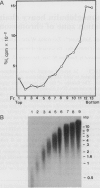
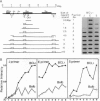
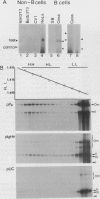
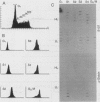
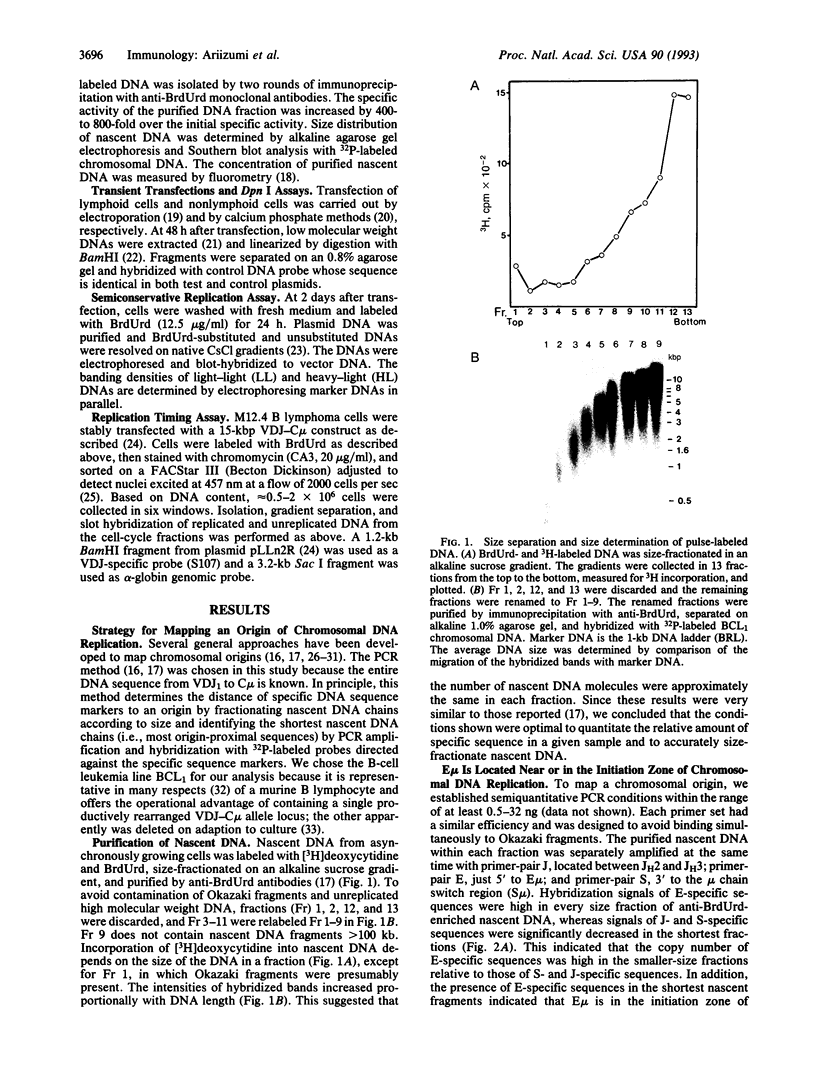
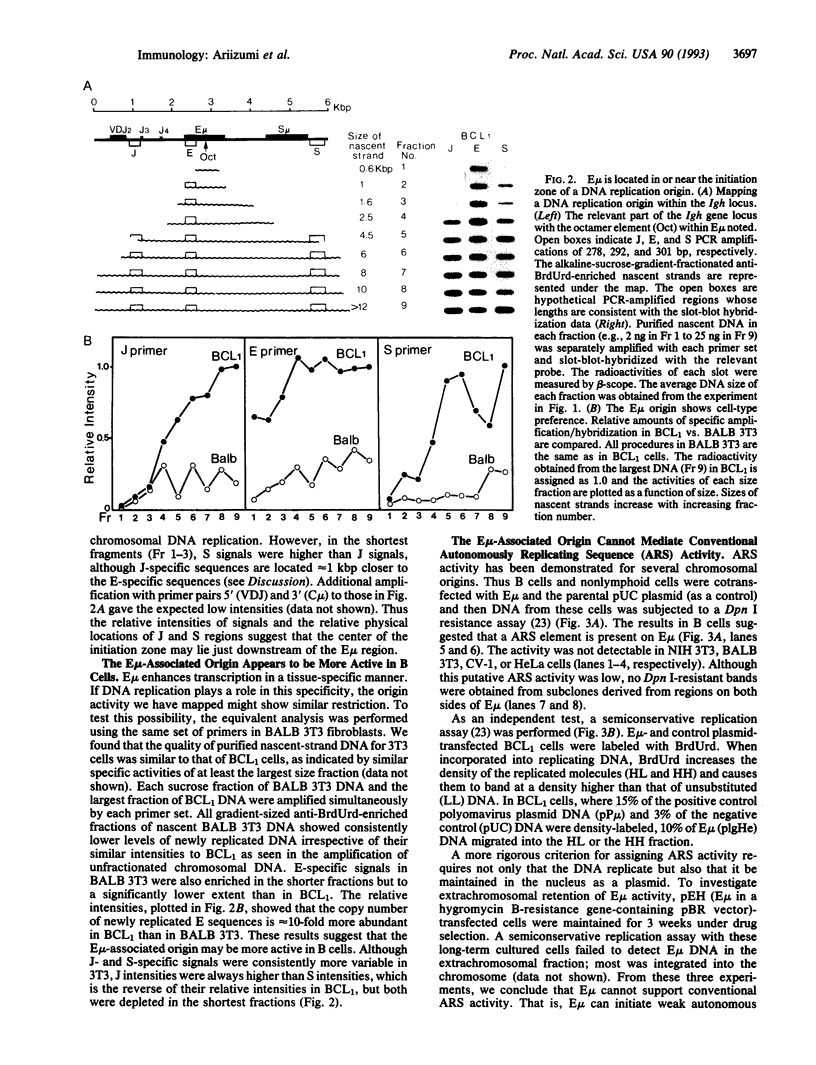
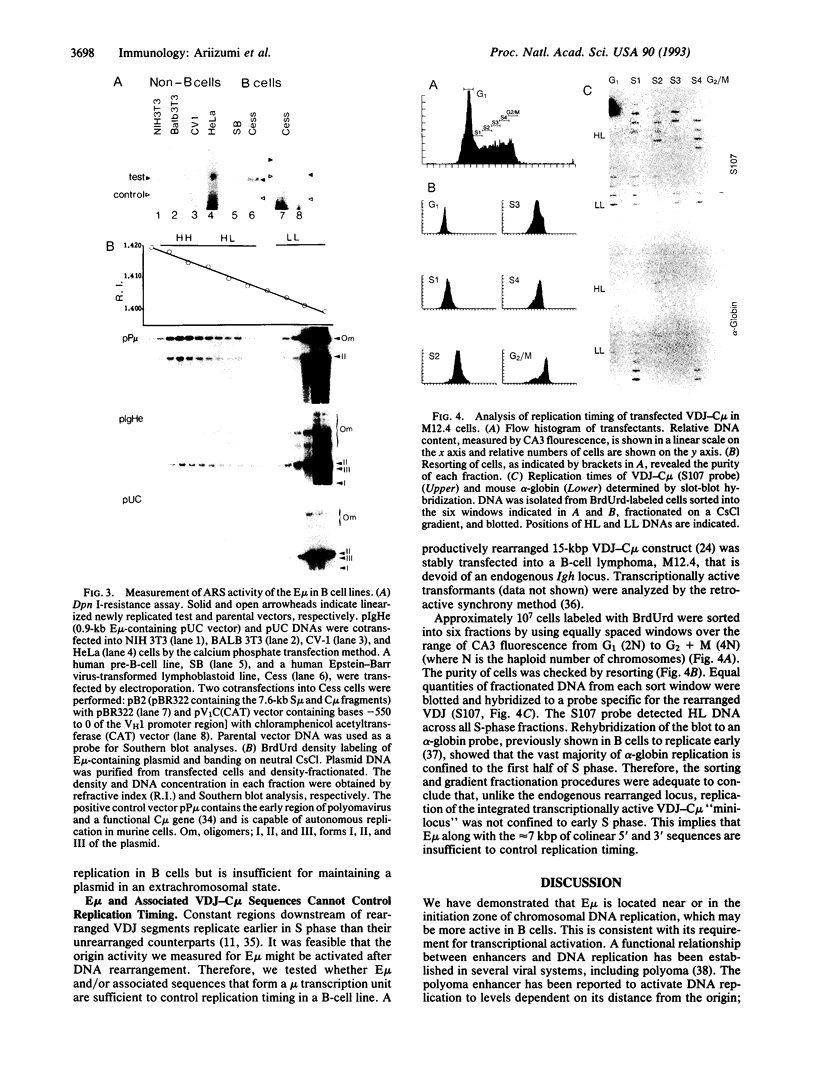
Abstract
In several animal viruses, enhancers have been implicated in both DNA replication and transcriptional activation. The linkage of the two mechanisms appears intimate, in that common DNA binding factors can be shared. The immunoglobulin heavy chain (Igh) intronic [heavy chain joining region (JH)-mu chain constant region (C mu)] enhancer (E mu) is required for tissue-specific transcription of Igh genes and is essential for somatic recombination of diversity (D) and J segments. We show here that E mu is located at or near an origin of chromosomal DNA replication, which is more active in B lymphocytes than fibroblasts. E mu does not fulfill two criteria demonstrated for some cellular origins. E mu can initiate but not maintain autonomous replicating activity in B cells. E mu is unable to impart early replication timing to a transfected VDJ-C mu Igh locus in B cells. Instead we propose that E mu-associated ori activity contributes to tissue-specific Igh expression through local effects on chromatin structure leading to subsequent accessibility of transcription and/or recombination factors for the enhancer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett-Cook E. R., Hassell J. A. Activation of polyomavirus DNA replication by yeast GAL4 is dependent on its transcriptional activation domains. EMBO J. 1991 Apr;10(4):959–969. doi: 10.1002/j.1460-2075.1991.tb08030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Boyes J., Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991 Mar 22;64(6):1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchini D., Reynaud C. A., Ripoche M. A., Grimal H., Jami J., Weill J. C. Rearrangement of a chicken immunoglobulin gene occurs in the lymphoid lineage of transgenic mice. 1987 Mar 26-Apr 1Nature. 326(6111):409–411. doi: 10.1038/326409a0. [DOI] [PubMed] [Google Scholar]
- Burhans W. C., Vassilev L. T., Wu J., Sogo J. M., Nallaseth F. S., DePamphilis M. L. Emetine allows identification of origins of mammalian DNA replication by imbalanced DNA synthesis, not through conservative nucleosome segregation. EMBO J. 1991 Dec;10(13):4351–4360. doi: 10.1002/j.1460-2075.1991.tb05013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., Gaudray P., De Rose M. L., Emery J. F., Meinkoth J. L., Nakkim E., Subler M., Von Hoff D. D., Wahl G. M. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987 May;7(5):1740–1750. doi: 10.1128/mcb.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Trotter J., Wahl G. M. Replication timing control can be maintained in extrachromosomally amplified genes. Mol Cell Biol. 1991 Sep;11(9):4779–4785. doi: 10.1128/mcb.11.9.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. W., Word C., Dev V., Uhr J. W., Vitetta E. S., Tucker P. W. Double isotype production by a neoplastic B cell line. II. Allelically excluded production of mu and gamma 1 heavy chains without CH gene rearrangement. J Exp Med. 1986 Aug 1;164(2):562–579. doi: 10.1084/jem.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Christ C., Tye B. K. Functional domains of the yeast transcription/replication factor MCM1. Genes Dev. 1991 May;5(5):751–763. doi: 10.1101/gad.5.5.751. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Dubey D. D., Davis L. R., Greenfeder S. A., Ong L. Y., Zhu J. G., Broach J. R., Newlon C. S., Huberman J. A. Evidence suggesting that the ARS elements associated with silencers of the yeast mating-type locus HML do not function as chromosomal DNA replication origins. Mol Cell Biol. 1991 Oct;11(10):5346–5355. doi: 10.1128/mcb.11.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler P., Roth P., Kim J. Y., Storb U. Factors affecting the rearrangement efficiency of an Ig test gene. J Immunol. 1991 Apr 15;146(8):2826–2835. [PubMed] [Google Scholar]
- Ferrier P., Krippl B., Blackwell T. K., Furley A. J., Suh H., Winoto A., Cook W. D., Hood L., Costantini F., Alt F. W. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 1990 Jan;9(1):117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. M. Temporal order of replication of Xenopus laevis 5S ribosomal RNA genes in somatic cells. Proc Natl Acad Sci U S A. 1986 May;83(9):2924–2928. doi: 10.1073/pnas.83.9.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Guise J. W., Lim P. L., Yuan D., Tucker P. W. Alternative expression of secreted and membrane forms of immunoglobulin mu-chain is regulated by transcriptional termination in stable plasmacytoma transfectants. J Immunol. 1988 Jun 1;140(11):3988–3994. [PubMed] [Google Scholar]
- Hatton K. S., Dhar V., Brown E. H., Iqbal M. A., Stuart S., Didamo V. T., Schildkraut C. L. Replication program of active and inactive multigene families in mammalian cells. Mol Cell Biol. 1988 May;8(5):2149–2158. doi: 10.1128/mcb.8.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. L., Lieber M. R. CpG methylated minichromosomes become inaccessible for V(D)J recombination after undergoing replication. EMBO J. 1992 Jan;11(1):315–325. doi: 10.1002/j.1460-2075.1992.tb05054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- James C. D., Leffak M. Polarity of DNA replication through the avian alpha-globin locus. Mol Cell Biol. 1986 Apr;6(4):976–984. doi: 10.1128/mcb.6.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Grosschedl R. Complex pattern of immunoglobulin mu gene expression in normal and transgenic mice: nonoverlapping regulatory sequences govern distinct tissue specificities. Genes Dev. 1991 Jun;5(6):932–943. doi: 10.1101/gad.5.6.932. [DOI] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Leffak M., James C. D. Opposite replication polarity of the germ line c-myc gene in HeLa cells compared with that of two Burkitt lymphoma cell lines. Mol Cell Biol. 1989 Feb;9(2):586–593. doi: 10.1128/mcb.9.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. 1985 Dec 5;318(6045):475–478. doi: 10.1038/318475a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- McWhinney C., Leffak M. Autonomous replication of a DNA fragment containing the chromosomal replication origin of the human c-myc gene. Nucleic Acids Res. 1990 Mar 11;18(5):1233–1242. doi: 10.1093/nar/18.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., Van der Vliet P. C. Nuclear factor I enhances adenovirus DNA replication by increasing the stability of a preinitiation complex. EMBO J. 1992 Feb;11(2):751–760. doi: 10.1002/j.1460-2075.1992.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Satake M., Yamaguchi-Iwai Y., Sakai M., Muramatsu M., Ito Y. The nuclear protooncogenes c-jun and c-fos as regulators of DNA replication. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3947–3951. doi: 10.1073/pnas.88.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawotka K. A., Huberman J. A. Two-dimensional gel electrophoretic method for mapping DNA replicons. Mol Cell Biol. 1988 Apr;8(4):1408–1413. doi: 10.1128/mcb.8.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen B., Hellman L., Sen R. The NF-kappa B-binding site mediates phorbol ester-inducible transcription in nonlymphoid cells. Mol Cell Biol. 1988 Aug;8(8):3526–3531. doi: 10.1128/mcb.8.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Lenardo M. J. Immunoglobulin gene transcription. Annu Rev Immunol. 1991;9:373–398. doi: 10.1146/annurev.iy.09.040191.002105. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Linskens M. H., Kowalski D., Huberman J. A. New beginnings in studies of eukaryotic DNA replication origins. Biochim Biophys Acta. 1989 Jan 23;1007(1):1–14. doi: 10.1016/0167-4781(89)90123-1. [DOI] [PubMed] [Google Scholar]
- Vassilev L. T., Burhans W. C., DePamphilis M. L. Mapping an origin of DNA replication at a single-copy locus in exponentially proliferating mammalian cells. Mol Cell Biol. 1990 Sep;10(9):4685–4689. doi: 10.1128/mcb.10.9.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L., Johnson E. M. An initiation zone of chromosomal DNA replication located upstream of the c-myc gene in proliferating HeLa cells. Mol Cell Biol. 1990 Sep;10(9):4899–4904. doi: 10.1128/mcb.10.9.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L., Johnson E. M. Mapping initiation sites of DNA replication in vivo using polymerase chain reaction amplification of nascent strand segments. Nucleic Acids Res. 1989 Oct 11;17(19):7693–7705. doi: 10.1093/nar/17.19.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P. Relationship of eukaryotic DNA replication to committed gene expression: general theory for gene control. Microbiol Rev. 1991 Sep;55(3):512–542. doi: 10.1128/mr.55.3.512-542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal L. P. Relationship of eukaryotic DNA replication to committed gene expression: general theory for gene control. Microbiol Rev. 1991 Sep;55(3):512–542. doi: 10.1128/mr.55.3.512-542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Brooks K., Chen Y. W., Isakson P., Jones S., Layton J., Mishra G. C., Pure E., Weiss E., Word C. T-cell-derived lymphokines that induce IgM and IgG secretion in activated murine B cells. Immunol Rev. 1984 Apr;78:137–157. doi: 10.1111/j.1600-065x.1984.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Wessel R., Müller H., Hoffmann-Berling H. Electron microscopic analysis of DNA forks generated by Escherichia coli DNA helicase II. Eur J Biochem. 1990 Sep 24;192(3):695–701. doi: 10.1111/j.1432-1033.1990.tb19278.x. [DOI] [PubMed] [Google Scholar]
- Yang L., Li R., Mohr I. J., Clark R., Botchan M. R. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature. 1991 Oct 17;353(6345):628–632. doi: 10.1038/353628a0. [DOI] [PubMed] [Google Scholar]