Abstract
Among immune cells in responding to sepsis, macrophages and neutrophils have been extensively studied, while the contribution of T lymphocytes and natural killer T (NKT) cells is less well characterized. Here we monitored tissue specific changes of T cell subsets in male C57BL/6 mice subjected to sham operation or cecal ligation and puncture (CLP) to induce polymicrobial sepsis. Thymus, spleen, liver, lungs and blood were processed and analyzed 20 h later. Total lymphocyte count showed a significant reduction in septic thymus, spleen and blood but not in lungs and liver. The septic thymi were hypocellular with severe reduction in cell numbers of immature CD4+CD8+ subset. CD4+ T and CD8+ T lymphocyte numbers in septic spleens were also significantly reduced, but the frequency of CD4+CD25+ Tregs was significantly increased. In addition, naïve and Tcm CD4+ T cell numbers were significantly reduced in the septic spleens. By contrast, in septic liver the CD8+ T cell numbers were significantly increased, whereas NKT cell numbers were reduced, but more activated with increased CD69 and CD25 expression. In the septic lungs, the CD4+ T and CD8+ T cell numbers showed no significant change, whereas they were severely reduced in the septic blood. Overall, this study provides important information on the alterations of different T-cell subsets in various tissues after sepsis.
Keywords: Sepsis, T-lymphocytes, natural killer T cells, Profiling, Tissue-specificity
1. Introduction
Sepsis is defined as an initial hyper-inflammatory response to systemic infection associated with a subsequent immune-suppression that can lead to multiple organ failure, immune dysfunctions, secondary infections and lethality. Sepsis afflicts >800,000 people annually in the United States alone and accounts for up to 39% of total hospital costs [1,2]. Unfortunately, its incidence appears to be raising with the mortality rates as high as 30% [3] and yet there are no effective therapies. Repeated failure of more than thirty clinical trials also indicates to a need for better understanding of the pathogenesis of sepsis [4,5].
The inflammatory roles of innate myeloid cell populations, such as macrophages and neutrophils, have always been the focus of the basic studies and target of clinical trials for sepsis [6]. Until recently, targeting immune-suppressive phase and boosting T-cell based immunity by administering interleukin (IL)-7 and IL-15 in septic mice and patients have showed promising results [7–11]. T lymphocytes develop from the bone marrow-derived precursors in the thymus through stages defined by the expression of the cell surface receptors CD4 and CD8 [12]. Maturing thymocytes start as CD4−CD8− double-negative populations and then progress to become CD4+CD8+ double-positive (DP) thymocytes constituting over 80% of the developing thymocytes [13]. DP thymocytes undergo a precise selection process to finally become CD4 single-positive (SP) or CD8 SP thymocytes, which exhibit MHC Class II or MHC Class I restriction, respectively [13].
Mature, nonself-reactive SP thymocytes are exported to the periphery as naïve CD4+ T and CD8+ T cells. These naïve T cells become activated on encountering antigen in the context of MHC presented by the antigen presenting cells and further develop into various subsets with distinct biological functions. CD4+ T cells differentiate into effector T helper (Th) cells or suppressive regulatory T cells (Tregs), and CD8+ T cells become cytotoxic CD8+ T cells [13]. Most of these effector subsets disappear at the end of the immune response with only a few becoming central memory T (Tcm) and effector memory T (Tem) cells which are ready to respond on reactivation with the same antigen [14]. The continuous output of naïve T cells from the thymus is essential for the maintenance of the diversity of the peripheral T cell repertoire and the ability of the host to respond to new antigens.
Natural killer T (NKT) cells are a heterogeneous and conserved unique subset of T cells co-expressing a T cell receptor (TCR) and NK1.1, a natural killer (NK) cell surface marker [15]. Thus, NKT cells share characteristics of conventional T cells and NK cells and bridge innate and adaptive immunity by rapidly producing cytokines and subsequently activating other cell types, e.g., dendritic and NK cells [16]. NKT cells comprise approximately 30% of the hepatic lymphoid population in mice, whereas a much smaller percentage is found in other organs, i.e., thymus, spleen, lymph nodes and peripheral blood [15,17].
The cellular composition of each organ, its micro-environment, and nature of the immune cell recruitment, has been known to influence local inflammation and tissue injury [18,19]. Even though sepsis is regarded as a systemic process, the pathophysiological events during sepsis differ from organ to organ and from organ to peripheral blood which has lead to the concept of compartmentalization [18]. Therefore, determining the redistribution of T and NKT cell populations in various organs after sepsis will help us to understand the contribution of each compartment in affecting T and NKT cells. In the present study, we used a mouse model of sepsis induced by cecal ligation and puncture (CLP), which is the most widely used and physiologically relevant experimental model for the induction of polymicrobial sepsis [20]. We then measured and compared the frequency and total numbers of CD4+ and CD8+ T lymphocytes, various CD4+ T subsets including Tregs, and NKT cells in various lymphoid and non-lymphoid organs, including thymus, spleen, liver and lungs, as well as the blood in healthy and septic mice. Our results clearly demonstrated that CLP-induced sepsis affected lymphocytes in a tissue-specific manner.
2. Materials and methods
2.1. Mice
Male 8- to 10-week-old C57BL/6 mice (20–25 g) were purchased from Taconic, Albany, NY. Mice were housed in a temperature-controlled room on a 12 hours light/dark cycle and fed a standard laboratory diet within the Feinstein Institute for Medical Research (Manhasset, NY, USA). All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD, USA) and were approved by the Institutional Animal Care and Use Committee (IACUC) at the Feinstein Institute for Medical Research.
2.2. Cecal ligation and puncture (CLP)
CLP was used as a model of polymicrobial sepsis as previously described [21]. The mice were anesthetized by isoflurane inhalation, and the abdomen was shaved and washed with 10% povidone iodine. A 1–2-cm midline incision was performed to allow exposure of the cecum and tightly ligated 1.5 cm from the tip with a 4–0 silk suture. Double puncture of the cecum was performed using a 22-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation sites and returned to the peritoneal cavity. The laparotomy site was then closed with 6–0 silk suture. Sham operated animals underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The CLP animals were resuscitated with 1 ml of isotonic sodium chloride solution by subcutaneous injection immediately after the surgery to improve dehydration after CLP. At 20 hours after CLP or sham operation, mice were anesthetized and blood, thymus, spleen, liver, and lung tissues were collected.
2.3. Lymphocyte preparation from thymus, spleen and blood
Single-cell suspensions were prepared from thymus, spleen and blood as per standard protocols. Briefly thymi and spleens were disrupted, homogenized and filtered in complete RPMI medium (with 10% FBS, 1 % Penn-Strep, 10 mM HEPES, 2 mM L-Glutamine, and 5×10−5 M β-mercaptoethanol) by crushing on 70 μm nylon filter (BD Falcon, Durham, NC, USA) using 3 ml syringe plunger. Red blood cell (RBC) lysis was conducted with splenic cell suspensions using RBC lysis solution (10 mM KHCO3, 155 mM NH4Cl, 0.1 mM EDTA, pH 8.0). After centrifugation at 1,100 rpm for 7 min, the cell pellets were resuspended in complete RPMI medium. Blood lymphocytes were isolated from fresh whole blood, collected via heart puncture and mixed well with EDTA, by doing RBC lysis.
2.4. Lymphocyte preparation from liver and lungs
Hepatic lymphocytes were isolated from livers that were minced and homogenized in PBS with 1% FBS, filtered through 70 μm nylon strainer and washed in PBS with 1% FBS. Cells were then resuspended in 44% Percoll (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), underlaid with 66% Percoll, and centrifuged for 20 min at 2,000 rpm. Cells at the interface were collected, washed, and counted. Lung lymphocytes were isolated by digesting minced lungs in complete RPMI medium containing 100 U/ml Collagenase type 1 (Worthington Biochemical, NJ, USA) and 20 U/ml DNase 1 (Roche Diagnostics, Mannheim, Germany) for 30 min at 37°C in shaker incubator followed by filtering, washing and Percoll gradient centrifugation done by same method as for liver.
2.5. Antibodies and flow cytometry
Single cell suspensions (1×106 cells per stain) obtained from different tissues were pre-incubated with anti-CD16/CD32 (93) to block FcγRII/III receptors and stained with desired antibodies on ice for 30 min and analyzed on FACSVerse (BD Bioscience, San Jose, CA, USA). The following anti-mouse antibodies conjugated to FITC, PE, peridinin chlorophyll protein-cyanine 5.5, allophycocyanin or PE-cyanine7 (all from Biolegend, San Diego, CA) were used for staining: anti-CD4 (GK1.5/RM4-5), anti-CD8α (53–6.7), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-TCRβ (H57–597), anti-NK1.1 (PK136), anti-CD25 (PC61), and anti-CD69 (H1.2F3). Dead cells were excluded by forward light scatter or forward light scatter plus propidium iodide. All the data were acquired and presented on log scale. Data were analyzed by FlowJo software (Tree Star, Ashland, OR, USA).
2.6. Quantification of cell numbers
The total cell numbers of lymphocytes isolated from each tissue type was determined by counting aliquots in a hemocytometer using trypan blue exclusion staining method. The frequency or percentage of each cell type in the tissues was identified by flow cytometric analysis using fluorescently labeled mAb directed against their respective CD surface markers. The cell numbers of each cell type in various tissues were determined using the following formula: Subpopulation cell numbers = total lymphocytes recovered × cell subpopulation percentage/100.
2.7. Statistical analysis
Data are expressed as means ± standard error mean (SEM) and analyzed using SigmaPlot11 graphing and statistical analysis software (Systat Software Inc., San Jose, CA, USA). Two groups were compared by Student’s t test. Differences in values were considered significant if p < 0.05.
3. Results
3.1. CD4+CD8+ DP cells decrease in the thymus after sepsis
To determine the impact of sepsis on T-lymphocytes, we compared the frequency and total numbers of T cell subsets in various organs between sham and septic mice at 20 h after CLP. We first prepared single-cell suspensions from the thymus and analyzed thymic subsets by flow cytometry. Total lymphocyte count showed a significant reduction by 73% in the septic thymi compared to sham (Fig. 1A). Among the thymic T cell subpopulations DP (CD4+CD8+) subset was significantly decreased in frequency by 30% (Fig. 1B and 1C) and severely depleted in absolute cell numbers by 81%, compared to sham (Fig. 1D). However, the frequency of DN (CD4−CD8−), and CD4+SP and CD8+ SP thymic subsets was significantly increased by 3.5-, 3.7-and 3.0-fold, respectively, because of the decreased percentage of DP subset (Fig. 1B and 1C). The absolute cell numbers of these subsets were not affected and were comparable to those in the sham (Fig. 1D). Thus, CLP-induced sepsis results in thymic hypocellularity due to a major loss of DP thymocyte subset without affecting the other thymic subsets.
Figure 1. Hypocellularity and CD4+ CD8+ double positive (DP) lymphocyte subset depletion in the thymi of septic mice.
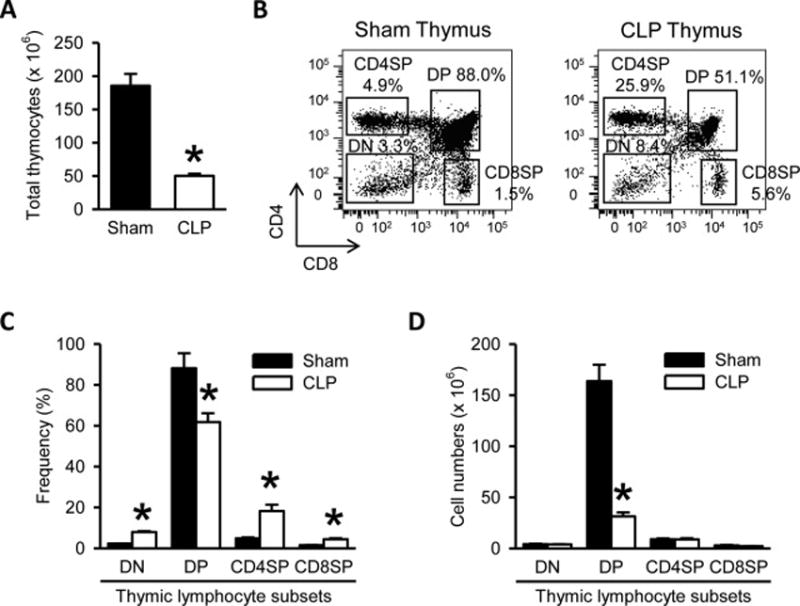
(A) Total numbers of thymocytes isolated from the thymi harvested after 20 h from sham- or CLP-operated mice are shown. (B) Flow cytometric analysis of surface CD4 and CD8 expression on the gated live lymphocytes isolated from the sham and CLP thymi. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of various thymic lymphocyte subsets as indicated. DP indicates CD4+CD8+ double positive thymocytes, DN indicates CD4−CD8− double negative thymocytes, CD4SP indicates CD4+ single positive thymocytes, and CD8SP indicates CD8+ single positive thymocytes. Data are representative of three independent analyses with a total of four to five mice in each group. (C–D) The graphs show the percentage (C) and absolute cell numbers (D) of the indicated thymic lymphocyte subsets. Data expressed as mean ± SEM (n = 5 per group). *P < 0.05 versus sham.
3.2. CD4+ and CD8+ T cell numbers decrease, Tregs frequency increases and naïve and Tcm CD4+ T cell numbers decrease in the spleen after sepsis
Lymphocytes isolated from the spleen of sham and septic mice were stained with anti-CD4 and anti-CD8 Abs. Total splenic lymphocyte count showed a significant reduction by 41% in the septic mice compared to sham (Fig. 2A). No significant differences in CD4+ and CD8+ T-cell frequency were noticed at 20 h after CLP as compared to the shams (Fig. 2B and 2C), although we noticed a decreasing trend in CD4 T cell frequency (Fig. 2C). However, CD4+ and CD8+ T cell numbers in the septic spleens were significantly decreased by 45% and 36%, respectively (Fig. 2D).
Figure 2. T cell depletion and increased frequency of CD4+ CD25+ regulatory T cells (Tregs) in the spleens of septic mice.
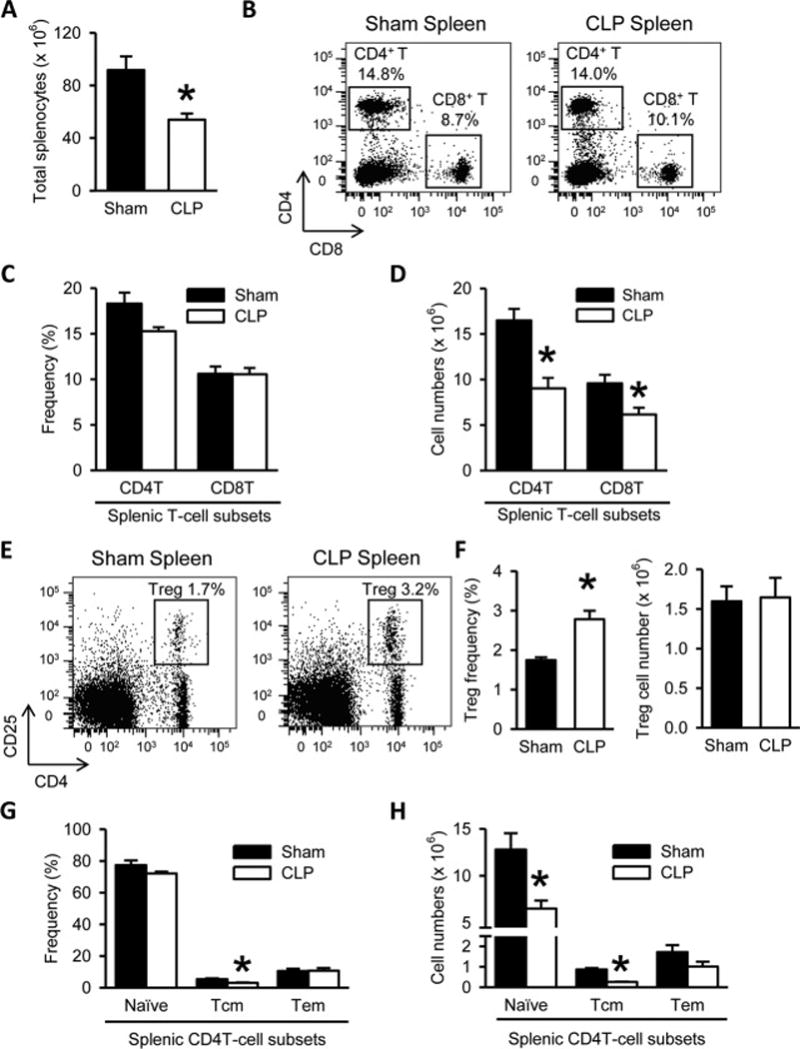
(A) Total numbers of splenocytes isolated from the spleens harvested after 20 h from sham- or CLP-operated mice are shown. (B) Flow cytometric analysis of surface CD4 and CD8 expression on the gated live lymphocytes isolated from the sham and CLP spleens. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of CD4+ T and CD8+ T cell subsets as indicated. Data are representative of three independent analyses with a total of four to five mice in each group. (C–D) The graphs show the percentage (C) and absolute cell numbers (D) of the indicated splenic T-cell subsets. (E) Flow cytometric analysis of surface CD4 and CD25 expression on the gated live lymphocytes isolated from the sham and CLP spleens. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of Tregs. Data are representative of three independent analyses with a total of four to five mice in each group. (F) The graphs show the percentage and absolute cell numbers of Tregs. (G–H) The graphs show the percentages (G) and absolute cell numbers (H) of CD4T cell subsets defined as naïve (CD44−CD62L+), central memory (Tcm; CD44+CD62L+) and effector memory (Tem; CD44+CD62L−) based on the flow cytometric analysis of the surface CD44 and CD62L expression on the gated splenic CD4T cells. Data expressed as mean ± SEM (n = 5 per group). *P < 0.05 versus sham for all graphs.
In our sepsis model, the frequency of CD4+ CD25+ Tregs in the spleen was significantly increased by 1.6-fold, compared to sham (Fig. 2E and 2F). However, the absolute Treg cell numbers in septic spleens were comparable to sham (Fig. 2F), indicating that the increase of their frequency was a result of the relative decrease in the effector CD4+ T cell populations. We further analyzed naïve (CD44−CD62L+), effector memory (Tem; CD44+CD62L−) and central memory (Tcm; CD44+CD62L+) CD4+ T cell subset distribution in the sham and septic spleens by flow cytometry. There was a significant 43.5% decrease in the frequency of Tcm CD4+ T cells in the septic spleens compared to sham (Fig. 2G). The frequencies of naïve and Tem CD4+ T cell subsets in the septic spleens were comparable to that in the sham spleens with only a decreasing trend in the naïve subset (Fig. 2G). However, the absolute cell numbers of both Tcm and naïve CD4 T cell subsets were significantly decreased by 70% and 49%, respectively after sepsis with no change in the Tem cell numbers in comparison with sham (Fig. 2H).
3.3. CD8+ T cells increase whereas NKT cells decrease but get activated in the liver after sepsis
In the septic mice, total liver leukocyte count was comparable to that from sham (Fig. 3A). We then examined the CD4+ T and CD8+ T cell subsets in the liver of the sham and septic mice by flow cytometry (Fig. 3B). The hepatic CD8+ T cells in the septic mice were significantly increased in frequency by 1.8-fold (Fig. 3C) and in absolute numbers by 1.9-fold (Fig. 3D), compared to sham. The hepatic CD4+ T cell frequency and absolute numbers after CLP also showed a trend towards increase but were not significantly different, compared to sham (Fig. 3C and 3D). The hepatic NK (NK1.1+ TCRβ−) cell population in the septic mice was also increased compared to sham (Fig. 3E).
Figure 3. Increased CD8+ T cells and decreased but activated natural killer T (NKT) cells in the livers of septic mice.
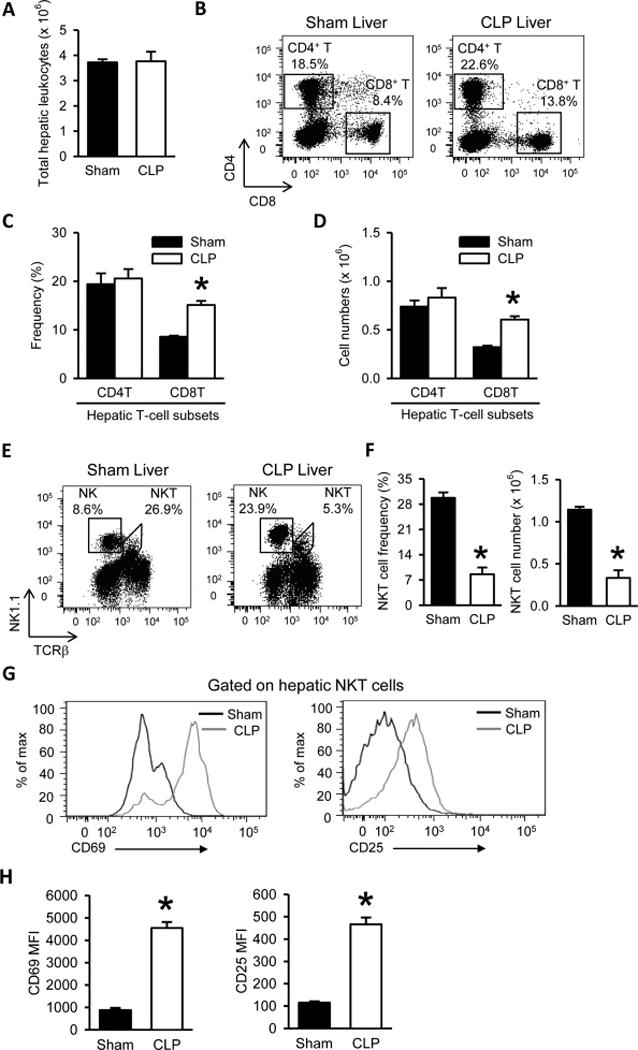
(A) Total numbers of leukocytes isolated from the livers harvested after 20 h from sham- or CLP-operated mice are shown. (B) Flow cytometric analysis of surface CD4 and CD8 expression on the gated live lymphocytes isolated from the sham and CLP livers. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of CD4 T and CD8 T cell subsets as indicated. Data are representative of three independent analyses with a total of four to five mice in each group. (C–D) The graphs show the percentage (C) and absolute cell numbers (D) of the indicated hepatic T-cell subsets. (E) Flow cytometric analysis of surface NK1.1 and TCRβ expression on the gated live lymphocytes isolated from the sham and CLP livers. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of NKT and natural killer (NK) cells. Data are representative of three independent analyses with a total of four to five mice in each group. (F) The graphs show the percentage and absolute cell numbers of hepatic NKT cells. (G) Representative histogram overlays show the flow cytometric analysis of surface CD69 and CD25 expression on the gated hepatic NKT cells from sham and CLP livers. Data are representative of three independent analyses with a total of four to five mice in each group. (H) The graphs show the mean fluorescence intensity (MFI) of CD69 and CD25 expression on gated hepatic NKT cells. Data expressed as mean ± SEM (n = 5 per group). *P < 0.05 versus sham for all graphs.
NKT cells in the liver were identified as leukocytes double-stained with NK1.1 and TCRβ surface markers (Fig. 3E). Both frequency and absolute cell number of hepatic NKT cells in the septic mice were significantly reduced by 71%, compared to sham (Fig. 3F). In contrast, the populations of the hepatic NKT cells stained with CD69 or CD25 in the septic mice were higher than that in the sham (Fig. 3G). Also the mean fluorescence intensities (MFI) of CD69 and CD25 expression per NKT cell in the septic mice were increased by 5.2- and 4.1-fold, respectively, compared to sham (Fig. 3H). Taken together, the data show that although the number of the hepatic NKT cells was reduced after sepsis, they became more activated.
3.4. CD4+ and CD8+ T cell frequencies decrease in the lungs after sepsis
Sepsis is known to cause acute lung injury (ALI) and lung dysfunction mediated by inflammatory immune cells, marked by neutrophil infiltration in majority [22,23]. We analyzed the total lung leukocyte count, which showed an increase in the septic mice, but was not significantly different, compared to sham (Fig. 4A). The frequencies of CD4+ and CD8+ T lymphocytes after sepsis were significantly reduced by 58% and 59% respectively (Fig. 4B and 4C). In accordance, there was a trend towards decrease in the absolute numbers of CD4+ and CD8+ T cells in the lungs of the septic mice, but it was not significantly different from sham (Fig. 4D).
Figure 4. No significant change but only a trend towards decrease in CD4+ and CD8+ T cell numbers in the lungs of septic mice.
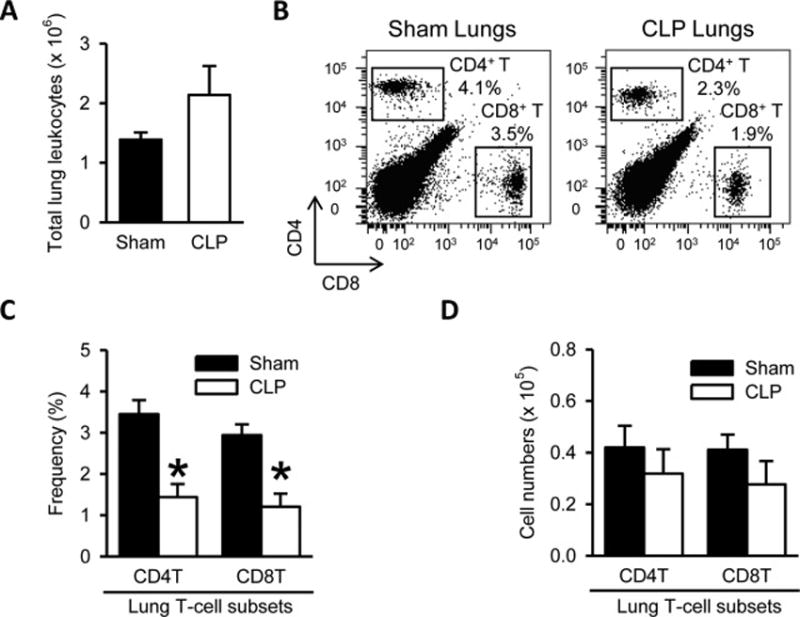
(A) Total numbers of leukocytes isolated from the lungs harvested after 20 h from sham- or CLP-operated mice are shown. (B) Flow cytometric analysis of surface CD4 and CD8 expression on the gated live lymphocytes isolated from the sham and CLP lungs. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of CD4+ T and CD8+ T cell subsets as indicated. Data are representative of three independent analyses with a total of four to five mice in each group. (C–D) The graphs show the percentage (C) and absolute cell numbers (D) of the indicated lung T-cell subsets.
3.5. CD4+ and CD8+ T cell depletion in the blood after sepsis
Next, we determined the impact of sepsis on circulating T-cell subsets in the blood from septic mice. Blood leukocytes per ml count showed a significant decrease by 62% in the septic mice, compared to sham (Fig. 5A). The frequencies of CD4+ and CD8+ T lymphocytes in the blood were significantly reduced by 46% and 27%, respectively, in comparison with sham (Fig. 5B and 5C). Combining the lymphocyte counts with the flow cytometric analysis indicated that the numbers of CD4+ and CD8+ T cells in per ml blood of the septic mice were decreased by 79% and 72%, respectively, compared to sham control (Fig. 5D).
Figure 5. CD4+ and CD8+ T cell depletion in the blood of septic mice.
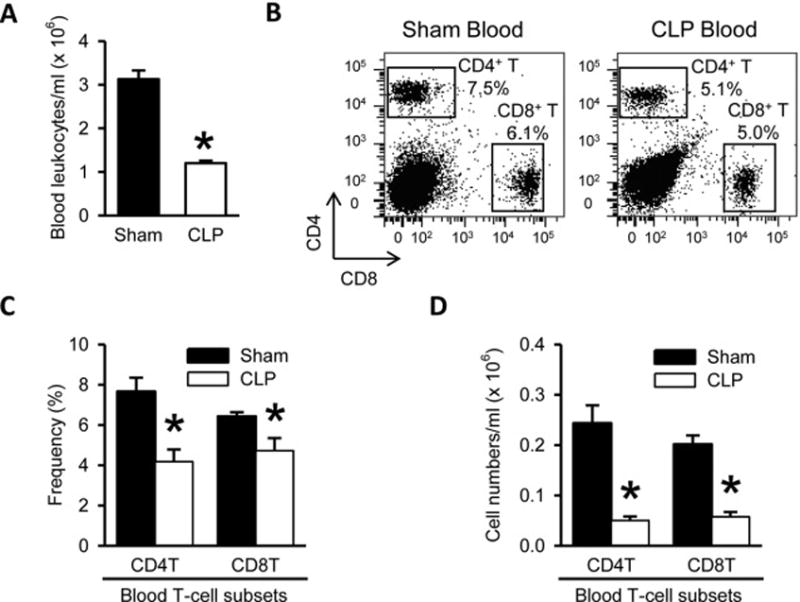
(A) Total numbers of leukocytes isolated from the blood harvested after 20 h from sham- or CLP-operated mice are shown. (B) Flow cytometric analysis of surface CD4 and CD8 expression on the gated live lymphocytes isolated from the sham and CLP blood. Numbers adjacent to the outlined areas in the representative dot plots show the percentage of CD4+ T and CD8+ T cell subsets as indicated. Data are representative of three independent analyses with a total of four to five mice in each group. (C–D) The graphs show the percentage (C) and absolute cell numbers (D) of the indicated blood T-cell subsets.
4. Discussion
Sepsis is a complex clinical condition defined by the dysregulation of the immune system in reaction to invading pathogens, which results in the systemic collateral injury [24]. T cells have been regarded as potent early mediators of the host response to sepsis [25,26]. They are known to play an important role in the development of a variety of adaptive and some innate immune functions [25]. In this study, by using a well-established mouse model of polymicrobial sepsis, we have studied the impact of sepsis on the profile of various T cell subsets, including NKT cells, in various organs at 20 h after CLP within one experimental set.
We first observed a dramatic decrease of T lymphocytes in the thymus after sepsis. The thymic cellularity was reduced after CLP-induced sepsis with a DP subset-specific loss of T cells in thymus. Since DP cells constitute the majority of total thymocytes in mice, a loss of DP cells would be responsible for the reduced cellularity. In our 20 h sepsis model, except for DP, other thymic subsets were not affected, most likely because DP cells are most susceptible to apoptosis [27]. Sepsis is known to cause acute thymic atrophy in the rodents which is associated with thymocyte apoptosis and characterized by a reduction in thymus size and weight caused by acute loss of immature DP cortical thymocytes [28–31]. Since mature CD4+ and CD8+ SP subsets arise from immature DP cells, decreased DP numbers at 20 h post-CLP would eventually result in reduced numbers of these mature subsets, as observed by Unsinger et al. [32]. Thus, our data, showing a dramatic and acute DP-subset specific loss of T cells in the thymus after sepsis, is in accordance with other studies. Such thymic atrophy would result in the reduced output of naïve T cells to the periphery and contribute to the development of a less-diverse, oligoclonal peripheral T cell repertoire and constricted host immunity.
Next, we show a significant reduction in the cellularity of spleens in the septic mice. This also reflected in a significant loss of CD4+ and CD8+ T cell numbers in the spleen 20 h after CLP-induced sepsis. We also noticed that in the spleen CD4+ T cells were affected more by sepsis compared to CD8+ T cells (Fig. 2D). The apoptotic loss of CD4+ and CD8+ T cells in the spleen after CLP has been reported previously [29,32–34], with maximum depletion observed by day 5 which is followed by near-normal recovery by 21 days post-CLP [32]. In contrast to splenic CD8+ T cells, we show a significant increase in the frequency and absolute numbers of the hepatic CD8+ T cells in the septic livers compared to sham livers. Similar to our findings, an earlier study reported a marked increase in hepatic CD8+ T cells after CLP resulting in liver-specific FasL mediated inflammation and hepatocyte apoptosis [35].
On the other hand, our analysis of lung lymphocytes showed that the CD4+ and CD8+ T lymphocyte frequencies were significantly decreased. However, the CD4+ and CD8+ T cell numbers in the septic lungs showed a decreasing trend but no significant change from sham lungs. We believe that this decrease in frequencies of CD4+ and CD8+ T lymphocytes was not due to loss of these lymphocytes, but was a result of the increased granulocyte/neutrophil infiltration in the lungs after sepsis (data not shown). Thus our data suggests that in the lungs T-cells are not the major immune cells affected by sepsis. Next, we determined the frequencies and absolute numbers of circulating CD4+ and CD8+ T lymphocytes in the blood of septic mice. Both frequencies and absolute numbers of CD4+ and CD8+ T lymphocytes were severely reduced in the circulating blood after sepsis. A similar decrease in circulating lymphocytes in sepsis patients has been reported previously [34].
In this study, hepatic NK cells were also increased in the livers along with hepatic CD8+ T cells after sepsis. We have also recently shown a similar combined increase in the hepatic CD8+ T and NK cells after CLP-induced sepsis specifically in the liver [36]. Furthermore, Sherwood et al. have shown that combined deficiency of CD8+ cells and NK cells is beneficial for survival in CLP-induced sepsis [37,38]. In agreement with the previous studies, these data collectively show that polymicrobial sepsis results in a combined increase in both hepatic CD8+ T cells and hepatic NK cells, suggesting a sepsis-induced influx of these T cell subsets to the liver.
NKT cells are a major T cell populations in the liver and are potent producers of IFN-γ and other pro-inflammatory mediators, which may contribute to the hyper-inflammatory response during sepsis [39]. So we analyzed hepatic NKT cells in the septic mice and found that their frequency and absolute cell numbers were dramatically decreased. However, the few NKT cells that were left in the septic livers were highly activated. A marked decline in NKT cell frequency in liver associated with their increased activation has also been previously reported after CLP-induced sepsis [40]. Also, NKT cell activation has been shown to have detrimental effects in polymicrobial sepsis [39–41]. Interestingly, unlike liver we did not find any change in NKT cells after CLP in any other organ studied (data not shown). These results suggest that CLP-induced decrease in NKT cells which are highly activated is liver-specific.
As CD4+ T-cell subsets including naïve, memory and Tregs decide the degree of success of immune response to any future pathogenic challenge [42], we also examined the changes in the frequency and numbers of these splenic CD4+ T cell subsets after CLP-induced sepsis. We show a significant increase in the frequency of CD4+CD25+ splenic Tregs in our CLP-induced sepsis model. However, absolute cell numbers of Tregs were not changed while total CD4+ T cell numbers were significantly decreased, indicating that the increased Treg frequency was due to relative decrease in the other subsets of CD4+ T cell population. Our data is in agreement with previous reports of increased Treg frequency in the spleen of mice after CLP-induced sepsis [43,44]. In consistence, similar increase in Treg frequency has also been reported in the periphery of septic patients [45–47], which was shown to be a result of the decreased effector populations of CD4+ T cells and resistance of Tregs to sepsis-induced apoptosis [48]. However, the role of Tregs in sepsis is still argued as increased Tregs have been accounted for decreased survival [49] as well as improved recovery [50,51]. In this study we also demonstrate a considerable decrease in the absolute cell numbers of naïve and Tcm subsets of splenic CD4+ T cells 20 h after CLP, with Tcm cell numbers being highly reduced in particular. However, Tem CD4+ T cell numbers in septic spleen remained comparable to sham. Further studies are needed to determine the impact of rapid depletion of these conventional T cell subsets but not Tregs and Tem memory CD4+ T cells.
In this study, we focus on determining the phenotypic alterations of the T cell subsets in various organs after sepsis. It has also been indicated that sepsis induces anergic state in CD4+ T cells with defects in proliferation and dysregulated Th1 and Th2 cytokine production attributed to chromatin remodeling [52]. Therefore, a future study is granted to identify the change of cytokine production and cellular function of T cell subsets in various organs after sepsis. In addition, bone marrow can function as a secondary lymphoid organ as well as a site of preferential homing and persistence for memory T cells [53]. However, it is very difficult to quantify the change of total cell number counts in bone marrow after sepsis. Whether sepsis affects the distribution and trafficking of the specific T cell subtypes in bone marrow needs further investigation. γδ T cells are another subset that has been studied in inflammation, which are present as a minor population in the lymphoid organs, but are enriched in mucosal epithelia such as skin, intestine, lungs and uterus [54]. Although we haven’t examined in this study, it has been reported that γδ T cells are increased in small intestine and lungs after sepsis [55, 56, 57]. However, their role as pathogenic or protective in sepsis is still very controversial with regard to IL-17 production [57, 58, 59].
Most of the clinical trials based on combating the hyper-inflammatory response have failed repeatedly [4–6]. By preventing T cell loss and speeding up numerical as well as functional T-cell recovery, it may provide another strategy to decrease mortality rates associated with the susceptibility to secondary infection in sepsis patients [9]. As each of the T cell subsets are differentially affected in a tissue-specific manner, it is likely that targeting multiple organs individually will be needed to restore their responses back to normal states. We recognize that the sepsis-induced changes within the T cell compartment in various organs that we have reported in our study are just a few of the many other organs affected by sepsis.
5. Conclusions
In conclusion, our study highlights the fact that there are differences in the T-cell subset profiles in various tissues after sepsis. The change of the frequency and total numbers of analyzed T cell subsets in various organs of septic mice is summarized in Table 1. This information is crucial in considering a strategy for the advancement of potential therapies to restore the normal function of immune system in sepsis patients.
Table 1.
Summary of CLP-induced changes in frequency and total numbers of T-cell subsets in various tissues
| T cell subsets | Frequency | Cell number |
|---|---|---|
| Thymus | ||
| Total lymphocytes | 73% ↓ | |
| CD4−CD8− DN | 3.5-fold ↑ | – |
| CD4+CD8+ DP | 30% ↓ | 81% ↓ |
| CD4+ SP | 3.7-fold ↑ | – |
| CD8+ SP | 3.0-fold ↑ | 28% ↓ |
| Spleen | ||
| Total lymphocytes | 41% ↓ | |
| CD4+ T | – | 45% ↓ |
| CD8+ T | – | 36% ↓ |
| CD4+CD25+ (Treg) | 1.6-fold ↑ | – |
| CD4+ CD62L+ CD44− (naïve) | – | 49% ↓ |
| CD4+ CD62L+ CD44+ (Tem) | – | – |
| CD4+ CD62L− CD44+ (Tcm) | 44% ↓ | 70% ↓ |
| Liver | ||
| Total leukocytes | – | |
| CD4+ T | – | – |
| CD8+ T | 1.8-fold ↑ | 1.9-fold ↑ |
| NKT | 71% ↓ | 71% ↓ |
| Lungs | ||
| Total leukocytes | – | |
| CD4+ T | 58% ↓ | – |
| CD8+ T | 59% ↓ | – |
| Blood | ||
| Total leukocytes | 62% ↓ | |
| CD4+ T | 46% ↓ | 79% ↓ |
| CD8+ T | 27% ↓ | 71% ↓ |
↑: Increase; ↓: Decrease; −: No significant change
Acknowledgments
We thank Christopher Colon and Herb Borrero from the flow cytometry facility at the Feinstein Institute for Medical Research for technical assistance. This study was supported by the National Institutes of Health (NIH) grants GM053008 and GM057468 (PW).
List of abbreviations
- CLP
cecal ligation and puncture
- DN
double negative thymocytes
- DP
double positive thymocytes
- MFI
mean fluorescence intensity
- NK
natural killer cells
- NKT
natural killer T cells
- SP
single positive thymocytes
- Tcm
central memory CD4+ T cells
- Tem
effector memory CD4+ T cells
- Tregs
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
AS, WLY, conceived and designed the experiments. AS performed the experiments. SM did the animal work. AS analyzed the data and drafted the manuscript. WLY critically revised the manuscript. PW reviewed the manuscript and supervised the whole project. All authors read and approved the final manuscript.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Contributor Information
Archna Sharma, Email: asharma11@nshs.edu.
Weng-Lang Yang, Email: wlyang@nshs.edu.
Shingo Matsuo, Email: Shingo.matsuo123@gmail.com.
Ping Wang, Email: pwang@nshs.edu.
References
- 1.Coopersmith CM, Wunsch H, Fink MP, Linde-Zwirble WT, Olsen KM, Sommers MS, et al. A comparison of critical care research funding and the financial burden of critical illness in the United States. Crit Care Med. 2012;40:1072–1079. doi: 10.1097/CCM.0b013e31823c8d03. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 4.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?*. Crit Care Med. 2014;42:1714–1721. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall JC. Sepsis: rethinking the approach to clinical research. J Leukoc Biol. 2008;83:471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- 7.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med. 2014;20:224–233. doi: 10.1016/j.molmed.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venet F, Foray AP, Villars-Mechin A, Malcus C, Poitevin-Later F, Lepape A, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–5081. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- 12.Shah DK, Zuniga-Pflucker JC. An overview of the intrathymic intricacies of T cell development. J Immunol. 2014;192:4017–4023. doi: 10.4049/jimmunol.1302259. [DOI] [PubMed] [Google Scholar]
- 13.Koch U, Radtke F. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol. 2011;27:539–562. doi: 10.1146/annurev-cellbio-092910-154008. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 15.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 17.Duwaerts CC, Gregory SH. Targeting the diverse immunological functions expressed by hepatic NKT cells. Expert Opin Ther Targets. 2011;15:973–988. doi: 10.1517/14728222.2011.584874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12:151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 19.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 21.Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected] J Immunol. 2009;183:5983–5990. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Taneja R, Razavi HM, Law C, Gillis C, Mehta S. Specific role of neutrophil inducible nitric oxide synthase in murine sepsis-induced lung injury in vivo. Shock. 2012;37:539–547. doi: 10.1097/SHK.0b013e31824dcb5a. [DOI] [PubMed] [Google Scholar]
- 23.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006;290:L51–58. doi: 10.1152/ajplung.00028.2005. [DOI] [PubMed] [Google Scholar]
- 24.Chen XH, Yin YJ, Zhang JX. Sepsis and immune response. World J Emerg Med. 2011;2:88–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Kasten KR, Tschop J, Adediran SG, Hildeman DA, Caldwell CC. T cells are potent early mediators of the host response to sepsis. Shock. 2010;34:327–336. doi: 10.1097/SHK.0b013e3181e14c2e. [DOI] [PubMed] [Google Scholar]
- 26.Kasten KR, Tschop J, Goetzman HS, England LG, Dattilo JR, Cave CM, et al. T-cell activation differentially mediates the host response to sepsis. Shock. 2010;34:377–383. doi: 10.1097/SHK.0b013e3181dc0845. [DOI] [PubMed] [Google Scholar]
- 27.Tadakuma T, Kizaki H, Odaka C, Kubota R, Ishimura Y, Yagita H, et al. CD4+CD8+ thymocytes are susceptible to DNA fragmentation induced by phorbol ester, calcium ionophore and anti-CD3 antibody. Eur J Immunol. 1990;20:779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- 28.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 29.Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 1996;87:4261–4275. [PubMed] [Google Scholar]
- 30.Barke RA, Roy S, Chapin RB, Charboneau R. The role of programmed cell death (apoptosis) in thymic involution following sepsis. Arch Surg. 1994;129:1256–1262. doi: 10.1001/archsurg.1994.01420360046005. [DOI] [PubMed] [Google Scholar]
- 31.Savino W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006;2:e62. doi: 10.1371/journal.ppat.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unsinger J, Kazama H, McDonough JS, Hotchkiss RS, Ferguson TA. Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J Leukoc Biol. 2009;85:382–390. doi: 10.1189/jlb.0808491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz M, Yang WL, Matsuo S, Sharma A, Zhou M, Wang P. Upregulation of GRAIL Is Associated with Impaired CD4 T Cell Proliferation in Sepsis. J Immunol. 2014;192:2305–2314. doi: 10.4049/jimmunol.1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 35.Wesche-Soldato DE, Chung CS, Gregory SH, Salazar-Mather TP, Ayala CA, Ayala A. CD8+ T cells promote inflammation and apoptosis in the liver after sepsis: role of Fas-FasL. Am J Pathol. 2007;171:87–96. doi: 10.2353/ajpath.2007.061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma A, Matsuo S, Yang WL, Wang Z, Wang P. Receptor-interacting protein kinase 3 deficiency inhibits immune cell infiltration and attenuates organ injury in sepsis. Crit Care. 2014;18:R142. doi: 10.1186/cc13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab Invest. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood ER, Lin CY, Tao W, Hartmann CA, Dujon JE, French AJ, et al. Beta 2 microglobulin knockout mice are resistant to lethal intraabdominal sepsis. Am J Respir Crit Care Med. 2003;167:1641–1649. doi: 10.1164/rccm.200208-950OC. [DOI] [PubMed] [Google Scholar]
- 39.Leung B, Harris HW. NKT cells: the culprits of sepsis? J Surg Res. 2011;167:87–95. doi: 10.1016/j.jss.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 40.Hu CK, Venet F, Heffernan DS, Wang YL, Horner B, Huang X, et al. The role of hepatic invariant NKT cells in systemic/local inflammation and mortality during polymicrobial septic shock. J Immunol. 2009;182:2467–2475. doi: 10.4049/jimmunol.0801463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee RJ, Carlton S, Lomas JL, Lane C, Brossay L, Cioffi WG, et al. Inhibition of CD1d activation suppresses septic mortality: a role for NK-T cells in septic immune dysfunction. J Surg Res. 2003;115:74–81. doi: 10.1016/s0022-4804(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 42.Cabrera-Perez J, Condotta SA, Badovinac VP, Griffith TS. Impact of sepsis on CD4 T cell immunity. J Leukoc Biol. 2014;96:767–777. doi: 10.1189/jlb.5MR0114-067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scumpia PO, Delano MJ, Kelly KM, O’Malley KA, Efron PA, McAuliffe PF, et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 45.Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, et al. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 46.Leng FY, Liu JL, Liu ZJ, Yin JY, Qu HP. Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells during early-stage sepsis in ICU patients. J Microbiol Immunol Infect. 2013;46:338–344. doi: 10.1016/j.jmii.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, et al. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 48.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, et al. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. Crit Care Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 49.Ono S, Kimura A, Hiraki S, Takahata R, Tsujimoto H, Kinoshita M, et al. Removal of increased circulating CD4+CD25+Foxp3+ regulatory T cells in patients with septic shock using hemoperfusion with polymyxin B-immobilized fibers. Surgery. 2013;153:262–271. doi: 10.1016/j.surg.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 50.Kuhlhorn F, Rath M, Schmoeckel K, Cziupka K, Nguyen HH, Hildebrandt P, et al. Foxp3+ regulatory T cells are required for recovery from severe sepsis. PLoS One. 2013;8:e65109. doi: 10.1371/journal.pone.0065109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, et al. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 52.Carson WF, 4th, Cavassani KA, Ito T, Schaller M, Ishii M, Dou Y, et al. Impaired CD4+ T-cell proliferation and effector function correlates with repressive histone methylation events in a mouse model of severe sepsis. Eur J Immunol. 2010;40:998–1010. doi: 10.1002/eji.200939739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Shibata K. Close link between development and function of gamma-delta T cells. Microbiol Immunol. 2012;56:217–227. doi: 10.1111/j.1348-0421.2012.00435.x. [DOI] [PubMed] [Google Scholar]
- 55.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of gammadelta T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338–343. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirsh M, Dyugovskaya L, Kaplan V, Krausz MM. Response of lung gammadelta T cells to experimental sepsis in mice. Immunology. 2004;112:153–160. doi: 10.1111/j.1365-2567.2004.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costa MF, de Negreiros CB, Bornstein VU, Valente RH, Mengel J, Henriques Md, et al. Murine IL-17+ Vγ4 T lymphocytes accumulate in the lungs and play a protective role during severe sepsis. BMC Immunol. 2015;16:36. doi: 10.1186/s12865-015-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 59.Xu R, Wang R, Han G, Wang J, Chen G, Wang L, et al. Complement C5a regulates IL-17 by affecting the crosstalk between DC and gammadelta T cells in CLP-induced sepsis. Eur J Immunol. 2010;40:1079–1088. doi: 10.1002/eji.200940015. [DOI] [PubMed] [Google Scholar]


