clear zone without rupture, and uniform cytoplasm were considered healthy and alive. After vitrification and thawing, the survived MII and GV oocytes were randomly selected for subsequent investigations.
3.3. In Vitro Maturation
GV oocytes were cultured in 20 μL of IVM culture medium overlaid with mineral oil (Sigma, Germany) in an incubator with 5% CO2 at 37°C. After 28 to 32 hours, oocytes were examined under inverted microscope. The oocytes with the first polar body were selected for further study.
3.4. In Vitro Fertilization
Cauda epididymal sperms of mature male of the NMRI strain aged 8 to 12 weeks were separated, and then transferred to culture medium for sperm preparation. Drops containing sperm were kept in incubator for 1.5 hours for sperms capacitation. About 1 - 2 × 106 sperms were transferred to each IVF Universal drop. Then, IVM-MII and OV-MII oocytes collected from experimental and control groups were transferred to the drops containing sperms and were kept in the incubator for 6 - 8 hours. Next, the embryos were transferred into ISM1 (2-cell to 4-cell) and blast assist mediums (8-cell stage to blastocyst) for further development and were kept in the incubator. The developmental rates of pronuclei, 2-cell, 4-cell, 8-cell, morula, and blastocyst stages were assessed under inverted microscope following 8, 24, 48, 72, 96, and 120 hours, respectively after inoculation (Figure 1).
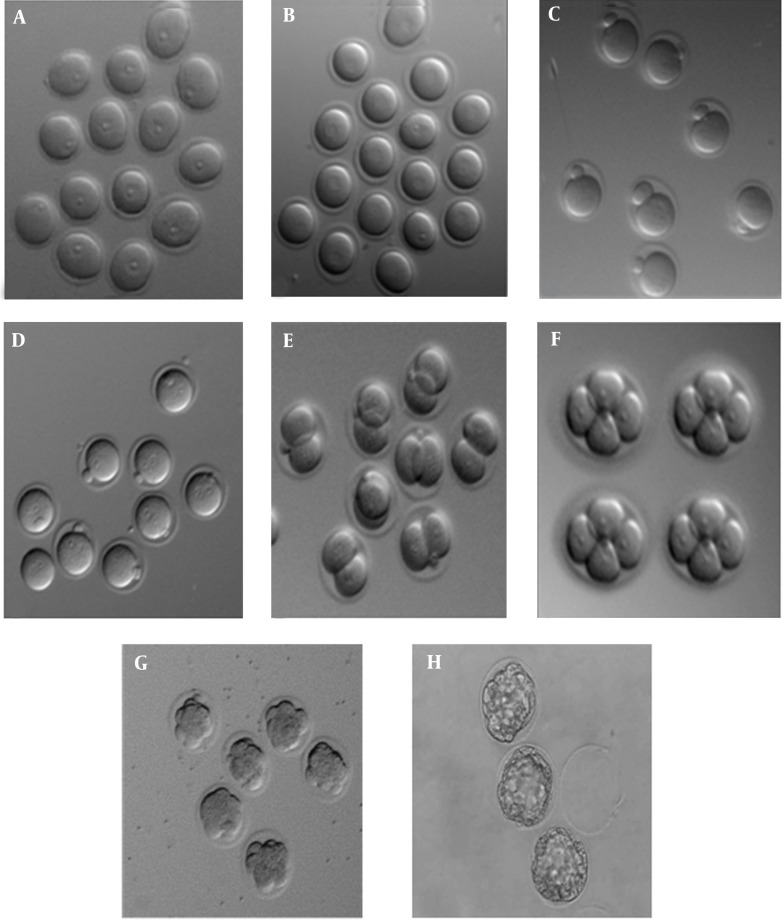
Figure 1. The Developmental Stages of Embryos Under Inverted Microscope.
A: GV oocyte, B: GVBD oocyte, C: MII oocyte, D: male and female pronuclei, E: 2-cell, F: 4-cell, G: morula, H: blastocyst were showed 8, 24, 48, 72, 96, and 120 hours, respectively after insemination.
3.5. Statistical Analysis
The maturation, fertilization and development rates between the oocytes in both control and experimental groups were analyzed using Chi-square test. P value of 0.05 or less was considered to be significant.
4. Results
4.1. In Vitro Maturation Rate
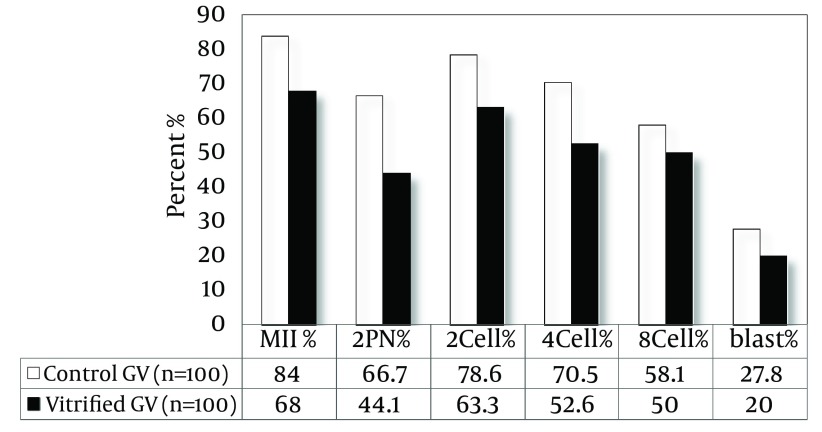
As shown in Figure 2, the maturation rate of GV oocytes after vitrification showed a significant decrease compared to control group (68% vs. 84%, P < 0.05).
Figure 2. The Maturation, Fertilization, and Developmental Rate in IVM-MII Oocytes After Vitrification.
4.2. Developmental Rate of IVM-MII Oocytes
As shown in Figure 2, the rate of fertilization in IVM-MII oocytes after vitrification and warming showed a significant difference compared to the control group (44.1% vs. 66.7%, P < 0.05). The percentages of embryos, 2-cell, 4-cell, 8-cell and blastocyst in control group were 78.6, 70.5, 58.1, 27.8, respectively and in the vitrified group they were 44.1, 63.3, 52.6, 50, and 20, respectively. The results of the statistical analysis showed that the developmental rates in vitrified IVM-MII oocytes were not significantly different compared to control oocytes.
4.3. Developmental Rate of OV-MII Oocytes
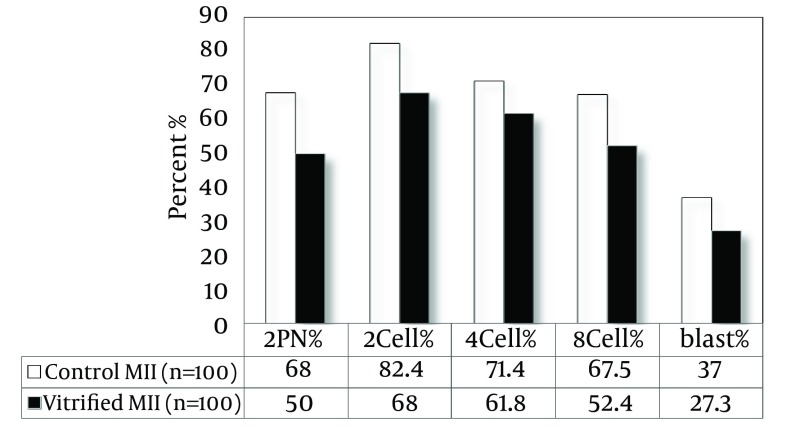
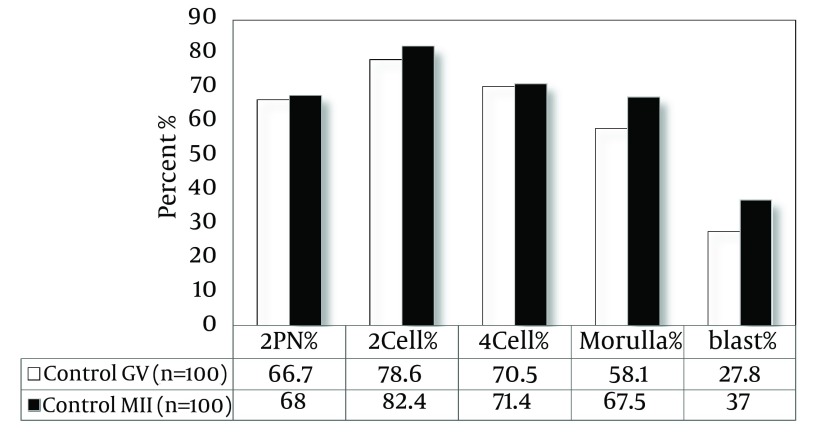
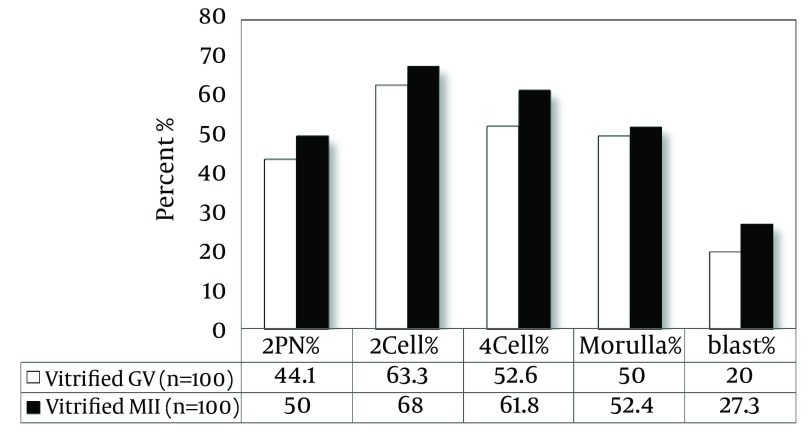
As shown in Figure 3, the rate of fertilization in OV-MII oocytes after vitrification and warming showed a significant difference compared to the control group (50% vs. 68%, P < 0.05). The percentages of embryos, 2-cell, 4-cell, 8-cell and blastocyst in the control group were 82.4, 71.4, 67.5, and 37, respectively and in vitrified group they were 68, 61.8, 52.4, and 27.3, respectively. The developmental rates in vitrified IVM-MII oocytes have no significant difference compared to control oocytes. There was no significant difference in fertilization and developmental rates of both vitrified and control oocytes (Figures 4 and 5).
Figure 3. The Fertilization and Developmental Rate in OV-MII Oocytes After Vitrification.
Figure 4. The Fertilization and Developmental Rate in OV-MII and IVM-MII Oocytes in the Control Group.
Figure 5. The Fertilization and Developmental Rate in OV-MII and IVM-MII Oocytes After Vitrification.
5. Discussion
The level of maturation in GV oocytes and the fertilization rate of IVM-MII and OV-MII cryopreserved oocytes were significantly decreased compared to the control group. Jee et al. (14) investigated the effect of multistep vitrification on the level of oocyte maturation of GV stage of the mouse and concluded that even multistep vitrification cannot improve this reduction in the rate of maturation and fertilization. Galeati et al. (15), after freezing and thawing of porcine oocytes using Cryotop showed that metaphase II stage oocytes are so sensitive to vitrification that it changes the organization of microtubules and affects the process of fertilization. In addition, several reports regarding human and mouse oocytes indicated the absence of meiosis spindle at metaphase II stage because of slow freezing and vitrification (16-24). Cha et al. (25) found no significant difference in the fertilization rate of mature oocytes after freezing and thawing among all groups. These data were not consistent with our results. This difference may be due to the use of slush nitrogen, carriers, different culture media, other vitrification protocols, and or other strains of animals. In the above mentioned study, liquid slush nitrogen (SN2) is used to increase cooling rate, while the samples in the present study froze in (LN2). Slush nitrogen has resulted from vacuumizing the liquid nitrogen, so it is considered part of nitrogen steam and remains colder (-205°C). After rapid immersion in liquid slush nitrogen, the sample is less evaporated and cools more rapidly (26). In the mentioned study, vitrified straw and electron microscope grids have been used. However, in the current study, Cryotop had been used as carrier. The most important factor in this difference is the use of different culture media. In the above mentioned study, G1, 2 medium had been used at culture stages. Also, time differences of the used protocols may have led to this difference. Furthermore, the current study has been performed on oocytes of NMRI mouse; while, in the above mentioned study, mice of ICR strain have been used.
As researches have shown, the survival rate of oocytes and frozen embryos are different because of not only vitrification methods but also the race of the animals, developmental stage, and even oocytes and embryos of different quality (27). Shen et al. (28) showed that deficiency in the organization of the spindle disturbs chromosome sequences which results in defects in the process of meiosis and fertilization (29-31). Since the most abundant organelles are found in mammalian oocytes, which severely affect any abnormalities and dysfunction of the oocyte developmental stages (32-34), freezing can disrupt maturation stages by affecting these intracellular structures, confirming the obtained results. However, the organelles of the oocytes in the nucleus and cytoplasm get damaged during freezing; therefore, disruption in the ability of normal growth, functions regarding stability maintenance of the intracellular environment (Homeostasis) and the ability of the frozen oocyte to continue to grow and develop due to damages caused by freezing are expected. The effects of the damages caused by freezing on cytoplasmic function of GV oocyte is closely associated with the phase of cell cycle because the ultra-structural state of the cytoplasm is constantly changing based on the meiosis stage. This matter applies, especially to the distribution and function of the cytoskeleton, which plays an essential role in normal separation of mitochondria, chromosomes, spindle rotation, cytokinesis, and formation of pronucleus, affecting the fertilization process. Thus, in addition to chromosomal changes, ultrastructural, cytoskeleton, mitochondrial, nuclear and cortical granules changes have been also observed in oocyte (35, 36). Researchers have shown that freezing may harden zona pellucid due to changes in biochemical nature (early withdrawal of cortical granules), which leads to the prevention of sperm penetration and interference with fertilizing process (6). All these factors together reduce pregnancy rates caused by oocyte vitrifying techniques (37, 38). However, according to the results, it seems that oocytes that passed freezing and thawing stages and fertilized, in the later stages of development till blastocyst stage, are not significantly different from the control group, which may be due to the use of a desirable culture medium for their development.
This study showed that vitrification using Cryotop and freezing medium can damage oocytes by reducing the maturation and fertilization rates in both developmental stages. Therefore, to advance oocyte freezing techniques, it is necessary to have an accurate understanding of cryobiological parameters.
Acknowledgments
We would like to thank Professor Christopher O’Neil, the Head of Human Reproduction Unit, Kolling Institute of Medical Research, University of Sydney for editing the manuscript and also Deputy of the Research Department of Qazvin University of Medical Sciences for financial support.
Footnotes
Authors’ Contributions:Freezing, thawing, and culturing of the oocytes and data collection: Neda Abedpour. Design, analysis, revision and supervision of the study: Farzad Rajaei.
Funding/Support:This study was supported by Deputy of the Research Department of Qazvin University of Medical Sciences, Qazvin, IR Iran.
References
- 1.Tucker MJ, Morton PC, Wright G, Sweitzer CL, Massey JB. Clinical application of human egg cryopreservation. Hum Reprod. 1998;13(11):3156–9. doi: 10.1093/humrep/13.11.3156. [DOI] [PubMed] [Google Scholar]
- 2.Cutting R, Barlow S, Anderson R. Human oocyte cryopreservation: evidence for practice. Hum Fertil (Camb). 2009;12(3):125–36. doi: 10.1080/14647270903132115. [DOI] [PubMed] [Google Scholar]
- 3.Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, et al. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: Case Report. Hum Reprod. 2008;23(2):336–9. doi: 10.1093/humrep/dem307. [DOI] [PubMed] [Google Scholar]
- 4.Chian RC, Kuwayama M, Tan L, Tan J, Kato O, Nagai T. High survival rate of bovine oocytes matured in vitro following vitrification. J Reprod Dev. 2004;50(6):685–96. doi: 10.1262/jrd.50.685. [DOI] [PubMed] [Google Scholar]
- 5.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11(5):608–14. doi: 10.1016/s1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 6.Mavrides A, Morroll D. Bypassing the effect of zona pellucida changes on embryo formation following cryopreservation of bovine oocytes. Eur J Obstet Gynecol Reprod Biol. 2005;118(1):66–70. doi: 10.1016/j.ejogrb.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Dobrinsky JR, Pursel VG, Long CR, Johnson LA. Birth of piglets after transfer of embryos cryopreserved by cytoskeletal stabilization and vitrification. Biol Reprod. 2000;62(3):564–70. doi: 10.1095/biolreprod62.3.564. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Lin XN, Tong XM, Li C, Liu L, Jin XY, et al. A frozen-thawed embryo transfer program improves the embryo utilization rate. Chin Med J (Engl). 2009;122(17):1974–8. [PubMed] [Google Scholar]
- 9.Goto S, Kadowaki T, Tanaka S, Hashimoto H, Kokeguchi S, Shiotani M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril. 2011;95(3):948–52. doi: 10.1016/j.fertnstert.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Suzuki J, Yu X, Kan FW, Qiao J, Chian RC. Cryo-survival, fertilization and early embryonic development of vitrified oocytes derived from mice of different reproductive age. J Assist Reprod Genet. 2010;27(11):605–11. doi: 10.1007/s10815-010-9450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon T. Live births after vitrification of oocytes in a stimulated in vitro fertilization–embryo transfer program. Fertil Steril. 2003;79(6):1323–6. doi: 10.1016/s0015-0282(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 12.Kasai M, Ito K, Edashige K. Morphological appearance of the cryopreserved mouse blastocyst as a tool to identify the type of cryoinjury. Hum Reprod. 2002;17(7):1863–74. doi: 10.1093/humrep/17.7.1863. [DOI] [PubMed] [Google Scholar]
- 13.Mukaida T, Nakamura S, Tomiyama T, Wada S, Kasai M, Takahashi K. Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless technique. Fertil Steril. 2001;76(3):618–20. doi: 10.1016/s0015-0282(01)01968-9. [DOI] [PubMed] [Google Scholar]
- 14.Jee BC, Chen HY, Chian RC, Suh CS, Kim SH, Moon SY. Vitrification of immature mouse oocyte using stepwise equilibration before or after in vitro maturation. Fertil Steril. 2009;92(3):1153–7. doi: 10.1016/j.fertnstert.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Galeati G, Spinaci M, Vallorani C, Bucci D, Porcu E, Tamanini C. Pig oocyte vitrification by cryotop method: effects on viability, spindle and chromosome configuration and in vitro fertilization. Anim Reprod Sci. 2011;127(1-2):43–9. doi: 10.1016/j.anireprosci.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Burgos M, Herrero L, Megias D, Salvanes R, Montoya MC, Cobo AC, et al. Vitrification versus slow freezing of oocytes: effects on morphologic appearance, meiotic spindle configuration, and DNA damage. Fertil Steril. 2011;95(1):374–7. doi: 10.1016/j.fertnstert.2010.07.1089. [DOI] [PubMed] [Google Scholar]
- 17.Chang CC, Lin CJ, Sung LY, Kort HI, Tian XC, Nagy ZP. Impact of phase transition on the mouse oocyte spindle during vitrification. Reprod Biomed Online. 2011;22(2):184–91. doi: 10.1016/j.rbmo.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Aman RR, Parks JE. Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro-matured bovine oocytes. Biol Reprod. 1994;50(1):103–10. doi: 10.1095/biolreprod50.1.103. [DOI] [PubMed] [Google Scholar]
- 19.Mandelbaum J, Anastasiou O, Levy R, Guerin JF, de Larouziere V, Antoine JM. Effects of cryopreservation on the meiotic spindle of human oocytes. Eur J Obstet Gynecol Reprod Biol. 2004;113 Suppl 1:S17–23. doi: 10.1016/j.ejogrb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Ciotti PM, Porcu E, Notarangelo L, Magrini O, Bazzocchi A, Venturoli S. Meiotic spindle recovery is faster in vitrification of human oocytes compared to slow freezing. Fertil Steril. 2009;91(6):2399–407. doi: 10.1016/j.fertnstert.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Sung LY, Lin CJ, Kort HI, Yang X, Tian XC, et al. The oocyte spindle is preserved by 1,2-propanediol during slow freezing. Fertil Steril. 2010;93(5):1430–9. doi: 10.1016/j.fertnstert.2009.01.106. [DOI] [PubMed] [Google Scholar]
- 22.Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Hum Reprod. 2001;16(11):2374–8. doi: 10.1093/humrep/16.11.2374. [DOI] [PubMed] [Google Scholar]
- 23.Chen CK, Wang CW, Tsai WJ, Hsieh LL, Wang HS, Soong YK. Evaluation of meiotic spindles in thawed oocytes after vitrification using polarized light microscopy. Fertil Steril. 2004;82(3):666–72. doi: 10.1016/j.fertnstert.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 24.Larman MG, Minasi MG, Rienzi L, Gardner DK. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online. 2007;15(6):692–700. doi: 10.1016/s1472-6483(10)60537-8. [DOI] [PubMed] [Google Scholar]
- 25.Cha SK, Kim BY, Kim MK, Kim YS, Lee WS, Yoon TK, et al. Effects of various combinations of cryoprotectants and cooling speed on the survival and further development of mouse oocytes after vitrification. Clin Exp Reprod Med. 2011;38(1):24–30. doi: 10.5653/cerm.2011.38.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedro PB, Zhu SE, Makino N, Sakurai T, Edashige K, Kasai M. Effects of hypotonic stress on the survival of mouse oocytes and embryos at various stages. Cryobiology. 1997;35(2):150–8. doi: 10.1006/cryo.1997.2034. [DOI] [PubMed] [Google Scholar]
- 27.Niemann H, Lucas-Hahn A, Stoffregen C. Cryopreservation of bovine oocytes and embryos following microsurgical operations. Mol Reprod Dev. 1993;36(2):232–5. doi: 10.1002/mrd.1080360217. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Betzendahl I, Tinneberg HR, Eichenlaub-Ritter U. Enhanced polarizing microscopy as a new tool in aneuploidy research in oocytes. Mutat Res. 2008;651(1-2):131–40. doi: 10.1016/j.mrgentox.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Kilani S, Cooke S, Kan A, Chapman M. Are there non-invasive markers in human oocytes that can predict pregnancy outcome? Reprod Biomed Online. 2009;18(5):674–80. doi: 10.1016/s1472-6483(10)60013-2. [DOI] [PubMed] [Google Scholar]
- 30.Lornage J, Salle B. Ovarian and oocyte cryopreservation. Curr Opin Obstet Gynecol. 2007;19(4):390–4. doi: 10.1097/GCO.0b013e328247f411. [DOI] [PubMed] [Google Scholar]
- 31.Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–95. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006;73(11):1454–62. doi: 10.1002/mrd.20579. [DOI] [PubMed] [Google Scholar]
- 33.Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril. 2009;91(4):1023–34. doi: 10.1016/j.fertnstert.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 34.Isachenko V, Isachenko E, Michelmann HW, Alabart JL, Vazquez I, Bezugly N, et al. Lipolysis and ultrastructural changes of intracellular lipid vesicles after cooling of bovine and porcine GV-oocytes. Anat Histol Embryol. 2001;30(6):333–8. doi: 10.1046/j.1439-0264.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu B, Tong J, Leibo SP. Effects of cooling germinal vesicle-stage bovine oocytes on meiotic spindle formation following in vitro maturation. Mol Reprod Dev. 1999;54(4):388–95. doi: 10.1002/(SICI)1098-2795(199912)54:4<388::AID-MRD9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Hyttel P, Vajta G, Callesen H. Vitrification of bovine oocytes with the open pulled straw method: ultrastructural consequences. Mol Reprod Dev. 2000;56(1):80–8. doi: 10.1002/(SICI)1098-2795(200005)56:1<80::AID-MRD10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 37.Fabbri R. Cryopreservation of human oocytes and ovarian tissue. Cell Tissue Bank. 2006;7(2):113–22. doi: 10.1007/s10561-005-1969-7. [DOI] [PubMed] [Google Scholar]
- 38.Tucker M, Morton P, Liebermann J. Human oocyte cryopreservation: a valid alternative to embryo cryopreservation? Eur J Obstet Gynecol Reprod Biol. 2004;113 Suppl 1:S24–7. doi: 10.1016/j.ejogrb.2003.11.006. [DOI] [PubMed] [Google Scholar]