Abstract
Introduction:
Seizures are symptoms associated with abnormal electrical activity in electroencephalogram (EEG). The present study was designed to determine the effect of absence seizure on heart rate (HR) changes in electrocardiogram (ECG).
Methods:
HR alterations were recorded simultaneous with spike and wave discharges (SWD) by EEG in 6 WAG/Rij rats as a well characterized and validated genetic animal epilepsy model. Moreover, 6 control rats were used to distinguish the differences of HR changes between various groups. Electrodes were placed on the skull and under the chest skin, minimizing time delay and signal attenuation. HR was calculated by an adaptable algorithm based on continues wavelet transform (CWT) particular for this study. Three main features of HR; minimum, maximum, and mean values were estimated for pre-ictal and ictal intervals for all seizures.
Results:
ECG beats detected with sensitivity of 99.9% and positive predictability of 99.8% based on CWT. HR deceleration was found in 86% of the seizures. There were statistically significant (P<0.001) reductions of these values from pre-ictal to ictal intervals. Interictal HR acceleration and ictal deceleration were the major feature of alterations and in 23% of seizures, this decrease had priority to the onsets.
Discussion:
These findings may lead to design a seizure alarm system based on HR and to obtain new insights about sudden unexpected death in epilepsy (SUDEP) phenomenon and side-effects of antiepileptic drugs (AED).
Keywords: Absence seizure, SWD, Heart rate, Continues wavelet transforms, Seizure detection
1. Introduction
Epilepsy is general term for a range of brain disorder characterized predominantly by recurrent seizures. An epileptic seizure is a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain (Fisher et al, 2005). Epilepsy is one of the most common neurological disorders in worldwide. According to world health organization (WHO), it accounts for 1% of the global burden of disease and 90% of epilepsy incidence is in the developing world (World Health Organization, 2005).
Based on the International League against Epilepsy (ILAE) classification, there are two fundamental groups of seizures, named partial and generalized, considering their originating location and circulation way in the brain (Angeles, 1981). Mainly generalized type is categorized in two convulsive and non-convulsive. Absence seizure which we have focused on, is one of the non-convulsive types of seizure and is marked with sudden loss of consciousness and occurrence of spike- and-wave discharges (SWDs) in the EEG. Human patients experience discharges with a frequency of 2.5–4 Hz, for 4–20 s, an amplitude of 200–1000 μV as well as outside sign of immobility (Crunelli & Leresche, 2002).
Devices analyzing EEG signals for seizure detection have been widely developed, which automatically monitor patients, 24-h without need to a neurophysiologist (Greene et al, 2007). These devices have some disadvantages such as lack of popularity due to invasive nature of placing electrodes in patients head. Moreover, there are some other constraints such as device size, weight, and unreliable electrodes attached to the scalp (Casson, et al, 2008).
Epileptic seizures are associated with autonomic symptoms mediated by a dysfunction of the central autonomic network (CAN) and may lead to different variations such as cardiovascular functionality changes. These effects change ECG signals morphology, HR and other related parameters that Sevcencu and Struijk have summarized these alterations in the literature (Sevcencu & Struijk, 2010). Opherk et al., have studied changes in HR and the occurrence of ECG abnormalities in 102 seizures, both generalized and non-generalized (Opherk et al, 2002). Some of studies have focused on HR and its features for seizure detection, which is a good choice for ambulatory conditions, since the effects of artifacts and noise are minimized. Leutmezer et al., have investigated HR changes at the transition from the preictal state to the ictal one (Leutmezer et al, 2003). Pradhan et al., have analyzed HR variability in patients with absence epilepsy (Pradhan et al, 2011).
For detection of electrocardiographic changes in patients with partial epilepsy, different methods have been used such as adaptive neuro-fuzzy inference system (ANFIS) with wavelet transform (WT) for feature extraction (Güler & Übeyli, 2004), lyapunov exponents with multilayer perception neural networks (MLPNN) (Übeyli & Güler, 2004), support vector machine (SVM) (Übeyli, 2008), recurrent neural networks (RNN) with composite features (wavelet coefficient and lyapunov exponents) (Übeyli, 2008), and eigenvector methods (Ubeyli, 2009). These algorithms were applied to the records of normal and partial epilepsy ECG signals from the MIT-BIH database (Goldberger et al, 2000). RR interval analysis for neonatal seizure detection has comparable results with those reported for EEG-based systems (Greene, et al, 2007).
In our study, WAG/Rij rats as a genetic animal model of absence epilepsy with comorbidity of depression, have been used instead of a human model. They share many EEG and behavioral characteristics with human patients; the most important one, SWDs in EEG resemble absence epilepsy. However, there are differences in frequency of discharges compared to human subjects. SWDs in WAG\Rij rats have the frequency of 7–11 Hz and amplitude varies from 300–1000 μV with mean duration of 8.0 ± 1.0 s (Sarkisova & Luijtelaar, 2011).
The aim of current study was to investigate the changes in ECG signs related to seizure onset, mostly HR, during pre-ictal, ictal, and post-ictal states and implementing an algorithm, CWT, for precise HR determination to analyze HR changes between pre-ictal, ictal, and post-ictal intervals.
2. Methods
Animal
Six adult male WAG/Rij rats and 6 normal Wistar rats with 250±25 g mean body weight and 6–8 month old were enrolled in the study. The animals were cared under laboratory condition [temperature 22 °C, light/dark (12/12 hours) cycle and unlimited access to food and water]. Before surgery, the rats were housed in small groups at one cage but after surgery they were housed singly. We worked on Tabriz University of medical sciences animal ethics.
Surgery
Rats were anaesthetized with the injection of ketamine (60 mg/kg, ip) and xylazine (20 mg/kg, ip) (Gorji, et al, 2011). Two monopolar stainless steel electrodes were implanted in the frontal cortex region (coordinates: AP: 0.22, L: 0.24, V: 0.26) and the other one, the ground electrode, was placed in occipital cortex (Coordinate: AP: −11.04, L: 4 mm) for EEG recording using a stereotaxic instrument underlying Paxinos and Watson atlas (Paxinos & Watson, 2006; Sadighi et al, 2013). The electrodes were fixed with dental cement and the socket was fixed to the skull.
For ECG recording, 2 electrodes were implanted under the chest skin in all WAG/Rij and normal Wistar rats and ECG signals was acquired from lead II (voltage between the right arm and the left leg). The ECG electrodes (BP disposable chest electrodes) were soldered to copper wires. To prevent wire damaging by the rats, they were conducted subcutaneously to back of the rat body and fixed in the socket.
Recording
One week after the surgery, all normal rats were located in a Faraday cage in a freely moving condition for signal recording. The same recording conditions such as recording time, duration, temperature, and lightness of environment were applied to both groups to compare HR results. The head and chest sockets of rats were connected to flexible and shielded copper cables for EEG and ECG recording. Prior to signal recording, we let the rats to adapt to with environment for 30 minutes. Signals were amplified by a DAM 80 AC amplifier (WPI Inc, USA) and digitized by a PowerLab instrument running the Chart software (ver. 05, AD Instrument, Australia) with a sampling frequency of 10 KHz, and finally they were saved as a lab chart formatted data in a computer. The animals were free to move in the plexiglas chamber, only restricted by attached cables. In WAG/Rij rats, SWDs occurs mainly during quiet awakens and drowsiness but rarely during rapid eye movement (REM) sleep or active wakefulness (Kostopoulos, 2009). To prevent the animals from sleeping, they were regularly stimulated with placid touch. Touching was also done for normal rats during drowsiness periods. EEG from normal rats and ECG signals from both types have been recorded simultaneously 7 successive days and 2 hours per day.
Analysis
In this study we have mainly focused on HR evaluation to find a clinical sign of seizure onset. For this purpose, we implemented a CWT based beat detection algorithm for HR determination. The first step for HR calculation is to find similar points in each ECG beat. One of the distinct points of an ECG beat is R-peak which is an important characteristic of ECG and there have been a great effort to find these points in noisy environments with good sensitivity and proper positive predictively (+P). These algorithms are commonly used to find R-R time or HR value. There are various algorithms for R-peak detection based on derivation (Balda et al, 1977: 197–205), digital filters (Borjesson et al, 1982), wavelets (Madeiro et al, 2007), hidden Markov method (HMM) (Andreão et al, 2006), Hilbert transform (Benitez et al, 2001), adaptive filters (Dandapat & Ray, 1997) and so on. The wavelet based algorithms are among those algorithms present desirable performance (Kohler et al, 2002). We have used continuous wavelet transform (CWT) in this study for our experimental databases with a new adaptive approach in which the ECG signal of each animals used to construct the mother wavelet, for the purpose of R-peak detection. To distinguish the effects of epileptic seizures on measures of HR, we have used nested mixed model analysis with nesting measures before and after seizure for each rat as the seizure times of individual rats are independent. Auto regressive 1 (AR1) has been used as the correlation structure.
Continuous wavelet transforms theory
Most of non-stationary biological signals such as ECG signal have different characteristics at different times or frequencies. To analyze these signals, a time-frequency representation of the signal is needed. Wavelet analysis provides a better representation of both time and frequency information of signal simultaneously (Mallat, 1999).
Beat detection algorithm
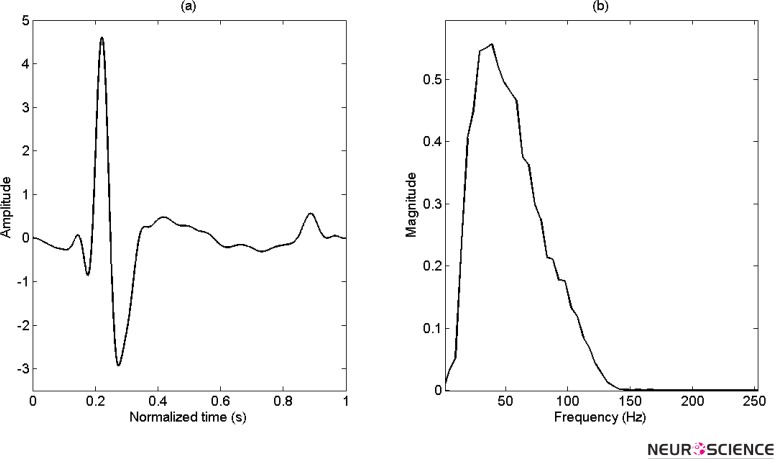
The first step in the beat detection algorithm based on wavelet is to select a mother wavelet. In our study, it is constructed from normal QRS complex signal of every rat that can be adapted for every animal and updated by the time. Figure 1 illustrates one of these normalized mother wavelets in normal condition adjusted to zero mean and restricted energy. For convenience, the frequency spectrum is also illustrated.
Figure 1.
(a) Adapted mother wavelet for specific rat, that normiliezed for 1 sec and (b) Its frequency spectrum.
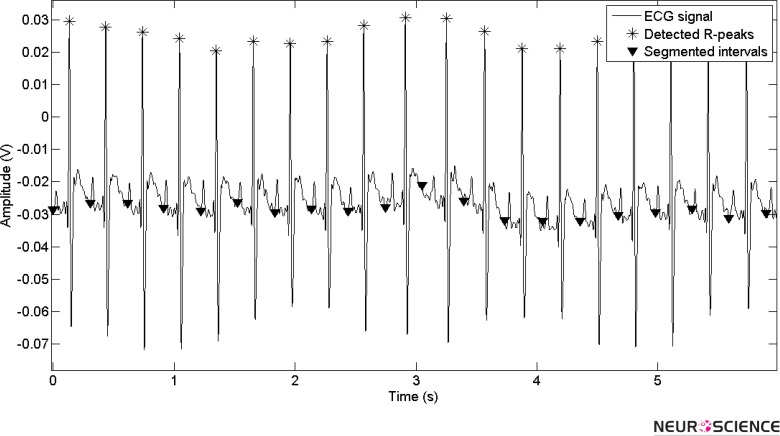
For beat detection, a time series of ECG signal should be transformed to time-frequency plan in special scales. The length of the selected segment for transformation is adapted by time intervals of passed beats and minimum HR of each rat. Each transformed frame is selected to have just one beat for high accuracy. Figure 2 shows the signal segmentation for a specific time of an ECG signal.
Figure 2.
Adapted segmentation based on prior beat points for new beat detection
To extract signal singularities, the wavelet modulus maxima is used for pre-calculated scale, which creates more correlation between wavelet and ECG beats and has the maximum absolute coefficient accorded to the R point.
Using modulus maxima tool and considering that a threshold should be applied to select apexes, the beat point is determined. The threshold is measured by analyzing the experimental data related to features of QRS complex signal of each rat.
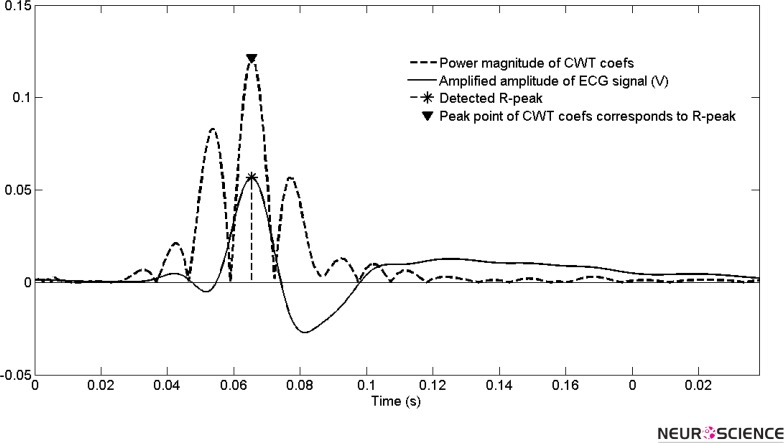
The extracted point is the R-peak of the QRS complex signal. Figure 3 illustrates the power of one period of heart beat at selected scale and its peak points. The modulus maxima tool selects the highest peak points as the R point.
Figure 3.
The power spectrum of wavelet cofficients for one beat and accordance of maximun power to R peak
As explained earlier, because of using ambulatory recording system in our study and being in a lab with typical noise signals, it have been required to measure the amount of noise level in signals. Since the ECG signal power is proportional to the square of HR, the SNR (signal to noise ratio) estimation algorithm introduced by Rooijakkers et al., is used to obtain a measure related to R-peak detection (Rooijakkers, et al, 2012).
Heart rate evaluation
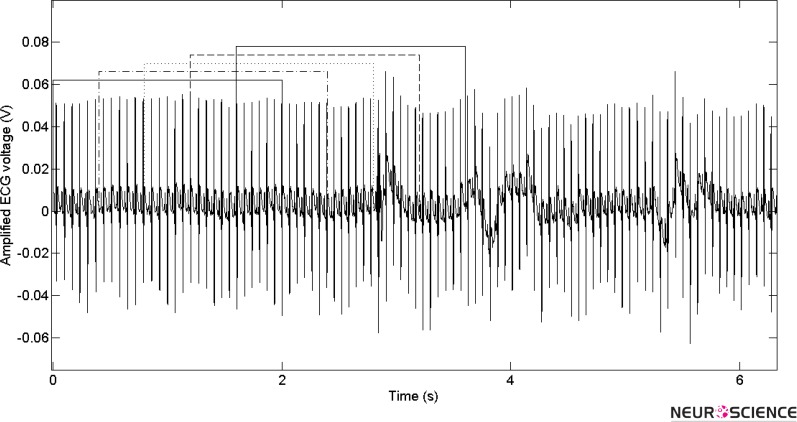
For evaluation of HR, a fixed length window is moved in time, overlapped with prior windows and the total number of beats per minutes (bpm) is counted for every second. Figure 4 shows moving of a fixed length window in time.
Figure 4.
Moving of a fixed length window, overlapping with prior windows through the signal for HR determination.
3. Results
In order to simultaneously investigate EEG signal and HR changes along the seizure times, it is necessary to evaluate the proposed algorithm by standard measurements, TP, the number of correctly detected beats (true positive), FN, the number of missed beats (false negative), FP, the number of falsely detected beats (false positive), and finally S_e and +P that are sensitivity and positive predictive values for all analyzed beats, respectively. These values for 6 WAG/Rij rats have been reported in Table 1. The average SNR for the measured signals was approximately 10dB.
Table 1.
Values of ; true positive, false negative, false positive, sensitivity and ; positive predictability for all analyzed beats.
| Rat number | Number of analyzed beats/1000 | TP | FN | FP | Se (%) | +P (%) |
|---|---|---|---|---|---|---|
| 1 | 309 | 308722 | 278 | 618 | 99.91 | 99.80 |
| 2 | 298 | 297553 | 447 | 566 | 99.85 | 99.81 |
| 3 | 311 | 310627 | 373 | 280 | 99.88 | 99.91 |
| 4 | 287 | 286770 | 230 | 402 | 99.92 | 99.86 |
| 5 | 272 | 271456 | 544 | 598 | 99.80 | 99.78 |
| 6 | 281 | 280691 | 309 | 337 | 99.89 | 99.88 |
| Total | 1758 | 1755819 | 2181 | 2801 | 99.87 | 99.84 |
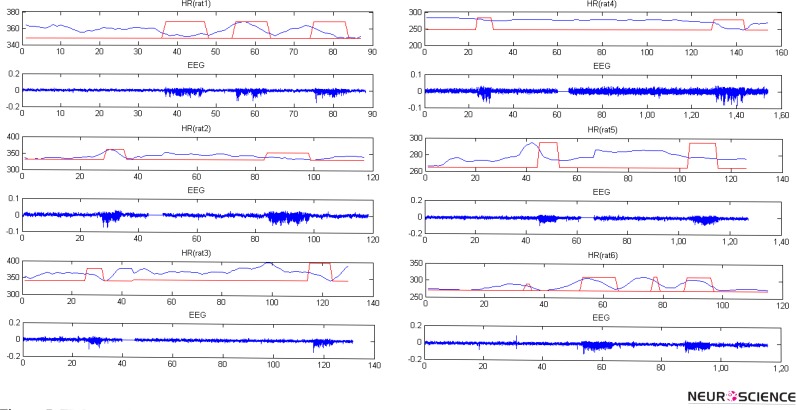
Figure 5 shows EEG signals and HR values for some of these seizures. They are illustrated with rectangular windows in HR plot. There are solid lines without any signals showing discontinuity in EEG and HR. To analyze HR changes from pre-ictal to ictal, it is essential to determine ictal period correctly. Twice the base line level of EEG signal was the criteria for SWDs identification and ictal onset detection.
Figure 5.
EEG signal and HR for 6 WAG\Rij rats. Onset and termination of seizures are shown by rectangular windows for HR. Solid lines show discontinuities in EEG and HR. Horizontal axis is the time of EEG and HR in second and vertical axis is bpm for HR and amplified EEG voltage for EEG plot.
The analysis reports for the WAG/Rij and control rats are summarized in Table 2 and 3, respectively. The mean, minimum, and maximum values of HR for all seizures of WAG/Rij rats at pre-ictal and ictal intervals and also their durations are summarized in Table 2. As shown in figure 5 and reported in this table, there are reductions in HR values (and as a result in its mean, minimum, and maximum) during the seizure compared to HR mean, minimum and maximum of preictal intervals.
Table 2.
HR Analysis results for 6 WAG\Rij rats. In columns 3 through 9, the values are mean of each parameter with their SD.
| Rat number | Number of seizures | Duration of Seizure (s) | Minimum preictal-HR (bpm) | Mean preictal-HR (bpm) | Maximum preictal-HR (bpm) | Minimum ictal-HR (bpm) | Mean ictal-HR (bpm) | Maximum ictal-HR (bpm) | Seizures with decrease (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 104 | 10±4 | 358±13 | 369±11 | 380±12 | 342±12 | 355±13 | 364±12 | 90 |
| 2 | 89 | 11±2 | 348±10 | 363±15 | 385±12 | 328±13 | 342±10 | 361±11 | 87 |
| 3 | 95 | 9±3 | 385±11 | 393±14 | 415±15 | 360±16 | 370±14 | 392±13 | 91 |
| 4 | 110 | 4±2 | 294±17 | 296±18 | 299±18 | 247±19 | 270±20 | 281±16 | 85 |
| 5 | 117 | 13±3 | 286±8 | 292±10 | 314±9 | 272±7 | 281±7 | 291±12 | 84 |
| 6 | 130 | 13±4 | 287±9 | 302±13 | 330±14 | 265±8 | 285±11 | 302±13 | 85 |
| Total | 645 | 10±4 | 322±15 | 331±13 | 350±13 | 298±18a | 313±15b | 327±16c | 86 |
Significant decrease from minimum preictal-HR P<0.001
Significant decrease from mean preictal-HR P<0.001
Significant decrease from maximum preictal-HR P<0.001
Table 3.
HR Analysis results for normal Wistar rats in durations of 10 second. In columns 3 through 5, the values are mean of each parameter with their SD.
| Rat number | Number of analyzed durations | Minimum HR (bpm) | Mean HR (bpm) | Maximum HR (bpm) |
|---|---|---|---|---|
| 1 | 93 | 272±6 | 277±5 | 283±5 |
| 2 | 88 | 265±4 | 270±6 | 272±5 |
| 3 | 105 | 284±7 | 288±6 | 295±8 |
| 4 | 100 | 297±3 | 299±5 | 305±9 |
| 5 | 85 | 280±9 | 286±7 | 293±10 |
| 6 | 97 | 293±6 | 298±4 | 300±5 |
| Total | 568 | 282±7 | 286±5 | 291±6 |
Percentages of the seizures following this change are also listed in Table 2. The results show statistical significance (P<0.001) for these three main measurements of HR at seizure time. The statistical analysis reports of HR changes are given in table 2.
The analysis reports for normal Wistar rats are listed in Table 3. The surveys show that there are no sudden changes for HR in normal Wistar rats. Moreover, they have smooth alterations with respect to even small changes in normal conditions.
4. Discussion
To reach the aim of this study, which is to find the possibility of ECG signal changes simultaneous with absence seizure onset, we have analyzed ECG signals in animal models for absence epilepsy and used EEG signals associated with SWDs with frequencies of 7–11 Hz as a seizure indicator. HR extracted from ECG is the most important parameter impressed by autonomic nervous system (ANS) and its sympathetic and para-sympathetic branches (Pradhan et al, 2011). Seizures influence these parts of neural system. In all of WAG/Rij rats, we observed HR alterations at seizure times.
To calculate HR from ECG signal, it is necessary to detect beats with high accuracy. For this purpose, we have implemented an adaptable algorithm to our experimental database which has 99.9% of sensitivity and is immune to noise and DC offset because of using time-frequency analysis. Since mother wavelets are constructed from the beats of each subject, the algorithm is adapted and customized to each rat.
There were HR changes in all of seizures in our study and the results are in accordance with those obtained in former studies (Leutmezer et al, 2003; Opherk et al, 2002; Pradhan et al, 2011). Analysis of all seizures recorded from the subjects have showed significant (P<0.001) reduction of minimum, mean and maximum of HR in preictal to ictal intervals and this reduction is observable in 86% of all seizures. HR deceleration had been reported in 66.7% of patients with complex partial and generalized seizures by Vaughn et al., (Vaughn et al, 1996). This deceleration may lead to ictal bradycardia (IB), which is in some cases (23%) arrives earlier than EEG and is in accordance with former works about human (Howell & Blumhardt, 1989) and animal (Nielsen et al, 2008: 290–293; Kerem et al, 2005) subjects.
Interictal tachycardia observed in signals, has been increased by shortening the interval, and leads to preictal tachycardia. This together with later ictal HR deceleration made parabolic curve patterns (figure 5 (rat 1, 6)). Like human subjects (Leutmezer et al, 2003) studied before, tachycardia has had priority over seizure onset and preictal tachycardia had been also reported by Vaughn et al., and Leutmezer et al, for 50% of patients (Vaughn et al, 1996) and for 75% of seizures (Leutmezer et al, 2003), respectively. Since special care is needed for patients with IB in clinics and there is no access to EEG signals from these patients (Tinuper et al, 2001) and implanted cardiac pacemakers are usually employed, IB have not been reported more often in literature. Studying animal models of different kinds of epilepsy instead of human subjects enables us to investigate properly those cases leading to dangerous ictal systoles. Implantable loop-recorders are also another efficient way of gathering valuable data from these patients, especially from animals because of its convenience. It has been reported by Rugg-Gunn et al., using these kinds of systems IB incidence was more than 7 times higher (36.7% vs. 5%) ((Rugg-Gunn et al, 2004). Therefore, probable role of cardiac alterations caused by seizures in SUDEP (Devinsky, 2011) can be studied precisely by these animal models.
To ensure that these HR changes occur due to epileptic seizures, the 2 rat groups were held in the same conditions with similar recordings, so the differences between HR variations show the effects of discharges in epileptic rats. HR for normal Wistar rats had maximum change of 4% in our study, which is low in comparison with patient ones. Therefore, tachycardia, bradycardia, and HR alterations have been defined clearly for animal, to design a complete model for deepening our knowledge about epileptic seizures, their effects on ECG, and SUDEP. This study is a suitable platform on the side-effects of AED on cardiovascular system and SUDEP for future experiments and it could help developing more efficient seizure alarm systems based on HR with non-invasive ECG probes.
Ictal cardiac changes are perhaps due to autonomic discharges triggered by epileptic seizures, and inputs from cortical structures to autonomic nervous system and changes in sympathetic and parasympathetic branches are possible reasons. Thalamus role in seizure generalization had been reported earlier (Steriade & Amzica, 2003) and its impression on HR decrease and increase has been shown in human and animal subjects (Mameli, et al, 2006). HR decrease in ictal and even in post-ictal periods are possible evidences for the involvement of thalamus subcortical circuit as the last region of electrical path in absence epilepsy, in HR change.
Interictal spikes with small durations can be seen in figure 5. Like other spikes, they can also arrive as inputs from cortical structures to ANS and make interictal cardiac alterations (fig. 5).
An algorithm for HR determination with sensitivity of 99.9% was developed based on the CWT and statistical features were investigated to distinguish ictal from preictal intervals. A complete experimental platform for investigations on ictal cardiovascular changes, SUDEP, side-effects of AEDs and seizure alarm systems have been proposed based on animal epilepsy models.
This study shows a decrement in HR value at seizure times for WAG\Rij rats, genetic animal models of absence epilepsy. More cases are needed to obtain comprehensive results. Moreover, human subjects will be investigated in the near future to validate the current results.
Acknowledgements
This research was financially supported by the School of Advance Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran (grant number: 91-6-13).
References
- Andreão R. V., Dorizzi B., Boudy J. (2006). ECG signal analysis through hidden Markov models. IEEE Transactions on Biomedical Engineering, 53 ( 8), 1541– 1549. [DOI] [PubMed] [Google Scholar]
- Angeles D. K. (1981). Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia, 22 ( 4), 489– 501. [DOI] [PubMed] [Google Scholar]
- Balda R. A., VanBemnel J. H., Willems J. L. (1977). Trends in Computer-Processed Electrocardiograms. In Proceedings of the IFIP Working Conference on Trends in Computer-Processed Electrocardiograms, Amsterdam, Holand. [Google Scholar]
- Benitez D., Gaydecki P. A., Zaidi A., Fitzpatrick A. P. (2001). The use of the Hilbert transform in ECG signal analysis. Computers in Biology and Medicine, 31 ( 5), 399– 406. [DOI] [PubMed] [Google Scholar]
- Borjesson P. O., Pahlm O., Sornmo L., Nygards M. E. (1982). Adaptive QRS detection based on maximum a posteriori estimation. IEEE Transactions on Biomedical Engineering, ( 5), 341–351 . [DOI] [PubMed] [Google Scholar]
- Casson A. J., Smith S., Duncan J. S., Rodriguez-Villegas E. (2008). Wearable EEG: what is it, why is it needed and what does it entail? Paper presented at the Engineering in medicine and biology society, 30th Annual International Conference of the Ieee . [DOI] [PubMed] [Google Scholar]
- Crunelli V., Leresche N. (2002). Childhood absence epilepsy: genes, channels, neurons and networks. Nature Reviews Neuroscience, 3 ( 5), 371– 382. [DOI] [PubMed] [Google Scholar]
- Dandapat S., Ray G. C. (1997). Spike detection in biomedical signals using midprediction filter. Medical and Biological Engineering and Computing, 35 ( 4), 354– 360. [DOI] [PubMed] [Google Scholar]
- Devinsky O. (2011). Sudden, unexpected death in epilepsy. New England Journal of Medicine, 365 ( 19), 1801– 1811. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Boas W. V. E., Blume W., Elger C., Genton P., Lee P., Engel J. (2005). Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia, 46 ( 4), 470– 472. [DOI] [PubMed] [Google Scholar]
- Goldberger A. L., Amaral L. A., Glass L., Hausdorff J. M., Ivanov P. C., Mark R. G., Stanley H. E., et al. (2000). Physiobank, physiotoolkit, and physionet components of a new research resource for complex physiologic signals. Circulation, 101 ( 23), e215– e220. [DOI] [PubMed] [Google Scholar]
- Gorji A., Mittag C., Shahabi P., Seidenbecher T., Pape H.-C. (2011). Seizure-related activity of intralaminar thalamic neurons in a genetic model of absence epilepsy. Neurobiology of Disease, 43 ( 1), 266– 274. [DOI] [PubMed] [Google Scholar]
- Greene B. R., Boylan G. B., Reilly R. B., de Chazal P., Connolly S. (2007). Combination of EEG and ECG for improved automatic neonatal seizure detection. Clinical Neurophysiology, 118 ( 6), 1348– 1359. [DOI] [PubMed] [Google Scholar]
- Greene B. R., de Chazal P., Boylan G. B., Connolly S., Reilly R. B. (2007). Electrocardiogram based neonatal seizure detection. IEEE Transactions on Biomedical Engineering, 54( 4), 673–682 . [DOI] [PubMed] [Google Scholar]
- Güler I. N., Übeyli E. D. (2004). Application of adaptive neuro-fuzzy inference system for detection of electrocardiographic changes in patients with partial epilepsy using feature extraction. Expert Systems with Applications, 27 ( 3), 323– 330. [Google Scholar]
- Howell S. J., Blumhardt L. D. (1989). Cardiac asystole associated with epileptic seizures: a case report with simultaneous EEG and ECG. Journal of Neurology, Neurosurgery & Psychiatry, 52 ( 6), 795– 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem D. H., Geva A. B. (2005). Forecasting epilepsy from the heart rate signal. Medical and Biological Engineering and Computing, 43 ( 2), 230– 239. [DOI] [PubMed] [Google Scholar]
- Kohler B. U., Hennig C., Orglmeister R. (2002). The principles of software QRS detection. Engineering in Medicine and Biology Magazine, IEEE, 21 ( 1), 42– 57. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G. (2009). Brain mechanisms linking epilepsy to sleep. Encyclopedia of Basic Epilepsy Research, 3, 1327– 1336. [Google Scholar]
- Leutmezer F., Schernthaner C., Lurger S., Pötzelberger K., Baumgartner C. (2003). Electrocardiographic changes at the onset of epileptic seizures. Epilepsia, 44 ( 3), 348– 354. [DOI] [PubMed] [Google Scholar]
- Madeiro J. P., Cortez P. C., Oliveira F. I., Siqueira R. S. (2007). A new approach to QRS segmentation based on wavelet bases and adaptive threshold technique. Medical Engineering & Physics, 29 ( 1), 26– 37. [DOI] [PubMed] [Google Scholar]
- Mallat S. (1999). A wavelet tour of signal processing: Academic press. [Google Scholar]
- Mameli O., Caria M. A., Pintus A., Padua G., Mameli S. (2006). Sudden death in epilepsy: an experimental animal model. Seizure, 15 ( 5), 275– 287. [DOI] [PubMed] [Google Scholar]
- Nielsen K. R., Sevcencu C., Rasmussen A., Struijk J. J. (2008, January). Prediction of epileptic seizures for on-demand vagus nerve stimulation . In 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics Springer Berlin Heidelberg . [Google Scholar]
- Opherk C., Coromilas J., Hirsch L. J. (2002). Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Research, 52 ( 2), 117– 127. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (2006). The rat brain in stereotaxic coordinates: hard cover edition: Academic press . [Google Scholar]
- Pradhan C., Sinha S., Thennarasu K., Jagadisha T. (2011). Quantitative analysis of heart rate variability in patients with absence epilepsy. Neurology India, 59 ( 1), 25. [DOI] [PubMed] [Google Scholar]
- Rooijakkers M. J., Rabotti C., Oei S. G., Mischi M. (2012). Low-complexity R-peak detection for ambulatory fetal monitoring. Physiological Measurement, 33 ( 7), 1135. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn F. J., Simister R. J., Squirrell M., Holdright D. R., Duncan J. S. (2004). Cardiac arrhythmias in focal epilepsy: a prospective long-term study. The Lancet, 364 ( 9452), 2212– 2219. [DOI] [PubMed] [Google Scholar]
- Sadighi M., Shahabi P., Oryan S., Pakdel F. G., Asghari M., Pshapour A. (2013). Effect of low frequency electrical stimulation on spike and wave discharges of perioral somatosensory cortex in WAG/Rij rats. Pathophysiology, 20 ( 3), 171– 176. [DOI] [PubMed] [Google Scholar]
- Sarkisova K., van Luijtelaar G. (2011). The WAG/Rij strain: a genetic animal model of absence epilepsy with comorbidity of depressiony. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35 ( 4), 854– 876. [DOI] [PubMed] [Google Scholar]
- Sevcencu C., Struijk J. J. (2010). Autonomic alterations and cardiac changes in epilepsy. Epilepsia, 51 ( 5), 725– 737. [DOI] [PubMed] [Google Scholar]
- Steriade M., Amzica F. (2003). Sleep oscillations developing into seizures in corticothalamic systems. Epilepsia, 44 ( s12), 9– 20. [DOI] [PubMed] [Google Scholar]
- Tinuper P., Bisulli F., Cerullo A., Carcangiu R., Marini C., Pierangeli G., Cortelli P. (2001). Ictal bradycardia in partial epileptic seizures Autonomic investigation in three cases and literature review. Brain, 124 ( 12), 2361– 2371. [DOI] [PubMed] [Google Scholar]
- Übeyli E. D., Güler İ. (2004). Detection of electrocardiographic changes in partial epileptic patients using Lyapunov exponents with multilayer perceptron neural networks. Engineering Applications of Artificial Intelligence, 17 ( 6), 567– 576. [Google Scholar]
- Übeyli E. D. (2008). Support vector machines for detection of electrocardiographic changes in partial epileptic patients. Engineering Applications of Artificial Intelligence, 21 ( 8), 1196– 1203. [Google Scholar]
- Übeyli E. D. (2008). Recurrent neural networks with composite features for detection of electrocardiographic changes in partial epileptic patients. Computers in Biology and Medicine, 38 ( 3), 401– 410. [DOI] [PubMed] [Google Scholar]
- Übeyli E. D. (2009). Eigenvector methods for automated detection of electrocardiographic changes in partial epileptic patients. IEEE Transactions on Information Technology in Biomedicine, 13 ( 4), 478– 485. [DOI] [PubMed] [Google Scholar]
- Vaughn B. V., Quint S. R., Tennison M. B., Messenheimer J. A. (1996). Monitoring heart period variability changes during seizures. II. Diversity and trends. Journal of Epilepsy, 9 ( 1), 27– 34. [Google Scholar]
- World Health Organization (2005). Atlas: epilepsy care in the world. [Google Scholar]