Abstract
Background
Vascular calcification independently predicts cardiovascular disease (CVD) and computed tomography (CT) is a useful tool to evaluate and quantify not only coronary but also thoracic aortic calcification (TAC). Previous TAC progression reports were limited to dialysis and renal transplant patients. This is the first study to evaluate TAC progression in a large multi-ethnic cohort without clinically evident CVD at entry.
Methods
Non-contrast enhanced cardiac CT were obtained in 5886 of 6814 MESA participants (mean age 62 years; 48% males; 40% white, 27% Black, 21% Hispanic, 12% Chinese. Baseline and follow-up TAC scores were derived.
Results
4308(73%) participants had no detectable baseline TAC. Mean follow-up duration was 2.4±0.8 years, during which 12% developed TAC. The overall incidence rate was 4.8%/year and was greater with age across gender and ethnic groups; TAC incidence was significantly lower in blacks than whites. After adjustment for follow-up duration, regression analyses showed age, systolic blood pressure, antihypertensives, and smoking were associated with incident TAC. 1578(27%) participants had TAC at baseline with a positive association between average annual TAC change and baseline age. While the overall median change was 32.9 (−1.4, 112.2) Agatston units, 27% showed an annual score change of ≥100 and blacks showed the lowest median across ethnic groups; 22.7 (−3, 86.8). Age, systolic BP, lipid-lowering medication, diabetes and smoking were associated with TAC progression.
Conclusion
In MESA, traditional CV risk factors were related to both TAC incidence and progression. Blacks had the lowest incidence and median change across ethnic groups, consistent with previous findings for coronary calcification.
1. Background
Vascular calcification has long been a major area of interest in cardiovascular medicine. Intimal calcification, a surrogate marker of atherosclerosis, has been associated with traditional and non-traditional (uremia-related) risk factors and predictive of future cardiovascular events1–3. Non-contrast computed tomography (CT) is the most sensitive method to quantify vascular calcification. Previous reports from the MESA study, have shown that traditional cardiovascular risk factors were associated with thoracic aortic calcification (TAC) with the highest prevalence in both white and Chinese populations4. Moreover, TAC was shown to be a significant predictor of future coronary events in women with increased event rate in symptomatic patients with stable angina5, 6. In contrast to Coronary Artery Calcium (CAC) progression, TAC progression reports were limited to dialysis and renal transplant patients. This is the first study to evaluate TAC progression in a large multi-ethnic cohort without clinically evident clinical CVD at entry. We evaluated the risk factors associated with both TAC incidence and progression.
2. Methods
2.1 Recruitment and baseline examination
The MESA cohort is a longitudinal, population-based study of 6814 men and women, free of clinical CVD, aged 45–84 at baseline recruited from six U.S communities: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN. Recruitment targeted four ethnic groups (white, black, Hispanic and Chinese).
The baseline visit took place between July 2000 and September 2002. Baseline medical history, anthropometric measurements, and laboratory data were taken from the first examination of the MESA cohort. Information about age, gender, ethnicity, and medical history were obtained by questionnaires. Resting blood pressure was measured three times in the seated position, and the average of the 2nd and 3rd readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of baseline blood pressure lowering medication. Body mass index (BMI) was calculated from the equation [weight(kg)/height(m2)]. Total and HDL-Cholesterol (HDL-C) were measured from blood samples obtained after a 12-h fast, LDL-Cholesterol (LDL-C) was estimated by the Friedewald equation and the use of lipid lowering medications was also noted. Smoking status was categorized into: Never, former and current where current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dl or use of hypoglycemic medications. Fibrinogen, creatinine, and high sensitivity C-reactive protein (HS-CRP) levels were also measured.
2.2 Measurement of TAC
Baseline and follow-up non-contrast enhanced cardiac CT scans were obtained in 5886 of the 6814 MESA participants. Follow-up TAC measurements were performed on half the cohort (randomly selected) at a second exam (September, 2002-January, 2004) and the other half of the cohort at a third exam (March, 2004-July, 2005) at an average of 1.6 and 3.2 years after the first examination, respectively with mean time between scans of 2.4±0.8 years. Three sites used an Electron beam CT (EBCT) scanner (GE–Imatron C–150XL, San Francisco, CA), and 3 sites used a 4-slice multidetector CT (MDCT) scanner. The method has been reported previously7. Image slices were obtained with the participant supine, with no couch angulation. A minimum of 35 contiguous images was obtained, starting above the left main coronary artery to the bottom of both ventricles. Each scan was obtained in a single breath hold. Section thickness of 3 mm, field of view of 35 cm, and matrix of 512 × 512 were used to reconstruct raw image data. The nominal section thickness was 3.0 mm for EBCT and 2.5 mm for 4–MDCT. Spatial resolution can be described by the smallest voxel, for the protocol for each system: 1.15 mm3 for 4–detector row CT (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 for EBCT (0.68 × 0.68 × 3.00 mm). Ascending and descending TAC ranged from the lower edge of the pulmonary artery bifurcation to the cardiac apex (imaged on every study of coronary calcium) were quantified by using the same lesion definition for coronary calcification.
2.3 Statistical Methods
All participants with both a baseline and a follow-up TAC measurement were included in the analysis. The presence of TAC was defined as an Agatston score greater than zero. The analysis strategy for this paper mirrors that used in previous MESA work on the progression of CAC8. Progression of TAC was defined in 2 ways: incident TAC defined as detectable TAC at the follow-up examination (either examination 2 or 3) in a participant free of detectable TAC at examination 1 and change in TAC score in participants who had detectable TAC at examination 1. Yearly incidence rates were estimated by gender and race/ethnicity. Similarly, median annual change in TAC (among those with existing TAC) were estimated by gender and race/ethnicity. The annual change was determined by the absolute between-scan change in Agatston scores divided by the interim time interval in years.
Risk factors included age, gender, race/ethnicity, education, income, systolic and diastolic blood pressures, use of antihypertensive medications, diabetes status, smoking (never, former, current), pack-years of smoking, alcohol consumption, exercise, BMI, LDL-C and HDL-C, triglycerides, use of lipid-lowering medication, fibrinogen, creatinine, and C-reactive protein (CRP).
Relative risk regression was used to model the probability of incident-detectable TAC among those free of TAC at examination 1. That is, the probability of incident TAC was modeled as a function of covariates using a generalized linear model with log link and Gaussian error distribution, with robust standard errors. Age- and follow-up time-adjusted models for each risk factor were estimated, followed by a multivariable model constructed via a backward elimination variable selection process. The time between scans was included as a covariate in all models, and interactions of each risk factor with gender and race/ethnicity tested. Among those with some detectable TAC at examination 1, we defined progression as the absolute difference between follow-up and examination 1 TAC, and this was treated as a continuous endpoint. Robust regression was used to model change, in order to account for outliers in the progression models. Scanner changes at some of the sites may also influence progression magnitude, and a term for scanner pair will be included in all the models for progression. The modeling strategy for progression will be analogous to that described for incident TAC. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
1. Sample size and baseline characteristics
After excluding those with missing baseline or follow-up TAC, the eligible sample size was 5886 participants with a mean follow-up of 2.4±0.8 years. A total of 4308 did not have TAC on baseline CT examination while 1578 had prevalent TAC. (figure 1)
Figure 1.
Flow diagram of MESA participants, categorized by TAC status
The study cohort was relatively young (mean age 62±10 years), ethnically diverse (60% non-white), and rather healthy (64% non-hypertensives, 88% non-diabetics). A total of 16% of the cohort was on lipid-lowering therapy, and baseline lipid levels were relatively normal (LDL 117±31 mg/dl; HDL 51±15 mg/dl; TG 131±86.5 mg/dl). Only 13% of the cohort were current smokers.
Compared to included participants, those excluded were slightly older (64 vs. 62 years), and more likely to have CAC at baseline (56% vs. 49%), higher systolic blood pressure (131 vs. 126 mm Hg), to be diabetics (18% vs. 12%) and current smokers (16% vs. 13%). (table 1)
Table 1.
Baseline Characteristics of included and excluded patients
| Included | Excluded | ||||
|---|---|---|---|---|---|
| Variable | Mean/frequency | Standard Deviation/percentage*100 | Mean/Frequency | Standard Deviation/Percentage*100 | |
| Age | 61.83 | 10.13 | 64.18 | 10.61 | |
| Systolic BP | 125.89 | 21.02 | 131.05 | 23.71 | |
| Diastolic BP | 71.86 | 10.17 | 72.29 | 10.79 | |
| Body Mass Index | 28.33 | 5.43 | 28.41 | 5.79 | |
| Packs of cigarettes per year | 11.18 | 22.18 | 12.82 | 22.67 | |
| LDL Cholesterol | 117.29 | 31 | 116.61 | 34.3 | |
| HDL Cholesterol | 50.98 | 14.72 | 50.85 | 15.49 | |
| Triglycerides | 130.89 | 86.5 | 136.04 | 102.16 | |
| C Reactive Protein | 3.67 | 5.36 | 4.53 | 8.48 | |
| Gender | 0: FEMALE | 3087 | 52.4 | 514 | 55.4 |
| 1: MALE | 2799 | 47.6 | 414 | 44.6 | |
| Race | 1:White | 2351 | 39.9 | 271 | 29.2 |
| 2:Chinese | 686 | 11.7 | 117 | 12.6 | |
| 3:AA | 1584 | 26.9 | 309 | 33.3 | |
| 4:Hispanic | 1265 | 21.5 | 231 | 24.9 | |
| Education | 1: Less than high school | 972 | 16.6 | 253 | 27.5 |
| 2: High school | 2746 | 46.8 | 427 | 46.4 | |
| 3: College | 1058 | 18 | 113 | 12.3 | |
| 4: Graduate school | 1095 | 18.7 | 127 | 13.8 | |
| Hypertension medication | No | 3750 | 63.7 | 525 | 56.6 |
| Yes | 2133 | 36.3 | 403 | 43.4 | |
| Lipid lowering medication | No | 4925 | 83.7 | 786 | 84.7 |
| Yes | 958 | 16.3 | 142 | 15.3 | |
| Cigarette smoking | 0: NEVER | 2958 | 50.4 | 460 | 50 |
| 1: FORMER | 2174 | 37 | 313 | 34 | |
| 2: CURRENT | 740 | 12.6 | 147 | 16 | |
| Diabetes | Normal/IFG | 5173 | 88.1 | 758 | 82.3 |
| Treated/untreated Diabetes | 696 | 11.9 | 163 | 17.7 | |
| Family history of heart attack | NO | 3157 | 57.1 | 504 | 58 |
| YES | 2369 | 42.9 | 365 | 42 | |
2. Incidence rate for participants free of TAC at baseline
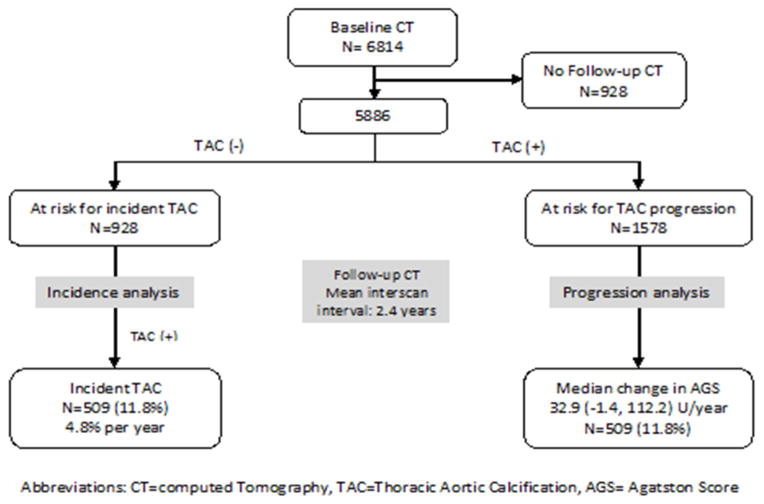
Of the 4308 participants without TAC at baseline, 509 (11.8%) developed TAC during the follow-up period, with an annual incidence rate of 4.8%/year. Compared to younger participants, there was a higher annual incidence rate for older participants (Figure 2a) with a similar positive correlation across genders (figure 2b) and different race subgroups (figure 2c).
Figure 2.
2a: Incidence rate of newly detectable TAC by age
2b: The association between incidence rate of TAC and age across gender
2c: The association between incidence rate of TAC and age across race subgroups
Blacks had a significantly lower incidence rate than whites in both males (3.9 vs 5.5% p=0.01) and females (4.8 vs 5.1% p=0.04). However, there was no racial difference across whites, Chinese and Hispanics and no significant gender difference within each racial group.
Assocation of traditional CVD risk factors and incidence of TAC
In analyses adjusted for follow-up time and age, risk factors associated with incident TAC were age (for each 10-year increment, the risk of incident TAC was 91% greater), follow-up time, systolic blood pressure, log triglycerides, antihypertensive and lipid-lowering medications and pack-years of smoking. However, in the multivariable model, log triglycerides and lipid-lowering medication were no longer associated with incident TAC (Table 2).
Table 2.
Relative risk of incident thoracic aortic calcium among those free of thoracic aortic calcium at baseline
| Variable | Age and Follow-up time adjusted model (n=4308) | Multivariable model (n=4252) | ||
|---|---|---|---|---|
| RR(95% CI) | P | RR(95% CI) | P | |
| Follow-up time | 1.35 (1.22,1,48) | <0.001 | 1.28 (1.16, 142) | <0.001 |
| Age (10 year) | 1.91 (1.75,2.08) | <0.001 | 1.78 (1.61,1.96) | <0.001 |
| BMI | 1.01 (0.99,1.02) | 0.506 | ||
| Systolic blood pressure (10 mm Hg) | 1.10 (1.06,1.14) | <0.001 | 1.11 (1.07, 1.15) | <0.001 |
| Diastolic blood pressure (10 mm Hg) | 1.06 (0.98, 1.16) | 0.15 | ||
| LDL-C (10 mg/dL) | 1.01 (0.98, 1.03) | 0.69 | ||
| HDL-C (10 mg/dL) | 0.98 (0.93, 1.04) | 0.50 | ||
| Log triglycerides (log mg/dL) | 1.43 (1.22, 1.68) | <0.001 | ||
| Fibrinogen (mg/dL) | 1.00 (1.00,1.00) | 0.77 | ||
| Log CRP (log mg/L) | 1.03 (0.96, 1.11) | 0.37 | ||
| Male gender | 0.90 (0.76, 1.06) | 0.21 | ||
| Race | ||||
| White | Reference | |||
| Chinese | 0.95 (0.72, 1.25) | 0.716 | 0.89 (0.66, 1.21) | 0.463 |
| African American | 0.70 (0.56, 0.86) | 0.001 | 0.6 (0.48,0.74) | <0.001 |
| Hispanic | 0.87 (0.70, 1.09) | 0.233 | 0.88 (0.71, 1.09) | 0.236 |
| Education | ||||
| Less than high school | Reference | |||
| High school | 0.94 (0.75, 1.18) | 0.598 | ||
| College | 0.92 (0.70, 1.21) | 0.553 | ||
| Graduate school | 0.94 (0.72, 1.24) | 0.659 | ||
| Income | ||||
| < 50,000 | Reference | |||
| 50,000–100,000 | 1.12 (0.88, 1.41) | 0.352 | ||
| >100,000 | 0.95 (0.76, 1.21) | 0.698 | ||
| Antihypertensive medication | 1.33 (1.12,1.57) | 0.001 | 1.32 (1.11, 1.57) | 0.001 |
| Lipid-lowering medication | 1.24 (1,1.52) | 0.046 | ||
| Diabetes status | ||||
| Normal/ Impaired fasting glucose | Reference | |||
| Treated/Untreated Diabetes | 1.24 (0.98, 1.58) | 0.075 | ||
| Family history of heart attack | 1.01 (0.85, 1.2) | 0.944 | ||
| Creatinine, mg/dL | ||||
| <=0.9 | 1.03 (0.82, 1.29) | 0.799 | ||
| 1 | Reference | |||
| >=1.1 | 0.82 (0.63, 1.06) | 0.125 | ||
| Alcohol | ||||
| Never | Reference | |||
| Former | 1.07 (0.83,1.38) | 0.595 | ||
| Current | 1.04 (0.83, 1.29) | 0.745 | ||
| Smoking | ||||
| Never | Reference | |||
| Former | 1.02 (0.85, 1.24) | 0.808 | 1.02 (0.85, 1.23) | 0.799 |
| Current | 1.15 (0.85, 1.56) | 0.361 | 1.28 (0.96, 1.72) | 0.094 |
| 10 pack-years of smoking* | 1.06 (1.03, 1.09) | <0.001 | 1.06 (1.03, 1.08) | <0.001 |
Model for pack-years includes adjustment for current and former smoking.
In both regression models, Chinese, Blacks and Hispanics all had lower rates of incident TAC as compared to whites, however only Blacks had significantly lower relative risk of incident TAC; 0.7 (0.56, 0.86) and 0.6 (0.48,0.74). We tested for the interaction between each risk factor and race and no significant interaction was found.
3. Annual TAC change for participants with prevalent TAC at baseline
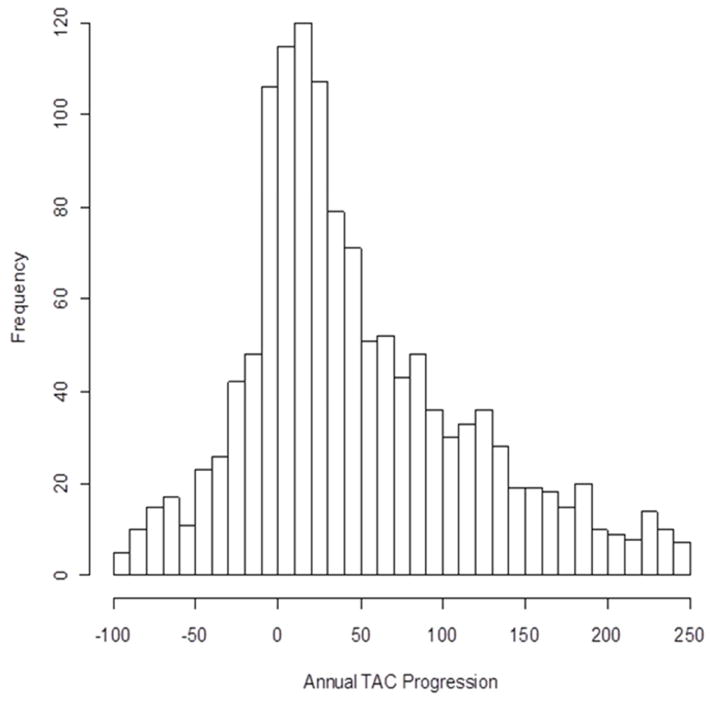
The distribution of annual TAC Agatston score change is shown in figure 3, where 123 participants had an annual change < 100 and 154 participants had an annual change >250. Of the 1578 with prevalent TAC at baseline, the median TAC change in Agatston scores was 32.9 (−1.4, 112.2) units/year.
Figure 3.
Distribution of annual TAC change among those with prevalent TAC at baseline.
Similar to incident TAC, there was a positive linear correlation between the average annual TAC change and age at baseline. The distribution and rate of TAC change is summarized in tables 3 and 4. There were443 (28.1%) participants that had negative annual TAC change., A large proportion of the population, 604 (38.3%) had mild annual progression (10–99 units), and 429 (27.2%) had moderate or larger annual progression (≥100).
Table 3.
Summary of Average Annual TAC change (Agatston Score) in participants with prevalent TAC at baseline
| Annual TAC change | Women N (%) | Men N (%) | Total N (%) |
|---|---|---|---|
| <0 | 242 (28.2) | 201 (27.9) | 443 (28.1) |
| 0 to 9 | 43 (5.0) | 59 (8.2) | 102 (6.5) |
| 10 to 99 | 341 (39.8) | 263 (36.5) | 604 (38.3) |
| 100 to 199 | 138 (16.1) | 89 (12.3) | 227(14.4) |
| >200 | 93 (10.9) | 109 (15.1) | 202 (12.8) |
Table 4.
Robust regression models for the change in TAC over time among participants with prevalent TAC at baseline.
| Variable | Robust Regression Model 1* (n=1578) | Robust Regression Model 2 (n=1550) | ||
|---|---|---|---|---|
| Difference in average progression (95% CI) | P value | Difference in average progression | P value | |
| Scanner type change | ||||
| EBCT to EBCT | Reference | |||
| EBCT to MDCT | −16.8 (−41,7.4) | 0.173 | −17 (−41.6, 7.5) | 0.174 |
| MDCT to MDCT | −23 (−31.7, −14.3) | <0.001 | −25.2 (−35.4, −15) | <0.001 |
| Follow-up time | 18.8 (13.8, 23.8) | <0.001 | 19.1 (14, 24.2) | <0.001 |
| Age (10 year) | 7.9 (2.5, 13.3) | 0.004 | 8.1 (2.4, 13.7) | 0.005 |
| BMI | 0.3 (−0.6, 1.2) | 0.512 | ||
| Systolic blood pressure (10 mm Hg) | 2.6 (0.6, 4.5) | 0.01 | 2.6 (0.7, 4.6) | 0.009 |
| Diastolic blood pressure (10 mm Hg) | 1.6 (−2.6, 5.7) | 0.465 | ||
| LDL-C (10 mg/dL) | −1.6 (−3, −0.2) | 0.028 | ||
| HDL-C (10 mg/dL) | −0.3 (−3.3, 2.7) | 0.838 | ||
| Log triglycerides (log mg/dL) | 4.0 (−4.4, 12.5) | 0.347 | ||
| Fibrinogen (mg/dL) | 0.1 (0.0, 0.1) | 0.048 | ||
| Log CRP (log mg/L) | 2.0 (−1.9, 5.8) | 0.316 | ||
| Male gender | −2.1 (−10.7, 6.5) | 0.632 | ||
| Race | ||||
| White | Reference | |||
| Chinese | −9.6 (−24.9, 5.7) | 0.217 | −5.9 (−21.5, 9.6) | 0.453 |
| African American | −9.7 (−21.1, 1.7) | 0.097 | −18.4 (−30.2, −6.6) | 0.002 |
| Hispanic | −14.8 (−27.2, −2.3) | 0.02 | −14.8 (−27.5, −2) | 0.023 |
| Education | ||||
| Less than high school | Reference | |||
| High school | −2.1 (−13.3, 9) | 0.706 | ||
| College | −9.1 (−23.7, 5.5) | 0.222 | ||
| Graduate school | −2.7 (−17.6, 12.2) | 0.723 | ||
| Income | ||||
| < 50,000 | Reference | |||
| 50,000–100,000 | 3.1 (−8.4, 14.7) | 0.593 | ||
| >100,000 | 3.5 (−8, 14.9) | 0.551 | ||
| Antihypertensive medication | 5.5 (−3.1, 14.1) | 0.212 | ||
| Lipid-lowering medication | 19.7 (9.8, 29.5) | <0.001 | 18.8 (8.8, 28.9) | <0.001 |
| Diabetes status | ||||
| Normal/ Impaired fasting glucose | Reference | |||
| Treated/Untreated Diabetes | 12.9 (1.2, 24.7) | 0.031 | 15.5 (3.5, 27.5) | 0.012 |
| Family history of heart attack | 6 (−2.9, 14.9) | 0.184 | ||
| Creatinine, mg/dL | ||||
| <=0.9 | −3.9 (−15.3, 7.6) | 0.505 | ||
| 1 | Reference | |||
| >=1.1 | −7.7 (−20.5, 5) | 0.236 | ||
| Alcohol | ||||
| Never | Reference | |||
| Former | −3.2 (−15.7, 9.4) | 0.621 | ||
| Currrent | −3.4 (−14.1, 7.4) | 0.54 | ||
| Smoking | ||||
| Never | Reference | |||
| Former | 5.8 (−4.6, 16.3) | 0.274 | 7.1 (−3.4, 17.5) | 0.184 |
| Current | 20.6 (4.6, 36.6) | 0.012 | 27 (10.9, 43) | 0.001 |
| 10 pack-years of smoking† | −0.5 (−2.1, 1.2) | 0.588 | −0.9 (−2.5, 0.8) | 0.302 |
Model 1 adjusted for scanner type change (EBCT to EBCT vs. EBCT to MDCT vs. MDCT to MDCT), age and follow-up time.
Model for pack-years also controls for smoking status (never, former, current).
4. Association of traditional CVD risk factors with TAC progression among those with prevalent TAC at baseline
In analyses adjusted for follow-up time, age and scanner type, risk factors associated with greater TAC progression included scanner type, follow-up time, age (each 10-year increment was associated wtih 7.9 units higher TAC progression), systolic blood pressure, Fibrinogen, Lipid-lowering medication, diabetes and current smoking. However, in the multivariable model, fibrinogen was no longer associated with TAC progression
Chinese, Blacks and Hispanics all had lower rates of TAC progression as compared to whites, though this was not significant for Chinese. In the multivariable model, the Hispanics had significantly lower TAC progression than whites by 14.8 units, and Blacks had lower progression than whites by 18.4 units. Among different ethnic groups, Blacks had the lowest median TAC change 22.7 (−3, 86.8), while Chinese had the highest median change 47.4 (12, 120.8). The median changes for whites and Hispanics were 34.6 (−1.5, 118.6) and 34.1 (−3.8, 112.8) respectively. We tested for the interaction between each risk factor and race and no significant interaction was found.
Discussion
In this analysis of the MESA cohort, we used quantitative TAC scores obtained from serial CT scans to characterize the incidence and progression rates of TAC as well as their prospective risk associations in this primary-prevention population.
Prevalence and Incidence of TAC
At baseline, TAC prevalence rate was 27%, this prevalence is similar to that of aortic calcifications reported in healthy control groups for hemodialysis patients, ranging from (17.3% in females and 22.1% in males) in one study from Japan9 and reaching 37.5% in a more recent European study10. A considerably higher prevalence of 63% was shown in Heinz Nixdorf Recall study where the participants had a worse cardiovascular risk profile, and TAC was defined to include both ascending, transverse and descending aorta rather than the ascending and descending aorta only in MESA11. In hemodialysis patients, a higher risk group with more metabolic derangements, a much higher prevalence of aortic calcification of >80% was shown in the “Calcification Outcome in Renal Disease” CORD study where the independent predictors were age, duration of dialysis and positive history of CVD12.
In our cohort, the annual incidence rate of developing new TAC was 4.8%. Whites had the highest incidence rate among different ethnicities while Blacks showed the lowest (30–40% lower than whites). Similarly, in earlier MESA reports, Blacks showed the lower risk for developing CAC and valvular calcifications compared to whites; 0.78 (95% CI 0.74–0.82) and 0.72 (95% CI 0.59–0.90) respectively13, 14.
The three main cardiovascular risk factors associated with the development of new TAC lesions in our study were age, hypertension and smoking, similar to those factors shown to be associated with other segments of the aorta, such as the aortic arch and abdominal aorta15, 16.
In a study by Raven and Sacks, the cohort was separated into younger and older participants revealing that elderly people (age ≥ 61 years) had more severe aortic calcification17. Our literature search did not locate any studies showing a negative or null correlation with age, indicating age as an important risk factor for aortic calcification. Matsushita et al. compared hypertensive and non-hypertensive participants based on the severity of calcification showing that calcifications of abdominal aortic aneurysms were more common in hypertensive males18. Whether hypertension predisposes to aortic calcification or vascular calcification causes higher blood pressure readings remains to be determined.
The majority of studies showed strong evidence to support smoking as a risk factor for aortic calcification17, 19, 20. Witteman et al. used radiographs to examine the relationship between smoking and aortic calcification in women in a population based 9-year follow-up study, the relative risk of those who smoked >20 cigarettes/day was 2.3 (95% CI 1.8–3.0) after adjustment for age and other cardiovascular risk factors 21.
Progression of TAC
Almost 73% of our study population showed negative or mild annual TAC changes, leaving only the smaller percentage with moderate or larger TAC progression rates (>100). This pattern reflects the fact our study population belongs to a lower risk asymptomatic group, a subset of patients that we commonly encounter in our everyday preventive clinical practice to further assess their current and future cardiovascular risk profile. Risk factors were associated with TAC progression in our study were age, systolic blood pressure, lipid lowering medication, diabetes and current smoking. Earlier MESA reports have found similar risk factors for progression of CAC8, while those that were associated with aortic valve calcification were male gender and the baseline Agatston score only22, 23. The risk of incident TAC was by 91% higher for each 10 year increment in age. It is worth noting that, for CAC, male gender was a significant risk factor with 43% higherCAC incidence and an average of 11 more Agatston units of progression when compared to women.8 As compared to peri-menopausal and post-menopausal women, male gender was not shown to be a significant risk factor for either the incidence or progression of TAC in our study.
In a recent longitudinal 4-year follow-up study, 94 subjects participating in health screening protocol were enrolled, both calcifications and inflammation (measured by 18F-FDG uptake on PET/CT scans) of the whole aorta significantly increased in the follow-up scans compared to the initial ones, the progression of 18F-FDG uptake and calcium score were significantly faster in the abdominal than in the thoracic aorta. Multiple regression analysis showed that progression of aortic calcifications was significantly associated with different atherogenic risk factors like age and smoking habit (P<0.001 and 0.0058 respectively)24.
Follow-up studies that evaluated dialysis patients and kidney transplant recipients have shown traditional cardiovascular risk factors like older age, male gender and higher pulse pressure to be associated with increased risk of aortic calcification progression and mortality25, 26.
White race was associated with more TAC progression than the other 3 ethnic groups. Blacks had the lowest annual median TAC change and the slowest rate of progression. This finding agrees with previous MESA reports on CAC and aortic valve calcification progression and might reflect a common pattern of racial distribution between coronary and extra-coronary calcifications8, 22.
Strengths and limitations
Strengths of the MESA study include the large sample size, inclusion of 4 racial/ethnic groups, and the community-based (as opposed to referral-based) nature of the sample. Additionally, the prospective nature of the study allowed TAC measurement and risk factor assessment to proceed in a standardized manner. This study has some limitations: (1) We only examined the aorta in the available range of CAC scans (excluding the aortic arch and the infrarenal abdominal aorta, two places with noted higher prevalence of calcification)19, 27, (2) There were some differences between included and excluded participants regarding their baseline characteristics. For example, excluded participants showed slightly greater prevalence of traditional risk factors (age, high blood pressure, diabetes, and smoking) and baseline CAC, this might had some influence on the incidence or progression rates in our study. (3) 123 participants had a decrease of TAC by 100 or more. We have previously evaluated the reproducibility of this measure in the MESA study, finding that TAC had an overall variability of 10%, with no difference between MDCT variability and EBCT variability (9.3 vs. 10.2%, respectively, P =NS). Agatston and volume scores were similar for each scanner type. We believe that TAC is most likely not reversible, so negative changes most likely represent measurement error from scan 1 to scan 2.
Conclusion
In the MESA cohort, both TAC incidence and progression were significantly associated with traditional cardiovascular risk factors and white race. Blacks demonstrated the lowest incidence and lowest median change compared to other ethnic groups. When compared to other reports from MESA and other studies, these findings have been consistent with those published for coronary calcification. The strongest risk factors for TAC incidence and progression were smoking, age, and hypertension. Since TAC has been demonstrated to have prognostic significance for future CV events, radiologists should report this finding when reading thoracic CT studies. It is important to note that TAC becomes quite common as patients age, and thus, evaluating incident TAC over time may prove important to better identify when atherosclerosis develops and anti-atherosclerotic therapies (both lifestyle and medications) can best be applied. Further study on the prognostic significance of TAC progression would be useful to determine whether reporting changes in TAC should be recommended.
Acknowledgments
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. and the Multi-Ethnic Study of Atherosclerosis and Air Pollution, funded by the Environmental Protection Agency (EPA) Science to Achieve Results (STAR) Program (grant RD 831697).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
George Youssef, LA Biomedical Research Institute at Harbor-UCLA. Torrance, California.
Mengye Guo, Department of Biostatistics, University of Washington, Seattle, Washington.
Robyn L. McClelland, University of Washington, Seattle, Washington.
David M Shavelle, Department of Medicine, Harbor-UCLA, Los Angeles, California.
Khurram Nasir, LA Biomedical Research Institute at Harbor-UCLA. Torrance, California; Department of Cardiology, Johns Hopkins University, Baltimore, Maryland; Center for Prevention and Wellness Research, Baptist Health Medical Group, Miami Beach, Florida; Department of Epidemiology, Robert Stempel College of Public Health, Florida International University, Miami, Florida; Department of Medicine, Herbert Wertheim College of Medicine, Florida International University, Miami, Florida.
Juan Rivera, Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore, Maryland; South Beach Preventive Cardiology, Miami, Florida.
J. Jeffrey Carr, Wake Forest University, Winston-Salem, NC.
Nathan D. Wong, Division of Radiology, University of California-Irvine, California.
Matthew J. Budoff, LA Biomedical Research Institute at Harbor-UCLA. Torrance, California.
References
- 1.Simon A, Levenson J. Early detection of subclinical atherosclerosis in asymptomatic subjects at high risk for cardiovascular disease. Clin Exp Hypertens. 1993;15(6):1069–76. doi: 10.3109/10641969309037094. [DOI] [PubMed] [Google Scholar]
- 2.Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94(5):1175–92. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 4.Takasu J, Katz R, Nasir K, et al. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) American heart journal. 2008;155(4):765–71. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budoff MJ, Nasir K, Katz R, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2011;215(1):196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen A, Tenenbaum A, Koren-Morag N, et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118(13):1328–34. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 7.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 8.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115(21):2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 9.Okuda K, Kobayashi S, Hayashi H, et al. Case-control study of calcification of the hepatic artery in chronic hemodialysis patients: comparison with the abdominal aorta and splenic artery. Journal of gastroenterology and hepatology. 2002;17(1):91–5. doi: 10.1046/j.1440-1746.2002.02653.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Garcia M, Gomez-Alonso C, Naves-Diaz M, et al. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(1):239–46. doi: 10.1093/ndt/gfn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erbel R, Delaney JA, Lehmann N, et al. Signs of subclinical coronary atherosclerosis in relation to risk factor distribution in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR) European heart journal. 2008;29(22):2782–91. doi: 10.1093/eurheartj/ehn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honkanen E, Kauppila L, Wikstrom B, et al. Abdominal aortic calcification in dialysis patients: results of the CORD study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(12):4009–15. doi: 10.1093/ndt/gfn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi- Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111(10):1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 14.Nasir K, Katz R, Takasu J, et al. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;198(1):104–14. doi: 10.1016/j.atherosclerosis.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA : the journal of the American Medical Association. 2000;283(21):2810–5. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 16.Jayalath RW, Mangan SH, Golledge J. Aortic calcification. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005;30(5):476–88. doi: 10.1016/j.ejvs.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Reaven PD, Sacks J. Reduced coronary artery and abdominal aortic calcification in Hispanics with type 2 diabetes. Diabetes care. 2004;27(5):1115–20. doi: 10.2337/diacare.27.5.1115. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita M, Nishikimi N, Sakurai T, et al. Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. International angiology : a journal of the International Union of Angiology. 2000;19(3):276–9. [PubMed] [Google Scholar]
- 19.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(2):331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell CJ, Chazaro I, Wilson PW, et al. Evidence for heritability of abdominal aortic calcific deposits in the Framingham Heart Study. Circulation. 2002;106(3):337–41. doi: 10.1161/01.cir.0000022663.26468.5b. [DOI] [PubMed] [Google Scholar]
- 21.Witteman JC, Grobbee DE, Valkenburg HA, et al. Cigarette smoking and the development and progression of aortic atherosclerosis. A 9-year population-based follow-up study in women. Circulation. 1993;88(5 Pt 1):2156–62. doi: 10.1161/01.cir.88.5.2156. [DOI] [PubMed] [Google Scholar]
- 22.Owens DS, Katz R, Takasu J, et al. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) The American journal of cardiology. 2010;105(5):701–8. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youssef G, Kalia N, Darabian S, et al. Coronary calcium: new insights, recent data, and clinical role. Current cardiology reports. 2013;15(1):325. doi: 10.1007/s11886-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu Y, Yoshida K, Suzuki Y, et al. Long-term changes of aortic (18)F-FDG uptake and calcification in health-screening subjects. Annals of nuclear medicine. 2012 doi: 10.1007/s12149-012-0679-z. [DOI] [PubMed] [Google Scholar]
- 25.Noordzij M, Cranenburg EM, Engelsman LF, et al. Progression of aortic calcification is associated with disorders of mineral metabolism and mortality in chronic dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(5):1662–9. doi: 10.1093/ndt/gfq582. [DOI] [PubMed] [Google Scholar]
- 26.Marechal C, Coche E, Goffin E, et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(2):258–69. doi: 10.1053/j.ajkd.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Takasu J, Mao S, Budoff MJ. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Academic radiology. 2003;10(6):631–7. doi: 10.1016/s1076-6332(03)80081-8. [DOI] [PubMed] [Google Scholar]
- 28.Budoff MJ, Katz R, Wong ND, Nasir K, Mao S, Takasu J, Kronmal R, Detrano RC, Carr JJ. Effect Of Scanner Type On The Reproducibility Of Extra-Coronary Measures Of Calcification: The Multi- Ethnic Study Of Atherosclerosis. Acad Radiol. 2007;14(9):1043–9. doi: 10.1016/j.acra.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of Coronary Calcium and Incident Coronary Heart Disease Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013;61(12):1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]