Abstract
In this study we aim to describe the characteristics of non-diabetic organ donors with circulating diabetes-associated autoantibodies collected within the Nordic Network for Islet Transplantation. One thousand and thirty organ donors have been screened in Uppsala for antibodies against glutamic acid decarboxylase (GADA) and islet antigen-2 (IA-2A). The 32 non-diabetic donors that tested positive for GADA (3·3% of all non-diabetic donors) were studied in more detail, together with 32 matched controls. Mean age among the autoantibody-positive donors was 52·6 (range 21–74), family history of type 1 diabetes (T1D) was unknown, and no donor was genetically predisposed for T1D regarding the human leucocyte antigen (HLA) locus. Subjects were analysed for islet cell antibodies (ICA), insulin autoantibodies (IAA) and zinc transporter 8 antibodies (ZnT8A), and pancreas morphology and clinical data were examined. Eight non-diabetic donors tested positive for two antibodies and one donor tested positive for four antibodies. No insulitis or other signs of a diabetic process were found in any of the donors. While inflammatory cells were present in all donors, subjects with high GADA titres had significantly higher CD45 cell numbers in exocrine tissue than controls. The extent of fibrosis was more pronounced in autoantibody-positive donors, even in subjects with lower GADA titres. Notably, it is possible that events not related directly to T1D (e.g. subclinical pancreatitis) may induce autoantibodies in some cases.
Keywords: autoantibodies, exocrine pancreas, fibrosis, inflammation, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is considered to be an immune-mediated disease targeting the insulin-producing beta cells in the pancreas, but the aetiology remains to be defined. The clinical manifestation is preceded by a preclinical phase, during which one or more autoantibodies directed towards islet antigens can be detected in the peripheral circulation 1,2. A higher number of autoantibodies is related to a greater T1D risk. However, these autoantibodies are not perceived as the culprit of beta cell destruction, but rather as a marker of the event. Islet autoantibodies serve as a diagnostic and prognostic marker for T1D. While the prevalence of T1D is approximately 0·1–2%, autoantibodies can be detected in 1–4% of the general population 3–5. Despite this, autoantibody-positive subjects are often referred to as ‘prediabetic’.
In 2007, In't Veld et al. described the finding of insulitis in two of 62 cases of autoantibody-positive subjects 5. Both subjects carried three autoantibodies, implying a high risk for progressing to T1D. Three other studies found no evidence of insulitis in autoantibody-positive subjects 6–8.
As T1D is viewed as a beta cell-specific disease, endocrine tissue has received the major attention in histopathological studies, perhaps overlooking the involvement of the exocrine tissue. Pancreatic weight and exocrine function is affected in patients with T1D 9–11, and autoantibodies also targeting exocrine epitopes, such as carbonic anhydrase II and lactoferrin, have been reported in patients with recent-onset diabetes 12. Exocrine fibrosis was observed in T1D patients already at the beginning of the 20th century 13, and morphological changes similar to those seen in chronic pancreatitis were later reported 14–16. The prevalence of diabetes in patients with chronic pancreatitis has been estimated to be 47–80%, depending on the time after onset, and up to half the patients with autoimmune pancreatitis (AIP) develop diabetes 17. A common or similar aetiology of the two diseases has therefore been suggested 18. Furthermore, excessive alcohol intake is related to both acute and chronic pancreatitis and, in some cases, leads to insulin-dependent diabetes 19.
In this study, we scanned 1030 pancreatic organ donors for autoantibodies in order to identify adult individuals testing positive for one or more beta cell-specific autoantibodies and to characterize these with regard to morphology and serology, in order to understand more clearly the underlying process leading to the production of diabetes-associated autoantibodies in non-diabetic subjects. We hypothesized that autoantibodies may arise as a result of pancreatic damage also affecting the exocrine pancreatic tissue, and thus that signs of exocrine damage and/or inflammation would be more common among autoantibody-positive subjects.
In addition, we aimed to assess the relevance of biobank materials from autoantibody-positive cohorts for increased understanding of the prediabetic disease process.
Materials and methods
Organ donors and clinical history
Between 2007 and 2015, 1030 brain dead organ donors enrolled into the Nordic Network for Islet Transplantation were screened for autoantibodies targeting glutamic acid decarboxylase (GAD) and islet antigen-2 (IA-2) at Uppsala University Hospital. Subjects were considered for inclusion in the study if consent for use in research was obtained by the donor's physician from the potential donor or from the relatives of the deceased donor. The study was approved by the Regional Ethics Committee in Uppsala, Sweden, according to the Act Concerning the Ethical Review of Research Involving Humans (2003:460; permit number Dnr 2009/043, 2009/371).
Sixty-one donors (6·3%) had T1D or T2D. Diagnosis of diabetes was made according to current World Health Organization (WHO) criteria. The diabetic donors were omitted from further analyses.
Sera from 32 of the 969 non-diabetic organ donors (3·3%) were positive for glutamic acid decarboxylase antibodies (GADA), three of which (0·31%) were double-positive for GADA and IA-2 antibodies (IA-2A) (Table1 and 2). For each non-diabetic autoantibody-positive donor, an autoantibody-negative control donor was chosen by selecting the following islet isolation where the donor was of the same sex and at an age within +/− 5 years.
Table 1.
Clinical information and islet quality testing.
| Variable | Autoantibody-positive donors | Control donors | All non-diabetic donors |
|---|---|---|---|
| Total no. of donors | 32 | 32 | 969 |
| Age (range) | 52·6 (21–74) | 52·1 (22–72) | 55·6 (1–84) |
| Sex | |||
| Female | 13 (40%) | 13 (40%) | 414 (43%) |
| Male | 19 (60%) | 19 (60%) | 549 (57%) |
| Unknown | 0 | 0 | 6 (0·62%) |
| Known excessive alcohol consumption (no of donors) | 9 (28%) | 4 (13%) | n.a. |
| Number of islets at isolation | 201 000 (27 000–480 000) | 220 000 (39 000–660 000) | 210 000 (7600–760 000) |
| Body mass index | 25 (18–34·6) | 27·3 (20·8–45·7) | 26·1 (13·9–45·7) |
| HbA1c (%) | 5·7 (5·1–7)* | 5·6 (4·8–6·2) | 5·7 (4·2–10)* |
| HbA1c (mmol/mol) | 39 (32–53)* | 38 (29–44) | 39 (22–86)* |
| Total no. of donors with HLA information available | 31 | 30 | |
| DR4 | 3 (9.7%) | 10 (33%) | n.a. |
| DR3 | 12 (38%) | 7 (23%) | n.a. |
| Neither DR4 nor DR3 | 16 (52%) | 13 (43%) | n.a. |
| DQ8 | 0 (0%) | 2 (6.7%) | n.a. |
| DQ2 | 16 (52%) | 11 (37%) | n.a. |
| Neither DQ8 nor DQ2 | 15 (48%) | 17 (57%) | n.a. |
| DR4–DQ8 | 0 | 0 | n.a. |
| Insulin (pmol/μg DNA) | 4·3 (0·39–15) | 4·9 (0·30–15) | 5.1 (0·095–26) |
| Tissue factor (10−15 mol/μg DNA) | 54 (2·0–170) | 43 (8·0–190) | 53 (0–640) |
| Monocyte chemoattractant Protein-1 (10−15 mol/μg DNA) | 9·1 (2·0–23) | 9·2 (1·0 −45) | 10 (0–120) |
| Interleukin-6 (10−15 mol/μg DNA) | 4·3 (0·1–52) | 1·7 (0·2–4·4) | 2.9 (0–93) |
| Interleukin-8 (10−15 pmol/μg DNA) | 60 (4·0–250) | 37 (8·0–120) | 50 (0–630) |
Glycated haemoglobin (HbA1c) values > 6·5% (48 mmol/mol) were noted in 11 of 969 control donors, and in one autoantibody-positive donor without diabetes diagnosis. Range is shown in parentheses unless specified otherwise. HLA = human leucocyte antigen.
Table 2.
Occurrence of pancreatic autoantibodies in serum from non-diabetic organ donors positive in screening for diabetes-associated autoantibodies and control subjects.
| Variable | Non-diabetic autoantibody-positive donors | Control donors |
|---|---|---|
| Islet autoantibodies (no. of donors) | ||
| Total no. of donors | 32 | 32 |
| GADA | 23 (72%) | 0 |
| IA-2A + GADA | 2 (6·3%) | 0 |
| ICA + GADA | 6 (19%) | 0 |
| ZnT8a + GADA + IA2A + ICA | 1 (3%) | 0 |
| ICA | 0 | 2 (6·3%) |
| IAA | 0 | 0 |
| Exocrine autoantibodies | ||
| (no. of donors) | ||
| Total no. of donors | 28 | 26 |
| CAII-abs | 2 (7·1%) | 1 (3·8%) |
| LF-antibodies | 3 (11%) | 1 (3·8%) |
| CAII-antibodies + LF-antibodies | 2 (7·1%) | 0 |
| Any exocrine autoantibodies | 7 (25%) | 2 (%) |
| Combinations of autoantibodies | ||
| CAII-abs + IA-2A | 1 (3·1%) | 0 |
| LF-antibodies + IA-2A | 0 | 0 |
| CAII + ICA | 1 (3·1%) | 0 |
| LF-antibodies + ICA | 1 (3·1%) | 0 |
GADA = glutamic acid decarboxylase antibodies; IA2A = islet antigen-2 antibodies; IAA = insulin autoantibodies; ZnT8A = zinc transporter 8-antibodies; ICA = islet cell antibodies; CAII-antibodies= carbonic anhydrase II-antibodies; LF-antibodies = lactoferrin antibodies.
The clinical history of each donor was screened at the time of the organ retrieval for information stating excessive alcohol consumption, which was noted in 13 of the organ donors. Information on human leucocyte antigen (HLA) alleles was available from 31 autoantibody-positive donors and 30 controls. The frequencies of HLA risk alleles among the donors are shown in Table1. The study was carried out according to the principles of the Declaration of Helsinki and the European Council's convention on Human Rights and Biomedicine.
Islet isolation and quality testing
Isolation of pancreatic islets for islet transplantation was carried out as described previously 20 on all autoantibody-positive donors and their controls. A clamp was used to compress the pancreatic duct at the head of the pancreas, and the tissue adjacent to the clamp was taken as a biopsy and stored in formalin. Glycated haemoglobin (HbA1c) was measured in peripheral blood from all donors. Quality tests were performed on homogenized isolated islets using the Gyrolab workstation (Gyros, Uppsala, Sweden). Tissue factor (CD142), insulin and the cytokines interleukin (IL)-6, IL-8 and monocyte chemoattractant protein-1 (MCP-1) were measured in islets and related to the DNA content. Islet function was assessed by dynamic glucose perifusion, with measurement of insulin secretion in response to glucose challenge with 1·67 mM and 16·7 mM glucose. Fractions were collected at 6-min intervals for 120 min, with insulin concentrations measured by enzyme-linked immunosorbent assay (ELISA) (Mercodia, Uppsala, Sweden).
GADA titres and screening for zinc transporter 8 antibodies (ZnT8A), insulin autoantibodies (IAA) and islet cell antibodies (ICA)
After initial GADA screening with ELISA, antibody titres were measured from all donors where initial screening was positive, using ELISA (ElisaRSRTM GADAb and IA-2Ab; RSR Limited, Cardiff, UK). GADA levels exceeding 5 IU and IA-2A levels exceeding 8 IU were considered positive, in line with clinical practice in Sweden. Serum from all donors carrying GADA and/or IA-2A, together with serum from the selected control donors, were sent to Mikael Knip's autoantibody laboratory in Helsinki to be analysed for IAA, ZnT8A and ICA. IAA and ZnT8A were analysed with specific radiobinding assays and ICA with indirect immunofluorescence 21.
Sectioning and staining
Formalin-fixed and paraffin-embedded tissue from the 60 donors from whom biopsies were accessible (29 autoantibody-positive donors and 31 controls) were cut into 6-μm sections. All antibodies used for staining, kits and reagents for visualization were bought from Dako (Glostrup, Denmark) if not specified otherwise. A standard protocol for immunohistochemistry staining was followed. Incubation time was 30 min for all primary antibodies. Subsequent to deparaffinization with xylene and ethanol, sections from all donors were double-stained for CD45 and insulin, using monoclonal mouse anti-human CD45, clone 2B11+PD7/26 (1 : 150) and polyclonal guinea pig anti-insulin antibody (1 : 200). Visualization of CD45 was performed using the Dako EnVision+ System-HRP (DAB). Insulin was visualized using the EnVision™ G|2 System/AP, rabbit/mouse (permanent red).
Consecutive sections were stained for collagen (Sirius red in saturated pikrin acid; HistoLab, Gothenburg, Sweden). After initial analyses of CD45/insulin stainings, 12 donors with high numbers and/or high local density of CD45-positive cells (six autoantibody-positive and six control donors) were selected and stained for CD8/insulin (monoclonal mouse anti-human, clone C8-144B, 1 : 2000), CD4 (monoclonal mouse anti-human CD4, clone 4B12, 1 : 60), regulatory T cells [anti-forkhead box protein 3 (FoxP3) antibody, 1 : 200; Abcam, Cambridge, UK] and neutrophils (MPO; polyclonal rabbit anti-human myeloperoxidase antibodies, 1 : 1200). Visualization was performed as mentioned above, using the EnVision+ system-HRP and DAB or alkaline phosphatase anti-alkaline phosphatase (APAAP). All sections were counterstained with Mayer's haematoxylin (HistoLab, Gothenburg, Sweden). Human spleen was used as a positive control for all immunohistochemical stainings.
Staining evaluation and analysis
All sections were blinded and evaluated using a Labophot-2 microscope (Nikon, Kingston upon Thames, UK).
Initially, all sections were screened for beta cell damage or other abnormalities at a magnification of ×10. Using a magnification of ×20, 50 islets were scrutinized in each section and the numbers of CD45+ cells present in the islet parenchyma and in close proximity to the islets were counted. Immune cell numbers were then related to the islet area, which was estimated using a 0·16-mm2 grid. Only islets with an area larger than 2·56 μm2 (the area of one hundredth of the grid size, corresponding to approximately five endocrine cells) were considered. In sections containing fewer than 50 islets, all islets in the section were examined. Average number of scrutinized islets was 30 in autoantibody-positive donors (range = 1–50) and 33 (4–50) in controls.
A 0·16-mm2 grid with 11 × 11 lines was used to estimate the distribution of tissues in each section, by noting what tissue was present in each of the 121 crossings. The number of immune cells was counted and related to the exocrine tissue, connective tissue and endocrine tissue areas, respectively. Fifteen adjacent grids were analysed in the centre part of the sections, constituting a total area of 2·4 mm2 in each section. If the exocrine or connective tissue area constituted ≤ 20% of the grid area, the area was considered non-reliable for analysis and excluded. The same type of analysis was applied on stainings for CD8, CD4, MPO and FoxP3.
Slides stained with Sirius red were scanned with the Aperio ScanScope system (Aperio Technologies). The collagen area was calculated using Aperio ImageScope version 12·1 software (Aperio Technologies, Oxford, UK) and the threshold function in ImageJ (National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay for carbonic anhydrase II and lactoferrin
Lactoferrin (LF) and carbonic anhydrase II (CAII) are antigens present in duct cells and in acinar cells, respectively, and serum antibodies against these were used as markers of an immune response targeting exocrine tissue. Carbonic anhydrase II, 0·5 µg (Sigma C6624) or 1 µg lactoferrin (Sigma L6793; Sigma, St Louis, MO, USA) was diluted in phosphate-buffered saline (PBS) for coating overnight. Ethylenediaminetetraacetic acid (EDTA (0·01 M, 1 h) was used for blocking; 50 µl of serum samples diluted 1 : 25 were added in triplicate. Goat anti-human immunoglobulin (Ig)G conjugated with HRP (1 : 60 000, 1 h) was used as a secondary antibody. 3,3′,5,5′-Tetramethylbenzidine (TMB) and subsequently 1 M H2SO4 was added and absorbance was read with a spectrophotometer at 450 nm. Optical density (OD) values higher than 2 standard deviations (s.d.) from the mean values for control donors were considered positive.
Statistical analysis
Differences between groups were analysed statistically using the Mann–Whitney U-test or the Kruskall–Wallis test. Categorical data were analysed with the χ2 test or Fisher's exact test. Mean and s.d. or range are presented in graphs and tables if not specified otherwise. All analyses were performed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA). The critical level of statistical significance was P < 0·05.
Results
Donor characteristics and quality of isolated islets
Clinical data are presented in Table1. There was no significant difference between the autoantibody-positive donors and control donors regarding donor characteristics, islet quality or islet content of cytokines (measured routinely in isolated islets). Insulin secretion in response to glucose perifusion did not differ between the groups (Fig. 1).
Figure 1.
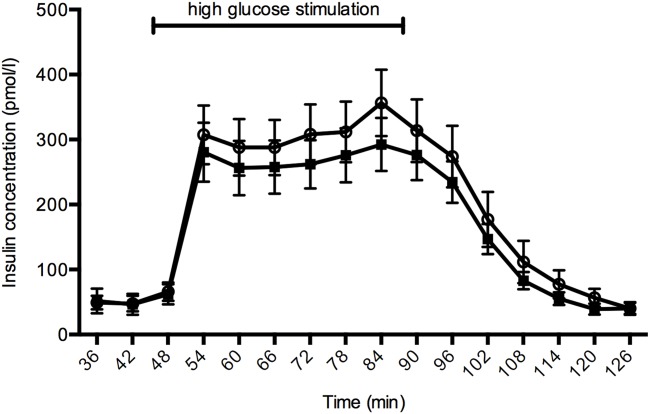
Insulin secreting function during glucose perifusion of autoantibody-positive islets (black squares, n = 27) and control islets (white circles, n = 31). There was no statistically significant difference in the area under the curve between the groups (P = 0·50). Error bars show the standard error of mean (s.e.m.).
Documented excessive alcohol consumption was noted in nine (28%) of the autoantibody-positive donors, compared to four (13%) of the control donors, but the difference was not statistically significant (P = 0·20). No histological differences were observed in pancreases from donors with alcohol over-consumption when compared with other donors.
Frequency and titre of T1D-associated autoantibodies and antibodies targeting exocrine pancreatic tissue
Serum was available from 28 control donors and 28 autoantibody-positive donors. Table2 shows the presence of endocrine and exocrine autoantibodies in donor serum. When including the results from the extended autoantibody analysis, eight donors were positive for two islet autoantibodies and one for four islet autoantibodies. In line with earlier results, the donors positive for ICA had significantly higher GADA titres than GADA+ donors without ICA reactivity; 5800 IU (s.d. = 7600, n = 6) versus 72 IU (s.d. = 75, n = 19), P < 0·005.
LF antibodies were detected in serum from five autoantibody-positive donors (18%) and CAII antibodies in four autoantibody-positive donors (15%), two of whom were double-positive for LF and CAII. In comparison, one control donor was positive for LF and one for CAII. However, the difference was not significant (P = 0·17).
Histopathological evaluation of immune cell stainings
Paraffin-embedded tissue from the head of the pancreas obtained at the time of islet isolation was available from 29 autoantibody-positive donors and 31 control donors. Results from the evaluations on CD45 cell stainings and tissue distribution are displayed in Table3.
Table 3.
Results from immunohistochemistry stainings on non-diabetic autoantibody-positive and control donors.
| Variable | Non-diabetic autoantibody-positive donors | Control donors | P | ||
|---|---|---|---|---|---|
| No. of donors with immune cells in the periphery of one or more islets | |||||
| 5–9 CD45+ cells | 8 | 8 | 0·60 | ||
| 10–14 CD45+ cells | 2 | 2 | 1·00 | ||
| ≥15 CD45+ cells | 0 | 2 | 0·50 | ||
| No. of donors with immune cells in the parenchyma of one or more islets | |||||
| 5–9 CD45+ cells | 3 | 1 | 0·30 | ||
| 10–14 CD45+ cells | 1 | 1 | 1·00 | ||
| ≥15 CD45+ cells | 0 | 1 | 1·00 | ||
| CD45+ cells/mm2 exocrine tissue | 72 | (s.d. = 74) | 63 | (s.d. = 56) | 0·20 |
| Endocrine area (%) | 1.5 | (s.d. = 1·1) | 1.8 | (s.d. = 1·4) | 0·50 |
| Connective tissue area (%) | 13.7 | (s.d. = 17·1) | 8.3 | (s.d. = 9·3) | 0·20 |
| Number of islets/mm2 exo-+endocrine tissue | 7.5 | (s.d. = 3·8) | 9.3 | (s.d. = 6·0) | 0·30 |
| Collagen area (%) | 5.2 | (s.d. = 3·9) | 2.8 | (s.d. = 1·8) | 0.014 |
No. of immune cells in the islet periphery and parenchyma are based on CD45 observations in the complete section. CD45+ cells in exocrine tissue, endocrine area, connective tissue area and number of islets/mm2 was evaluated in 2·4 mm2 of each section. s.d. = standard deviation.
CD45+ cells were present in all donors, scattered principally in the exocrine and connective tissue.
Beta cell damage was not noted in any donor. No insulitis, defined as ≥ 15 CD45+ cells in at least three islets, was seen in any of the donors. Two autoantibody-positive and one control donor fulfilled the previously commonly used definition of more than five infiltrating immune cells in three or more islets. The proportion of islets with one or more CD45+ cells infiltrating the parenchyma was similar between the groups, as was the number of islets with CD45+ cells present in the islet periphery (Fig. 2a). Notably, in both autoantibody-positive and control donors, CD45+ cells were more numerous in the direct periphery of islets than inside the islet parenchyma (Fig. 2a). Immune cells present in the direct periphery of the islets made up 76% (s.d. = 25) of all infiltrating cells in autoantibody-positive donors and 79% (s.d. = 24) in control donors (P = 0·60).
Figure 2.
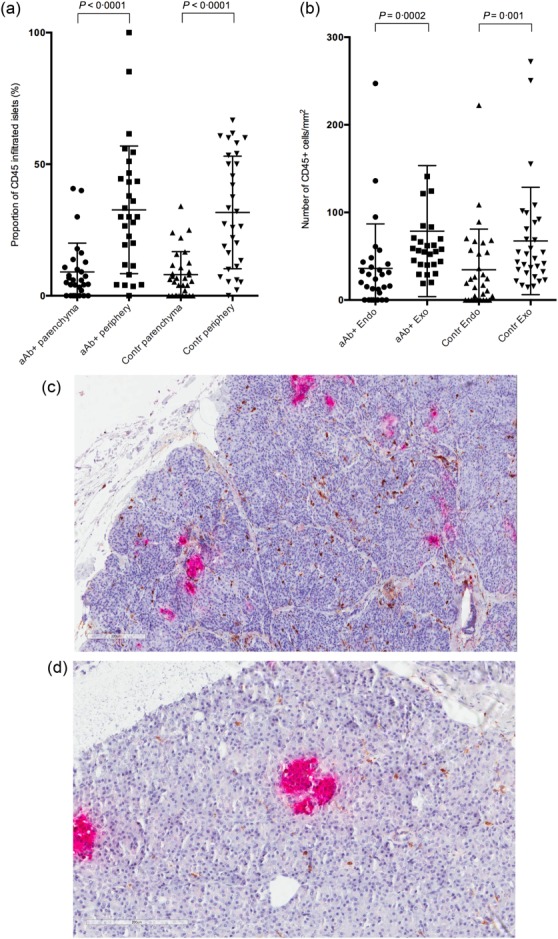
Results from immunohistochemical analysis on CD45 stainings. Autoantibody-positive donors and control donors are plotted according to the proportion of pancreatic islets with CD45+ cells present in the islet parenchyma and peripheral area, respectively (a). (b) The distribution of CD45+ cells/mm2 in endocrine and exocrine tissue in autoantibody-positive and control donors. There was no significant difference in the distribution of CD45 between the groups (c–d). Immunohistochemical stainings for insulin (red) and CD45 (brown) on formalin-fixed paraffin embedded pancreatic sections. Numbers of CD45+ cells infiltrating exocrine tissue were higher in donors with high glutamic acid decarboxylase antibodies (GADA) titres (c) compared to controls (d). No difference was seen between the autoantibody-positive and the control group as a whole. aAb+ = autoantibody-positive donors; Contr = control donors; Endo = endocrine tissue; Exo = exocrine tissue.
CD45+ cells were present at a significantly higher density in the exocrine than in the endocrine tissue in both autoantibody-positive and control donors (P < 0·005) (Fig. 2b-d). When comparing the donors with GADA titres in the 4th quartile to control donors and donors with GADA titres in the 1st–3rd quartiles, the former presented significantly higher numbers of CD45+ cells in exocrine tissue (227 ± 109 CD45/mm2 versus 61 ± 48 CD45/mm2, P = 0·0001). However, there was no difference between the autoantibody-positive group as a whole and the control donors regarding the CD45+ density in either exocrine or endocrine tissue. There were no significant differences in the tissue distribution between autoantibody-positive and control donors (Table3). In a number of cases, extensive amounts of CD45+ cells infiltrated the connective tissue.
Twelve donors with high numbers and/or high local density of CD45+ cells in exocrine tissue were selected for further analyses of leucocyte subtypes: CD8+ T cells, CD4+ T cells, neutrophils and regulatory T cells (Fig. 3, Table4). CD8 cells were present to the greatest extent in exocrine tissue in all donors, followed quantitatively by neutrophils and CD4+ T cells. Regulatory T cells were present to a very limited extent. There were no statistically significant differences between autoantibody-positive and control donors regarding these immune cell phenotypes.
Figure 3.
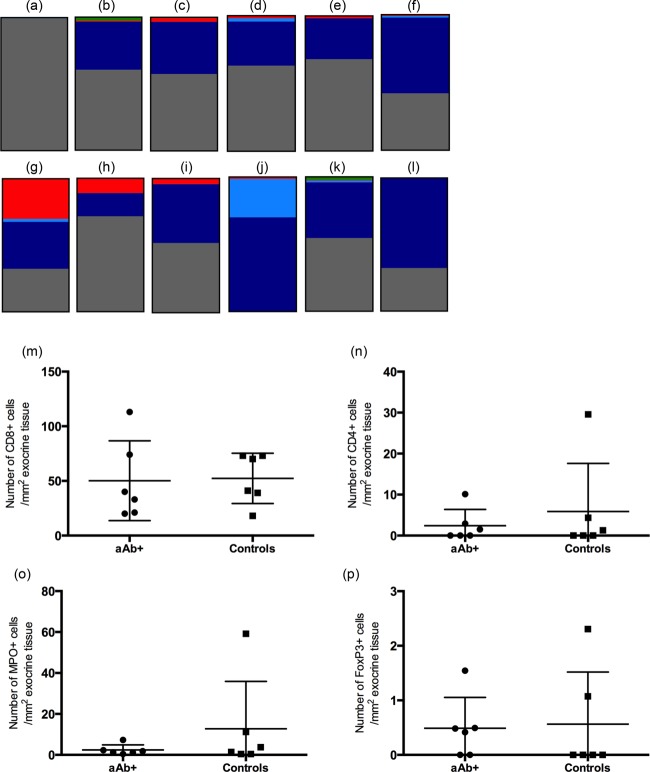
Coloured boxes (a–l) display the estimated proportion of the total immune cell count constituted by regulatory T cells [forkhead box protein 3 (FoxP3)+, green], neutrophils [myeloperoxidase (MPO), red], CD4+ (light blue) and CD8+ T cells (dark blue) from autoantibody-positive (a–f) and control donors (g–l), selected for high numbers of CD45+ cells. CD45+ cells not verified as any of the tested phenotypes are referred to as ‘other’ (grey). The category ‘other’ shows the difference between the total number of CD45 cells and the sum of cells positive for any of the subgroups. In the scatter-plots (m–p), the number of CD8+ (m), CD4+ (n), MPO+ (o) and FoxP3+ (p) cells in exocrine pancreatic tissue from six autoantibody-positive donors and six control donors are plotted. aAb+ = autoantibody-positive; controls = autoantibody-negative control donors.
Table 4.
Frequency of CD8+ T cells, CD4+ T cells, neutrophils and regulatory T cells in exocrine tissue from the 12 donors with the highest numbers of CD45 cells in exocrine tissue. Average number of cells is calculated on the donors where the relevant immune cells are present.
| Autoantibody-positive donors with high CD45 density (n = 6) | Donors double-positive for GADA and IA-2A (n = 3) | Control donors (n = 6) | ||||
|---|---|---|---|---|---|---|
| Cell type | n (%) | Average no of cells/mm2 (s.d.) | n (%) | Average no of cells/mm2 (s.d.) | n (%) | Average no of cells/mm2 (s.d.) |
| CD8+ | 6 (100) | 33 (23) | 3 (100) | 47 (57) | 6 (100) | 52 (23) |
| CD4+ | 3 (50) | 1·6 (1.2) | 1 (33) | 2·0 (n.a.) | 3 (50) | 12 (16) |
| Neutrophils | 6 (100) | 1·4 (0.63) | 3 (100) | 9·2 (7·3) | 6 (100) | 13 (23) |
| Regulatory T cells | 3 (50) | 0·84 (0.61) | 2 (67) | 0·56 (0·63) | 2 (33) | 1.7 (0·88) |
GADA = glutamic acid decarboxylase antibodies; IA2A = islet antigen-2 antibodies; n.a. = not applicable; s.d. = standard deviation.
Distribution of collagen
The proportion of the section area made up by collagen was significantly higher in the autoantibody-positive than in the control donors (P = 0·014) (Fig. 4, Table 3). Collagen was present to some extent in all donors and was present primarily around ducts and scattered diffusely in the exocrine tissue, but could also often be seen within islets.
Figure 4.
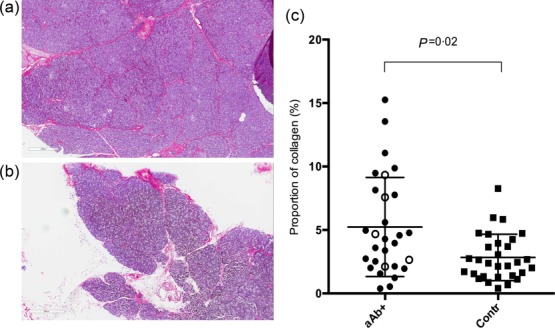
Immunohistochemical stainings for collagen (red) stained with Sirius red on pancreatic tissue from an autoantibody-positive donor (a) and a control donor (b). The proportion of collagen-containing area in sections from autoantibody-positive donors was significantly higher than in control donors, as shown in the scattered plot (c). Donors with glutamic acid decarboxylase antibodies (GADA) titres in the 4th quartile are shown as white circles. aAb+ = autoantibody-positive donors; Contr = control donors.
Donor with multiple autoantibodies
Nine donors were positive for multiple (≥ 2) autoantibodies. These donors had a higher titre of GADA in serum (mean = 4400, s.d. = 6700 versus 58, s.d. = 74 μ/ml, P = 0·015), which was also connected to a higher number of CD45+ cells infiltrating exocrine pancreatic tissue (mean = 140, s.d. = 120 versus 70, s.d. = 63 CD45+ cells/mm2, P = 0·03). No other significant differences in histological observations, donor information, including frequency of HLA risk alleles, or presence of exocrine antibodies were seen in these donors compared to single-positive or control donors.
One of the autoantibody-positive donors tested positive for four autoantibodies: GADA, IA2A, ICA and ZnT8A. The donor was a 51-year-old male with a body mass index (BMI) of 22·8 kg/m2 and an HbA1c of 5·1 mmol/l. Excessive alcohol consumption was noted in the transplantation information and the pancreas was remarkably small in size and weight. During islet isolation, 120 000 islets were obtained, which was within the normal range. When subjected to immunohistochemistry, a large amount of CD45+ cells were observed in both endocrine (140 cells/mm2) and exocrine (320 cells/mm2) tissue. Thirteen of the 29 islets present in the scrutinized section were infiltrated with more than 5 CD45+ immune cells, but no islet was infiltrated with more than 14 cells. However, further characterization of the immune cells, using stainings for CD8, CD4, MPO and FoxP3, revealed only the immunophenotype of a few cells. Thus, the phenotype of the vast majority of immune cells in this donor remains unknown (Fig. 3a).
Discussion
The number of immune cells in exocrine tissue was increased significantly in the autoantibody-positive donors with the highest GADA titres (concentrations in the 4th quartile) compared to control donors without diabetes-associated antibodies. As a group, however, the autoantibody-positive donors did not display increased immune cell infiltration of the pancreases when compared with controls, which contrasts with results from earlier studies 8,22. The currently applied detection limit for GADA-positivity (5 IU) is set based on clinical practice in Sweden, which is lower than, for example, the network for Pancreatic Organ Donors (nPOD): 18 IU. With the nPOD detection limits, 11 of the included autoantibody-positive donors would have been considered autoantibody-negative, which may explain the discrepancy with earlier studies. Interestingly, the number of CD45 cells/mm2 was significantly lower in the endocrine than in the exocrine tissue. Furthermore, the collagen-containing area was significantly higher in the autoantibody-positive group, as would be expected in a chronic inflammatory process. High GADA titres may thus be related to an ongoing inflammatory process in the exocrine pancreas. It has been demonstrated that the affinity of GADA is related to T1D risk, whereas titres or the mere presence of GADA is of less significance 23,24. It would therefore be of future interest to look closer at GADA affinity when studying potentially prediabetic subjects.
Insulitis is a feature connected classically to T1D, defined currently by a minimum of three pancreatic islets infiltrated by at least 15 CD45+ cells 25. It is hypothesized that the infiltration of immune cells precedes the clinical presentation of T1D. Therefore, it has been of interest to search for insulitis in potentially ‘prediabetic’ individuals. In the present study, no insulitis was seen in any of the autoantibody-positive subjects, while two controls presented CD45 cell infiltration with > 15 immune cells in one islet. Nevertheless, the above-mentioned criteria for insulitis were not fulfilled. In addition, the number of CD45+ cells/area was higher in the exocrine tissue, as well as in the direct periphery of the islet, than in the islet parenchyma. This was observed in both autoantibody-positive and control subjects. Peri-insulitis is a common feature in T1D, but the reason for this typical localization of immune cells around islets is unknown. Here we show that, in non-diabetic donors, the relative number of immune cells is also higher in the islet periphery than inside the islets.
CD8 cells were the most commonly occurring immune cells in exocrine tissue in all donors, followed quantitatively by neutrophils and CD4+ T cells, while regulatory T cells were present to only a limited extent. There was no significant difference between autoantibody-positive and control donors regarding the numbers of any of the immune cells in the pancreas, further arguing against an ongoing prediabetic process. Furthermore, no signs of the diabetes-characteristic pseudoatrophic islets were found; islet size and number did not differ between the groups, and no beta cell loss was noted during the initial screenings of the whole sections. However, T1D lesions have been reported, at least in the early phases, to appear in some but not other lobes of the pancreas. Due to this focal disease progression, and the limitation within our material where only a single biopsy was available from each pancreas, we cannot exclude the possibility of ongoing morphological changes in other parts of the pancreas 26. In addition, while an average of 30 islets were screened in each section, a few biopsies presented with only few islets, which obviously entails a risk of missing potential insulitis in these donors.
All human pancreatic tissues in this and earlier histological studies were obtained from brain dead organ donors, a condition known to affect immune cell infiltration in all organs, including the pancreas, and entails a risk of the characteristics of premortal pancreatic morphology being confused with changes due to preterminal clinical conditions 27. However, the pancreases were obtained and procured in the same manner as for clinical transplantation, minimizing postmortal changes severely affecting the tissue quality in PAD studies of the pancreas.
Circulating antibodies directed towards duct cells and acinar cells, as well as alcohol over-consumption, were present in a larger number of autoantibody-positive donors than control donors. However, the difference was not statistically significant in our limited material. Information on alcohol over-consumption was obtained from the clinical information noted in organ transplantation situation, based on the donors’ medical record, and is therefore more probably an under- than an over-estimation of the true alcohol consumption. Excessive intake of alcohol is the most common cause of pancreatitis, but chronic pancreatitis is also a frequent finding in diabetic patients 28. In addition, antibodies targeting exocrine tissue are known to be more common in patients with T1D than in the general population 12. If verified in a larger study, our observations would be of interest considering the increased collagen areas seen in the autoantibody-positive donors. These results indicate that pancreatic damage may induce or enhance the development of both endocrine and exocrine autoantibodies.
Positivity for multiple (≥ 2) disease-associated autoantibodies is a strong risk marker for progression to clinical T1D, as approximately 70% of genetically predisposed subjects with multiple autoantibodies progress to clinical T1D over the next 10 years [1]. In this study, six donors were double-positive for GADA and ICA, two for GADA and IA-2A, and one donor was positive for GADA, ICA, IA-2A and ZnT8A. It should, however, be considered that the ICA positivity could be due to high GADA titre and therefore not represent true reactivity against multiple islet autoantigens. It should also be noted that donors positive for IAA, ICA and/or ZnT8A without reactivity towards GAD and IA-2 would be missed due to the initial screening.
The presence of multiple antibodies was linked in the current study to higher GADA titres and increased numbers of CD45+ cells infiltrating exocrine tissue, but these donors differed in no other way from donors with a single or no antibody, nor in the donor testing positive for four autoantibodies were any prediabetic signs noted, such as increased HbA1c, decreased or destroyed islets or insulitis. This donor had a history of heavy alcohol over-consumption and was significantly older (51 years) than the typical age at T1D onset. Furthermore, the pancreas was remarkably small in size, and histological analysis revealed a high number of infiltrating cells in all pancreatic tissues. Together, these findings present an interesting case supporting the thesis of general pancreatic damage (e.g. by alcohol consumption) as a generator of islet antibodies. One could speculate that the presence of autoantibodies in subjects with T1D may also arise due to a general pancreatic damage, and not merely beta cell-specific damage.
A limitation common to most studies targeting organ donors is the relatively old age of organ donors, the average age being 53 years in our study population, an age when T1D incidence is low. Thus these autoantibody-positive donors do not represent a typical ‘prediabetic’ patient group, either with regard to genetics or age. Conclusions from this study should therefore not be applied to subjects with a statistically high risk of T1D progression with regard to heritability, genetics and age.
In conclusion, T1D-associated autoantibodies are often present in non-diabetic organ donors without any signs of pancreatic damage or morphological findings reminiscent of those commonly observed in T1D. Pancreata from autoantibody-positive donors contained a larger amount of collagen than controls, and donors with high GADA titres had an elevated number of CD45 cells in the exocrine pancreas. However, no signs of endocrine tissue damage were observed. Interestingly, production of beta cell autoantibodies seems to occur in response to unspecific pancreatic lesions. The notion that autoantibody-positive organ donors might be regarded as prediabetic and provide insight into the early injurious events of T1D should, based on the results presented here, be applied with caution.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (65X-12219-15-6, K2015-54X-12219-19-4) and EU-FP7-Health 2010 PEVNET 261441, the Novo Nordisk Fund, Diabetes Wellness Sweden, the Swedish Diabetes Foundation, the Juvenile Diabetes Research Foundation (Barndiabetesfonden), the Family Ernfors Fund and the Academy of Finland (Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012–2017, Decision no. 250114). Human pancreatic islets and tissue samples were obtained from the Nordic Network for Clinical Islet Transplantation supported by the Swedish national strategic research initiative EXODIAB (Excellence of Diabetes Research in Sweden) and the Juvenile Diabetes Research Foundation.
Author contributions
This study was designed by A. W., O. S. and O. K. Immunostainings were carried out by A. G. and S. I. A. W. and A. G. collected the data. A. W. performed the statistical analysis, interpreted the results, wrote the manuscript and reviewed it critically for intellectual content. T. H. and M. K. were responsible for the autoantibody analyses performed in Helsinki. O. S., O. K. and M. K. reviewed and edited the manuscript. All the authors have read and edited the manuscript and approved the version to be published. A. W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
The authors have no conflicts of interest to declare.
References
- Ziegler AG, Eisenbarth GS, Rewers M, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarn AC, Thomas JM, Dean BM, et al. Predicting insulin-dependent diabetes. Lancet. 1988;1:845–50. doi: 10.1016/s0140-6736(88)91601-7. [DOI] [PubMed] [Google Scholar]
- Jaeger C, Hatziagelaki E, Petzoldt R, Bretzel RG. Comparative analysis of organ-specific autoantibodies and celiac disease – associated antibodies in type 1 diabetic patients, their first-degree relatives, and healthy control subjects. Diabetes Care. 2001;24:27–32. doi: 10.2337/diacare.24.1.27. [DOI] [PubMed] [Google Scholar]
- Harrison LC. Risk assessment, prediction and prevention of type 1 diabetes. Pediatr Diabetes. 2001;2:71–82. doi: 10.1034/j.1399-5448.2001.002002071.x. [DOI] [PubMed] [Google Scholar]
- In't Veld P, Lievens D, Grijse JD, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56:2400–4. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- Gianani R, Putnam A, Still T, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab. 2006;91:1855–61. doi: 10.1210/jc.2005-1171. [DOI] [PubMed] [Google Scholar]
- Wagner R, Mcnally JM, Bonifacio E, et al. Lack of immunohistological changes in the islets of nondiabetic, autoimmune, polyendocrine patients with p-selective GAD-specific islet cell antibodies. Diabetes. 1989;43:851–6. doi: 10.2337/diab.43.7.851. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Calvo T, von Herrath GM. Increased immune cell infiltration of the exocrine pancreas: a possible 2 contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–90. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt PD, Krauss A, Bretz L, et al. Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol. 2000;37:105–10. doi: 10.1007/s005920070011. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308:2337–9. doi: 10.1001/jama.2012.15008. [DOI] [PubMed] [Google Scholar]
- Williams AJK, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E2109–13. doi: 10.1210/jc.2012-1815. [DOI] [PubMed] [Google Scholar]
- Hardt PD, Ewald N, Bröckling K, et al. Distinct autoantibodies against exocrine pancreatic antigens in European patients with type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. JOP. 2008;9:683–9. [PubMed] [Google Scholar]
- Cecil RL. A study of the pathological anatomy of the pancreas in ninety cases of diabetes mellitus. J Exp Med. 1909;11:266–90. doi: 10.1084/jem.11.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt PD, Killinger A, Nalop J, Schnell-Kretschmer H, Zekorn T, Klör HU. Chronic pancreatitis and diabetes mellitus. A retrospective analysis of 156 ERCP investigations in patients with insulin-dependent and non-insulin-dependent diabetes mellitus. Pancreatology. 2002;2:30–3. doi: 10.1159/000049445. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Kobayashi T, Miyashita H, et al. Exocrine pancreatic ductograms in insulin-dependent diabetes mellitus. Am J Gastroenterol. 1994;89:762–6. [PubMed] [Google Scholar]
- Bilgin M, Balci NC, Momtahen AJ, Bilgin Y, Klör H-U, Rau WS. MRI and MRCP findings of the pancreas in patients with diabetes mellitus: compared analysis with pancreatic exocrine function determined by fecal elastase 1. J Clin Gastroenterol. 2009;43:165–70. doi: 10.1097/MCG.0b013e3181587912. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger G, Layer P. Exocrine pancreatic function in diabetes mellitus. Eur J Gastro Hepatol. 1995;7:740–6. [PubMed] [Google Scholar]
- Sjoberg RJ, Kidd GS. Pancreatic diabetes mellitus. Diabetes Care. 1989;12:715–24. doi: 10.2337/diacare.12.10.715. [DOI] [PubMed] [Google Scholar]
- Goto M, Eich TM, Felldin M, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78:1367–75. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- Knip M, Virtanen SM, Seppä K, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010;363:1900–8. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese A, Vendrame F, Reijonen H, Atkinson MA, Campbell-Thompson M, Burke GW. New insight on human type 1 diabetes biology: nPOD and nPOD-transplantation. Curr Diab Rep. 2014;14:530. doi: 10.1007/s11892-014-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C, Schlosser M, Christen U, Ziegler AG, Achenbach P. GAD autoantibody affinity in schoolchildren from the general population. Diabetologia. 2014;57:1911–8. doi: 10.1007/s00125-014-3294-9. [DOI] [PubMed] [Google Scholar]
- Achenbach P, Warncke K, Naserke HE, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53:384–92. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56:2541–3. doi: 10.1007/s00125-013-3043-5. [DOI] [PubMed] [Google Scholar]
- Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. 1986;1:267–74. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- In't Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59:1702–8. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseda H, Okawa-Takatsuji M, Shinmura W, Hasimoto N, Ozaki Y, Ikeda Y. Potential for differential diagnosis of autoimmune pancreatitis and pancreatic cancer using carbonic anhydrase II antibody. 2008;37:1–7. doi: 10.1097/MPA.0b013e318162cb3a. [DOI] [PubMed] [Google Scholar]