Abstract
Background
Both parasitic load and resource availability can impact individual fitness, yet little is known about the interplay between these parameters in shaping body condition, a key determinant of fitness in wild mammals inhabiting seasonal environments.
Methods
Using partial least square regressions (PLSR), we explored how temporal variation in climatic conditions, vegetation dynamics and sarcoptic mange (Sarcoptes scabiei) severity impacted body condition of 473 Iberian ibexes (Capra pyrenaica) harvested between 1995 and 2008 in the highly seasonal Alpine ecosystem of Sierra Nevada Natural Space (SNNS), southern Spain.
Results
Bottom-up regulation was found to only occur in healthy ibexes; the condition of infected ibexes was independent of primary productivity and snow cover. No link between ibex abundance and ibex body condition could be established when only considering infected individuals.
Conclusions
The pernicious effects of mange on Iberian ibexes overcome the benefits of favorable environmental conditions. Even though the increase in primary production exerts a positive effect on the body condition of healthy ibexes, the scabietic individuals do not derive any advantage from increased resource availability. Further applied research coupled with continuous sanitary surveillance are needed to address remaining knowledge gaps associated with the transmission dynamics and management of sarcoptic mange in free-living populations.
Keywords: Capra pyrenaica, Host-parasite relationships, Iberian Ibex, Density-dependence, Remote sensing, Sarcoptes scabiei
Background
Body condition, i.e. energetic state and fat stores of an animal, is a major determinant of individual performance in most vertebrate species [1], including ungulates [2]. In particular, the increase of body condition in anticipation of food shortages is one of the most common mechanisms displayed by herbivores to prevent starvation in highly seasonal environments [3]. Parasites typically have a deleterious effect on body condition, mainly because infected hosts try to reduce the intensity or length of infestation by allocating resources in the activation of the immune response; they may also try to alleviate the damages caused by the infestation by investing energy in tissue repair and detoxification [4]. Because of this, one can expect the energetic costs of infestation are more pronounced in periods of food shortage [5].
Sarcoptic mange caused by the mite Sarcoptes scabiei is an excellent study model to evaluate how parasites may interfere with the bottom-up regulation of body condition in wild mammals inhabiting highly seasonal ecosystems. This mite is responsible for severe epizootic disease outbreaks in a broad range of mammals, sometimes causing increases in mortality rates. Infected animals typically suffer from dramatic structural and functional changes in the skin, becoming listless, dehydrated, emaciated and eventually dying from the infestation [6]. Notwithstanding the increasing knowledge about the immune response [7] and pathology [8] of sarcoptic mange, little is known about the relationship between environmental conditions, mange severity and body condition. This topic is of particular importance for the management of the disease, as hosts facing hard environmental conditions could experience increased mange severity. The energetic demands on the host, coupled with the induced physiological changes, make this parasitic disease particularly worrying for free-living populations inhabiting such environments.
Sarcoptic mange in the Iberian ibex
Iberian ibex (Capra pyrenaica Schinz, 1838) is a medium-sized endemic mountain ungulate commonly affected by sarcoptic mange [9]. Mange outbreaks can result in devastating short-term mortality of ibexes, as happened in the Sierras de Cazorla, Segura y Las Villas Natural Park, southern Spain, in the late 80s. There, approximately 95 % of ibexes were killed by the parasite [9]. Since 1992, sarcoptic mange has become endemic in the ibex population of the Sierra Nevada Natural Space (SNNS, hereafter). One particularity of this extreme Alpine ecosystem is the strong seasonal climatic variation found in the area, with snow cover present for six months of the year. SNNS ibex are adapted to such contrasted conditions and do display an income breeder strategy, i.e. increasing adipose tissues during the summer in anticipation of food deprivation during winter times [10].
Previous studies have addressed the consequences of mange infestation in SNNS Iberian ibex population: e.g. immune response to first and second exposures [11], effects on individual growth [12], reproductive allocation [13] and seasonal variation of hematology and biochemistry among scabietic ibexes [14]. To date, however, no information exists on how climatic conditions, vegetation dynamics and mange severity impact the body condition of these free-ranging ibexes. Although body weight reduction is detectable at early stages of infestation [14], 80 % of ibexes recover totally from mange [15]. Thus, we can hypothesize that aside from the individual factors that shape resistance to Sarcoptes scabiei infestation, the effects of sarcoptic mange on ibex body condition will be influenced by the local environmental conditions (e.g. food availability, winter harshness) experienced during infestation.
Taking advantage of thirteen years (1995–2008) of data on sarcoptic mange monitoring in the Iberian ibex population of SNNS, we explored whether disease severity interacted with primary productivity, snow cover and ibex abundance in determining body condition. The strength of these interactions was assessed during two periods of contrasted vegetation dynamics and snow cover: green (March – October) and dormant period (November – February). We hypothesized that the deleterious effects of mange on ibex body condition will be compensated by favorable environmental conditions, e.g. high primary productivity, little snow cover and low population abundance. To the best of our knowledge, this study provides the first explicit assessment of how a parasitic disease shapes bottom-up processes in a large mammal.
Methods
Ethics statement
This study complies with the Spanish and the Andalusian laws regarding bioethics and animal welfare. The Sierra Nevada National Park approved this study.
Study area
The SNNS covers an area of approximately 2.000 km2 and is characterized by a heterogeneous orography, with an altitudinal range between 860 and 3.482 m.a.s.l (Fig. 1). According to the Köppen–Geiger classification system, the SNNS experiences a Mediterranean Subartic climate [16]. Annual average precipitation is about 600 mm [17]. Minimum and maximum average monthly temperatures vary between −5 °C in February and 17 °C in July, with pronounced summer drought. The average annual temperature decreases from 12 to 16 °C below 1.500 m to 0 °C above 3.000 m. Snow generally covers a significant part of the study area between December and May; vegetation growth mainly occurs between June and August. SNNP encompasses the largest and best-known population of Iberian ibex in Andalusia [9].
Fig. 1.
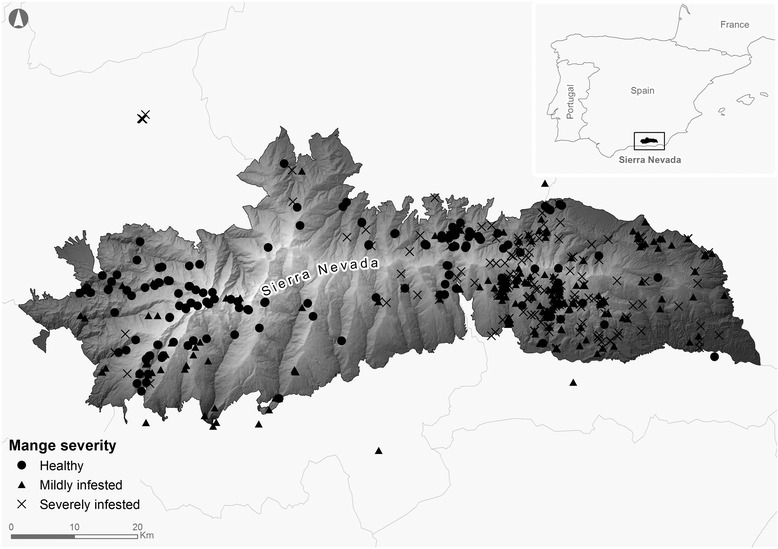
Location of the study area, the Sierra Nevada Natural Space. The spatial distribution of shot-harvested animals and the degree of mange severity (healthy, mildly and severely infested) are also showed
Vegetation greenness and snow cover data
Two environmental variables were considered while assessing the role of bottom-up processes on the body condition of ibex: the normalized difference vegetation index (NDVI, Fig. 2) used as a proxy of vegetation productivity, and snow cover [18, 19]. The former information was extracted from the MODIS repository (Moderate Resolution Imaging Spectroradiometer; http://modis.gsfc.nasa.gov) at a spatial resolution of 500 m and bi-monthly temporal resolution. Snow cover (percentage of surface covered by snow) was retrieved from the Observatorio Cambio Global - Sierra Nevada website (http://obsnev.es/linaria.html). Only NDVI values associated with shrubs and herbaceous layers were considered, since ibex primarily feed in areas encompassed by these landcover types in SNNS [20]. Mean monthly NDVI and snow cover values were computed for each year.
Fig. 2.
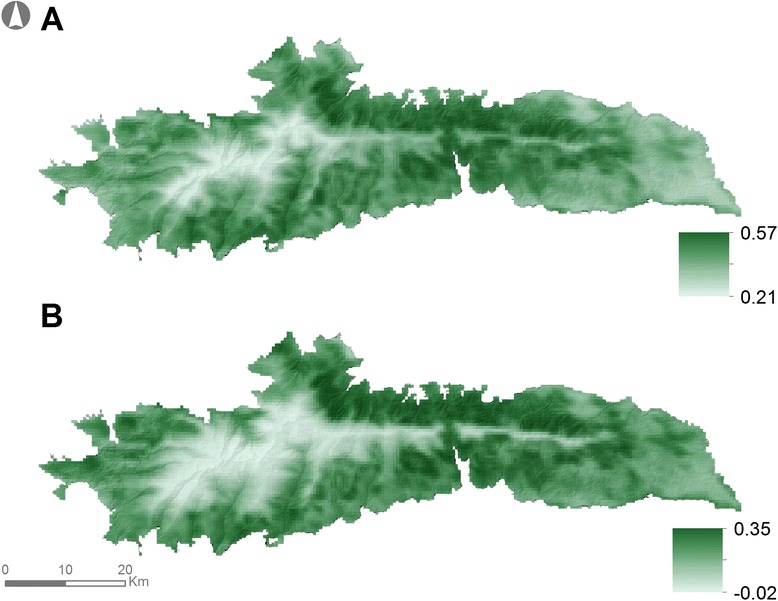
Spatial variability of NDVI during the (a) green (March – October) and (b) dormant (November – February) periods in SNNS. Pixel values in each map correspond to the pixel average for the relevant season for the period 2000–2008
Ibex data
We used block counts to estimate ibex abundance during the study period. In brief, a set of line transects were systematically placed in order to provide an equal coverage of habitats that occur in the study area. Thanks to this approach, the bias related to the systematic prospection of suitable areas was reduced. The survey was conducted every month by a fixed number of teams. Ibexes were observed by means of 8 × 40 binoculars and 20–60 × 65 spotting scopes. For modelling purposes, counts were expressed as the number of ibexes recorded in a given itinerary on a seasonal basis.
A total of 243 male and 230 female Iberian ibexes older than two years were shot between 1995 and 2008, in the context of a mange control program carried out in the SNNS during this period. Sex was determined by visual inspection and the age in years was determined from horn-segment counts [21].
Mange severity was visually assessed using three categories, based on the percentage of skin surface affected by mites [22]: healthy = ibexes without skin lesions, mildly infested = skin surface affected ≤ 50 % and severely infested when skin surface affected > 50 %. Animals were weighed to the nearest 0.1 kg; the kidneys were removed and transported to the laboratory in a cold box at 4 °C. Kidney fat reserves were assessed following Serrano et al. [10] recommendations. Both fat-free kidney mass (KM) and associated peripheral fat (KF) are positively correlated to nutritional status and body condition of a wide range of mammals including Iberian ibex [23]. Therefore, we used the residuals from the linear regression between KM and KF as a proxy of body condition.
Statistical analysis
Partial least square regressions (PLSR) were used to assess the influence of disease severity, resource dynamics, intra-specific competition and snow cover on body condition. Carrascal et al. [24] defined the PLSR method as an extension of multiple linear regressions in which a response variable (body condition) is modelled through the analysis of linear combinations among predictors (NDVI, snow cover and ibex abundance). This technique is distribution-free and well suited to handle multicollinearity issues, preventing possible misinterpretations of regression coefficients [25]. A PLSR model was developed for each combination of time period (green and dormant period) and mange severity (healthy, mildly and severely infested). Results are presented and interpreted under the assumption that body condition of severely infected ibexes is lower than in healthy animals and of those in early stages of infestation ([26]). The sex of Iberian ibexes was excluded from the analysis because the lack of sex-biased effect of mange on ibexes’ condition ([26]). To minimize the potential effects of body growth on body condition, only individuals close to the final body size were retained in our analysis (≥3 years old [21, 27]). The significance of PLSR models was assessed through the Stone-Geisser Q2 test, a cross-validation redundancy measure created to evaluate the predictive significance of the exogenous variables. Test values greater than 0.0975 indicate that the exogenous variables are statistically significant for the response, whereas values below this threshold reveal no significance. The R2 was performed to measure the explanatory performance of models developed. Radar plots were used to explore the correlations between the variables and the first two axes associated to the first two components. All calculations were performed in R (version 3.2.0, R Development Core Team 2013). The package “plspm” was used to develop and visualize the PLSR outputs [28].
Results
Information on the body condition of 473 Iberian ibex was considered in our analyses: 43 (32 males and 11 females) of these individuals were classified as healthy, 217 (92 males and 125 females) as mildly infested and 213 (119 males and 94 females) as severely infested.
The monthly variations in the vegetation productivity, snow cover and ibex counts are shown in the Fig. 3. As would be expected, vegetation productivity and snow cover were characterized by opposite trends. The number of Iberian ibexes recorded a slight increase during the dormant period comprising the winter and the early spring.
Fig. 3.
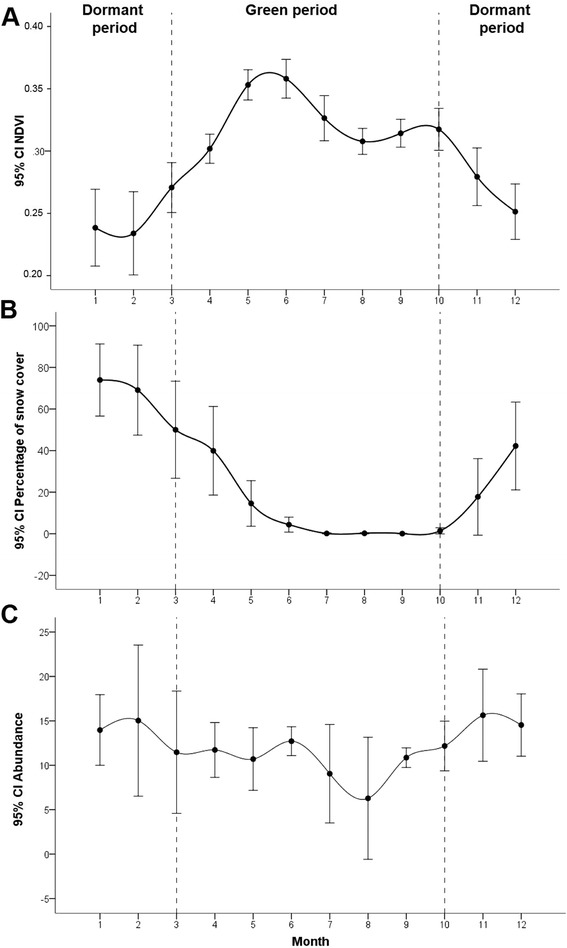
Intrannual variations of NDVI (a), snow cover (b) and number of ibexes observed (c) in SNNS. The error bars represent the inter-annual (1995–2008) fluctuations of variables values
Summaries of PLSR analyses for each combination of time period and mange severity are presented in the Tables 1 and 2. Through Stone-Geyser’s Q2 test we identified that bottom-up regulations of ibex condition only occurred in healthy ibexes (Q2 > 0.0975, Table 1). For healthy individuals, the considered environmental predictors as well as population abundance explained 25.14 % and 16.86 % of the observed variability in ibex body condition in the green and dormant period, respectively. Body condition of healthy animals was positively correlated with vegetation productivity (as indexed by average NDVI) and was negatively influenced by snow cover (Fig. 4). Primary productivity (Load = 0.68, w = 0.62) and snow cover (Load = −0.71, w = −0.77) were the main drivers of body condition of healthy animals in the green period whereas the ibexes abundance had greater relevance during the dormant season (Load = −0.62, w = −0.57) (Table 2, Fig. 5). None of the environmental and population factors analyzed influenced body condition of diseased ibexes, either at the mild or severe mange stages of infestation (Table 1). Thus, neither higher primary productivity nor winter harshness and population abundance influenced the impact of mange on ibex body condition.
Table 1.
R-squared and Stone-Geyser’s Q 2 test values for the partial least squares regression (PLSR) analysis. Each model results from the combination between the two time-periods of contrasted vegetation productivity, and mange severity categories. A component is considered significant if Q 2 ≥ 0.0975
| Period | Severity | R 2 (%) | Q 2 |
|---|---|---|---|
| Green | Healthy | 25.14 | 0.14 |
| Mildly infested | 3.37 | 0.01 | |
| Severely infested | 0.2 | −0.02 | |
| Dormant | Healthy | 16.86 | 0.11 |
| Mildly infested | 8.43 | 0.06 | |
| Severely infested | 0.01 | −0.02 |
Table 2.
Predictor weights and loads for the first component of partial least squares regression (PLSR) analysis. The contribution of each environmental predictor to the PLSR’s axis X is represented by the predictor weights
| Period | Severity | Predictors | Loads | Weights |
|---|---|---|---|---|
| Green | Healthy | NDVI | 0.68 | 0.62 |
| Snow | −0.71 | −0.77 | ||
| Abundance | −0.35 | −0.04 | ||
| Mildly infested | NDVI | −0.66 | −0.66 | |
| Snow | 0.55 | 0.25 | ||
| Abundance | 0.60 | 0.70 | ||
| Severely infested | NDVI | 0.67 | 0.59 | |
| Snow | −0.65 | 0.75 | ||
| Abundance | −0.38 | 0.38 | ||
| Dormant | Healthy | NDVI | 0.59 | 0.55 |
| Snow | −0.52 | −0.62 | ||
| Abundance | −0.62 | −0.57 | ||
| Mildly infested | NDVI | −0.39 | −0.29 | |
| Snow | 0.91 | 0.96 | ||
| Abundance | 0.66 | 0.02 | ||
| Severely infested | NDVI | −0.62 | −0.61 | |
| Snow | 0.42 | 0.36 | ||
| Abundance | 0.67 | 0.70 |
Fig. 4.
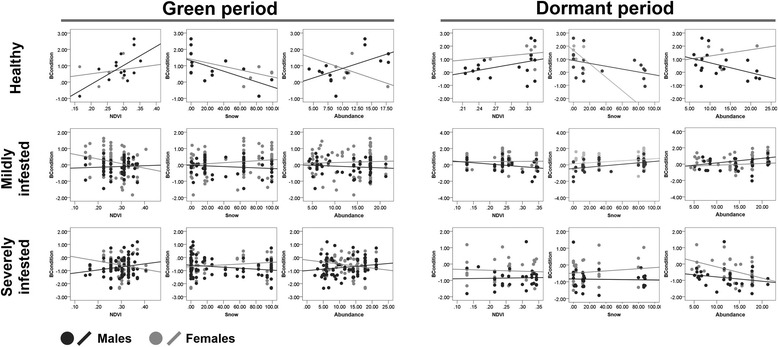
Relation between body condition (males and females) and the three environmental predictors in healthy, mildly and severely infested animals across two time periods of contrasted vegetation productivity (green and dormant)
Fig. 5.
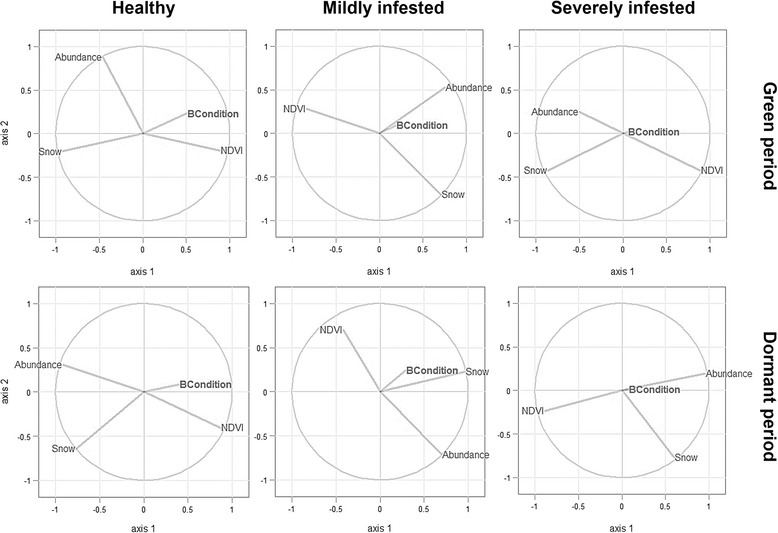
Correlation of the environmental predictors and the response with the first two components. Each segment represents a variable. Longer segments, i.e. closer to the perimeter of the circle, indicate that the corresponding variable is better represented. Segments close to each other represent highly and positively correlated variables. On the other hand, segments in opposite extremes indicate negative correlation. Orthogonal segments mean no correlation among predictors
Discussion and conclusions
This study provides the first known quantification of the impact of sarcoptic mange on the bottom-up regulation of body condition in a mountain ungulate. Our analyses led to three main results: i) an increase in primary productivity clearly triggers an increase in body condition in healthy ibexes, ii) sarcoptic mange can disrupt the link between environmental conditions and body condition, and iii) body condition is independent from ibex abundance parameter in scabietic ibexes.
The absence of a bottom-up regulation of body condition in scabietic individuals may be related to the physiopathology of this parasitic disease, which is characterized by emaciation, muscle mass losses and anemia [6, 29]. Even at the early/mild stages of infestation, mange can result in anemia (i.e. RBC, Hb, hematocrit reduction), accelerating the net catabolism of the body protein storage in ibex (i.e. increased blood urea and decreased creatine concentration) [14]. Such pathological and physiological changes have been reported in several other species inhabiting a wide range of environments (coyotes (Canis latrans), [30]; rabbits (Oryctolagus cuniculus), [31]; red foxes (Vulpes vulpes), [32]; wombats (Vombatus ursinus), [33]). For instance, Skerrat et al. [33] showed that wombats were using their body stores to cope with the energetic costs of sarcoptic mange. Likewise, Arlian et al. [31] concluded that the energy demand in rabbits is driven by mange severity i.e. severity increases the energetic costs to handle weight loss. These costs probably hinder the restoration of energy reserves in scabietic ibexes with severe hyperkeratotic lesions. However, in Iberian ibex the negative effects of sarcoptic mange on the capability to restore reserves was also present in the mildly stages of the disease. Indeed, sarcoptic mange exerts a negative and seasonal effect on body weight of infested Iberian ibexes [14], compromising the daily weight gain.
When overabundance coincides with limited food availability, the ability of the hosts to cope with infestations can be compromised [34]. Here, we failed to detect density-dependence in the bottom-up regulation of body condition in scabietic ibexes (see also Fernández-Morán et al. [35] who reported the absence of correlation between host population density and mange prevalence in Cantabrian chamois (Rupicapra pyrenaica parva). It has been suggested that host’s abundance predisposes the population to mange infestation [36, 37], since host-to-host transmission is favored in crowded host populations [15, 38]. In fact, aggregation improves disease maintenance [39] and host susceptibility increase when high host densities coincide with limited food availability [5]. Despite all these evidences, once infected, food shortage due to intra-specific competition appears to have no effect on the body condition of infected individuals.
In healthy ibexes, the negative relationship between population abundance and body condition is less pronounced in the green period than in the dormant season. This result could be explained by the habitat use of Iberian ibex. As with other closely related Caprinae species [40], ibexes can be easily seen around alpine meadows and pastures looking for sites free from snow and with fresh vegetation. However, during the dormant period the strong and negative relationship between population abundance and body condition can be explained by the fact that available resources are probably not sufficient to cover ibex’ energetic needs. Such a period also coincides with a reduction of forage intake and the typical increase of energy expenditure due to rut [41]. Therefore, whereas food shortage in the dormant period would act as a classical population bottom-up regulator, higher food availability during the high production green period would allow the Iberian ibex population to thrive.
Here, we have shown how scabietic ibexes do not take advantage of increases in resource availability, which is a drawback to the implementation of management practices, at least in the short term. A very recent work [15] underlined the ability of ibexes to survive from mange infestation, which opens a new window to disease management. Further research should be focused on the effects of habitat management on the progression of mange and the survival of scabietic individuals.
Acknowledgments
Thanks are due to Apolo Sánchez, José López Pérez, Isidro Puga González, Elías Martínez Ortíz, Manuela Cárdenas Fernández, Francisco Casado Felipe, Antonio Rodríguez Dueñas and Antonio Rodríguez Huete for their assistance in fieldwork. The research was supported by the projects 173/2009/M/00 and 861/11/M/00 (CMA, Andalusian Government), CGL2004-03171, CGL2012-40043-C02-01 and CGL-40043-C02-02 (MEC, Spanish Government), RTA 2009-00114-00-00 (INIA), RNM-6400, and Proyecto de Excelencia (Junta de Andalucia, Spain). João Carvalho was supported by a PhD Grant (SFRH/BD/98387/2013) co-financed by Fundação para a Ciência e a Tecnologia (FCT), European Social Fund (ESF) and Ministério da Educação e Ciência (MEC) National Funds. Emmanuel Serrano was supported by the postdoctoral program (SFRH/BPD/96637/2013) of the Fundação para a Ciência e Tecnologia, Portugal. This study was supported by the National Foundation for Science and Technology, through CESAM: UID/AMB/50017/2013.
Footnotes
Competing interests
The authors declare no conflict of interest.
Authors’ contributions
Designed the study: JC, JEG, JRLO, JMP, RCS, CF, NP, ES. Performed the ibex sampling: JEG, JCM, PF, RCS, JE, AR. Necropsies were performed by: JEG, JMP. Analysed the data: JC, NP, CF, ES. Wrote the paper: JC, CF, NP, ES. All authors read and approved the final version of the manuscript.
Contributor Information
João Carvalho, Phone: +351 234370350, Email: jlcarvalho@ua.pt.
José E. Granados, Email: josee.granados.ext@juntadeandalucia.es
Jorge R. López-Olvera, Email: jordi.lopez.olvera@uab.cat
Francisco Javier Cano-Manuel, Email: franciscoj.canomanuel@juntadeandalucia.es.
Jesús M. Pérez, Email: jperez@ujaen.es
Paulino Fandos, Email: pfandos@agenciamedioambienteyagua.es.
Ramón C. Soriguer, Email: soriguer@ebd.csic.es
Roser Velarde, Email: roser.velarde@uab.cat.
Carlos Fonseca, Email: cfonseca@ua.pt.
Arian Ráez, Email: arian.raez@gmail.com.
José Espinosa, Email: jcerrato@ujaen.es.
Nathalie Pettorelli, Email: nathalie.pettorelli@ioz.ac.uk.
Emmanuel Serrano, Phone: +351 234370350, Email: emmanuel.serrano.ferron@gmail.com.
References
- 1.Green AJ. Mass/length residuals: measures of body condition or generators of spurious results? Ecology. 2001;82(5):1473–83. doi: 10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2. [DOI] [Google Scholar]
- 2.Serrano E, Alpizar-Jara R, Morellet N, Hewison AJM. A half a century of measuring ungulate body condition using indices: is it time for a change? Eur J Wildl Res. 2008;54:675–80. doi: 10.1007/s10344-008-0194-7. [DOI] [Google Scholar]
- 3.Parker KL, Barboza PS, Gillingham MP. Nutrition integrates environmental responses of ungulates. Func Ecol. 2009;23:57–69. doi: 10.1111/j.1365-2435.2009.01528.x. [DOI] [Google Scholar]
- 4.Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Phil Trans R Soc B. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beldomenico PM, Begon M. Disease spread, susceptibility and infestation intensity: vicious circles? Trends Ecol Evol. 2009;25(1):21–7. doi: 10.1016/j.tree.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Pence DB, Ueckerman E. Sarcoptic mange in wildlife. Rev sci tech Off int Epiz. 2002;21(2):385–98. [PubMed] [Google Scholar]
- 7.Mounsey KE, Murray HG, Bielefeld-Ohmann H, Pasay C, Holt DC, Currie BJ, Walton SF, McCarthy JS. Prospective study in a porcine model of Sarcoptes scabiei indicates the association of Th2 and Th17 pathways with the clinical severity of scabies. PLoS Negl Trop Dis. 2015;9(3) doi: 10.1371/journal.pntd.0003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmervoll H, Hoby S, Robert N, Lommano E, Welle M, Ryser-Degiorgis MP. Pathology of sarcoptic mange in red foxes (Vulpes vulpes): macroscopic and histologic characterization of three disease stages. J Wildl Dis. 2013;49:91–102. doi: 10.7589/2010-11-316. [DOI] [PubMed] [Google Scholar]
- 9.Pérez JM, Granados JE, Soriguer RC, Fandos P, Márquez FJ, Crampe JP. Distribution, status and conservation problems of the Spanish Ibex, Capra pyrenaica (Mammalia: Artiodactyla) Mammal Rev. 2002;32:26–39. doi: 10.1046/j.1365-2907.2002.00097.x. [DOI] [Google Scholar]
- 10.Serrano E, Granados JE, Sarasa M, González FJ, Fandos P, Soriguer RC, Pérez JM. The effects of winter severity and population density on body stores in the Iberian wild goat (Capra pyrenaica) in a highly seasonal mountain environment. Eur J Wildl Res. 2011;57:45–55. doi: 10.1007/s10344-010-0398-5. [DOI] [Google Scholar]
- 11.Sarasa M, Rambozzi L, Rossi L, Meneguz PG, Serrano E, Granados JE, González FJ, Fandos P, Soriguer RC, Gonzalez G, Joachim J, Pérez JM. Sarcoptes scabiei: specific immune response to sarcoptic mange in the Iberian ibex Capra pyrenaica depends on previous exposure and sex. Exp Parasitol. 2010;124:265–71. doi: 10.1016/j.exppara.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Serrano E, Granados JE, Pérez JM. Sarcoptic mange and metapodial development in growing male Iberian ibex (Capra pyrenaica) Vet Parasitol. 2007;144:375–9. doi: 10.1016/j.vetpar.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Sarasa M, Serrano E, Soriguer RC, Granados JE, Fandos P, Gonzalez G, Joachim J, Pérez JM. Negative effect of the arthropod parasite, Sarcoptes scabiei, on testes mass in Iberian ibex, Capra pyrenaica. Vet Parasitol. 2011;175:306–12. doi: 10.1016/j.vetpar.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Pérez JM, Serrano E, Soriguer RC, González FJ, Sarasa M, Granados JE, Cano-Manuel FJ, Cuenca R, Fandos P. Distinguishing disease effects from environmental effects in a mountain ungulate: seasonal variation in body weight, hematology, and serum chemistry among Iberian ibex (Capra pyrenaica) affected by sarcoptic mange. J Wildl Dis. 2015;51(1):148–56. doi: 10.7589/2014-01-008. [DOI] [PubMed] [Google Scholar]
- 15.Alasaad S, Granados JE, Fandos P, Cano-Manuel FJ, Soriguer RC, Pérez JM. The use of radio-collars for monitoring wildlife diseases: a case study from Iberian ibex affected by Sarcoptes scabiei in Sierra Nevada, Spain. Parasit Vectors. 2013;6:242. doi: 10.1186/1756-3305-6-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World Map of the Köppen-Geiger climate classification updated. Meteorol Z. 2006;15:259–63. doi: 10.1127/0941-2948/2006/0130. [DOI] [Google Scholar]
- 17.Pérez-Luque AJ, Bonet FJ, Pérez-Pérez R, Aspizua R, Lorite J, Zamora R. Sinfonevada: Dataset of Floristic diversity in Sierra Nevada forests (SE Spain) PhytoKeys. 2014;35:1–15. doi: 10.3897/phytokeys.35.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. doi: 10.1038/386698a0. [DOI] [Google Scholar]
- 19.Pettorelli N. The Normalized Difference Vegetation Index. 1st ed. Oxford: Oxford University Press; 2013
- 20.Martínez T. Diet selection by Spanish ibex in early summer in Sierra Nevada. Acta Theriol. 2000;45(3):335–46. doi: 10.4098/AT.arch.00-33. [DOI] [Google Scholar]
- 21.Fandos P. La cabra montés Capra pyrenaica en el Parque Natural de las Sierras de Cazorla, Segura y Las Villas. Madrid: ICONA-CSIC; 1991. [Google Scholar]
- 22.Pérez JM, Granados JE, Sarasa M, Serrano E. Usefulness of estimated surface area of damaged skin as a proxy of mite load in the monitoring of sarcoptic mange in free-ranging populations of Iberian wild goat, Capra pyrenaica. Vet Parasitol. 2011;176:258–64. doi: 10.1016/j.vetpar.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Serrano E, González FJ, Granados JE, Moço G, Fandos P, Soriguer RC, Pérez JM. The use of total serum proteins and tryglycerides for monitoring body condition in the Iberian wild goat (Capra pyrenaica) J Zoo Wildl Med. 2008;39:646–9. doi: 10.1638/2007-0088.1. [DOI] [PubMed] [Google Scholar]
- 24.Carrascal LM, Galván I, Gordo O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos. 2009;118:681–90. doi: 10.1111/j.1600-0706.2008.16881.x. [DOI] [Google Scholar]
- 25.Mevik BH, Wehrens R. The pls package: principal components and partial least squares regression in R. J Stat Softw. 2007;18:1–24. doi: 10.18637/jss.v018.i02. [DOI] [Google Scholar]
- 26.López-Olvera JR, Serrano E, Armenteros A, Pérez JM, Fandos P, Carvalho J, et al. Sex-biased severity of sarcoptic mange at the same biological cost in a sexually dimorphic ungulate. Parasit Vectors (in evaluation) Parasit Vectors 2015 (in press); doi:10.1186/s13071-015-1186-6. [DOI] [PMC free article] [PubMed]
- 27.Serrano E, Pérez JM, Christiansen P, Gállego L. Sex-difference in the ossification rate of the appendicular skeleton in Capra pyrenaica Schinz, 1838, and its utility in the sex identification of long bones. Anat Histol Embryol. 2006;35:69–75. doi: 10.1111/j.1439-0264.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez G, Trinchera L, Russolillo G. Package “plspm”: Tools for Partial Least Squares Path Modeling (PLS-PM). 2015. http://cran.r-project.org/web/packages/plspm/index.html Accessed 7 March 2015.
- 29.Bornstein S, Mörner T, Samuel WM. Sarcoptes scabiei and sarcoptic mange. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wild mammals. 2. Ames: Iowa State University Press; 2001. pp. 107–19. [Google Scholar]
- 30.Pence DB, Windberg LA, Pence BC, Sprowls R. The epizootiology and pathology of sarcoptic mange in coyotes Canis latrans from south Texas. J Parasitol. 1983;69:1100–15. doi: 10.2307/3280873. [DOI] [PubMed] [Google Scholar]
- 31.Arlian LG, Ahmed M, Vyszenski-Moher DL. Effects of S. scabiei var. canis (Acari: Sarcoptidae) on blood indexes of parasitized rabbits. J Med Entomol. 1988;25:360–9. doi: 10.1093/jmedent/25.5.360. [DOI] [PubMed] [Google Scholar]
- 32.Little SE, Davidson WR, Rakich PM, Nixon TL, Bounous DI, Nettles VF. Responses of red foxes to first and second infestation with Sarcoptes scabiei. J Wildl Dis. 1998;34:600–11. doi: 10.7589/0090-3558-34.3.600. [DOI] [PubMed] [Google Scholar]
- 33.Skerratt LF, Middleton D, Beveridge I. Distribution of life cycle stages of Sarcoptic scabiei var. wombati and effects of severe mange on common wombats in Victoria. J Wildl Dis. 1999;35:633–46. doi: 10.7589/0090-3558-35.4.633. [DOI] [PubMed] [Google Scholar]
- 34.Gortázar C, Acevedo P, Ruiz-Fons F, Vicente J. Disease risks and overabundance of game species. Eur J Wildl Res. 2006;52:81–7. doi: 10.1007/s10344-005-0022-2. [DOI] [Google Scholar]
- 35.Fernández-Morán J, Gómez S, Ballesteros F, Quirós P, Benito JL, Feliu C, Nieto JM. Epizootiology of sarcoptic mange in a population of cantabrian chamois (Rupicapra pyrenaica parva) in Northwestern Spain. Vet Parasitol. 1997;73:163–71. doi: 10.1016/S0304-4017(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 36.Rossi L, Meneguz PG, De Martin P, Rodolfi M. The epizootiology of sarcoptic mange in chamois, Rupicapra rupicapra, from the Italian eastern Alps. Parasitologia. 1995;37:233–40. [PubMed] [Google Scholar]
- 37.Oleaga A, Casais R, González-Quirós P, Prieto M, Gortázar C. Sarcoptic mange in red deer from Spain: Improved surveillance or disease emergence? Vet Parasitol. 2008;154:103–13. doi: 10.1016/j.vetpar.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 38.León-Vizcaíno L, Ruíz de Ybáñez M, Cubero MJ, Ortíz JM, Espinosa J, Pérez L, Simón MA, Alonso F. Sarcoptic mange in Spanish ibex from Spain. J Wildl Dis. 1999;35(4):647–59. doi: 10.7589/0090-3558-35.4.647. [DOI] [PubMed] [Google Scholar]
- 39.Rossi S, Fromont E, Pontier D, Crucière C, Hars J, Barrat J, Pacholek X, Artois M. Incidence and persistence of classical swine fever in free-ranging wild boar (Sus scrofa) Epidemiol Infect. 2005;133:559–68. doi: 10.1017/S0950268804003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grignolio S, Parrini F, Bassano B, Luccarini S, Apollonio M. Habitat selection in adult males of Alpine ibex, Capra ibex ibex. Folia Zool. 2003;52:113–20. [Google Scholar]
- 41.Brivio F, Grignolio S, Apollonio M. To feed or not to feed? Testing different hypotheses on rut-induced hypophagia in a mountain ungulate. Ethology. 2010;115:1–10. [Google Scholar]


