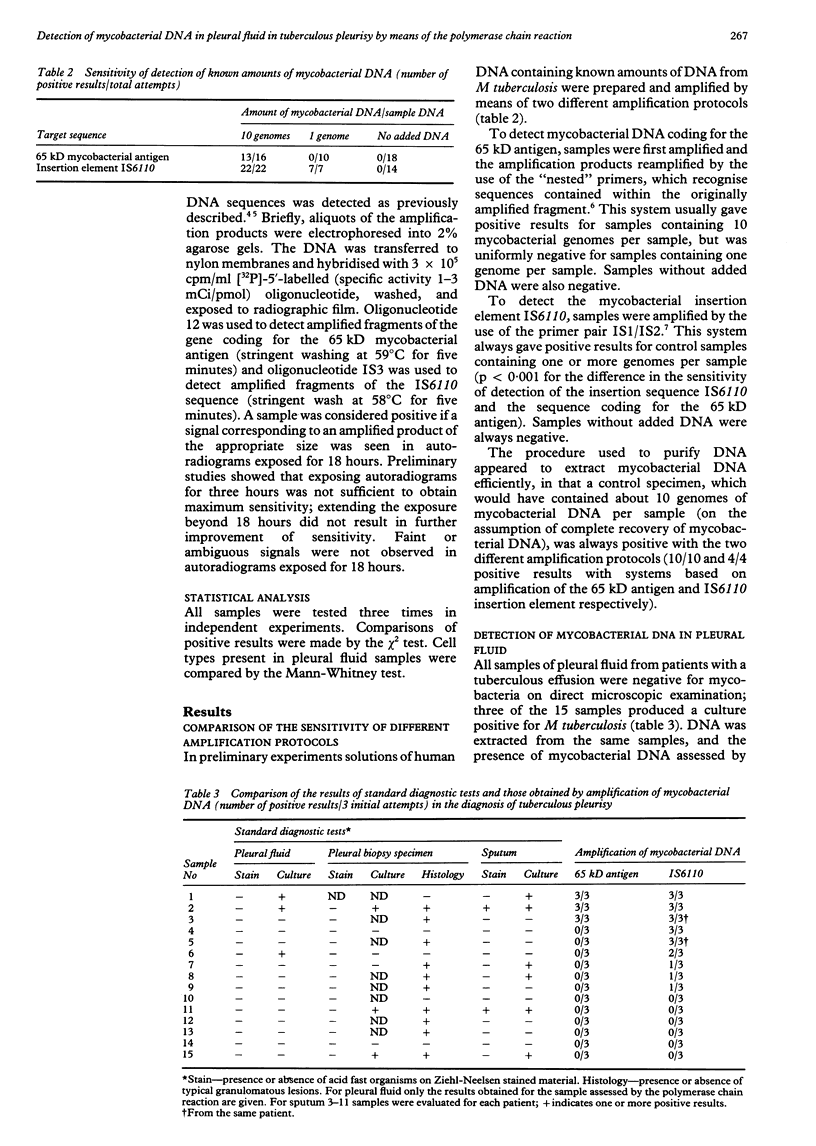
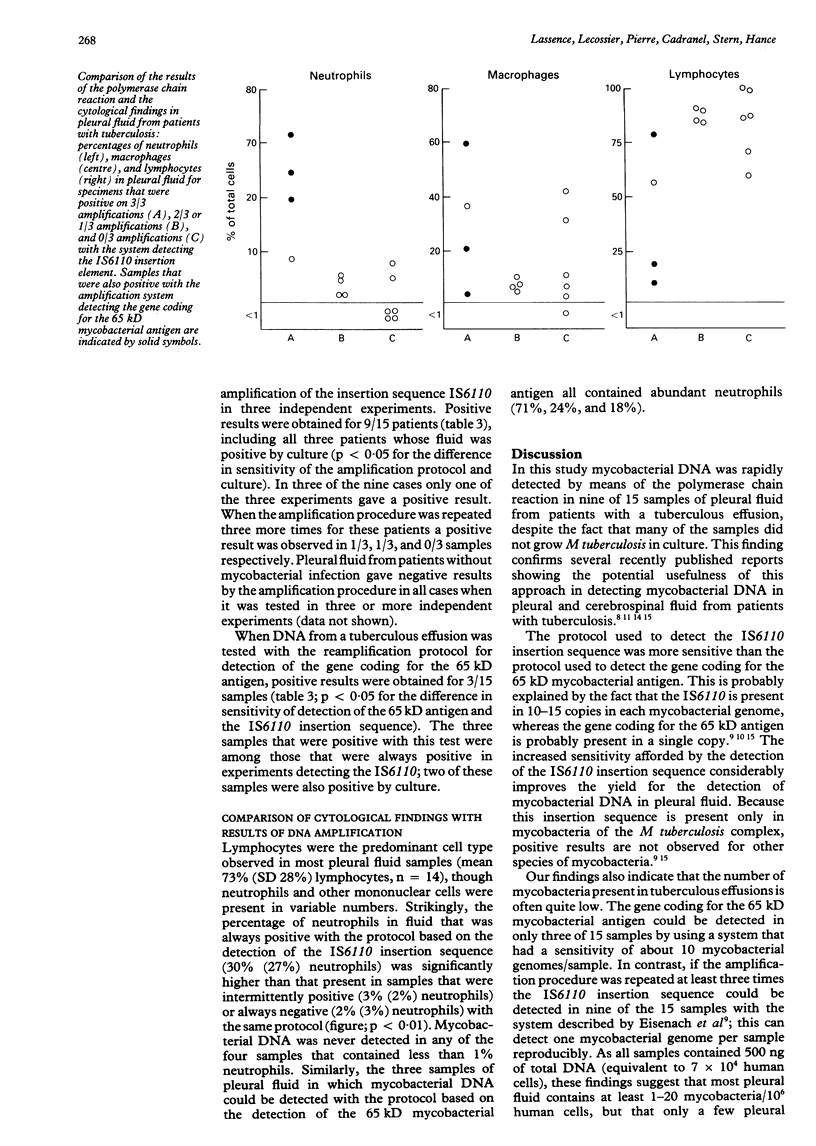
Abstract
BACKGROUND: The detection of mycobacterial DNA in clinical samples on the basis of the polymerase chain reaction is a promising approach for the rapid diagnosis of tuberculous infections. No consensus exists, however, about which protocols are most sensitive, and the usefulness of this approach in the diagnosis of tuberculous effusions has been assessed in few patients. METHODS: The sensitivity of two protocols was compared for the detection of DNA from Mycobacterium tuberculosis in samples containing known amounts of mycobacterial DNA and in DNA extracted from 15 tuberculous pleural effusions. The results obtained for pleural fluid have been compared with cytological findings and with results obtained by standard microbiological techniques. RESULTS: Mycobacteria could be detected by acid fast staining in none and by culture in three of the 15 pleural fluid samples. A protocol based on the detection of the IS6110 insertion element (which could detect one mycobacterial genome/sample reproducibly) gave a positive result in nine of the 15 tuberculous effusions, though some samples were only intermittently positive (p less than 0.05 compared with culture). In contrast, a protocol based on the detection of the gene coding for the 65 kD mycobacterial antigen (which could detect mycobacterial genomes only if there were at least 10/sample) gave a positive result in three of the 15 tuberculous effusions. Pleural fluid that was always positive with the amplification procedure detecting the IS6110 sequence contained more neutrophils (30% (SD 27%)) than samples that were intermittently positive or always negative (3% (3%)); mycobacterial DNA was never detected in the four samples containing less than 1% neutrophils. CONCLUSIONS: The amplification of the IS6110 insertion element represents a rapid and sensitive means of detecting M tuberculosis in tuberculous effusions. The enrichment of cells containing mycobacteria (possibly neutrophils) before DNA extraction may be required to improve the sensitivity of this approach.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniskis D., Amin K., Barnes P. F. Pleuritis as a manifestation of reactivation tuberculosis. Am J Med. 1990 Oct;89(4):447–450. doi: 10.1016/0002-9343(90)90374-m. [DOI] [PubMed] [Google Scholar]
- Baess I. Determination and re-examination of genome sizes and base ratios on deoxyribonucleic acid from mycobacteria. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):209–211. doi: 10.1111/j.1699-0463.1984.tb02822.x. [DOI] [PubMed] [Google Scholar]
- Berger H. W., Mejia E. Tuberculous pleurisy. Chest. 1973 Jan;63(1):88–92. doi: 10.1378/chest.63.1.88. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D., Steyn L., Shoemaker S., Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990 Nov;28(11):2437–2441. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H., Metzger W., Lacera D., Kay L. Diagnosis of tuberculous pleurisy by culture of pleural biopsy specimen. Arch Intern Med. 1970 Aug;126(2):269–271. [PubMed] [Google Scholar]
- Levine H., Szanto P. B., Cugell D. W. Tuberculous pleurisy. An acute illness. Arch Intern Med. 1968 Oct;122(4):329–332. [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Yen T. S., You J. B., Maa J. S., Fiss E. H., Chang C. H. Detection and identification of Mycobacterium tuberculosis by DNA amplification. J Clin Microbiol. 1990 Sep;28(9):1877–1880. doi: 10.1128/jcm.28.9.1877-1880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. J., Fries J. W., Piessens W. F., Wirth D. F. Sequence analysis and amplification by polymerase chain reaction of a cloned DNA fragment for identification of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Mar;28(3):513–518. doi: 10.1128/jcm.28.3.513-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Kvach J. T., Mounts P. Isolation and restriction endonuclease analysis of mycobacterial DNA. J Gen Microbiol. 1986 Feb;132(2):541–551. doi: 10.1099/00221287-132-2-541. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. B., Gelber R. H., Shinnick T. M. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J Clin Microbiol. 1990 Sep;28(9):1913–1917. doi: 10.1128/jcm.28.9.1913-1917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn S. A. State of the art. The pleura. Am Rev Respir Dis. 1988 Jul;138(1):184–234. doi: 10.1164/ajrccm/138.1.184. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]



