Abstract
End of study analyses of the phase III trials of prophylactic human papillomavirus (HPV) virus-like particle (VLP) vaccines in young women are now largely completed. Two distinct vaccines were evaluated, Gardasil® (Merck & Co., Whitehouse Station, NJ USA) a quadrivalent vaccine containing VLPs of types 6, 11, 16 and 18 and Cervarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium), a bivalent vaccine containing VLPs of types 16 and 18. Both vaccines exhibited excellent safety and immunogenicity profiles. The vaccines also demonstrated remarkably high and similar efficacy against the vaccine-targeted types for a range of cervical endpoints from persistent infection to cervical intraepithelial neoplasia grade 3 (CIN3) in women naïve to the corresponding type at the time of vaccination. However, protection from incident infection or disease from non-vaccine types was restricted, and the vaccines had no effect on prevalent infection or disease. Gardasil® also demonstrated strong protection against genital warts and vulvar/vaginal neoplasia associated with the vaccine types. In other trials, Gardasil® protected mid-adult women from incident infection and CIN caused by the vaccine types and protected men for incident infection, genital warts and anal intraepithelial neoplasia by the vaccine types. Cervarix® protected against vaccine-targeted anal infections in women in an end of study evaluation. For practical reasons, efficacy studies have not been conducted in the primary target populations of current vaccination programs, adolescent girls and boys. However, immunogenicity bridging studies demonstrating excellent safety and strong immune responses in adolescence, coupled with the documentation of durable antibody responses and protection in young adults, leads to an optimistic projection of the effectiveness of the vaccines in adolescent vaccination programs. Taken together, the excellent clinical trial results strongly support the potential of the vaccines as high value public health interventions and justify their widespread implementation to prevent anogenital HPV infections and their associated neoplasia. This article forms part of a special supplement entitled “Opportunities for comprehensive control of HPV infections and related diseases” Vaccine Volume 30, Supplement X, 2012.
Keywords: Human papillomavirus, HPV, Prophylactic vaccine, Virus-like particle, Gardasil®, Cervarix®, Clinical trials, Cervical cancer, Genital warts, CIN, VIN, VaIN
1. Introduction
This article provides a broad overview of clinical trial results for the two licensed prophylactic human papillomavirus (HPV) vaccines, Cervarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium) and Gardasil® (Merck & Co., Whitehouse Station, NJ USA), concentrating on studies published since 2008. It emphasizes the end of study analyses of the pivotal phase III trials in young women that have led to widespread licensure and subsequent uptake of the vaccines. A review of earlier publications on the subject can be found in a previous monograph in this series [1]. The results of efficacy studies in mid-adult women and men that, in some instances, have led to additional indications for the vaccines, are also presented. In addition, safety/immunogenicity studies involving alternative dosing schedules, other populations, or combined administration with other licensed vaccines are outlined. Finally, potential second generation vaccines are briefly discussed. A companion article in this monograph is devoted to the implementation issues related to the introduction of these vaccines (Markowitz LE et al., Vaccine, this issue) [2].
2. Vaccine formulations
2.1. Currently available HPV vaccines
Both Cervarix® and Gardasil® are non-infectious subunit vaccines composed primarily of virus-like particles (VLPs). The VLPs spontaneously self-assemble from 360 copies of L1, the major structural protein of the virion [3]. Although referred to as “virus-like”, the VLPs are completely non-infectious and non-oncogenic, since they do not contain the viral DNA genome or specific viral genes required for these activities. VLP vaccines are based on the concept of forming a structure that sufficiently resembles the outer shell of an authentic HPV virion such that antibodies that are induced to it react with and inactivate the authentic virus [4]. The specifics of how these antibodies are induced, how they reach the site of HPV infection, and how they prevent HPV infection, are the subject of an accompanying article in this monograph (Stanley M et al., Vaccine, this issue)[5].
2.2. Formulation differences
Although conceptually similar, Cervarix® and Gardasil® differ in several aspects, including valency, dose, production system, and adjuvant (Table 1). Cervarix® is a bivalent vaccine, containing the VLPs of HPV16 and 18, the two types that cause 70% of cervical cancer worldwide, and even greater proportions of HPV-associated vulvar, vaginal, penile, anal, and oropharyngeal cancers [6,7] (see Forman D et al., Vaccine, this issue for details on type-specific HPV disease burden) [8]. Gardasil® targets the same two cancer-causing types, but in addition contains VLPs of HPV6 and 11, which cause approximately 90% of external genital warts in both men and women [9]. The VLPs for Cervarix® are produced in insect cells infected with L1 recombinant insect virus vectors [10]. Gardasil®’s VLPs are produced in baker’s yeast (Saccharomyces cerevisiae) expressing L1 [11]. Each VLP type is produced and purified separately and the different types are mixed during final formulation. Both vaccines must be refrigerated, but not frozen. Delivery of both vaccines is via three intramuscular injections in the deltoid area over a 6-month period, but the recommended timing of the second dose differs slightly (Table 1).
Table 1.
Characteristics of HPV VLP vaccines
| Gardasil® | Cervarix® | |
|---|---|---|
| Manufacturer | Merck | GlaxoSmithKline |
| VLP Types | 6/11/16/18 | 16/18 |
| Dose of L1 Protein | 20/40/40/20 µg | 20/20 µg |
| Producer Cells |
Saccharomyces cerevisiae (baker’s yeast) expressing L1 |
Trichoplusia ni (Hi 5) insect cell line infected with L1 recombinant baculovirus |
| Adjuvant | 225 µg aluminum hydroxyphosphate sulfate |
500 µg aluminum hydroxide, 50 µg 3-O-deacylated-4’- monophosphoryl lipid A |
| Injection Schedule | 0, 2, 6 months | 0, 1, 6 months |
Gardasil® (Merck & Co., Whitehouse Station, NJ USA).
Cervarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium).
HPV: human papillomavirus; VLP: virus-like particle.
Data from reference [1].
Like other protein subunit vaccines, the two HPV VLP vaccines are formulated with adjuvants to increase their immunogenicity. Gardasil® contains a simple aluminum salts adjuvant (aluminum hydroxyphosphate sulfate), whereas Cervarix® contains a more complex adjuvant system, designated AS04, consisting of monophosphoryl lipid A (MPL) and an aluminum salt (aluminum phosphate) [12]. MPL is a detoxified form of bacterial lipopolysaccharide and is a toll-like receptor (TLR)-4 agonist. TLRs are an evolutionarily conserved class of host sensors of microbial constituents that activate innate and adaptive immune responses to invading microbes. It is noteworthy that AS04 is the first TLR agonist-containing prophylactic vaccine adjuvant to be licensed by the United States (U.S.) Food and Drug Administration (FDA). Neither vaccine contains a preservative.
3. Phase III trial design
3.1. Design of phase III clinical trials
Phase III efficacy trials of the VLP vaccines in young women were primarily designed to demonstrate efficacy in preventing incident vaccine-related HPV infection and the preneoplastic lesions caused by incident persistent infections related to vaccine HPV types. Initiation of these trials was predicated on successful completions of a series of preceding studies including development of industrial scale manufacturing processes, validation of type-restricted measures of antibody responses to the VLPs, and promising safety, immunogenicity and preliminary efficacy results in preclinical and early phase I/II trials [10,13]. Two phase III studies, FUTURE I [14] and FUTURE II [15], evaluated Gardasil® and two, PATRICIA [16] and the Costa Rica HPV Vaccine Trial (CVT) [17] evaluated Cervarix®. All of the trials were relatively large (5,500–18,500 vaccinees), blinded, randomized and controlled trials of young women (mean age 20, range 15–26) (Table 2). The CVT was a U.S. government sponsored community-based trial, centered in the Guanacaste province of Costa Rica [17], whereas the other trials were company-sponsored and multi-centric, involving multiple trial sites in Europe, North, Central and South America, and Asia Pacific, including Australia. With the exception of the CVT and the Finnish subjects in PATRICIA, there was a restriction on the number of lifetime sexual partners. This restriction was used to limit the number of women with prevalent infections and/or prevalent genital lesions at enrollment, in keeping with the primary goal of evaluating immunoprophylaxis. However, women were not excluded from the trials if they had prevalent infection at enrollment, as measured by the presence of genital tract HPV DNA by sensitive PCR-based techniques, or evidence of prior exposure, as measured by serum antibodies reactive to the VLPs, or in some cases by finding an abnormal cervical cytology at baseline. Their inclusion permitted an evaluation of the safety, immunogenicity, and prophylactic efficacy of the vaccine in women with prior or current HPV exposure, and also the possibility that the vaccines may have therapeutic activity.
Table 2.
Characteristics of phase III efficacy studies in young women
| Characteristic | Future I | Future II | PATRICIA | CVT |
|---|---|---|---|---|
| Vaccine | Gardasil® | Gardasil® | Cervarix® | Cervarix® |
| Funding source | Merck & Co., Inc. | Merck & Co., Inc. | GlaxoSmithKline | National Cancer Inst. |
| No. study sites | 62 | 90 | 135 | 7 |
| Countries included | 16 | 13 | 14 | 1 |
| Length | 4 years | 4 years | 4 years | 4 years |
| Control | 225 µg Aluminum hydroxyphosphate sulfate |
225 µg Aluminum hydroxyphosphate sulfate |
Hepatitis A Vaccine | Hepatitis A Vaccine |
| Age | 16–24 | 15–26 | 15–25 | 18–25 |
| Lifetime no. sexual partners | ≤4 | ≤4 | ≤6a | No restriction |
| Exclusion criteria | Pregnancy, history of abnormal Pap smear or genital warts |
Pregnancy, history of abnormal Pap smear |
Pregnancy, breastfeeding, history of colposcopy, autoimmume disease or immunodeficiency |
Pregnancy, breastfeeding, history of immunosuppression, hysterectomy, hepatitis A vaccination |
| Primary endpoints | Incident HPV6/11/16/18- associated genital warts, CIN1-3, VIN1-3, VaIN1- 3, AIS and cervical, vaginal or vulvar cancer |
Incident HPV16/18 – associated CIN2-3, AIS or cervical cancer |
Incident HPV16/18 – associated CIN2+ |
Incident 12 mo. persistent HPV16/18 infection |
3.2. Trial endpoints
The outcome of most interest, prevention of cervical or other anogenital cancers, was not a reasonable endpoint for these trials. Trial size and duration would be unmanageable, since cancer is a rare outcome of persistent oncogenic HPV infection, and it usually takes more than a decade for cancers to develop from incident infection [18]. In addition, a cancer endpoint would be unethical. Women undergoing active follow-up in clinical trials were monitored closely for the development of high-grade premalignant lesions that must be removed before they progress to cancer. Consequently, the two largest trials, FUTURE II and PATRICIA employed a precancer primary efficacy endpoint of high-grade dysplasia otherwise known as cervical intraepithelial neoplasia (CIN) grade II or III (CIN2+), adenocarcinoma in situ (AIS), or cervical cancer associated with HPV16/18 (Table 2). This endpoint was recommended by a U.S. FDA advisory committee, and other national regulatory agencies, for a vaccine indication of prevention of cervical cancer [19]. Importantly, end of study analyses also included reasonably powered evaluation of the efficacy against CIN III, the most immediate and widely accepted precursor of cervical cancer. FUTURE I had co-primary efficacy endpoints of HPV6/11/16/18-associated CIN1+ and external genital lesions, which included genital warts and vulvar/vaginal intraepithelial neoplasia (VIN/VaIN). The primary endpoint for CVT was cervicovaginal HPV16/18 infection that persisted for at least 1 year. All four trials were designed to have at least 4 years of follow-up. However, interim analyses were conducted in the FUTURE I, Future II and PATRICIA trials, based on an accrual of a pre-specified total number of primary endpoint events [14–16]. These interim analyses led to regulatory approval for both vaccines prior to completion of the trials. However, end of study analyses including additional endpoint events have recently been published for all four studies. To improve statistical power for secondary analyses, data from phase II/III trials employing the same vaccine and similar study designs were combined in some recent publications [20].
3.3. Cohorts analyzed
Interpreting the results from these trials can be confusing because they often involve analyses of various sub-cohorts of the trial participants (summarized in Table 3), and the composition of these subsets can greatly influence the calculated vaccine efficacy. In addition, conceptually similar cohorts often have different designations in the publications for the two vaccines. Intention-to-Treat (ITT) cohorts, also designated Total Vaccine Cohort (TVC), are the most inclusive, including all individuals that are randomized and participate in the trial. For vaccine trials “participation” is usually defined as receiving at least one dose of the vaccine. These cohorts include women with evidence of prior HPV exposure and hence current infection/lesions by vaccine-targeted as well as other HPV types. ITT analyses can be viewed as an approximation of the effectiveness of the vaccine in general use, at least for individuals with similar demographic and risk characteristics as the subjects in the trial. The most restrictive cohorts are According to Protocol (ATP), also designated Per Protocol Efficacy (PPE). ATP analyses are restricted to individuals who adhere to all aspects of the study protocol: for example, they received the three vaccine doses within specified intervals, and events are not counted until after receiving all three doses. Importantly, individuals included in ATP cohorts have no evidence of exposure to the vaccine-targeted type under analysis. Thus ATP analyses can be viewed as the best-case scenario for the effectiveness of a prophylactic vaccine. Modified Intention-To-Treat (MITT) analyses fall somewhere in between ITT and ATP, allowing for some deviation from the ideal protocol. One interesting MITT cohort is designated TVC-naïve or ITT-naïve. These cohorts include all participating individuals with no evidence at baseline of cervical cytology abnormalities, prevalent infection by any of the genital HPV types evaluated (up to 14 types) or serological evidence of past exposure to the vaccine-targeted types. These cohorts are currently the best approximation for the primary target group for the vaccines, pre- and early-adolescent girls who have not yet become sexually active. Finally, it is always import to note whether the efficacy against lesion development is restricted to those specifically related to vaccine-targeted types or irrespective of HPV type. As discussed below, protection from infection by the L1 VLP vaccines is type restricted and so efficacy is generally higher in the analyses restricted to the vaccine-targeted types.
Table 3.
Phase III efficacy studies in young women: End of study cohorts
| Future I/II - Gardasil® | PATRICIA - Cervarix® | CVT - Cervarix® | ||||
|---|---|---|---|---|---|---|
| Vaccine No. | Control No. | Vaccine No. | Control No. | Vaccine No. | Control No. | |
| ATP/PPE | Received 3 doses within a year, Remained DNA-negative to HPV6/11/16/18 or HPV16/18 through mo. 7, seronegative to HPV6/11/16/18 at baseline; no protocol violations; could have abnormal Pap at baseline |
Received 3 doses, seronegative at baseline and DNA-negative to HPV16/18 through mo. 6, normal or low grade cervical cytology at baseline, no protocol violation |
Received 3 doses within defined window, HPV DNA-negative for the corresponding type through mo. 6, no biopsy/treatment through mo. 6 |
|||
| 7864a | 7865a | 7338 | 7305 | 2635–2643b | 2677–2697b | |
| ITT/TVC-naïve2 | Received ≤1 dose, baseline HPV DNA negative for 14 types testedc seronegative for HPV6/11/16/18, and cytology negative |
Received ≤1 dose, baseline HPV DNA negative for 14 types testedd seronegative for HPV16/18, and cytology negative |
Not evaluated | |||
| 4616–4689b | 4680–4735b | 5466 | 5452 | Not reported | Not reported | |
| ITT/TVC | Received ≤1 dose, regardless of compliance, enrollment HPV DNA or serology status or evidence of anogenital neoplasia |
Received ≤1 dose, regardless of compliance, enrollment cytology, HPV-DNA or HPV serology status |
All randomized women regardless of compliance or enrollment HPV DNA status |
|||
| 8562 | 8598 | 8694 | 8708 | 3727 | 3793 | |
Includes subjects in Phase IIb trial 007.
Number of subjects varies depending on endpoint or HPV type under analysis.
HPV6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59.
HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.
3.4. Measures of health impact
Most publications have concentrated on reporting vaccine efficacy, which can be thought of as the percent reduction in an individual’s probability of acquiring a given endpoint if s/he received the experimental vaccine versus the control. However, analyses of rate reductions in disease or treatment, generally reported using the denominator of per 100 subject-years, have also been reported in some of the more recent publications. Rate reductions can sometimes be more useful indicators of the potential for health impact of an intervention. As noted below, there are instances in which cohorts with markedly different vaccine efficacy measurements can have similar vaccine-induced reductions in the rate of disease. However, absolute reductions in disease rates can be difficult to compare across trials, since, in addition to efficacy, they are dependent on attack rates, which can vary depending upon the sexual activity (of the individual as well as their partner), pre-existing immunity and other variables of the cohorts.
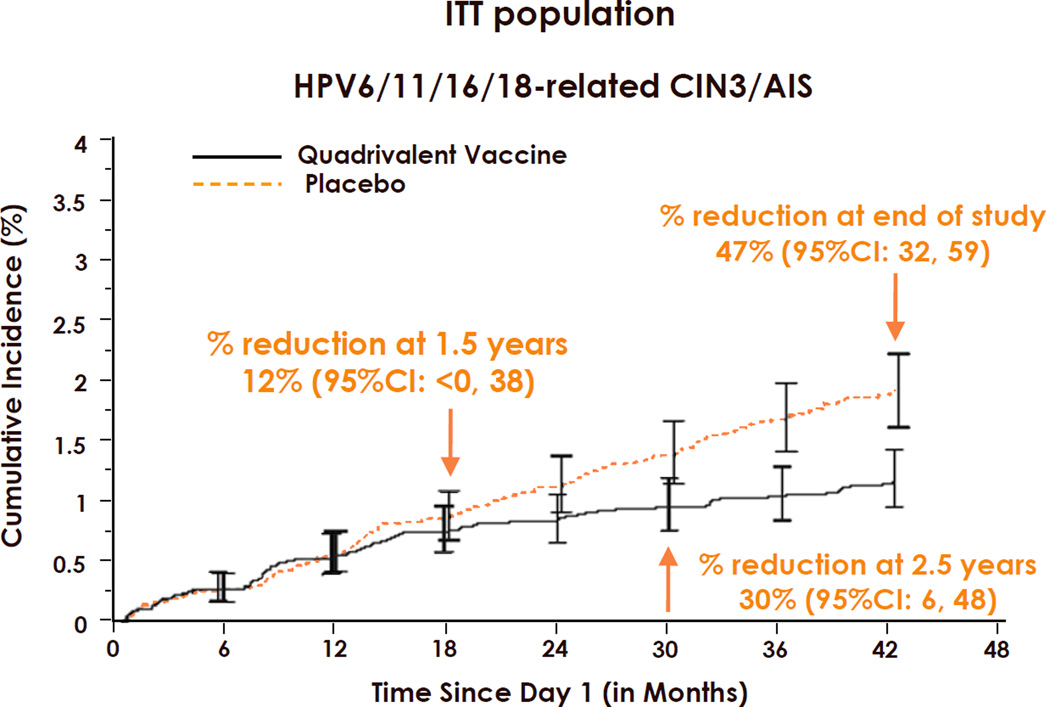
It is important to note that for prophylactic HPV vaccine trials, neither efficacy nor rate reduction is an absolute measure of a vaccine’s performance. Rather, they are time dependent variables. The time dependency is more pronounced in ITT than ATP analyses and for high-grade disease than low-grade disease or infection endpoints. The phenomenon is best illustrated in time-to-event curves. Fig. 1 shows the time-to-event curves for HPV6/11/16/18-related CIN3/AIS in Gardasil® and placebo vaccinated young women in an ITT cohort [21]. No reduction in incidence disease was seen in the first year of the trial, whereas steadily increasing disease reduction was observed thereafter, up to 47% after 3.5 years. The lack of significant efficacy or rate reduction during the initial months can be explained by the fact that it normally takes many months for neoplasia, especially CIN3, to develop from incident infection [22]. It follows that most early CIN3 cases will result from prevalent, not incident infection. Because the subjects were randomized, the percent of vaccine and placebo subjects with prevalent infection should be approximately equal. It is only after a substantial number of disease cases have developed from incident infection that the preferential prevention of incident infection in the vaccinated subjects can lead to a significant divergence of the two curves. Similar trends were seen in the Cervarix® efficacy trials [23]. This phenomenon makes it difficult to compare vaccine performance across trials with different attack rates and length of follow-up, apart from methodological differences in colposcopy referral, DNA detection and attribution of causal HPV for cervical lesions. If the follow-up of the trials were extended past 4 years, the expectation is that cumulative efficacy/rate reduction would continue to increase, providing the vaccines continued to protect from incident infection. However, in many countries, the rate of divergence of the curves would likely be reduced in later years as the cohorts move beyond their peak years of HPV acquisition. The time dependency effect is less pronounced for ATP analyses since subjects in whom prevalent infection or disease is detected are excluded. However, nascent prevalent infections that are undetected at baseline and later emerge can lead to a more modest increase in efficacy with time in ATP analyses as well.
Figure 1.
Rate reduction and vaccine efficacy are time dependent variables. Time-to-event curves for acquisition of HPV6/11/16/18-related CIN3/AIS in Gardasil® (Merck & Co., Whitehouse Station, NJ USA) and placebo recipients in the ITT cohort. AIS: Adenocarcinoma in situ; CIN3: Grade 3 cervical intraepithelial neoplasia; ITT: Intention-to-Treat. Taken with permission from reference [21].
4. Prophylactic efficacy in young women
In the end of study analyses of the pivotal phase III efficacy trials in young women, prophylactic efficacy against vaccine type-associated primary and secondary endpoints was uniformly high in ATP and ITT-naïve cohorts (Tables 4–6).
Table 4.
Protection of young women against genital disease by Gardasil® in Future I and II Trials
| A. Genital disease related to HPV6/11/16/18 | ||
| % Efficacy (95% CI) | Rate reductiona | |
| ATPb | ||
| CIN2 | 100 (94.7–100) | 0.5 |
| CIN3 | 96.8 (88.1–99.6) | 0.3 |
| AIS | 100 (30.9–100) | <0.1 |
| VIN2/3 or VaIN2/3+ | 100 (82.6–100) | 0.1 |
| Unrestricted susceptible | ||
| CIN2 | 100 (88.4–100) | 0.3 |
| CIN3 | 100 (90.5–100) | 0.2 |
| AIS | 100 (<0–100) | <0.1 |
| VIN2/3 or VaIN2/3 | 95.4 (71.5–99.9) | 0.1 |
| Genital warts | 96.4 (91.4–98.9) | 0.8 |
| ITT | ||
| CIN2 | 54.8 (40.8–65.7) | 0.3 |
| CIN3 | 45.1 (29.8–57.3) | 0.3 |
| AIS | 60.0 (<0–87.3) | <0.1 |
| VIN2/3 or VaIN2/3+ | 78.5 (55.2–90.8) | <0.1 |
| Genital warts | 79.5 (73.0–84.6) | 0.8 |
| B. Genital disease irrespective of HPV type | ||
| % Efficacy (95% CI) | Rate reductiona | |
| ITT naive | ||
| CIN2 | 42.9 (20.2–59.5) | 0.3 |
| CIN3 | 43.0 (13.0–63.2) | 0.2 |
| AIS | 100 (<1–100) | <0.1 |
| VIN2/3 or VaIN2/3 | 77.1 (47.1–91.5) | 0.1 |
| Genital warts | 82.8 (74.3–88.8) | 0.8 |
| Unrestricted susceptible | ||
| CIN2 | 19.3 (5.7–31.0) | 0.2 |
| CIN3 | 16.4 (0.4–30.0) | 0.2 |
| AIS | 62.5 (<0–88.0) | <0.1 |
| VIN2/3 or VaIN2/3 | 50.7 (22.5–69.3) | 0.1 |
| Genital warts | 62.0 (53.5–69.1) | 0.8 |
per 100 women years.
includes final results of trial 007.
Table 6.
Protection of young women against 1-year persistent HPV16/18 cervicovaginal infection by Cervarix® in the Costa Rica Vaccine Trial
| A. Stratified by age at vaccination | ||
| % Efficacy (95% CI) | Rate reductiona | |
| ATP | ||
| All ages | 90.9 (82.0–95.9) | 3.0 |
| 18–19 | 95.9 (78.5–99.8) | 2.9 |
| 20–21 | 86.6 (59.2–96.8) | 2.9 |
| 22–23 | 95.7 (77.4–99.8) | 3.8 |
| 24–25 | 82.2 (43.9–95.9) | 2.5 |
| ITT | ||
| All ages | 49.0 (38.1–58.1) | 3.9 |
| 18–19 | 68.9 (53.1–79.9) | 5.2 |
| 20–21 | 42.8 (17.9–60.6) | 3.6 |
| 22–23 | 51.5 (28.4–67.7) | 4.7 |
| 24–25 | 21.8 (16.9–47.9) | 1.6 |
| B. Stratified by time since enrollment | ||
| % Efficacy (95% CI) | Rate reductiona | |
| ATP | ||
| 10–22 mo. | 71.2 (25.6–90.5) | 0.8 |
| 22–34 mo. | 91.9 (76.6–98.0) | 1.6 |
| 34–46 mo. | 100 (81.0–100) | 1.4 |
| 46+ mo. | 100 (78.6–100) | 1.6 |
| ITT | ||
| 10–22 mo. | 15.6 (8.1–34.2) | 0.7 |
| 22–34 mo. | 59.7 (36.5–75.0) | 1.3 |
| 34–46 mo. | 83.5 (69.9–91.7) | 1.8 |
| 46+ mo. | 94.3 (80.1–99.1) | 1.6 |
per 100 women vaccinated
ATP: According to Protocol; CI: Confidence interval; ITT: Intention-to-Treat.
Data from reference [26].
4.1. Gardasil®: efficacy against CIN3, VIN2/3, VaIN2/3 and genital warts
High efficacy against vaccine HPV type-related CIN3 was observed in the final ITT-naïve analyses (called unrestricted susceptible population) of the Gardasil® phase III trials, 100% [21]. As expected, efficacy was considerably lower in the ITT analysis, 45.1%, since it included women with prevalent infection at entry and VLP vaccines do not appear to induce regression of established infections (discussed below) [20] (Table 4). Efficacy against CIN3 was notably lower in the analyses irrespective of HPV type, 43.0% and 16.4% in the ITT-naïve and ITT cohorts, respectively. However, rate reduction in CIN3 was consistently 0.2 to 0.3 across the various cohorts (Table 4). Greater than 95% efficacy and greater than 75% efficacy was also observed against vaccine type-related VIN2/3 or VaIN2/3 and genital warts in the ITT-naïve and ITT cohorts, respectively. Efficacy against these endpoints was also high in the analyses irrespective of HPV type, reflecting the predominance of HPV6/11/16/18 in external genital lesions in young women. Rate reductions were particularly high for genital warts (0.8) [21], due to their relatively high incidence and relatively rapid progression from incident infection to clinical disease. The latter finding supports the observations in preliminary effectiveness studies suggesting that genital warts will be the first substantial public health benefit detected after implementing Gardasil® vaccination programs with high population coverage [24].
4.2. Cervarix®: efficacy from the PATRICIA trial
In the PATRICIA trial, efficacy against HPV16/18-related CIN3 in the TVC-naïve analysis was 100% [23] (Table 5). As expected, efficacy was lower in the full TVC analysis, 45.7%. However the reduction in the rate of CIN3 in both cohorts was 0.13 per 100 women years. A recent conference abstract reported significant protection against HPV16/18 associated VIN1+ or VaIN1+ in the TVC-naïve and full TVC.
Table 5.
Protection of young women against incident cervical disease by Cervarix® in the PATRICIA trial
| A. HPV16 or HPV18-related endpoints | ||
| % Efficacy (95% CI) | Rate reductiona | |
| ATP-E | ||
| CIN2+ | 94.9 (87.7–98.4) | 0.38 |
| CIN3+ | 91.7 (66.6–99.1) | 0.09 |
| AIS | 100 (−8.2–100) | 0.02 |
| TVC-naive | ||
| CIN2+ | 99.0 (94.2–100) | 0.47 |
| CIN3+ | 100 (85.5–100) | 0.13 |
| AIS | 100 (15.5–100) | 0.03 |
| TVC | ||
| CIN2+ | 60.7 (49.6–69.5) | 0.43 |
| CIN3+ | 45.7 (22.9–62.2) | 0.13 |
| AIS | 70 (−16.6–94.7) | 0.02 |
| B. Endpoints irrespective of HPV DNA | ||
| % Efficacy (95% CI) | Rate reductiona | |
| TVC-naive | ||
| CIN2+ | 64.9 (52.7–74.2) | 0.54 |
| CIN3+ | 93.2 (78.9–98.7) | 0.20 |
| AIS | 100 (31.0–100) | 0.03 |
| TVC | ||
| CIN2+ | 33.1 (22.2–42.6) | 0.44 |
| CIN3+ | 45.6 (28.8–58.7) | 0.22 |
| AIS | 76.9 (16.0–95.8) | 0.03 |
per 100 women years
AIS: Adenocarcinoma in situ; ATP-E: According to Protocol for efficacy; CI: Confidence interval; CIN: Cervical intraepithelial neoplasia; HPV: Human papillomavirus.
Data from reference [23].
The 93.2% efficacy against CIN3 in the TVC-naïve analysis, irrespective of HPV type, has received considerable attention. However, the long-term effectiveness of both Cervarix® and Gardasil® in adolescent vaccination campaigns is unlikely to equal the high a level of efficacy against any CIN3 seen in the clinical trials. HPV16 and 18, and to a lesser extent some of the types to which the vaccines exhibits cross-protection (discussed below), are more frequently present in CIN3 lesions that appear relatively early after incident infection [22]. CIN3 caused by types for which the vaccines apparently offer no protection generally appear later, and so are less likely to contribute to this endpoint in a 4-year trial than they will during a women’s lifetime. In addition, it is possible that protection against non-vaccine types will wane more rapidly than against vaccine targeted types [25] (discussed below).
4.3. Cervarix®: efficacy from the CVT
Efficacy against the primary endpoint of the CVT, one-year persistent HPV16/18 infection, was 90.9% in the ATP cohort and 49.0% in the ITT [26] (Table 6). The protection Cervarix® afforded against this endpoint was similar in the PATRICIA trial, 92.4% (95% confidence interval [CI]: 88.6–95.2) in the TVC-naïve and 57.5 (95% CI: 51.7–62.8) in the TVC [23]. While efficacy and rate reduction in the CVT was similar across ages in the ATP cohort, they were age dependent in the ITT cohort, despite the relatively small age range in the trial (Table 6). Efficacy fell from 68.9% in 18–19 year-olds to 21.8% in 24–25 year-olds (p for trend = 0.005). Similarly, the rate reduction in persistent infections per 100 women years fell from 5.2 to 1.6. Similar declines in efficacy and rate reductions were seen when the women were stratified according to time since first sexual intercourse. These decreases probably are due to a combination of higher prevalent HPV16/18 infection and decreased acquisition rates (due to immunity and reduced exposure) in the older women. The results exemplify the effectiveness of the vaccine at preventing new infection, independent of age, but the decreased overall benefits of vaccination with age in a population of mostly sexually active young women.
Protection from persistent infection increased dramatically with time since vaccination in the ITT cohort in the CVT, where it increased from a non-significant 15.6% in the interval 10–22 mo. after vaccination to 94.3% after 46 mo. since vaccination (Table 6) [26]. This finding is likely the result of the resolution of most prevalent infections by 4 years coupled with the durability of protection from incident infection over this time period. Interestingly, there was also a trend for lower efficacy (and also rate reduction) early after vaccination in the ATP cohort, from 71.2% (95% CI: 25.6–90.5) during mo. 10–22 to 100% (78.6–100) starting 46 mo. post vaccination. The findings suggest that some prevalent infections were undetected at baseline and then emerged during the first two years of the trial. Undetected prevalent infections likely account for many of the “breakthrough” infections detected in other Cervarix® and Gardasil® trials. However, the effect might be greater in the CVT because of the greater likelihood of HPV exposure at entry due to the higher minimum age and no limit to the number of lifetime sex partners for enrollees.
4.4. Data on less than three doses from the CVT
Protection from cervical HPV infection by less than three doses of Cervarix® was also evaluated in the CVT [27]. Approximately 11% of vaccine and control recipients received two doses and approximately 5% received only one dose. Perhaps surprisingly, protection in the ATP cohort from 12 month persistent HPV16/18 infection after 4 years of follow-up did not significantly differ depending on number of doses. Vaccine efficacy after three, two, or one dose was 80.9 (95% CI: 71.1–87.7), 84.1% (95% CI, 50.2–96.3) and 100% (95% CI: 66.5–100), p for trend = 0.21. These results must be interpreted with some caution because the number of women receiving less than three doses was limited and the study was not formally randomized by number of doses, nor been followed beyond four years. However, these observations should encourage ongoing and future trials to more rigorously evaluate long-term efficacy of less than three doses of the two vaccines (discussed in Markowitz LE et al., Vaccine, this issue[2]).
4.5. Efficacy against anal HPV infection from the CVT
In the CVT, anal swab specimens were obtained from consenting women at the year 4 exit visit and assessed for HPV DNA status. Anal HPV DNA status was not evaluated at enrollment. Vaccine efficacy against single time anal HPV16/18 DNA was substantial, 62.0% (95% CI: 47.1–73.1) but less than the efficacy against single time detection at exit for the cervix, 76.4% (95% CI: 67.0–83.5) [28]. However, protection at the anus and cervix was similar in the cohort restricted to women who were negative for cervical HPV16/18 DNA and antibodies at enrollment, 83.6% (95% CI: 66.7–92.8) and 87.9 (95% CI: 77.4–94.9) at the anus and cervix, respectively. Therefore, it appears that Cervarix® strongly protects against anal HPV infection in young women, particularly among those most likely to be HPV16/18 naïve at entry.
5. Cross-type protection
Although none of the phase III studies was specifically designed to evaluate cross-type protection, both vaccines have been evaluated for protection against infection and cervical disease associated with oncogenic types, particularly those most closely related phylogenetically to types 16 and 18 (A9 and A7, respectively), that are not specifically targeted by inclusion of the corresponding VLP type in the vaccine. Cross-protection against non-vaccine types is an important consideration since non-vaccine types are associated with approximately 30% of cervical cancers worldwide [6].
5.1. Cross-protection against persistent infection
Analysis of cross-protection from persistent infection is relatively straightforward, provided that infection by one type does not substantially reduces the sensitivity of PCR-based detection of other types. Both Gardasil® and Cervarix® provided significant protection against infection by HPV16-related types (A9 species), 21.9% and 27.6%, respectively [29,30]. Cervarix® demonstrated significant efficacy against three individual A9 types, HPV31, 33, and 52, whereas Gardasil® demonstrated significant efficacy only against HPV31 (Table 7). Cervarix®, but not Gardasil®, also demonstrated significant protection against infection by HPV18-related A7 species, 22.3% and 14.8, respectively. Most notably, Cervarix® provided relatively strong protection against HPV45, 79.0%, but Gardasil® did not, 7.8%. Partial protection against HPV45 and HPV31 in Cervarix® vaccinees was also observed in the CVT [26]. Overall, the cross-protection results from PATRICIA and CVT were in general agreement. The exception is that weak protection against HPV51, which is not closely related to HPV16 or 18, was measured in PATRICIA (16.6%; 95% CI: 3.6–27.9 in ATP) while potential enhancement of infection was observed in CVT (−56.1%; 95%CI: −114–−14.2). This discrepancy may well represent a chance finding that can sometimes occur when multiple comparisons are made within a data set. In the CVT, partial cross- protection against anal infection at study exit was also observed in a combined analysis of HPV31, 33, or 45, for example 49.4% (95% CI: 30.3– 63.6) in the full cohort [28].
Table 7.
Cross-type protection against 6-month persistent infection
| Efficacy (95% CI) | |||
|---|---|---|---|
| Trial: | FutureI/II | PATRICIA | CVT |
| Vacccine: | Gardasil® | Cervarix® | Cervarix® |
| Cohort: | ITT-Naive | TVC-Naive | ATP |
| Mean | 3.6 yrs | 3.3 yrs | 4 yrs |
| Follow-up: | |||
| HPV31 | 46.2 (15.3–66.4) | 77.1 (67.2–84.4) | 64.7 (42.6–78.9) |
| HPV33 | 28.7 (45.1–65.4) | 43.1 (19.3–60.2) | 32.1 (41.1–68.2) |
| HPV35 | 17.8 (77.1–62.5) | 21.8 (102.5–26.2) | 25.0 (40.6–60.6) |
| HPV52 | 18.4 (−20–45.0) | 18.9 (3.2–32.2) | 19.6 (8.1–40.4) |
| HPV58 | 5.5 (54.3–42.2) | 6.2 (44.0–21.6) | 2.8 (48.0–36.2) |
| Non-Vaccine A9 | 21.9 (0.6–38.8) | 27.6 (17.6–36.5) | NR |
| HPV39 | NRa | 20.9 (2.3–39.9) | 30.8 (109.2–17.6) |
| HPV45 | 7.8 (67.0–49.3) | 79.0 (61.3–89.4) | 73.0 (45.3–87.8) |
| HPV59 | 18.7 (22.8–46.4) | 3.9 (61.7–33.1) | 30.3 (130.3–25.6) |
| HPV68 | NR | 8.9 (18.8–30.1) | NR |
| Non-vaccine A7 | 14.8 (19.9–39.6)b | 22.3 (8.4–34.2) | NR |
| HPV51 | NR | 25.5 (12.0–37.0) | 56.1 (114.3–14.2) |
| HPV56 | NR | 1.4 (24.8–22.0) | 25.8 (12.7–51.4) |
| HPV66 | NR | 1.5 (29.3–20.3) | 1.6 (41.0–31.3) |
Interestingly, while cross-protection against cervical infection by non-vaccine types was clearly observed in CVT women receiving three doses of Cervarix®, there was no indication of cross-protection in those receiving two doses [27]. For instance, efficacy in the ATP cohort against 12 month persistent infection with HPV31, 33, and 45 combined was 41.3% (95% CI: 18.9–57.9) in women receiving three doses and −25.9% (95% CI: −334–66.1) in those receiving two doses. There were too few non-vaccine type infections in the women receiving one dose to meaningfully evaluate cross-protection in this group.
Evidence from a long-term follow up of a phase IIb trial of Cervarix® suggests that cross-protection might preferentially wane over time [31]. Protection from incident HPV16/18 infection remained consistently high (>90%) throughout the 6.4 years of follow up, with a cumulative efficacy of 95.3% (95% CI: 87.3–99.6). In contrast, protection from HPV31 and HPV45 infection was 100% through the first 3 years, but then incident infections began to appear over the next 3 years, yielding cumulative efficacies of 59.8% (95% CI: 20.5–80.7) and 77.7% (95% CI: 39.3–93.4) for HPV31 and HPV45, respectively. It will be important to evaluate in long-term field studies the public health impact of cross-protection afforded by the two vaccines.
5.2. Cross-protection against disease endpoints
Evaluating cross-protection against disease endpoints is complicated by the fact that many women with cervical disease are infected with more than one HPV type. Causal inferences can be made by determining the specific type(s) in a lesion biopsy or by assuming that the preceding most persistent infection is responsible for the CIN, but these approaches have limitations. Complicating the issue is the fact that infections by HPV16 and 18, the vaccine types, tend to progress to CIN more rapidly than infections by other high risk types [22]. Thus, in a 4-year trial, the probability that the lesion in a co-infected woman will be due to the non-vaccine type is less than the probability that it will be due to a vaccine type. A conservative approach used in the PATRICIA trial to address this issue was to evaluate cross-protection after excluding cases that were co-infected with vaccine types [30]. This exclusion consistently results in lower efficacy estimates against non-vaccines type-associated lesions. For instance, for the composite endpoint of CIN2+ associated with any of 12 non-vaccine types, efficacy in the TVC-naïve cohort was 56.2% (95% CI: 37.2–65.0) if HPV16/18 co-infections were included and a nonsignificant 17.1% (95% CI,: −25.5–45.4) if HPV16/18 co-infections were excluded. However, the corresponding efficacies against CIN3+ were significant in both cases, 91.4% (95% CI: 65.0–99.0) and 81.9% (95% CI: 17.1–98.1), respectively. For individual types, CIN2+ efficacy estimates with 95% CIs above zero were noted for types 31, 33, 45, 51, 52, and 56 when HPV16/18 co-infections were included but only for types 31 and 33 when HPV16/18 co-infections were excluded. This difference may be due, in part, to the low number of disease endpoints for many types when HPV16/18 co-infections were excluded.
Cross-protection against cervical disease endpoints was also observed for Gardasil® in the combined FUTURE I/II analysis [29]. Efficacy against CIN2+ associated with any one of the 10 most common oncogenic non-vaccine types was 32.5% (95% CI: 6.0–51.9). Of the 69 cases in the placebo arm, 22 (31.9%) occurred in women who also had an HPV16/18-related CIN2+. HPV31 was the only individual type for which significant protection against CIN2+ was observed, 70% (95% CI: 32.1–88.2). Efficacy against non-vaccine A9 species (types 31,33, 35, 52, or 58) in aggregate was 35.4% (95% CI: 4.4–56.8) and, for non-vaccine A7 species (types 39, 45 or 59) in aggregate, efficacy was a nonsignificant 47.0% (95% CI: −15.0–76.9). Efficacy estimates excluding infections by vaccine types were not reported.
6. Efficacy in women with exposure to vaccine type infections
Prior exposure to the HPV types targeted by the vaccine will be minimal in the primary focus of vaccination campaigns, 10–14 year old girls. However, vaccine safety and efficacy after HPV16/18 infection is an issue for young women targeted by catch up vaccination programs because they are expected to have appreciable exposure at the time of vaccination. This expectation was met in the phase III clinical trials. For instance, in the PATRICIA trial, approximately 6–7% were positive for cervical HPV16 or HPV18 DNA at enrollment and 18–19% of women had serologic evidence of HPV16 and/or HPV18 infection at enrollment [32]. In a combined FUTURE I/II analysis, 19.8% of the study population was seropositive for HPV6/11/16/18 and 26.8% were either PCR DNA-positive or seropositive for at least one of the vaccine types [33]. It is important to note that serologic measures of prior exposure to genital HPV infections substantially underestimate true exposure rates since many women with evidence of cervicovaginal infection will not seroconvert and some seropositive women will become seronegative over time [34].
Vaccine efficacy in PATRICIA was high for CIN2+ related to HPV16 or HPV18 in women with evidence of current infection (as measured by HPV DNA detectability) by the other vaccine type at enrollment, 90.0% (95% CI: 31.8–99.8) [32]. Among HPV16/18 DNA-negative women, vaccine efficacy against HPV16/18 infection was somewhat lower in those seropositive from natural infection than in those seronegative, 72.3 (96.1% CI: 53.0–84.5) and 90.3 (96.1% CI: 87.3–92.6), respectively. A greater probability of latent infection (susceptible to reactivation) in seropositives might explain this difference. The notably lower rate reductions in seropositives than seronegatives (2.66 vs 1.01 and 0.31 vs 0.16 for 6-month persistent infection and CIN2+, respectively) were driven by lower attack rates in the seropositive controls than in the seronegative controls (1.5 vs. 2.95 for 6-month persistent infection; 0.20 vs. 0.39 for CIN2+).
In a FUTURE I/II analyses of HPV6, 11,16, and/or 18 DNA positive women, Gardasil® was 100% (95% CI: 78.6–100) effective in preventing incident CIN2+ associated with a vaccine type for which the women were DNA negative at enrollment [33]. Efficacy against vaccine type-related external genital and vaginal lesions in this study group was 93.8% (95% CI: 80.7–98.8). Gardasil® was also shown to protect seropositive women against subsequent disease from the corresponding vaccine type [35]. In a MITT analysis of combined FUTURE I, FUTURE II and 007 data, efficacy in women DNA negative and seropositive for the corresponding type was 100% (95% CI: 28.7–100.0) against CIN1+ and 100% (95% CI: 28.3–100.0) against external genital lesions. However attack rates in controls, and therefore rate reductions, were low, 0.2 for CIN1+ and external genital lesions (EGLs) and 0.1 for CIN+2. In comparison, a similar analysis of seronegatives in this combined study group reported a CIN2+ attack rate of 0.5 in controls [20].
From these studies, it is clear that prevalent infection by one type does not impede vaccine-induced protection from incident infection by another vaccine type. In addition, the results also seemingly indicate that the antibody responses to natural infection do not fully protect women from reinfection, in contrast to antibodies induced by vaccination. The generally much lower antibody titers detected after infection likely account for this difference in protection (discussed below). Consistent with this explanation, most seropositive controls who subsequently became DNA positive had antibody titers that were below the geometric mean titer [32,35]. Also supporting this interpretation, a recent analysis of seropositive controls in the CVT found that women with relatively high antibody titers at enrollment were mostly protected from incident infection (as measured by DNA detection) whereas those with low titers were not [36]. The 2- to 5-fold lower attack rates in seropositives vs. seronegatives supports the conclusion that antibodies induced by infection play a substantial role in protection from reinfection, or are a surrogate marker for cell-mediated immune protection.
It is important to note that the above analyses might be subject to substantial misclassification. Relatively low cut points for seropositivity were used in the vaccine trials, because of the desire to exclude, as much as possible, women with prior exposure to the vaccine types in the primary ATP analyses. It is possible that the low titers in some women might be due to non-specific or cross-reactive antibody rather than indications of prior vaccine-type infection.
The findings that even women with evidence of prior infection can benefit from vaccination have been used to support arguments for the implementation of more vigorous vaccination programs in adult women. The substantially lower attack rates in seropositives are an important consideration that should not be ignored in these discussions.
7. Therapeutic efficacy
Therapeutic efficacy of the vaccines was not specifically evaluated in the end of study publications, in large measure because there was no evidence for it in interim analyses. Although the clinical trials were primarily designed to evaluate immunoprophylaxis, the fact that women who had prevalent cervicovaginal infection or low grade disease were not excluded at entry provided a cohort to evaluate therapeutic efficacy. In the CVT, time to clearance of prevalent infection was evaluated. There was no difference in the rate of clearance of vaccine or non-vaccine types in Cervarix® vaccinees and control [37]. For example, 48.9% and 49.8% of HPV16/18 infections were cleared after 12 months in vaccine recipients and controls, respectively. The therapeutic activity of Gardasil® was evaluated in FUTURE II [15]. No significant difference in the rate of progression of HPV16/18 infection to CIN2+ was observed in VLP vaccinees versus controls, 11.1% and 11.9%, respectively. Thus the VLP vaccines do not appear to alter the course of established cervicovaginal HPV infection or disease.
8. Safety
Both vaccines exhibited excellent safety profiles in the clinical trials. Mild to moderate injection-site symptoms, headache and fatigue were the most common adverse events in Cervarix® and Gardasil® vaccinees and controls. Injection-site pain ranged from 83.0–93.4% in vaccine groups and from 75.5–87.25% in control groups [14,15,38,39]. Headache and fatigue was reported in 50–60% of participants in both groups. These solicited symptoms were transient and resolved spontaneously and did not increase with number of doses. Symptoms were not notably different in women with evidence of prevalent or past infection [32,35]. In a randomized control trial directly comparing the two vaccines, injection-site pain was somewhat higher with Cervarix® than with Gardasil®; 92.9% (95% CI: 90.4–95.0) and 71.6% (95% CI: 67.5–75.4) respectively [40]. Grade 3 severity was reported in 17.4% (95% CI: 14.2–20.9) and 3.4% (95% CI: 2.0–5.4) in Cervarix® and Gardasil® groups respectively. However, compliance rates with the three-dose schedule were similarly high (>84%). The inclusion of the immune stimulating component MPL in the Cervarix® adjuvant might account for somewhat higher reactogenicity of the vaccine [38]. For both Cervarix® and Gardasil®, vaccine and control groups experienced similar rates of serious adverse events (SAEs) (Table 8). The numbers of SAEs judged to be possibly related to vaccine injection was low for both vaccines and similar to the numbers in the control groups (Table 8).
Table 8.
Assessment of serious adverse events
| Outcome | Study | Vaccine | % Vaccine | % Control | Relative risk (95% CI) |
|---|---|---|---|---|---|
| SAE | |||||
| FUTURE I | Gardasil® | 1.8 | 1.7 | 1.07 (0.71–1.60) | |
| FUTURE II | Gardasil® | 0.7 | 0.9 | 0.83 (0.56–1.24) | |
| PATRICIA | Cervarix® | 7.5 | 7.5 | 1.00 (0.91–1.11) | |
| Injection-related SAE | |||||
| FUTURE I | Gardasil® | 0.03 | 0.0 | 3.00 (0.12–73.58) | |
| FUTURE I | Gardasil® | 0.05 | 0.03 | 1.50 (0.25–8.99) | |
| PATRICA | Cervarix® | 0.12 | 0.06 | 1.83 (0.68–4.96) | |
CI: Confidence interval; SAE: serious adverse event. Data from reference [38].
Pregnancy outcomes have received special attention, given the target ages of catch up vaccination programs. A pooled analysis of the PATRICIA and CVT found no significant increase in miscarriages in the Cervarix® arm (11.5%) compared to the control arm (10.2%) [41]. However, there is the need to follow up a nonsignificant trend toward an increase rate of miscarriage for pregnancies conceived within 3 months of Cervarix® vaccination. Similarly, in a combined analysis of phase III trials involving Gardasil®, the proportions of women with live births, spontaneous abortions and congenital abnormalities where similar in the vaccine and control groups [15,42]. For example, the rate of spontaneous abortion was 21.9% and 23.3% in the Gardasil® and control groups, respectively. The congenital abnormalities observed were diverse and consistent with those generally seen in young women. Several post-licensure safety studies have been conducted or are ongoing [43–45]. To date, the findings are consistent with those of the clinical trials.
9. Efficacy in mid-adult women
The end of study results (median follow-up of 4 years) of a multi-centric Gardasil® trial in 3819 mid-adult women, ages 24–45, were recently published [46]. The results confirm and extend an interim analysis of this trial in establishing that older women without evidence of prior exposure to the vaccine types can benefit from the vaccine [47]. In the ATP population, efficacy against a combined primary endpoint of 6-month persistent infection, CIN of any grade or EGL related to the vaccine types was 88.7% (Table 9). Similar efficacies were observed for CIN, EGL and persistent infection individually. There was a trend for protection against vaccine type CIN2/3 in the ATP analysis, but the study was not powered for this endpoint and the efficacy was not statistically significant. Vaccine efficacies against these endpoints irrespective of HPV type were not reported.
Table 9.
Efficacy of Gardasil® against HPV6/11/16/18-related endpoints in mid-adult women
| Endpoint | ATP % Efficacy (95% CI) |
ITT % Efficacy (95% CI) |
|---|---|---|
| Persistent infection, CIN or EGL | 88.7 (78.1–94.8) | 47.2 (33.5–58.2) |
| Persistent infection | 89.6 (79.3–95.4) | 49.0 (35.5–59.9) |
| CIN – any grade | 94.1 (62.5–99.9) | 47.5 (16.3–67.7) |
| CIN2/3 | 83.3 (37.6–99.6) | 22.4 (42.5–58.3) |
| EGL | 100 (30.8–100) | 8.5 (126.6–63.4) |
ATP: According to Protocol; CI: Confidence interval; CIN: Cervical intraepithelial neoplasia; EGL: External genital lesion; ITT: Intention-to-treat. Data from reference [46].
In the case of mid-adult women, ATP and ITT naïve analyses have limited public health implications, since prescreening women and vaccination of only HPV DNA/seronegatives is not being seriously contemplated. This is in contrast to the trials in young women in which these cohorts provide the best approximation for the primary target for the vaccines, girls prior to the onset of sexual activity. Of more practical relevance, the efficacy for the combined primary endpoint in the ITT population was 47.2% for vaccine-targeted types [46]. From a public health perspective, perhaps the most relevant analysis was the overall vaccine impact on cervical and external genital procedures regardless of HPV type in the ITT population. There were modest nonsignificant rate reductions in colposcopy, biopsy, and definitive treatment of 6.8, 6.4, and 2.4%, respectively. The safety profile in mid-adult women was similar to that seen in younger women, with a somewhat greater number of Gardasil® vaccinees having adverse injection-site experiences compared to controls (76.7% vs 64.2%).
A recent conference abstract related to a Cervarix® trial in mid-adult women up to age 55 years outlined encouraging efficacy results against HPV16/18 persistent infection and CIN1+, but the findings have not yet been published. The published safety and immunogenicity results from this trial are discussed below [48].
Extension of recommendations and public financing to include vaccination of mid-adult women is debatable, based on the trial results and current knowledge of the epidemiology of genital HPV infection [49]. In most populations, immunity to vaccine-related types is expected to increase with age while the rates of incident infection, and the probability of infection progressing to cervical cancer, are expected to decrease. Consequently, cost modeling studies have indicated that vaccination becomes less cost effective with increasing age [50]. Interestingly, both vaccines are licensed by the European Medicines Agency (EMA) for use from the age of 9 onwards, but neither is licensed for women over age 26 in theU.S. However, the vaccine is not routinely provided to mid-adult women in publically financed programs in Europe. Nevertheless, it is clear from the trials that some mid-adult women could potentially benefit from the vaccine, and it seems reasonable to permit them to purchase it on an individual basis. However vaccination cannot replace screening in mid-adult women.
10. Efficacy in males
The efficacy of Gardasil® was examined in a placebo-controlled, double-blind trial in 4065 men ages 16–26 from 18 countries [51]. The primary endpoint of the study was protection from HPV6, 11, 16 or 18-associated incident EGLs, defined as external genital warts (condylomata acuminata) or penile, perianal or perineal intraepithelial neoplasia (PIN) of any grade, or cancer at these sites. Protection against this combined endpoint was 90.4% in the ATP population and 65.8 in the ITT population. Of the EGLs, 28 of 31 and 72 of 77 were genital warts in the ATP and ITT cohorts, respectively, and most were associated with HPV6 or HPV11 infections. Significant protection against EGLs was also observed in both populations, irrespective of the HPV type in the lesion (Table 10), reflecting the large proportion of genital warts caused by the vaccine types 6 and 11. Similar efficacy against persistent infection endpoints was reported in the ATP analysis (Table 10). The results of this study have led to the licensure of Gardasil® for the prevention of EGL in men in several countries.
Table 10.
Efficacy of Gardasil® in men
| ATP % Efficacy (95% CI) |
ITT % Efficacy (95% CI) |
|
|---|---|---|
| External genital lesions | ||
| Any Typea | 83.8 (61.2–94.4) | 60.2 (40.8–73.8) |
| HPV6, 11, 16, 18 | 90.4 (69.2–98.1) | 65.5 (45.8–78.6) |
| Persistent genital infectionb | ||
| HPV6, 11, 16, 18 | 85.6 (73.4–92.9)c | 27.1 (16.6–36.3) |
| HPV6 | NR | 35.1 (20.3–47.3) |
| HPV11 | NR | 43.2 (18.7–60.7) |
| HPV16 | 78.7(55.5–90.9) | 28.0 (12.9–40.7) |
| HPV18 | 96.0 (75.6–99.9) | 33.9 (13.0–50.1) |
| AIN-any grade | ||
| Any Typea | 54.9 (8.4–79.1) | 25.7 (−1.1–45.6) |
| HPV6, 11, 16, 18 | 77.5 (39.6–93.3) | 50.3 (25.7–67.2) |
| AIN2/3 | 74.9 (8.8–95.4) | 54.2 (18.0–75.3) |
| Persistent anal infectionb | ||
| HPV6, 11, 16, 18 | 94.9 (80.4–99.4) | 59.4 (43.0–71.4) |
| HPV6 | 92.1 (47.2–99.8) | 62.5 (37.5–78.2) |
| HPV11 | 100 (−15.5–100) | 53.7 (7.5–78.0) |
| HPV16 | 93.8 (60.0–99.9) | 54.0 (23.9–2.9) |
| HPV18 | 100 (51.5–100) | 49.5 (11.3–72.1) |
Seronegative to HPV6, 11, 16, 18 and DNA negative to 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 56, 58, and 59 at enrollment.
Persistence defined as detection of the same type on consecutive visits at least 6 (+/−1) months apart.
97.5% CI
AIN: Anal intraepithelial neoplasia; AIN 2/3: Anal intraepithelial neoplasia grade 2 or 3; ATP: According to Protocol; CI: Confidence interval; HPV: Human papillomavirus; ITT: Intention-to-treat; NR: Not reported.
A subset of 602 men in the above trial who reported having sex with men was concurrently enrolled in a study of anal infection and anal intraepithelial neoplasia (AIN). After 3 years, Gardasil® was 78.6% (95% CI: −0.4–97.7) effective against HPV16/18 (the two types that cause most anal cancers) and 77.5% (95% CI: 39.6–93.3) effective at preventing HPV6/11/16/18-related AIN of any grade in the ATP population. It was 54.9% (95% CI: 8.4–79.1) effective for preventing AIN of any grade caused by any HPV type [52]. Efficacy against AIN2+ for this population was 74.9 (95% CI: 8.8–95.4). An efficacy of 94.9% (95% CI: 80.4–99.4) was observed against persistent infection by the vaccine-targeted types. As expected, efficacies for these endpoints were lower in the ITT population, but were significant for AIN of any grade, AIN2+ and persistent infection by the vaccine types (Table 10). The differences in vaccine efficacy in the two populations reinforce the desirability of vaccinating males before they become sexually active.
The findings of the anal disease/infection substudy led to U.S. FDA approval of Gardasil® for the prevention of AIN and anal cancer in both men and women. Approval for women was based on the argument that the risk factors for HPV-related anal cancer are similar and its development is biologically indistinguishable in the two sexes. The trial results likely also contributed to the recent changes in government guidelines for male vaccination in the U.S. and Australia to policies of routine vaccination of both boys and girls. However, these findings have not resulted in EMA approval of AIN/anal cancer indications for either sex.
11. Immunogenicity analyses
Immunogenicity analyses in vaccine trials are important for several reasons. They help to determine the range of responses and provide insights into the potential for long term protection of the current vaccines and the probability of efficacy of second-generation vaccines. They have also been used to evaluate the relative potency of the competing vaccines. Most importantly, safety/immunogenicity analyses can be used in bridging studies to extend vaccination recommendations to groups that are difficult to evaluate specifically in efficacy trials, such as children, in whom clinical outcomes for HPV-related disease cannot be measured in the immediate time frame.
11.1. Assay considerations
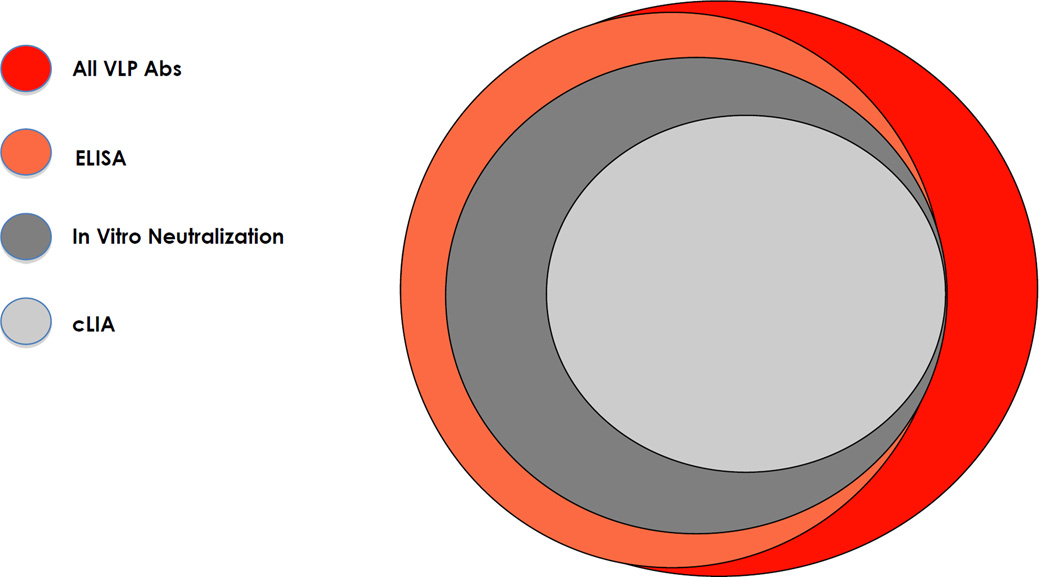
There is no standard assay for assessing immunogenicity in HPV VLP vaccine trials [53]. For most analyses, the two companies have used different assays that measure different subsets of the constellation of antibodies induced by VLP vaccination, making direct comparisons difficult. Three types of assays have commonly been used [54]. Enzyme-Linked Immunosorbant Assays (ELISAs) that employ VLPs as antigen measure the largest subset of vaccine-induced antibodies, namely all VLP-specific ones that have sufficient affinity to remain bound through the several wash steps (Fig. 2). In vitro neutralizing assays measure the biologically relevant subset of virion capsid-binding antibodies that can prevent infection of cultured cells. Competitive Luminex Immunoassays (cLIA) measure the subset of antibodies that compete with a type-specific neutralizing monoclonal antibody for binding to one epitope on the VLPs. GlaxoSmithKline has routinely used an ELISA and Merck a cLIA in their trials. Both have used in vitro neutralizing assays to a more limited extent, in large measure because it is more difficult to conduct with large numbers of samples. ELISAs and in vitro neutralizing results have similar analytic sensitivities and correlate well for individual women [55]. cLIAs have the virtue of being very type specific, since the monoclonal antibodies used in them were specifically selected to not inhibit infection by even closely related types [56]. However, assays based on reactivity of a single monoclonal antibody do not correlate quite as well with the other two assays. In particular, it is not uncommon for sera to be negative in a monoclonal antibody competition assay and positive in a less restrictive assay [55,57]. A likely explanation for this observation is that the dominant antibody response in some individuals is to epitopes that do not overlap with the epitope recognized by the competing monoclonal antibody [58].
Figure 2.
Relationships among VLP-specific antibodies detected in ELISA, cLIA, and neutralization assays. Shown is a schematic representation of the constellation of antibodies detected in VLP antibody assays. Red = all VLP antibodies induced; Orange = ELISA detected; Grey = in vitro neutralizing assay detected; Light grey = cLIA detected. cLIA: Competitive Luminex® immunoassay; VLP antibodies: Virus-like particle-specific antibodies. Adapted from reference [53].
11.2. Data on immunogenicity analyses
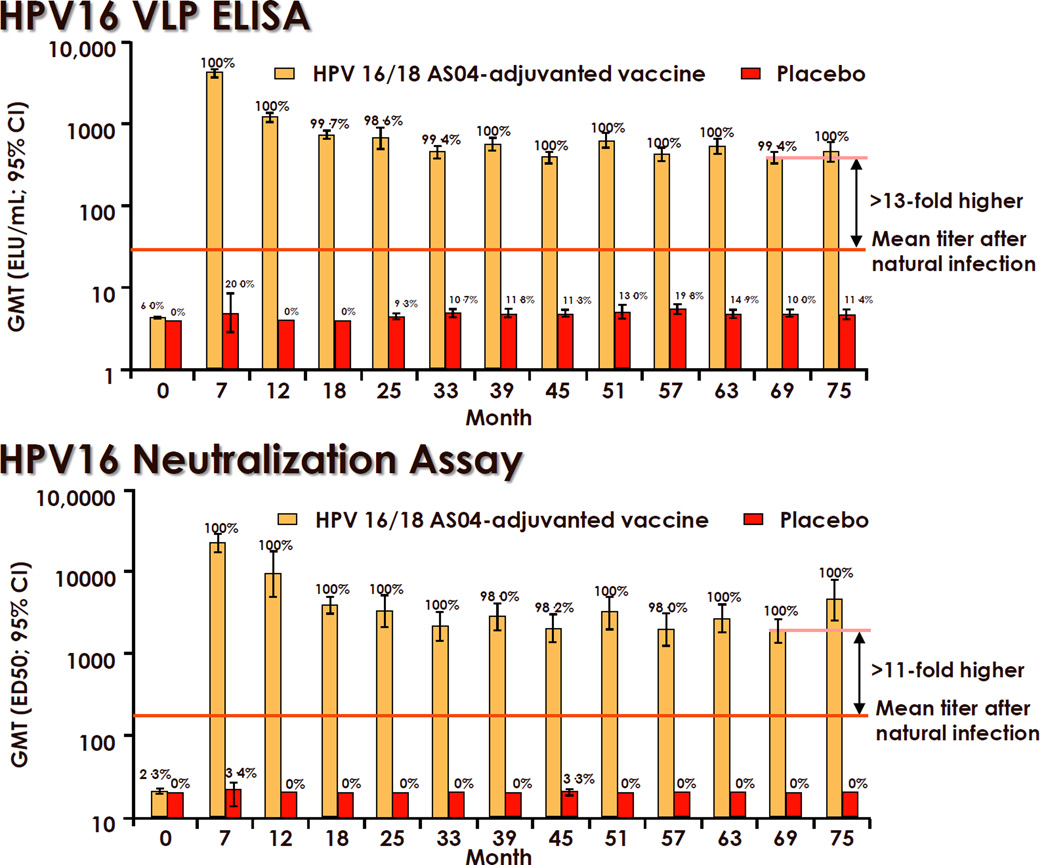
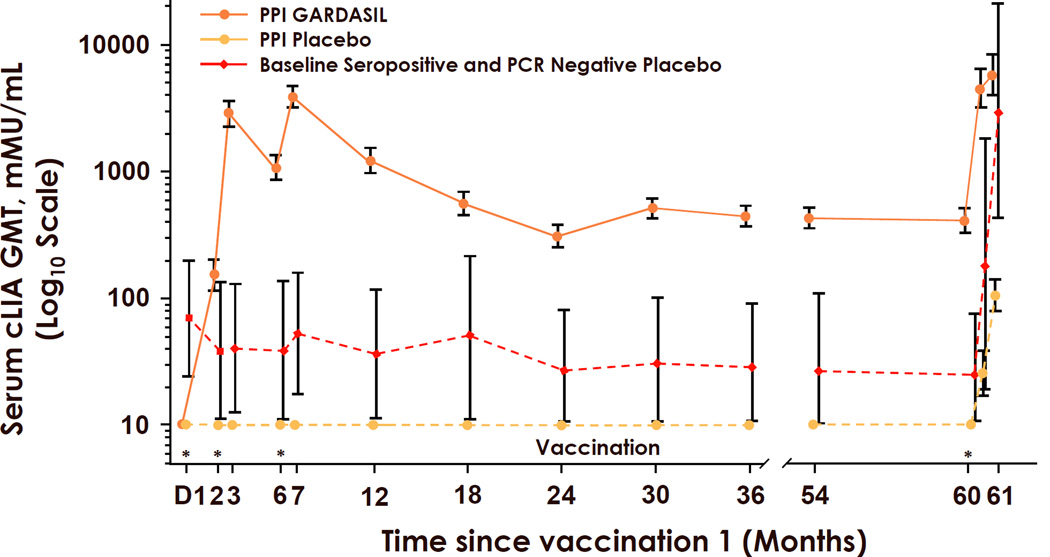
Regardless of the assay used, studies in young women have demonstrated consistent, strong, and durable antibody responses to each type in the vaccine. Seroconversion rates approach or equal 100% for each type in the vaccines [31,57,59,60]. Peak geometric mean titers (GMTs) one month after the third dose were at least 100-fold higher than after natural infection and then decline approximately 10-fold to a plateau level in the next 2 years. Virtually all women maintain stable detectable responses for more than 4 years. For Cervarix®, maintenance of plateau levels above the levels detected after natural infection for up to 8.4 years have been observed [31,61] (Fig. 3). Similar results were reported for Gardasil®, with the additional evidence for immune memory in that antibody responses could be boosted by revaccination at month 60 (Fig. 4) [62]. The notable exception is that about one third of the vaccinees became seronegative for HPV18 in the cLIA assay used in the Gardasil® trials [60]. This exception is more likely due primarily to the HPV18-specific monoclonal antibody not competing effectively with the vaccine-induced antibodies in some women than due to the absence of protective antibodies. Most of the cLIA-negative women were positive in a less restricted assay that measures total VLP IgG, and there is no sign of preferential waning of HPV18 immunity in the Gardasil® trials [57,60]. Moreover and importantly there is still protection from HPV18-related disease in these women.
Figure 3.
Durability of antibody responses to Cervarix® in young women. Orange Bars = GMTs in Cervarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium) vaccinees. Red bars = GMTs in control vaccinees. Numbers above bars indicate the percent seropositive at the indicated month since vaccination. CI: Confidence interval; cLIA: Competitive Luminex® immunoassay; ED50: Serum dilution causing a 50% reduction in secreted alkaline phosphatase activity compared with a control without serum; ELU: ELISA units; GMT: Geometric mean titer; VLP: Virus-like particle. Taken with permission from reference [31].
Figure 4.
Durability of HPV16 antibody responses to Gardasil® in young women. cLIA: Competitive Luminex® immunoassay geometric mean titer; GMT: Geometric mean titer; mMU: milliMerck Units. PCR: Polymerase chain reaction; PPI: Per Protocol Immunogenicity cohort. * Gardasil® (Merck & Co., Whitehouse Station, NJ USA) vaccination (0, 2, 6, and 60 months). Taken with permission from reference [61].
There has been one randomized trial in women 18–45 years old that directly compared the immunogenicity of Gardasil® and Cervarix®. Cervarix® induced significantly higher peak GMTs of neutralizing antibodies than Gardasil®, 2.3–4.8-fold for HPV16 and 6.8–9.1-fold for HPV18, depending upon age [40]. Similar significant differences in HPV16 and HPV18 GMTs for the two vaccines were also observed at month 24 [59]. Higher HPV16/18 VLP-specific IgG levels in the serum of Cervarix® vaccinated women was reflected in correspondingly higher levels of HPV16/18 VLP-specific IgG in cervicovaginal secretions through month 24. The greater antibody (and also T helper) responses to Cervarix® compared to Gardasil® is most likely the result of increase immune activation by the TL4 ligand MPL in the Cervarix®’s AS04 adjuvant [12].
Higher antibody responses would, in general, seem desirable. However, it is currently unclear whether the differences in antibody responses to the two vaccines will translate into differences in long-term protection for disease, in part, because the minimum level of serum antibodies needed to protect women from genital infection has not been established, and, in part, due to the small number of breakthrough infections detected in the trials. Perhaps of relevance is the finding that mice are protected from cervicovaginal challenge with HPV16 pseudovirions even if they have serum levels of VLP antibodies that are 500-fold lower than the minimum that can be detected in an in vitro neutralization assay [63]. This observation raises the possibility that detection of any vaccine-induced serum antibodies in women using standard assays indicates levels that are well above the minimum needed for protection.
Detection of neutralizing antibodies in vitro to a non-vaccine type has generally corresponded with partial protection against infection by that type in clinical trials [25]. Therefore, the above trial compared cross-reactive immune responses to HPV31 and HPV45 induced by Cervarix® and Gardasil® [64]. For both types, the two vaccines induced similar levels of neutralizing and VLP ELISA reactive antibodies. This is in contrast to Cervarix®’s apparently greater degree of cross-protection against HPV45 infection in the efficacy trials. One interpretation of this result is that cross-protection is not antibody mediated. However, cross-reactive responses were very low, generally less than 1% the responses to homologous types. Therefore, it may be that the current serologic assays are simply not sufficiently accurate measures of cross-type protective antibody responses.
12. Immunobridging trials
12.1. Data in pre-adolescent girls and boys
Safety and immunogenicity bridging studies were critical in extending regulatory approval for the vaccines to pre- and early adolescent girls and boys. Gardasil® induced geometric mean titers (GMTs) in 10–15 year old girls and boys that were 1.7–2.0 and 1.8–2.7-fold higher, respectively, than the titers induced in 16–23 year-old women, as measured by cLIA [65]. Similarly, Cervarix® induced GMTs in 10–14 year old girls that were 2.1–2.5-fold higher than those induced in 15–25 year-old women, as measured by ELISA [66]. Titers were also higher in 10–18 year old boys [67]. Higher titer antibody responses in younger individuals are also generally seen in trials of other vaccines.
The higher responses in children led to the comparison of two- and three-dose vaccination protocols. Two doses of Gardasil® in 9–13 year-old girls delivered at 0 and 6 months was judged non-inferior to three doses in 16–26 year old women delivered at 0, 2, and 6 months, as measured by peak titers in HPV16- and HPV18-specific vitro neutralization assays [68]. Similarly, two doses of Cervarix® delivered at 0 and 6 months to 9–14 year-old girls was non-inferior to the standard three doses at 0, 1, and 6 month in 15–25 year-old young women, as measured in HPV16 and HPV18 VLP ELISAs at one month and 18 months after the last vaccination [69]. Follow-up studies will be needed to determine if the durability of the responses to two and three doses remain comparable. However, these results have already prompted some jurisdictions to initiate programs that delay administration of the third dose for at least 5 years, with an interim assessment to determine if it is needed.
12.2. Data in mid-adult women
The immunogenicity of Gardasil® and Cervarix® was also assessed in mid-adult women. In the Gardasil® efficacy trial, peak titers trended modestly downward with age when stratified into 16–23, 24–34 and 35–45 age groups [46]. However, seroconversion rates, measured one month after the third dose in cLIA assays, were greater than 97% for all vaccine types. At month 48, seropositive rates in 24–45 year-olds were 91.5%, 92.0%, 97.4 and 47.9% for HPV6, 11, 16, and 18, respectively. The loss of seropositivity to HPV18 in half of the mid-adult women mirrors the loss in approximately one third of young women [60]. As mentioned above, this finding may be more related to the specific performance of the HPV18 cLIA used in the analysis, than lower immunogenicity of the HPV18 VLPs used in the vaccine.
In a Cervarix® trial of women ages 15–55, all women seroconverted to both HPV16 and 18 at one month after the last dose, as measured in a VLP ELISA [48]. Although peak and plateau titers were higher for the 15–25 year-old group than the 26–45 and 46–55 year-old groups, all women remained seropositive at month 24. GMTs in the 46–55 year-olds remained 16-fold (HPV16) and 8-fold (HPV18) higher than the GMTs elicited by natural infection. Thus, mid-adult women are able to mount robust antibody responses to both vaccines.
12.3. Data in HIV-infected individuals
HIV-infected individuals have an increased risk of persistent HPV infection, HPV-associated benign lesions and HPV-associated cancers. It is therefore of interest to determine the immune response to the vaccines in HIV-infected individuals. Safety and immunogenicity of Gardasil® was assessed in separate studies of adult males (ages 22– 61) and children (ages 7–12) [70,71]. The vaccine was safe and well tolerated in both studies, with no adverse effects on CD4+ cell counts or plasma HIV RNA levels. Seroconversion rates were greater than 95% and antibody titers were approximately 50% of those measured in HIV-uninfected individuals of similar age. These findings encourage targeted vaccination programs for young HIV positive individuals.
12.4. Data on co-administration with other vaccines
Since several other vaccines are routinely given to adolescents, it is important to determine if they can be co-administered with the HPV vaccines. Recent studies have demonstrated safety and non-inferior immune responses when Gardasil® was coadministered with Recombivax HB® (hepatitis B; Merck & Co., Whitehouse Station, NJ USA) [72], Repevax® (diphtheria, tetanus, acellular pertusis, inactive polio; Sanofi Pasteur MSD, Lyon France) [73], or Menactra® (meningococcal conjugate; Sanofi Pasteur, Inc., Swiftwater, PA USA) plus Adacel® (diphtheria, tetanus, acellular pertusis; Sanofi Pasteur, Inc., Swiftwater, PA, USA) [74]. Safety and immune response non-inferiority has been demonstrated for co-administration of Cervarix® and Boostrix®-IPV (diphtheria, tetanus, acellular pertusis, inactivated polio; GlaxoSmithKline Biologicals, Rixensart, Belgium) [75]. These encouraging results might eventually lead to co-formulation of HPV and other vaccines, particularly with hepatitis B where vaccination schedules and adjuvants appear most compatible.
13. Second-generation vaccines
Several second-generation HPV prophylactic vaccines are under development with the goal of addressing some of the inherent limitations of the current vaccines. The approach that is by far the most advanced is to simply increase the valency of an L1 VLP vaccine to address the issue of type-restricted protection. Merck appears to be well advanced in a Phase III efficacy trial of a nonavalent vaccine, which, in addition to the four types in Gardasil®, contains L1 VLPs of types 31, 33, 45, 52 and 58 [76]. Even if the vaccine is entirely type-specific, it would have the potential to prevent approximately 85% of cervical cancer-associated HPV infections [6].
Vaccines based on L1-pentameric subunits produced in E. coli have been generated to address the cost of production in eukaryotic cells [77]. These capsomere-based vaccines have demonstrated protection from experimental challenge in animal models [78]. Phase I clinical trials of a capsomere-based vaccine are anticipated in the near future [76]. Alternatives for lowering the cost of manufacturing being investigated include the generation of the L1 VLPs in alternative yeast production systems, such as Pichia pastoris [79], or in plants [80]. Live recombinant viral and bacterial vectors, such a measles [81], adeno-associated virus [82] and Salmonella typhi [83], expressing L1 have also generated promising results in preclinical studies.
Vaccines based on the minor virion protein, L2, have generated increasing interest in recent years (reviewed in [84]). L2 contains some remarkably broad cross-type neutralizing epitopes. These epitopes are able to induce antibodies that prevent infection by genital and cutaneous HPV types both in cultured cells and animal models. Simple L2 polypeptides generated in E. coli or synthetically can elicit these broadly cross-neutralizing antibodies, raising the possibility of an inexpensive monovalent vaccine with the potential to be broadly protective. However, neutralizing antibody titers to L2-based immunogens are invariably lower than homologous type neutralizing titers elicited by VLP-based immunogens. There have been a number of strategies employed to increase L2-induced neutralizing titers, including virus-like display approaches and fusion to immunogenic peptides. Whether the responses will be sufficient to induce long-term type specific and cross-type protection remains to be determined.
It is important to note that the path and timeline for clinical development and licensure of these second generation vaccines is uncertain. It would be imprudent to delay introduction of the current vaccines in the hopes that a more attractive product might be forthcoming in the future. Since it is unlikely that the next generation of vaccines will have therapeutic efficacy, the opportunity to protect the current cohort of girls (and boys) from HPV-associated cancers would likely be lost if the introduction of the available vaccines were delayed.
14. Conclusions
The basic profiles of the two licensed HPV VLP vaccines are now well established (Table 11). They are generally safe, with minor injection-site symptoms the principal adverse events reported. They are highly immunogenic, inducing high peak titers of antibodies in virtually all vaccinees, and measurable serum antibody responses persist for years. They are highly efficacious at preventing incident anogenital infection and subsequent neoplastic disease by the types specifically targeted by the vaccines. To date there are no signs of waning protection. They induce partial cross-protection against infection and disease caused by a limited number of phylogenetically-related non-vaccine types. Infection by one vaccine type does not inhibit prevention of infection by another vaccine type. However, the vaccines do not act therapeutically to induce regression or prevent progression of established infections.
Table 11.
Key findings from clinical trials of HPV VLP vaccines
| Study group | Outcome | Gardasil® | Cervarix® |
|---|---|---|---|
| Young women | Infection efficacy | Proven | Proven |
| CIN2+ efficacy | Proven | Proven | |
| CIN3 efficacy | Proven | Proven | |
| VIN/VaIN 2/3 efficacy | Proven | Provena | |
| Genital warts efficacy | Proven | Not a target | |
| Anal infection efficacy | Not proven | Proven | |
| Partial cross-protection infection | Proven | Proven | |
| Partial cross-protection CIN2+ | Proven | Proven | |
| Therapeutic efficacy | None | None | |
| Safety | No concerns | No concerns | |
| Mid-adult women | Infection efficacy | Proven | Provena |
| CIN2+ efficacy | Proven | Not proven | |
| Immunogenicity | Proven | Proven | |
| Safety | No concerns | No concerns | |
| Young men | Infection efficacy | Proven | Not proven |
| Genital wart efficacy | Proven | Not a target | |
| Anal infection | Proven | Not proven | |
| AIN2+ efficacy | Proven | Not proven | |
| Safety | No concerns | No concerns | |
| Children | Infection efficacy | Not proven | Not proven |
| Disease efficacy | Not proven | Not proven | |
| Immunogenicity Safety | Proven No concerns | Proven No concerns | |
Meeting abstract but not yet published.
CIN: Cervical intraepithelial neoplasia; HPV: Human papillomavirus; VIN/VaIN: Vulvar/vaginal intraepithelial neoplasia; VLP: Virus-like particle.
Several gaps in our understanding of the vaccines’ performance remain. Most importantly, the duration of protection has not yet been established. The continued persistence of serum antibodies for up to 8.4 years now for Cervarix® [61] without a significant drop in titer after 2 years encourages an optimistic projection for continued strong efficacy through the peak years of anogenital HPV acquisition and perhaps lifelong. The stable long-term antibody titers observed after L1 VLP vaccination are reminiscent of the antibody responses to virion proteins in live virus vaccines that routinely provide life-long protection [85]. We are less optimistic about the prospects for durable cross-type protection. The planned long-term follow up of vaccinated cohorts should provide answers to these questions [86].
Efficacy in pre- and early-adolescents, the primary targets for vaccination, has not been demonstrated. Trials in this age group are logistically challenging, since the vaccinees would require active follow-up for many years to accrue sufficient numbers of sexually transmitted infections or resulting disease endpoints. It is unlikely that a formal efficacy trial in pre- and early-adolescents will ever be conducted. Now that the vaccine is approved for this age group, it is doubtful that a placebo-controlled trial would be permitted. The best evidence will likely come from effectiveness studies in adults vaccinated as adolescents. This type of data should be forthcoming in the next 5–10 years. Given the strong immunogenicity results in adolescents, the expectations are high that the findings will be favorable.
In contrast to the extensive data on anogenital infection (Table 11), there are no data to date on vaccine efficacy against oropharyngeal HPV infections. This deficit is an important consideration, since the incidence of HPV-associated oropharyngeal cancer (mostly attributable to HPV16) appears to be increasing dramatically, at least in industrialized countries [87]. It is uncertain whether a trial to specifically evaluate oropharyngeal efficacy will be conducted. The premalignant precursors of oropharyngeal cancer cannot be routinely identified, making it difficult to contemplate a trial with intraepithelial neoplasia as a surrogate endpoint [88]. Current routine HPV DNA sampling methods appear to have relatively low sensitivity for detecting oropharyngeal infections, making trials using persistent infection endpoints difficult as well. Finally, approval of a trial with a placebo-controlled trial might be difficult, given that the vaccines are approved for other indications in the prospective study populations. Regarding other oral lesions, it would helpful to establish a surveillance system for recurrent respiratory papillomatosis, since its frequency in infants of Gardasil®-vaccinated women is likely to decrease.
In conclusion, the profiles of the HPV VLP vaccines established in the randomized clinical trials illustrate their potential as high value public health interventions and strongly support their wide spread implementation to prevent anogenital HPV infections and their associated neoplasia. The primary focus must now be on implementation issues to maximize the rapid, effective and cost-efficient delivery of the vaccines to those individuals that are most likely to benefit from them.
The analyses of human papilloma virus/virus-like particle vaccine phase III efficacy trials in young women are largely completed.
High efficacy was observed for incident infection and disease by vaccine types.
Other clinical trials demonstrated protection against genital warts and anal dysplasia in men.
Immunobridging studies have extended vaccine recommendations to adolescents.
Acknowledgments
JTS: Named inventor on U.S. government-owned HPV vaccine-related patents that are licensed to Merck & Co., GlaxoSmithKline, Sanofi Pasteur and Shantha Biotechnics and is entitled to limited royalties as specified by federal law.
XC: Has received travel and speaker honoraria and institutional research grants from Merck & Co. Inc., GlaxoSmithKline, and Sanofi Pasteur MSD.
SMG: Has received advisory board fees and grant support from Commonwealth Serum Laboratories and GlaxoSmithKline, and lecture and consultancy fees from Merck & Co.
She has received grant support through her institution from Merck & Co. and GlaxoSmithKline to do clinical trials for HPV/cervical cancer vaccines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosed potential conflicts of interest
References
- 1.Schiller JT, Castellsague X, Villa L, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26S:K53–K61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markowitz EL, Tsu V, Deeks SL, Cubie H, Wang S, Vicardi A, et al. Human papillomavirus vaccine introduction – The first five years. Vaccine. doi: 10.1016/j.vaccine.2012.05.039. this issue. [DOI] [PubMed] [Google Scholar]
- 3.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer. 2010;5:19. doi: 10.1186/1750-9378-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines – Immune responses. Vaccine. doi: 10.1016/j.vaccine.2012.04.106. this issue. [DOI] [PubMed] [Google Scholar]
- 6.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010 Nov;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008 Nov 15;113(10 Suppl):3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman D, L L Bruni, Ferlay F, Bray F, Franceschi S, Roura E, et al. Burden of human papillomavirus infections and related diseases. Vaccine. doi: 10.1016/j.vaccine.2012.07.055. this issue. [DOI] [PubMed] [Google Scholar]
- 9.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009 Mar 15;199(6):805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 10.Inglis S, Shaw A, Koenig S. Chapter 11: HPV vaccines: Commercial Research & Development. Vaccine. 2006 Aug;21(24 Suppl 3):S99–S105. doi: 10.1016/j.vaccine.2006.05.119. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, Sings HL, Bryan JT, Wang B, Wang Y, Mach H, et al. GARDASIL: prophylactic human papillomavirus vaccine development--from bench top to bedside. Clin Pharmacol Ther. 2007 Feb;81(2):259–264. doi: 10.1038/sj.clpt.6100055. [DOI] [PubMed] [Google Scholar]
- 12.Garcon N, Wettendorff M, Van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther. 2011 May;11(5):667–677. doi: 10.1517/14712598.2011.573624. [DOI] [PubMed] [Google Scholar]
- 13.Lowy DR, Frazer IH. Chapter 16: Prophylactic human papillomavirus vaccines. J Natl Cancer Inst Monogr. 2003;31):111–116. doi: 10.1093/oxfordjournals.jncimonographs.a003472. [DOI] [PubMed] [Google Scholar]
- 14.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007 May 10;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 15.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007 May 10;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 16.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007 Jun 30;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 17.Herrero R, Hildesheim A, Rodriguez AC, Wacholder S, Bratti C, Solomon D, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008 Sep 2;26(37):4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 Sep 8;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 19.Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine. 2004 Dec 16;23(5):569–578. doi: 10.1016/j.vaccine.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009 Oct;2(10):868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 21.Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010 Mar 3;102(5):325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010 Oct 6;102(19):1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2011 Nov 8; doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 24.Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011 Jan;11(1):39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 25.Kemp TJ, Hildesheim A, Safaeian M, Dauner JG, Pan Y, Porras C, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011 Mar 3;29(11):2011–2014. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discovery. 2011;1(5):408–419. doi: 10.1158/2159-8290.CD-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011 Oct 5;103(19):1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, Rodriguez AC, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol. 2011 Sep;12(9):862–870. doi: 10.1016/S1470-2045(11)70213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009 Apr 1;199(7):926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler CM, Castellsague X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2011 Nov 8; doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 31.Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009 Dec 12;374(9706):1975–1985. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 32.Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2011 Aug 19; doi: 10.1002/ijc.26362. [DOI] [PubMed] [Google Scholar]
- 33.Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007 Nov 15;196(10):1438–1446. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 34.Dillner J. The serological response to papillomaviruses. Semin Cancer Biol. 1999 Dec;9(6):423–430. doi: 10.1006/scbi.1999.0146. [DOI] [PubMed] [Google Scholar]
- 35.Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009 Oct;5(10):696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 36.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and −18 infections. J Natl Cancer Inst. 2010 Nov 3;102(21):1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007 Aug 15;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 38.Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009 May;5(5):332–340. doi: 10.4161/hv.5.5.7211. [DOI] [PubMed] [Google Scholar]
- 39.Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11:13. doi: 10.1186/1471-2334-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009 Oct;5(10):705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 41.Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ. 2010;340:c712. doi: 10.1136/bmj.c712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: a combined analysis of five randomized controlled trials. Obstet Gynecol. 2009 Dec;114(6):1179–1188. doi: 10.1097/AOG.0b013e3181c2ca21. [DOI] [PubMed] [Google Scholar]
- 43.Dana A, Buchanan KM, Goss MA, Seminack MM, Shields KE, Korn S, et al. Pregnancy outcomes from the pregnancy registry of a human papillomavirus type 6/11/16/18 vaccine. Obstet Gynecol. 2009 Dec;114(6):1170–1178. doi: 10.1097/AOG.0b013e3181c2a122. [DOI] [PubMed] [Google Scholar]
- 44.Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009 Aug 19;302(7):750–757. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 45.Souayah N, Michas-Martin PA, Nasar A, Krivitskaya N, Yacoub HA, Khan H, et al. Guillain-Barre syndrome after Gardasil vaccination: data from Vaccine Adverse Event Reporting System 2006–2009. Vaccine. 2011 Jan 29;29(5):886–889. doi: 10.1016/j.vaccine.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011 Jun 28;105(1):28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009 Jun 6;373(9679):1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009 Jan 22;27(4):581–587. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 49.Wright TC, Jr, Huh WK, Monk BJ, Smith JS, Ault K, Herzog TJ. Age considerations when vaccinating against HPV. Gynecol Oncol. 2008 May;109(2 Suppl):S40–S47. doi: 10.1016/j.ygyno.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Westra TA, Rozenbaum MH, Rogoza RM, Nijman HW, Daemen T, Postma MJ, et al. Until which age should women be vaccinated against HPV infection? Recommendation based on cost-effectiveness analyses. J Infect Dis. 2011 Aug 1;204(3):377–384. doi: 10.1093/infdis/jir281. [DOI] [PubMed] [Google Scholar]
- 51.Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011 Feb 3;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011 Oct 27;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 53.Frazer IH. Measuring serum antibody to human papillomavirus following infection or vaccination. Gynecol Oncol. 2010 Jun;118(1 Suppl):S8–S11. doi: 10.1016/j.ygyno.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus viruslike-particle vaccine trials. J Infect Dis. 2009 Jul 15;200(2):166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008 Nov-Dec;4(6):425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 56.Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin. 2008 Mar-Apr;4(2):134–142. doi: 10.4161/hv.4.2.5261. [DOI] [PubMed] [Google Scholar]
- 57.Brown DR, Garland SM, Ferris DG, Joura E, Steben M, James M, et al. The humoral response to Gardasil(R) over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin. 2011 Feb 1;7(2) doi: 10.4161/hv.7.2.13948. [DOI] [PubMed] [Google Scholar]
- 58.Carter JJ, Wipf GC, Madeleine MM, Schwartz SM, Koutsky LA, Galloway DA. Identification of human papillomavirus type 16 L1 surface loops required for neutralization by human sera. J Virol. 2006 May;80(10):4664–4672. doi: 10.1128/JVI.80.10.4664-4672.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: Follow-up from months 12–24 in a phase III randomized study of healthy women aged 18–45 years. Hum Vaccin. 2011 Dec 1;7(12) doi: 10.4161/hv.7.12.18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008 Dec 9;26(52):6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 61.Roteli-Martins C, Naud P, De Borba P, Teixeira J, De Carvalho N, Zahaf T, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012 Mar 1;8(3) doi: 10.4161/hv.18865. [DOI] [PubMed] [Google Scholar]
- 62.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007 Jun 21;25(26):4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 63.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011 Dec;85(24):13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18–45 years. Hum Vaccin. 2011 Dec 1;7(12) doi: 10.4161/hv.7.12.18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006 Nov;118(5):2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007 Jun;40(6):564–571. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Petaja T, Keranen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health. 2009 Jan;44(1):33–40. doi: 10.1016/j.jadohealth.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clin Vaccine Immunol. 2011 Mar;18(3):418–423. doi: 10.1128/CVI.00489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared to the licensed 3-dose schedule: Results from a randomized study. Hum Vaccin. 2011 Dec 1;7(12) doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA, 3rd, Read JS, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010 Oct 1;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010 Oct 15;202(8):1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheeler CM, Bautista OM, Tomassini JE, Nelson M, Sattler CA, Barr E. Safety and immunogenicity of co-administered quadrivalent human papillomavirus (HPV)-6/11/16/18 L1 virus-like particle (VLP) and hepatitis B (HBV) vaccines. Vaccine. 2008 Jan 30;26(5):686–696. doi: 10.1016/j.vaccine.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 73.Vesikari T, Van Damme P, Lindblad N, Pfletschinger U, Radley D, Ryan D, et al. An open-label, randomized, multicenter study of the safety, tolerability, and immunogenicity of quadrivalent human papillomavirus (types 6/11/16/18) vaccine given concomitantly with diphtheria, tetanus, pertussis, and poliomyelitis vaccine in healthy adolescents 11 to 17 years of age. Pediatr Infect Dis J. 2010 Apr;29(4):314–318. doi: 10.1097/INF.0b013e3181c177fb. [DOI] [PubMed] [Google Scholar]
- 74.Reisinger KS, Block SL, Collins-Ogle M, Marchant C, Catlett M, Radley D, et al. Safety, tolerability, and immunogenicity of gardasil given concomitantly with Menactra and Adacel. Pediatrics. 2010 Jun;125(6):1142–1151. doi: 10.1542/peds.2009-2336. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Sicilia J, Schwarz TF, Carmona A, Peters K, Malkin JE, Tran PM, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine coadministered with combined diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine to girls and young women. J Adolesc Health. 2010 Feb;46(2):142–151. doi: 10.1016/j.jadohealth.2009.11.205. [DOI] [PubMed] [Google Scholar]
- 76.Peres J. For cancers caused by HPV, two vaccines were just the beginning. J Natl Cancer Inst. 2011 Mar 2;103(5):360–362. doi: 10.1093/jnci/djr053. [DOI] [PubMed] [Google Scholar]
- 77.Chen XS, Casini G, Harrison SC, Garcea RL. Papillomavirus capsid protein expression in Escherichia coli: Purification and assembly of HPV11 and HPV16 L1. J Mol Biol. 2001;307(1):173–182. doi: 10.1006/jmbi.2000.4464. [DOI] [PubMed] [Google Scholar]
- 78.Yuan H, Estes PA, Chen Y, Newsome J, Olcese VA, Garcea RL, et al. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol. 2001;75(17):7848–7853. doi: 10.1128/JVI.75.17.7848-7853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanumantha Rao N, Baji Babu P, Rajendra L, Sriraman R, Pang YY, Schiller JT, et al. Expression of codon optimized major capsid protein (L1) of human papillomavirus type 16 and 18 in Pichia pastoris; purification and characterization of the virus-like particles. Vaccine. 2011 Oct 6;29(43):7326–7334. doi: 10.1016/j.vaccine.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rybicki EP, Williamson AL, Meyers A, Hitzeroth II. Vaccine farming in Cape Town. Hum Vaccin. 2011 Mar;7(3):339–348. doi: 10.4161/hv.7.3.14263. [DOI] [PubMed] [Google Scholar]
- 81.Cantarella G, Liniger M, Zuniga A, Schiller JT, Billeter M, Naim HY, et al. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine. 2009 May 26;27(25–26):3385–3390. doi: 10.1016/j.vaccine.2009.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuck D, Lau T, Leuchs B, Kern A, Muller M, Gissmann L, et al. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J Virol. 2006 Mar;80(6):2621–2630. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, et al. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol. 2007 Oct;14(10):1285–1295. doi: 10.1128/CVI.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karanam B, Jagu S, Huh WK, Roden RB. Developing vaccines against minor capsid antigen L2 to prevent papillomavirus infection. Immunol Cell Biol. 2009 May-Jun;87(4):287–299. doi: 10.1038/icb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007 Nov 8;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 86.Bonanni P, Cohet C, Kjaer SK, Latham NB, Lambert PH, Reisinger K, et al. A summary of the post-licensure surveillance initiatives for GARDASIL/SILGARD. Vaccine. 2010 Jul 5;28(30):4719–4730. doi: 10.1016/j.vaccine.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 87.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011 Nov 10;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal "pap-test equivalent" in high-risk populations. Cancer Prev Res (Phila) 2011 Sep;4(9):1378–1384. doi: 10.1158/1940-6207.CAPR-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]