Abstract
Introduction:
The hippocampus (HIP), the primary brain structure related to learning and memory, receives sparse but comprehensive dopamine innervations and contains dopamine D1/D2-like receptors. It is demonstrated that dopamine receptors in dentate gyrus (DG) region of HIP have a remarkable function in spatial reward processing. Much less is known about the involvement of HIP and its D1/D2 dopamine receptors in drug-seeking behaviors, more particularly, in the morphine extinguished conditioned place preference (CPP).
Methods:
To find out the role of D1/D2-like receptors within the DG in morphine-seeking behaviors, forty adult male albino Wistar rats weighing 220–280g were unilaterally implanted by a cannula into the DG. The CPP paradigm was done; conditioning score and locomotors activity were recorded by Ethovision software. All drugs/vehicles were microinjected one day after extinction (just before the CPP test) into the DG as reinstatement day.
Results:
The results showed that intra-DG administration of different dose of SCH23390 (0.25, 1 and 4μg/0.5μl saline), as a selective D1-like receptor antagonist and sulpiride (0.25, 1 and 4μg/0.5μl DMSO), as a selective D2-like receptor antagonist dose-dependently attenuated the morphine-extinguished CPP reinstated by priming injection of morphine (1 mg/kg;sc).
Discussion:
It can be concluded that D1/D2-like receptors within this region have an important role in morphine-seeking behaviors in extinguished rats.
Keywords: Reward, D1-like receptor, D2-like receptor, Dentate gyrus, Reinstatement, Morphine
1. Introduction
Relapsing to opioid after abstinence therapy is a major clinical problem in addicted people who were undergoing detoxification (O’Brien, 1997). Drug desiring is a subjective feeling which results in drug seeking and can produce relapse to drug abuse even long-time after withdrawal. It has clarified that the dopamine mesolimbic (DA) system is the principal pathway that can cause opioids’ psychological dependency (Wise, 2002). Many studies have revealed the mesocorticolimbic DA system contribution to the acute reinforcing effects of opioids (Imenshahidi, Qaredashi, Hashemzaei, & Hosseinzadeh, 2014). It is now well-established that almost all drugs converge on a common circuitry in the brain which includes ventral tegmental area (VTA), nucleus accumbens (NAc), hippocampus (HIP), hypothalamus and prefrontal cortex (Koob & Simon, 2009). The mesolimbic DA system is necessary for the acquisition of morphine-induced CPP (Wise, 2002). The HIP and VTA form a dopaminergic loop, which may regulate some kind of information into the long-term memory that affects hippocampal-dependent learning (Lisman & Grace, 2005). Dopamine D2-like receptors have an important role in functional interaction between the VTA and HIP in the expression of morphine CPP (O’Donnell, 2003). Activation of the VTA increases DA release in the HIP and seems to have a pivotal role in hippocampal plasticity. Furthermore, systemic administration of selective D2-like dopamine receptor agonists promotes reinstatement of drug-seeking (De Vries, Schoffelmeer, Binnekade, Raaso, & Vanderschuren, 2001) while selective D2-like dopamine receptor antagonists attenuate the cocaine priming-induced reinstatement (Schenk & Gittings, 2003). Similarly, systemically administered D1-like dopamine receptor antagonists attenuate reinstatement of drug-seeking elicited by a priming injection of cocaine (Schenk & Gittings, 2003).
Dentate granule cells are considered the “gatekeepers” of the hippocampal formation (Hamilton et al., 2010). Anatomical, electrophysiological, behavioral and computational evidence supports the heterogeneity of the hippocampal regions with regard to information processing and memory function, with the dentate gyrus (DG) being most probably involved in sustaining a primary representation of space during learning and conveying a new pattern of neural activity to CA3 for each context-dependent instance of episodic memory (Hernández-Rabaza, Barcia, Llorens-Martín, Trejo, & Canales, 2007). The VTA dopaminergic fibers are unique among biogenic amines innervating the HIP in that they participate in a reward detection system that promotes learning. During exploratory behaviors, when gamma-frequency activity occurs in the DG fire at high rates, the reward salience of an external stimulus is signaled by release of DA from the VTA projections to the molecular layer of the DG (Hamilton et al., 2010). In addition, the HIP has an important role in reward-related learning task such as conditioned place preference (CPP) paradigm (Riahi, Khodagholi, & Haghparast, 2013). CPP is a model of context conditioned reward (Hernandez-Rabaza et al., 2008) used in animal experiments to evaluate performance for stimuli of environment that have been associated with a positive or negative reward (Liu, Jiang, Zhong, Wu, & Luo, 2010). Moreover, CPP is widely used to study the rewarding effects of morphine and is extensively used to assess the reward-related learning in rats. It was reported that the injection of morphine directly into the HIP induced CPP (Corrigall & Linseman, 1988) and also, it has been shown that the acquisition and expression of cocaine-induced CPP are intercepted by colchicine-induced lesion of the DG (Hernandez-Rabaza et al., 2008). Considering the mechanisms underlying the effects of D1- and D2-like receptors on the CPP remain unclear, we tried to find out the role of these receptors within the DG in the morphine-seeking behaviors of the priming-induced reinstatement of morphine-CPP in its extinguished rats.
2. Methods
2.1. Animals
Forty adult male albino Wistar rats (Pasteur Institute, Iran), weighing 220–280g were used in this study. The animals were randomly kept in groups of three with free access to chow and tap water. The vivarium was maintained at 12:12h light/dark cycle and controlled temperature (23±1ºc). All experiments were executed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
2.2. Stereotaxic surgery
For stereotaxic implantation of cannulae, the rats were deeply anaesthetized with intraperitoneal injection of a mixture of Ketamine and Xylazine (100 and 10 mg/kg, respectively) and placed in a stereotaxic apparatus (Stoelting, USA). An incision was made along the mid-line, the scalp was retracted, and the area surrounding bregma was cleaned and dried. Stainless steel guide cannulae were unilaterally implanted into 1mm above the DG according to atlas of the rat brain (Paxinos & Watson, 2007). Stereotaxic coordinates for the DG were AP=3.85±0.15 mm caudal to bregma, Lat=± 0.9 mm lateral to midline, DV=3.1±0.2 mm ventral from the skull surface (cannula 23-gauge, 9mm). The guide cannulae were secured in their places using two stainless steel screws anchored to the skull and dental acrylic cement. After the cement was completely dried and hardened, a stainless steel obdurator was inserted into each guide cannula to prevent occlusion. After 5–7 days of recovery animals were used for the experiments.
2.3. Drugs
In this study, the used drugs were: Morphine sulfate (TEMAD, Iran), SCH23390 (Tocris Bioscience, UK), selective D1-like receptor antagonist, and Sulpiride (Tocris Bioscience, UK), selective D2-like receptor antagonist which were dissolved in sterile saline (0.9%) and 12% dimethyl sulfoxide (DMSO; Sigma Aldrich, Germany), respectively. In separate control groups, animals received either saline or 12% DMSO as a vehicle into the DG.
2.4. Conditioning place preference paradigm
CPP paradigm was used to evaluate the effects of intra-DG administration of SCH23390 and sulpiride on morphine priming-induced reinstatement in conditioned place preference. This paradigm has been described in our previous works in detail (Karimi et al., 2014). However, in this study, it is explained briefly.
2.4.1. Apparatus
The CPP apparatus was made of plastic and consisted of three compartments divided by sliding doors; two compartments were identical in size (30×30×40 cm) but differed in shading and texture. One compartment had vertical stripes on the walls with smooth floor and the other had horizontal wall stripes with a textured floor. Time spent in each compartment and motor activity were monitored and recorded by a 3CCD camera (Panasonic Inc., Japan) that put 2 m above the apparatus.
2.4.2. Protocol
The CPP schedule consisted of three phases (pre-conditioning, conditioning, and post-conditioning phases). In pre-conditioning day 1, each animal was placed in the start box with the guillotine door removed and rats were allowed to move freely in all compartments for 10-min period. Conditioning phase started the day after pre-conditioning test and consisted of six, 30-min sessions (three saline and three drug pairing) in a 3-day schedule (Fig. 1A). Animals received single subcutaneous (sc) dose of morphine (5 mg/kg) or saline (1 ml/kg) in the morning or evening session every day in counterbalance manner (Riahi et al., 2013), and immediately confined to the drug-or saline-paired compartment for 30 min by closing the removable wall, respectively. On post-conditioning day (day 5), in a morphine free state, rats were placed in the CPP box and were tested for CPP with free access to all compartments. The time spent in each compartment during the 10-min period was recorded by a 3CCD camera and analyzed using the Ethovision software (Version 7). Difference(s) of the time spent in the morphine- and saline-paired compartments considered as conditioning score (CPP score). No injection was given on the test day. Total distance traveled (locomotor activity) by each animal was also recorded in control and experimental groups.
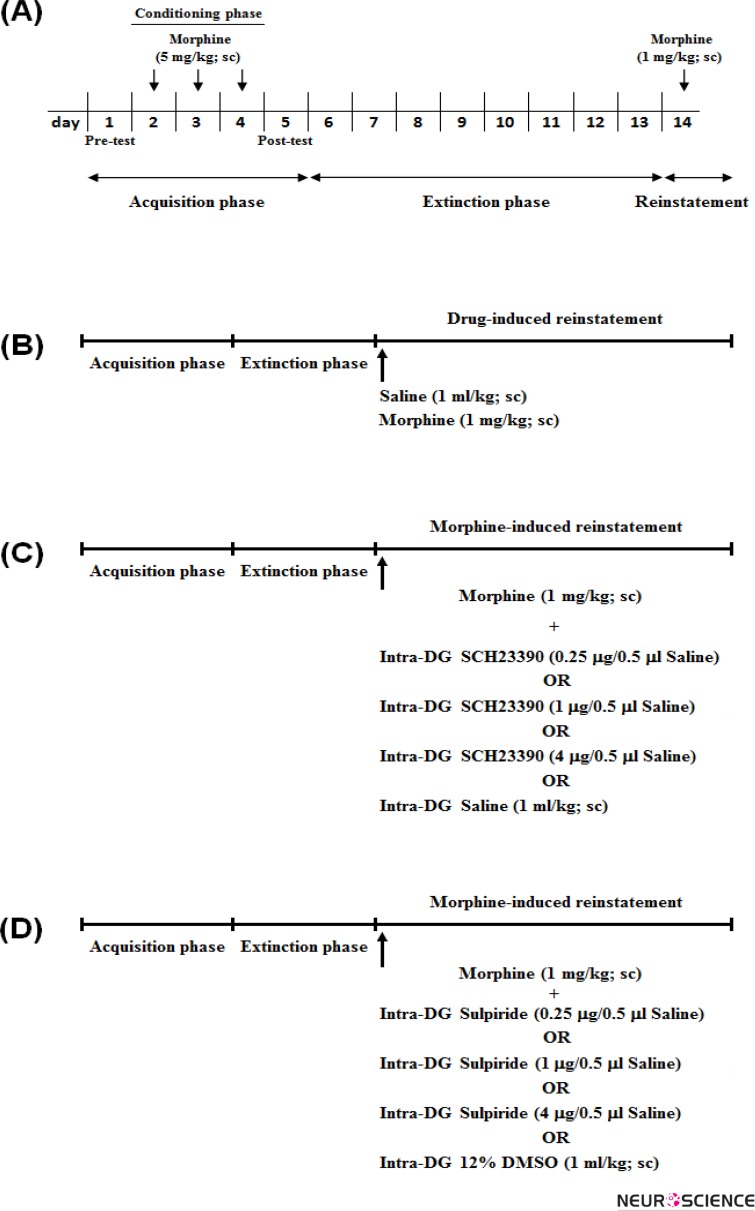
Figure 1.
The control and experimental groups, used in the present study, received their treatments (vehicles or D1/D2-like receptor antagonists) during the reinstatement phase. (A) Schematic time-line for showing the conditioned place preference (CPP) protocol and its phases. Animals in all control and experimental groups received the effective dose of subcutaneous (sc) morphine (5 mg/kg) during 3-day conditioning phase for induction of reward-related behaviors in the CPP paradigm. Then, in the extinction phase (eight days), animals had access to all compartments in the CPP apparatus without any drug injection for 30 min each day. The day after extinction was reinstatement phase. In the reinstatement phase, the preference to drug-paired chamber can be reinstated by using (B) priming/ineffective dose of morphine (5 mg/kg; sc), (C) different doses of SCH-23390/vehicle, and (D) different doses of sulpiride/vehicle in the morphine-extinguished rats.
2.4.3. Extinction (Maintenance) experiment
Following establishment of CPP, in a morphine free state, the rats were daily placed into the CPP box and the time spent in each compartment was recorded by Ethovision every day (Fig. 1A). This procedure was repeated for each animal in control and experimental groups until the calculated CPP score in two consecutive days during extinction period became similar to those on the preconditioning day (Attarzadeh-Yazdi, Arezoomandan, & Haghparast, 2014). Thus, the criterion for maintenance of morphine rewarding properties in all groups was a lack of significant differences in CPP scores between two consecutive days in the extinction period and CPP score on pre-conditioning day.
2.4.4. Reinstatement experiment
In this set of experiment, 24h after the last extinction day, rats received a priming dose of morphine (1mg/kg,sc) and were immediately placed in the CPP box with access to the entire apparatus for 10-min period (Fig. 1B). The CPP score and locomotors activity for each animal were separately recorded in each compartment during this period.
2.5. Experimental design
2.5.1. Effect of D1-like receptor antagonist micro-injected into the DG on reinstatement of morphine-extinguished CPP in rats
To find out the role of D1-like receptor within the DG in morphine priming-induced reinstatement, 24h after the last extinction day, animals received different doses of SCH23390 (0.25, 1 and 4μg/0.5μl saline) into the DG, 5 min before injection of priming dose of 1 mg/kg morphine (Fig. 1C). In control group, saline was injected into the DG, and immediately after injection of priming dose of morphine, animals were placed in the CPP box with free access to the entire apparatus, and time spent and distance traveled were recorded in each compartment for 10-min period.
2.5.2. Effect of D2-like receptor antagonist micro-injected into the DG on reinstatement of morphine-extinguished CPP in rats
In this set of experiments, we examined the effect of intra-DG administration of D2-like receptor antagonist on seeking behaviors in morphine-extinguished rats. 24h after the last extinction day, animals received different doses of sulpiride (0.25, 1 and 4μg/0.5μl DMSO) into the DG, 5 min before injection of priming dose of morphine (Fig. 1D). In control group, animals received 12% DMSO instead of sulpiride into the DG, and were placed in the CPP box for 10-min period after injection of priming dose of morphine. The time spent and distance traveled in each compartment, and conditioning score were recorded and analyzed during this period.
2.6. Histological verification
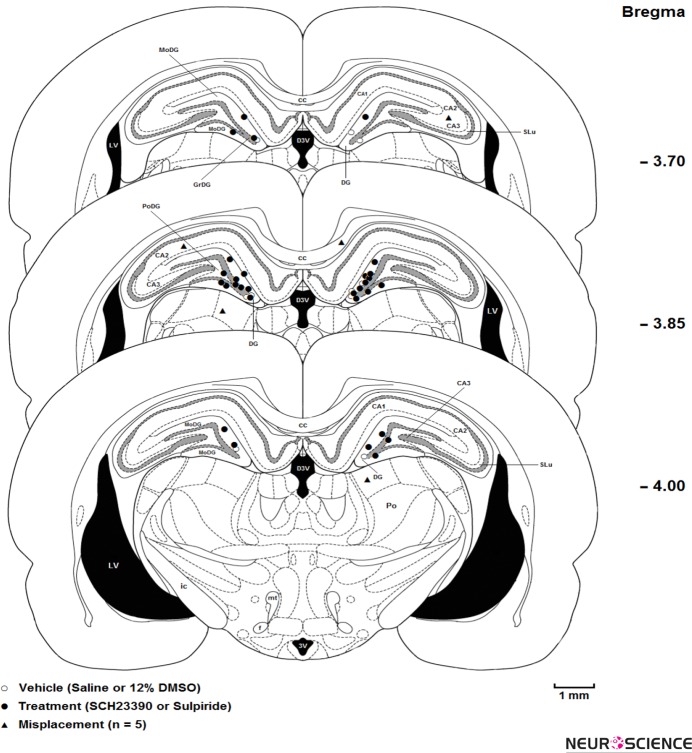
After completion of experimental sessions, animals were deeply anesthetized with Ketamine and Xylazine. Then, they were transcardially perfused with 0.9% saline and 10% formalin solution. The brains were removed, blocked and cut coronally in 50-μm sections through the cannula placements. Sections were examined to determine the location of the cannulae aimed for the DG according to the rat brain atlas (Paxinos & Watson, 2007; Fig. 2). Data from animals with injections sites located outside the DG region were excluded (n=5) from the statistical analysis.
Figure 2.
Three coronal schematic microinjection sites in the dentate gyrus (○ = saline or DMSO microinjection and • = SCH23390 or Sulpride microinjection and ▴ = misplacement). All microinjections were performed bilaterally. cc, corpus callosum; CA1, field CA1 of hippocampus; CA2, field CA2 of hippocampus; CA3, field CA3 of hippocampus; 3V, 3rd ventricle; D3V, dorsal 3rd ventricle; LV, lateral ventricle; and DG, dentate gyrus; SLu, stratum lucidum of the hippocampus; GrDG, granular layer of the dentate gyrus; MoDG, molecular layer of dentate gyrus; PoDG, polymorph layer of dentate gyrus, Po; posterior thalamic nuclear group; ic, internal capsule; mt, mammillothalamic tract; f, fornix. Scale bare is 1mm.
2.7. Statistics
Data are expressed as mean ±SEM (standard error of mean). CPP score represents the difference of time spent in the morphine- and saline-paired compartments. Locomotors activity is expressed as total distance traveled (mean ±SEM) that the rats passed through the entire of CPP apparatus. The data were processed by commercially available software Graph Pad Prism®5.0. In order to compare the CPP scores and distance traveled obtained in all vehicle and experimental, repeated measures/block randomized one-way analysis of variance (ANOVA) followed by post-hoc analysis (Dunnett’s or Newman-Keuls’ test) was used, as appropriated. P-value less than 0.05 (P<0.05) was considered to be statistically significant.
3. Results
3.1. Effect of D1-like receptor antagonist microinjected into the DG on reinstatement of morphine-extinguished CPP in rats
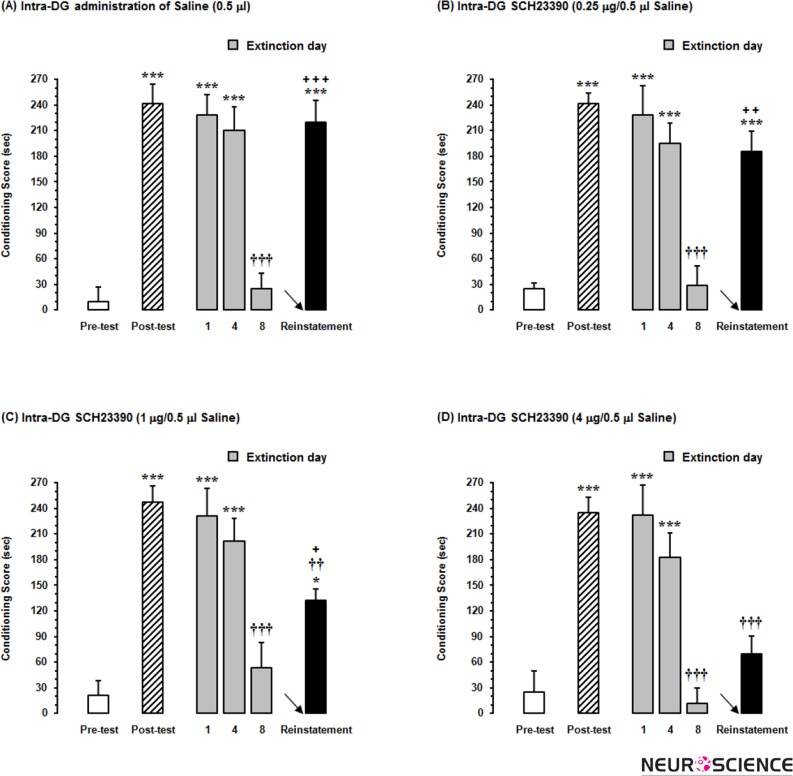
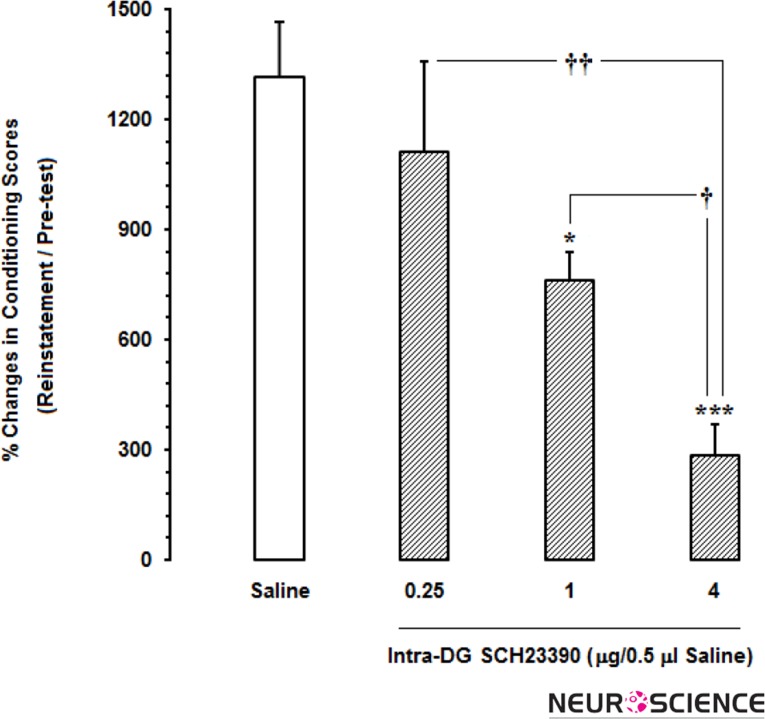
In this set of experiments, we examined the effects of different doses of SCH23390 as D1-like receptor antagonist microinjected into the DG on seeking behaviors in morphine-extinguished rats. As shown in Fig. 3A, microinjection of saline as a vehicle into the DG (0.5μl) just 5 min prior to administration of the priming dose of morphine (1 mg/kg;sc) didn’t affect the reinstatement of morphine-extinguished CPP, and the conditioning scores in this phase significantly (P<0.001) increased after the extinction [F(5,29)=22.04, P<0.0001]. Nevertheless, repeated measures one-way ANOVA followed by Dunnett’s multiple comparison test revealed that the concurrent intra-DG administration of different doses of SCH23390 and priming dose of morphine decreased CPP scores in reinstatement phase as compared to post-test day in a dose-dependent manner. Although the lowest dose of this antagonist could not decrease the place preference of morphine-extinguished rats in reinstatement day as compared to that in post-test day (Fig. 3B), this effect was statistically significant at the dose of 1 μg [F(5,29)=19.9, P<0.0001; Fig. 3C] and 4 μg [F(5,29)=16.58, P<0.0001; Fig. 3D] SCH23390 in rats. On the other hand, block randomized one-way ANOVA followed by Newman-Keuls test [F(3,19)=8.53, P=0.0013] showed that % changes of conditioning scores calculated in reinstatement day to pre-test day dose-dependently decreased by increase in dosage of this antagonist (Fig. 4).
Figure 3.
The effects of administration of (A) saline as a vehicle and different doses of SCH23390 as selective D1-like receptor antagonist, (B) 0.25μg (C) 1μg and (D) 4μg per 0.5μl saline into the dentate gyrus (DG), 5 min before injection of priming dose of morphine (1 mg/kg;sc) in reinstatement day (24h after the last extinction day) dose-dependently decreased the conditioning score as compared to that in post-test day. Each column represents the mean ±SEM of 5–6 rats.
*P<0.05 and ***P<0.001 compared with pre-test day
††P<0.01 and †††P<0.001 compared with post-test day
+P<0.05, ++P<0.01 and +++P<0.001 compared with the last day of extinction period
Figure 4.
The effects of administration of different doses of SCH23390 as selective D1-like receptor antagonist into the dentate gyrus (DG) on the percentage of CPP scores calculated in reinstatement day to pre-test day.
*P<0.05 and ***P<0.001 compared with saline-treated group
†P<0.05 and ††P<0.01
3.2. Effect of D2-like receptor antagonist microinjected into the DG on reinstatement of morphine-extinguished CPP in rats
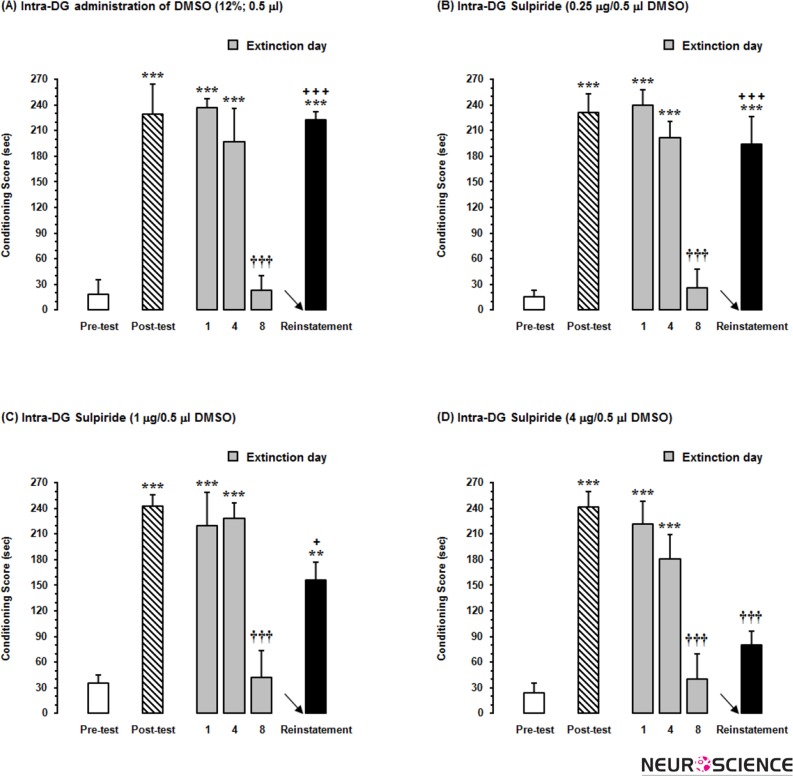
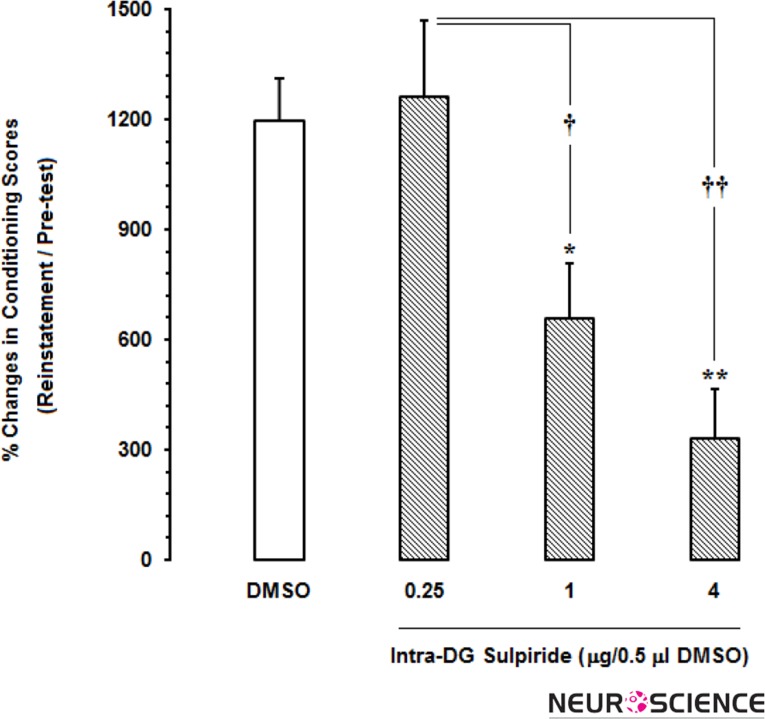
As shown in Fig. 5, to determine the role of D2-like receptor within the DG in the reinstatement of morphine-induced CPP, after conditioning and 24h after the last extinction day, in separate groups of animals, different doses of sulpiride (0.25, 1 and 4μg/0.5μl DMSO) were administered into the DG prior to injection of priming dose of morphine (1mg/kg,sc) in the reinstatement day. Statistical analysis showed that intra-DG administration of 12% DMSO as a vehicle didn’t affect the reinstatement of morphine-induced conditioned place preference as compared to post-test (Fig. 5A). However, repeated measures one-way ANOVA followed by Dunnett’s multiple comparison test showed that there was a difference in the magnitude of conditioning scores (place preference) in reinstatement day and post-test day in all experimental groups. However, this difference was not significant in two lower doses of sulpiride (Fig. 5B and 5C). Statistical analysis [F(5,29)=18.11, P<0.0001] showed that only the highest dose of sulpiride (4μg) decreased drug-seeking behaviors in reinstatement phase as compared to post-test day (P<0.001; Fig. 5D). Notably, block randomized one-way ANOVA followed by Newman-Keuls test [F(3,19)=8.53, P=0.0013] showed that % changes of conditioning scores calculated in reinstatement day to pre-test day decreased at the two higher doses of sulpiride (Fig. 6) in this study.
Figure 5.
The effects of administration of (A) 12% DMSO as a vehicle and different doses of sulpiride as selective D2-like receptor antagonist, (B) 0.25μg (C) 1μg and (D) 4μg per 0.5μl DMSO into the dentate gyrus (DG), 5 min before injection of priming dose of morphine (1 mg/kg;sc) in reinstatement day (24h after the last extinction day) decreased the conditioning score as compared to that in post-test day. Each column represents the mean ±SEM of 5–6 rats.
**P<0.01 and ***P<0.001 compared with pre-test day
†††P<0.001 compared with post-test day
+P<0.05 and +++P<0.001 compared with the last day of extinction period
Figure 6.
The effects of administration of different doses of sulpiride as selective D2-like receptor antagonist into the dentate gyrus (DG) on the percentage of CPP scores calculated in reinstatement day to pre-test day.
*P<0.05 and **P<0.01 compared with DMSO-treated group
†P<0.05 and ††P<0.01
4. Discussion
In the present study, we demonstrated that antagonism of dopaminergic receptors within the DG region of the hippocampus attenuates the reinstatement of morphine-extinguished CPP in the rats. However, this decrease in D1-like receptor antagonist-treated groups was more significant than that in sulpiride-treated animals. Morphine-induced CPP is one of the complicated phenomenon, in which many mechanisms such as dopaminergic, GABAergic and opioidergic systems, and many brain structures such as the VTA, NAc and HIP are involved (Cami & Farré, 2003). The HIP plays an important role in the expression and acquisition of CPP in response to morphine, cocaine, and nicotine (Vetulani, 2001). Local infusion of morphine into the HIP (Corrigall & Linseman, 1988) and methamphetamine into dorsal HIP induce drug-seeking behaviors in a CPP paradigm (Ricoy & Martinez Jr, 2009). Moreover, the HIP is known to participate in associative processes such as declarative memory, and not typically considered an integral component of the reward pathway, but it might be expected to play a significant role in the mechanism leading to development of drug addiction. In fact, the HIP plays an important role in the formation of contextual memory between the environment and the rewarding effect of abused drugs.
The interaction between activation of dopaminergic receptors and drug abuse phenomenon has been proved. Acquas et al., (1989) showed that systemic administration of dopamine antagonists block the motivational properties of rewarding as well as aversive stimuli (Acquas, Carboni, Leone, & Di Chiara, 1989). In agreement with our results, Maldonado et al., (1997) indicated that the D2 receptor plays a crucial role in the motivational component of drug addiction (Maldonado et al., 1997). On the other hand, it has been shown that the standard selective D1-like receptor antagonist (SCH23390) blocked the place preference induced by morphine, nicotine and diazepam (Acquas et al., 1989). Furthermore, it demonstrated that D1/D2-like receptor antagonists microinjected into the CA1 region of the HIP prevented the acquisition of morphine-induced CPP (Esmaeili, Kermani, Parvishan, & Haghparast, 2012). It seems that these kinds of receptors in the HIP mediate such memory. It did not initiate the development of rewarding effects of many abused drugs when dopamine receptors are blocked in the HIP, and it suggests that this region by itself is not a place of rewarding but it cooperates with other parts (Rezayof, Zarrindast, Sahraei, & Haeri-Rohani, 2003). Hippocampal excitatory outputs regulate the activity of VTA dopaminergic neurons through a polysynaptic pathway (Gholami, Haeri-Rohani, Sahraie, & Zarrindast, 2002). Recently, Tanaka et al., (2011) showed that the protein level of the D1 receptor and its prerequisite mRNA in the HIP increases in animals that show a clear preference for the environment paired with cocaine. They concluded that the alteration of D1 receptor in this area is related to the induction of drug-induced contextual memory (Haghparast et al., 2013). Hernandez-Rabaza et al., (2008) showed that the DG is essential for generating contextual memories of drug-induced reward that it necessitates the activation of a circuit including the dorsal DG (Hernandez-Rabaza et al., 2008). This author documented a slower acquisition rate in rats with lesions of the DG and suggest that the short-term expression (2h after conditioning) of contextual memory is impaired by the DG lesions (Hernández-Rabaza et al., 2007).
These studies are in line with our results that administration of both D1 and D2 receptor antagonists into the DG reduced conditioning scores induced by priming morphine in the reinstatement day and have led us to investigate if the memory is formed during conditioning phase (acquisition phase), is there possibility to be affected by blockade of the dopamine receptors on the reinstatement phase? In conclusion, our findings showed that administration of dopamine receptor antagonists into the DG decreased the reward-associated learning of the CPP paradigm, which in turn affected the reinstatement of morphine-induced CPP in the rats. Additionally, it seems that in this region, D1-like receptor has more important role than D2-like receptor in morphine-seeking behaviors. However, more investigations with molecular and electrophysiological approaches are needed to clarify the exact mechanisms.
References
- Acquas E., Carboni E., Leone P., Di Chiara G. (1989). SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology, 99( 2), 151–155 . [DOI] [PubMed] [Google Scholar]
- Attarzadeh-Yazdi G., Arezoomandan R., Haghparast A. (2014). Minocycline, an antibiotic with inhibitory effect on microglial activation, attenuates the maintenance and reinstatement of methamphetamine-seeking behavior in rat. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 53, 142– 148. [DOI] [PubMed] [Google Scholar]
- Cami J., Farré M. (2003). Drug addiction. New England Journal of Medicine, 349 ( 10), 975– 986. [DOI] [PubMed] [Google Scholar]
- Corrigall W. A., Linseman M. A. (1988). Conditioned place preference produced by intra-hippocampal morphine. Pharmacology Biochemistry and Behavior, 30 ( 3), 787– 789. [DOI] [PubMed] [Google Scholar]
- De Vries T. J., Schoffelmeer A. N., Binnekade R., Raaso H., Vanderschuren L. J. (2001). Relapse to cocaine-and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmalogy, 26, 15– 26. [DOI] [PubMed] [Google Scholar]
- Esmaeili M. H., Kermani M., Parvishan A., Haghparast A. (2012). Role of D1/D2 dopamine receptors in the CA1 region of the rat hippocampus in the rewarding effects of morphine administered into the ventral tegmental area. Behavioural Brain Research, 231 ( 1), 111– 115. [DOI] [PubMed] [Google Scholar]
- Gholami A., Haeri-Rohani A., Sahraie H., Zarrindast M.-R. (2002). Nitric oxide mediation of morphine-induced place preference in the nucleus accumbens of rat. European Journal of Pharmacology, 449 ( 3), 269– 277. [DOI] [PubMed] [Google Scholar]
- Haghparast A., Esmaeili M. H., Taslimi Z., Kermani M., Yazdi-Ravandi S., Alizadeh A.-M. (2013). Intrahippocampal administration of D2 but not D1 dopamine receptor antagonist suppresses the expression of conditioned place preference induced by morphine in the ventral tegmental area. Neuroscience Letters, 541, 138– 143. [DOI] [PubMed] [Google Scholar]
- Hamilton T. J., Wheatley B. M., Sinclair D. B., Bachmann M., Larkum M. E., Colmers W. F. (2010). Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proceedings of the National Academy of Sciences, 107 ( 42), 18185– 18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Rabaza V., Barcia J., Llorens-Martín M., Trejo J. L., Canales J. (2007). Spared place and object-place learning but limited spatial working memory capacity in rats with selective lesions of the dentate gyrus. Brain Research Bulletin, 72 ( 4), 315– 323. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V., Hontecillas-Prieto L., Velazquez-Sanchez C., Ferragud A., Perez-Villaba A., Arcusa A., Canales J., et al. (2008). The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiology of Learning and Memory, 90 ( 3), 553– 559. [DOI] [PubMed] [Google Scholar]
- Imenshahidi M., Qaredashi R., Hashemzaei M., Hosseinzadeh H. (2014). Inhibitory effect of berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur-Journal of Natural Pharmaceutical Products, 9 ( 3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi S., Attarzadeh-Yazdi G., Yazdi-Ravandi S., Hesam S., Azizi P., Razavi Y., Haghparast A. (2014). Forced swim stress but not exogenous corticosterone could induce the reinstatement of extinguished morphine conditioned place preference in rats: Involvement of glucocorticoid receptors in the basolateral amygdala. Behavioural Brain Research, 264, 43– 50. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Simon E. J. (2009). The neurobiology of addiction: Where we have been and where we are going. Journal of drug issues, 39 ( 1), 115– 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Grace A. A. (2005). The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron, 46 ( 5), 703– 713. [DOI] [PubMed] [Google Scholar]
- Liu F., Jiang H., Zhong W., Wu X., Luo J. (2010). Changes in ensemble activity of hippocampus CA1 neurons induced by chronic morphine administration in freely behaving mice. Neuroscience, 171 ( 3), 747– 759. [DOI] [PubMed] [Google Scholar]
- Maldonado R., Saiardi A., Valverde O., Samad T. A., Roques B. P., Borrelli E. (1997). Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature, 388 ( 6642), 586– 589. [DOI] [PubMed] [Google Scholar]
- O'Brien C. P. (1997). A range of research-based pharmacotherapies for addiction. Science, 278 ( 5335), 66– 70. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. (2003). Dopamine gating of forebrain neural ensembles. European Journal of Neuroscience, 17 ( 3), 429– 435. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson W. C. (2007). The rat brain in stereotaxic coordinates ( 6th ed. ). London UK : Academic Press . [Google Scholar]
- Rezayof A., Zarrindast M. R., Sahraei H., Haeri-Rohani A. (2003). Involvement of dopamine receptors of the dorsal hippocampus on the acquisition and expression of morphine-induced place preference in rats. Journal of Psychopharmacology, 17 ( 4), 415– 423. [DOI] [PubMed] [Google Scholar]
- Riahi E., Khodagholi F., Haghparast A. (2013). Role of dorsal hippocampal orexin-1 receptors in associating morphine reward with contextual stimuli. Behavioural Pharmacology, 24 ( 4), 237– 248. [DOI] [PubMed] [Google Scholar]
- Ricoy U. M., Martinez J. L., Jr (2009). Local hippocampal methamphetamine-induced reinforcement. Frontiers in Behavioral Neuroscience, 3 ( 11), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S., Gittings D. (2003). Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN 35,428 in rats. Psychopharmacology, 168 ( 1–2), 118– 123. [DOI] [PubMed] [Google Scholar]
- Vetulani J. (2001). Drug addiction. Part II. Neurobiology of addiction. Polish Journal of Pharmacology, 53 ( 4), 303– 318. [PubMed] [Google Scholar]
- Wise R. A. (2002). Brain reward circuitry: insights from unsensed incentives. Neuron, 36 ( 2), 229– 240. [DOI] [PubMed] [Google Scholar]