Abstract
Blood Brain Barrier (BBB) exposed to realistic concentrations (comparable to a chronic heavy smoker) of Cigarette Smoke Extract (CSE) triggers a strong endothelial inflammatory which can lead to the onset of neurological disorders. The involvement of Reactive Oxygen Species (ROS) in this inflammatory cascade is evident from the up-regulation of nuclear factor erythroid 2 related factor 2 (Nrf-2), a transcription factor involved in anti-oxidant response signaling in CSE exposed endothelial cells. We have shown that pre-treatment with α-tocopherol and/or ascorbic acid is highly protective for the BBB, thus suggesting that, prophylactic administration of antioxidants can reduce CSE and/or inflammatory-dependent BBB damage. We have assessed and ranked the protective effects of 5 popular OTC antioxidants (Coenzyme Q10, Melatonin, Glutathione, Lipoic acid and Resveratrol) against CSE-induced BBB endothelial damage using hCMEC/D3 cells. The analysis of pro-inflammatory cytokines release by ELISA revealed that, resveratrol, lipoic acid melatonin and Co-Q10 inhibited the BBB endothelial release of pro-inflammatory cytokines IL-6 & IL-8, reduced (not Co-Q10) CSE-induced up-regulation of Platelet Endothelial Cell Adhesion Molecule -1 (PECAM-1), Vascular Endothelial Cell Adhesion Molecule-1 (VCAM-1) & E-selectin and inhibited monocytes-endothelial cell adhesion. The anti-inflammatory effects correlated with the anti-oxidative protection endowed by these compounds as evidenced by upregulation of NADPH: Quinone Oxidoreductase 1 (NQO1) and reduced cellular oxidative stress. CSE-induced release of Vascular Endothelial Growth Factor (VEGF) was inhibited by all tested compounds although the effect was not strictly dose-dependent. Further in vivo studies are required to validate our results and expand our current study to include combinatorial treatments.
Keywords: Blood-Brain Barrier, Smoking, Oxidative stress, Inflammation, Countermeasure, Antioxidant, Alternative
2. Introduction
Smoking cigarettes is the principal but least understood risk factor for tilting the intrinsic dynamic balance between pro and anti-inflammatory mechanisms towards impaired vascular endothelial function (Adams, Jessup, and Celermajer, 1997; Chen, Chien, Chaung, Lii, and Wang, 2004; Davis, 1990; Hossain, Sathe, Fazio, Mazzone, Weksler, Janigro, Rapp, and Cucullo, 2009; Nagy, Demaster, Wittmann, Shultz, and Raij, 1997; Naik, Fofaria, Prasad, Sajja, Weksler, Couraud, Romero, and Cucullo, 2014; Noronha-Dutra, Epperlein, and Woolf, 1993; Raij, Demaster, and Jaimes, 2001a). The extent to which smoking can trigger vascular endothelial dysfunction is proportional to the frequency and number of cigarettes smoked (Gill, Shipley, Tsementzis, Hornby, Gill, Hitchcock, and Beevers, 1989). Among the more than 4000 chemicals present in cigarette smoke (CS), reactive oxygen species (ROS) (Hanna, 2006; Panda, Chattopadhyay, Ghosh, Chattopadhyay, and Chatterjee, 1999), nicotine (Arnson, Shoenfeld, and Amital, 2010; Batkin and Rayner, 1976; Catanzaro, Zhou, Chen, Yu, Catanzaro, De Lorenzo, Subbaramaiah, Zhou, Pratico, Dannenberg, and Weksler, 2007; Das, Gautam, Dey, Maiti, and Roy, 2009; Paulson, Yang, Selvaraj, Mdzinarishvili, Van der Schyf, Klein, Bickel, and Abbruscato, 2010; Wang, Kittaka, Sun, Schreiber, and Zlokovic, 1997), and associated pro-inflammatory activity (Howard, Wagenknecht, Cai, Cooper, Kraut, and Toole, 1998) are linked to this injurious effect. Smoking increases the risk of silent cerebral infarction (SCI) (Shinton and Beevers, 1989) and stroke by approximately 50% (Mannami, Iso, Baba, Sasaki, Okada, Konishi, and Tsugane, 2004; Miller, Bauer, Cooper, and Rosenberg, 1998) due to its pro-coagulant and atherogenic effects (Mast, Thompson, Lin, Hofmeister, Hartmann, Marx, Mohr, and Sacco, 1998) and is currently considered a major public health concern, accounting for ≥ 400,000 deaths/year in US. Recently, our group has reported that Blood Brain Barrier (BBB) exposure to Cigarette Smoke Extract (CSE) mimicking physiological concentrations of a chronic heavy smoker initiates a strong endothelial inflammatory response. We have also shown that loss of BBB function and integrity following an ischemic/reperfusion injury is significantly worsened by CSE and, under the same experimental conditions, and pre-treatment with α-tocopherol and/or ascorbic acid proved highly protective for the BBB (Hossain, Mazzone, Tierney, and Cucullo, 2011a; Willcox, Ash, and Catignani, 2004a). This strongly suggests that prophylactic administration of antioxidants can reduce CSE and/or inflammatory-dependent BBB damage. The Food and Nutrition Board of the National Academy of Sciences has established a higher recommended dietary allowance (RDA) of vitamin C for smokers (over 200 mg/day versus the recommended 90 mg/day for non-smokers) based on the fact that chronic smokers suffer from antioxidants shortages caused by increased anti-oxidative mobilization evoked by CS (Dietrich, Block, Norkus, Hudes, Traber, Cross, and Packer, 2003; Sobczak, Golka, and Szoltysek-Boldys, 2004; Tsuchiya, Asada, Kasahara, Sato, Shindo, and Inoue, 2002). We have evaluated and ranked the protective effects of 5 popular, highly commercialized and readily available over the counter (OTC) antioxidants (Coenzyme Q10, Melatonin, Glutathione, Lipoic acid and Resveratrol) to prevent BBB endothelial damage caused by exposure to soluble CSE. Although protective effects of these antioxidants against CS induced injury in different medical conditions have been reported in a few occasions, melatonin, for example has been proven to be a useful against CS induced kidney damage (Ozan, Sonmez, Ozan, Colakoglu, Yilmaz, and Kuloglu, 2007), vasculopathy (Rodella, Filippini, Bonomini, Bresciani, Reiter, and Rezzani, 2010), inflammation of the larynx (Donmez, Yigit, Bilici, Dursun, Gul, Dastan, and Uzun, 2015) and arterial occlusion (Yang, Li, Wang, Liu, Ye, Ni, Zeng, Miao, Wang, and Liu, 2014) while resveratrol and lipoic acid have been shown to have beneficial roles against CS induced pulmonary inflammation (Liu, Ren, Chen, Huang, Li, Zhang, and Wang, 2014) and oxidative damage respectively (Jia, Liu, Sun, Miller, Ames, Cotman, and Liu, 2007). For the first time, our lab is reporting the protective effects of all five of these popular antioxidants against CS induced damage to BBB.
3. Results
3.1 Effect of antioxidants and CSE exposure on hCMEC/D3 cell viability
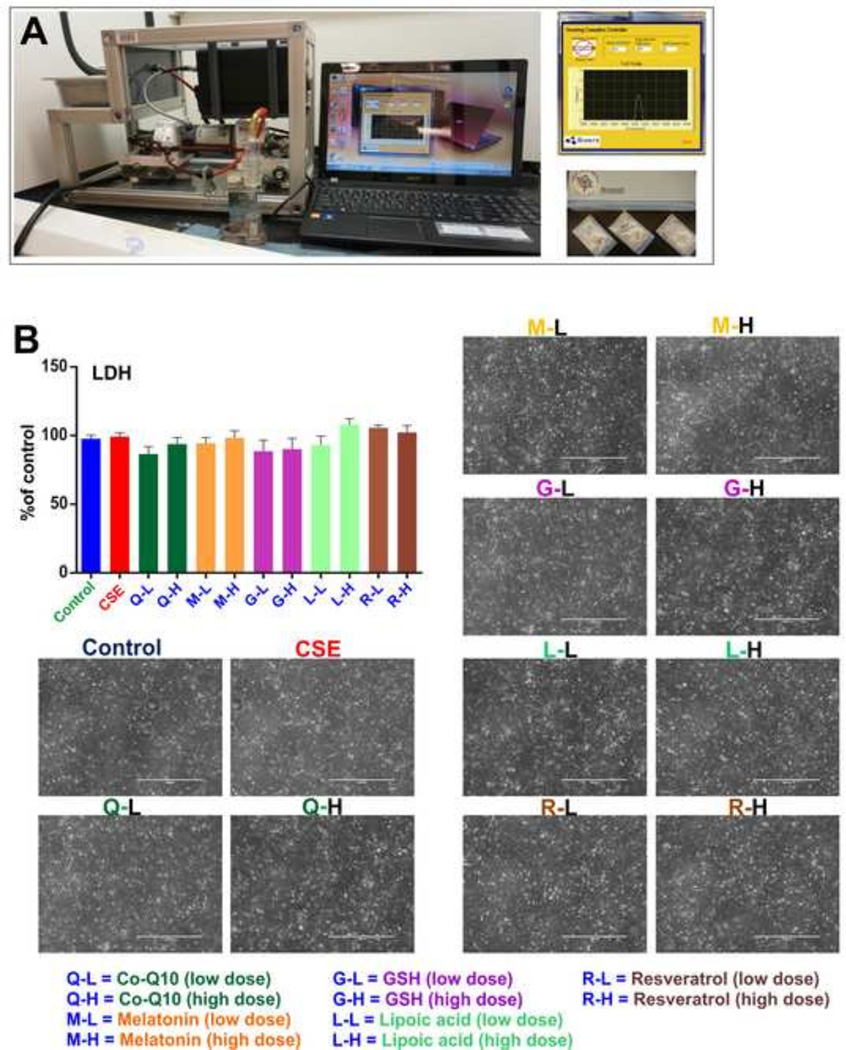
Viability and integrity of the cell monolayers were confirmed by Lactate Dehydrogenase (LDH) release measurements versus control conditions. No significant alteration of LDH release (Fig-1B) was observed to indicate possible cellular damage. Results were further confirmed using a Transmitted Light microscope (Evos FL) at 10× resolution which clearly demonstrated the presence of intact cell monolayers in all the experimental conditions.
Figure 1. CSE preparation and endothelial viability assessment following antioxidant treatments.
Panel A) Concentrated Smoke Extract (CSE) preparation by a Single Cigarette Smoking Machine according to ISO/FTC protocol. (Puff volume- 35ml, duration-2s, interval-58s; 8 puffs/cigarette); Smoking protocol is computer controlled. Panel B) results from the lactate dehydrogenase (LDH) assay using the Pierce™ LDH cytotoxicity kit. No significant change in LDH release was observed in all exposure conditions in comparison to the control. Microscopic images of intact monolayers of hCMEC/D3 cells after treatments. n = 5 biological replicates.
3.2 Antioxidant treatments counteract the pro-inflammatory effects of CSE exposure on BBB endothelial cells
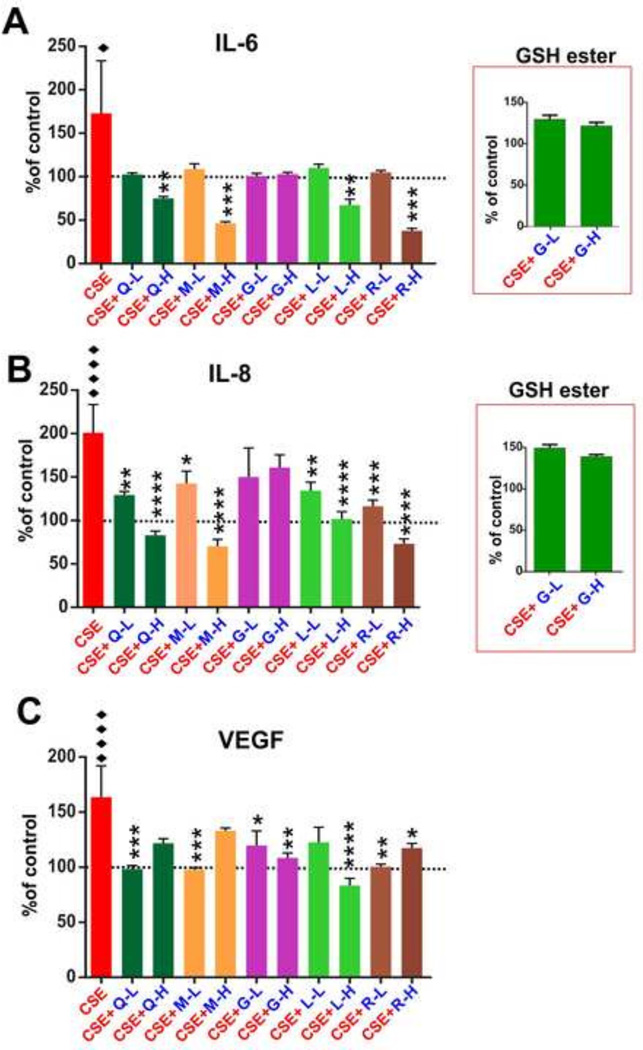
The analysis of pro-inflammatory cytokines by ELISA revealed that, resveratrol, melatonin, lipoic acid and Co-Q10 decreased IL-6 (Fig-2A) release by the endothelial monolayer in response to CSE exposure (“♦” p<0.05). The effect was clearly dose-dependent demonstrating a statistical significance (“**”p < 0.01; “***” p<0.001) only at the highest dose tested (10 µg/mL). Glutathione, both in free (reduced) or ester (more lipophilic) forms were ineffective. Resveratrol and melatonin seemed to be the most effective although no statistically significant differences were observed compared to lipoic acid and Co-Q10. A similar anti-inflammatory trend was observed for IL-8 (see Fig. 2B). Statistically significant anti-inflammatory activity of all antioxidants were observed for both low and high doses tested with the exception of glutathione (both reduced free and ester forms) which did not elicit any significant effect even at a high dose. However, low doses of lipoic acid was ineffective in repressing CSE-induced VEGF release (see Fig. 2C) while other compounds (such as Co-Q10, Melatonin and Resveratrol) were very effective at low dose and equally effective or ineffective (Co-Q10 and Melatonin) at high dose. A similar statistically significant effect was observed in hCMEC/D3 cultures treated with Co-Q10, melatonin and resveratrol. However, the anti-angiogenic effect was already significant at a low dose.
Figure 2. Effect of antioxidant treatments on release of pro-inflammatory cytokines and VEGF.
Panel A) ELISA of IL-6. Resveratrol, lipoic acid, melatonin and Co-Q10 reduced IL-6 release at a higher dose after CSE exposure. Glutathione was inactive in both doses. The antioxidative efficacy of glutathione ester was similar to that of its free form in both doses.. Panel B) ELISA of IL-8. Resveratrol, melatonin, lipoic acid and Co-Q10 decreased CSE induced IL-8 release. Glutathione in both forms and doses was ineffective. Panel C) Inhibition of CSE-induced VEGF release by antioxidants. n = 3 biological replicates; “♦” = p<0.05, “♦♦♦♦” = p<0.0001 against control; “*” = p < 0.05, “**” = p < 0.01, “***” = p<0.001, “****” = p<0.0001 against CSE.
3.3 Antioxidants counteract CSE-promoted oxidative stress in HCMEC/D3 cells
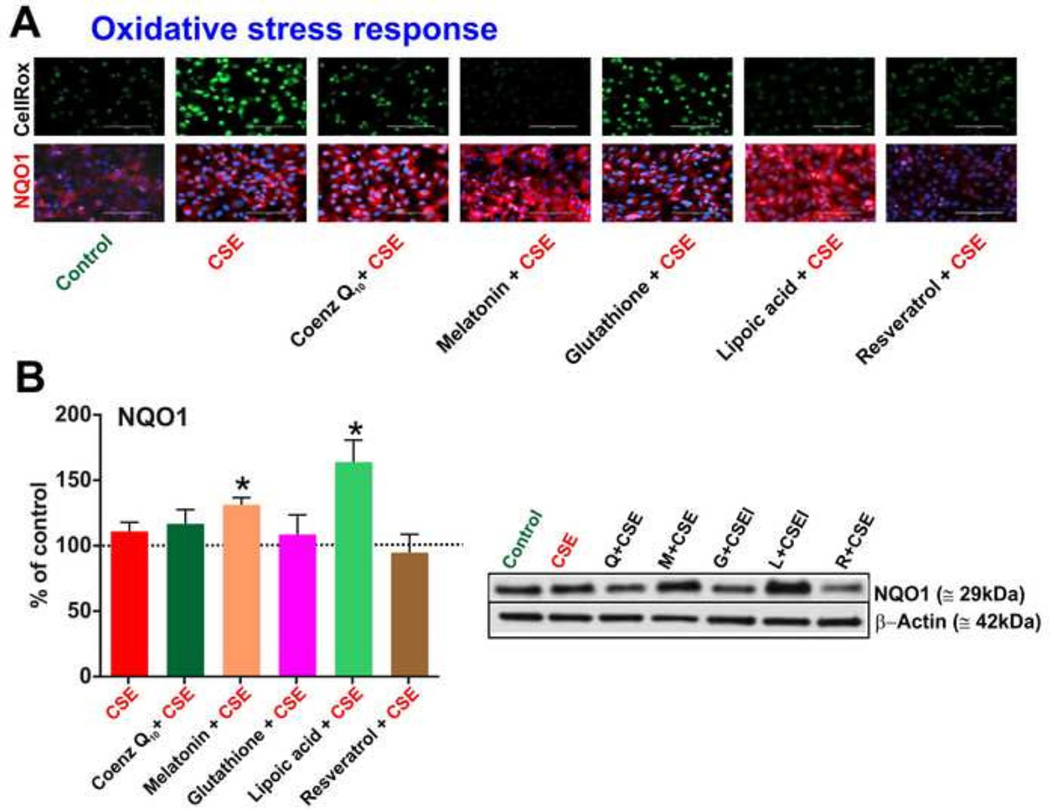
Measurements by CellROX fluorogenic probes (see Fig-3A) clearly showed the ability of melatonin, resveratrol and lipoic acid to effectively counteract the CSE-induced oxidative stress responses in hCMEC/D3 cells. By contrast, the effect of Co-Q10 was marginal whereas no measurable protective effect was noted in response to glutathione. NADPH:Quinone Oxidoreductase-1 (NQO1) gets up-regulated when cells are under oxidative stress to detoxify ROS such as those contained in CSE (Naik, Sajja, Prasad, and Cucullo, 2015). An increase in the expression of NQO1 was observed in hCMEC/D3 cultures that received either lipoic acid or melatonin when compared to cultures exposed to CSE but without antioxidant treatments. Results were confirmed by corresponding western blot analyses of the total protein content revealing statistically significant differences (p < 0.05) (see Fig 3B) between CSE cultures and both CSE + lipoic acid and CSE + melatonin. NQO1 expression did not change in response to Co-Q10, glutathione and resveratrol.
Figure 3. Oxidative stress cellular responses to antioxidant treatments.
Panel A) Nuclear oxidative stress was measured in hCMEC/D3 by CellROX fluorogenic probes (Life Technologies) following 3 hours of CSE exposure w/wo antioxidants. Note melatonin, lipoic acid and resveratrol reduce CSE induced oxidative stress in D3 cells whereas glutathione and Co-Q10 failed to elicit a significant protective effect. (n=3 biological replicates). Panels A&B) Immunofluorescence images & western blots of downstream detoxifying antioxidative enzyme NQO1 induced by CSE. Lipoic acid significantly up-regulated NQO1 expression. Although the effect of melatonin was significant but to a lesser extent compared to lipoic acid. No effects were noted in response to Co-Q10, glutathione and resveratrol. n= 3 biological replicates; “*” = p < 0.05 against CSE..
3.4 Antioxidants counteract CSE-promoted inflammatory responses in hCMEC/D3 cells
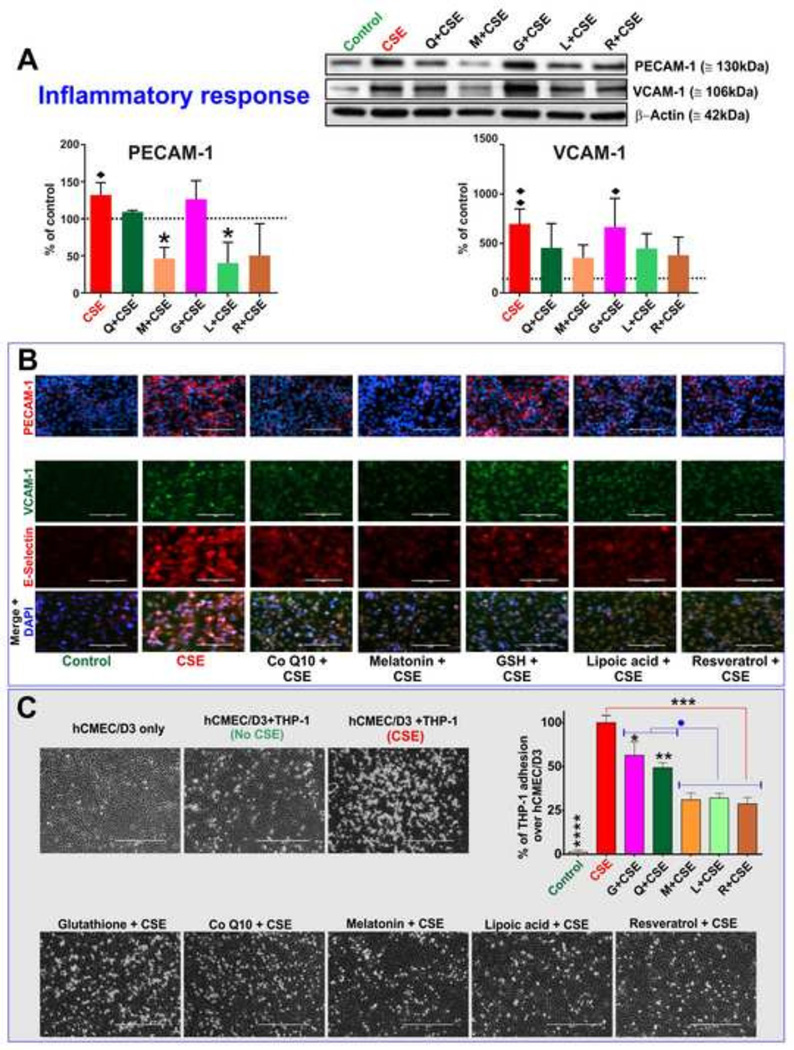
Pretreatment with melatonin and lipoic acid significantly (p<0.05) reduced CSE-induced up-regulation of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) as shown by the immunofluorescent analysis in Fig. 4A and confirmed by corresponding western blots. A similar, yet marginal, effect was also observed in resveratrol-treated CSE cultures while both glutathione and Co-Q10 were ineffective. Parallel to PECAM-1, expression levels of Vascular Endothelial Cell Adhesion Molecule -1 (VCAM-1; Fig. 4A) & E-selectin (see Fig. 4B) in CSE-exposed endothelial cells were marginally decreased by all tested antioxidants when compared to CSE with the exception of glutathione which was completely ineffective. In agreement with these results, adhesion of monocytes (THP-I cells) on the endothelial monolayer (Hossain, Mazzone, Tierney, and Cucullo, 2011a) upon CSE exposure was significantly inhibited by resveratrol, lipoic acid, melatonin and, to a lower extent, by Co-Q10 (Fig. 4C) when compared to CSE cultures. The effect of glutathione on THP-1 adhesion although statistically significant, was the most modest of the antioxidants tested herein.
Figure 4. Cellular inflammatory response to CSE exposure and counteractive effects of antioxidant treatments.
Immunofluorescence images (Panel A) & western blot (Panel B) of cell adhesion molecules, PECAM-1, VCAM-1 and E-selectin induced by CSE exposure. Pretreatment with lipoic acid, resveratrol and melatonin significantly reduced the endothelial inflammatory response to CSE. The effect of Co-Q10 was moderate at best whereas no effects were observed in response to GSH administration. (n=3 biological replicates). Panel C) Adhesion of THP-1 on endothelial monolayer upon CSE exposure was differentially inhibited by antioxidant treatments with the most noticeable effects observed in response to resveratrol, lipoic acid & melatonin administration. n=3 biological replicates; “♦” = p<0.05, “♦♦” = p<0.01 against control; “*” = p < 0.05, “**” = p < 0.01, “***” = p<0.001, “****” = p<0.0001 against CSE; “●” = p<0.05 against GSH and Co-Q10.
4. Discussion
Although there is still no unequivocal evidence that an increased intake of antioxidant nutrients can fully counteract CS toxicity, there are many supporting data suggesting that antioxidants may prove to be effective scavengers of exogenous-derived ROS (Traber, van, V, Reznick, and Cross, 2000). For example, vitamin C prevents histamine release and increases the detoxification of histamine (Johnston, Martin, and Cai, 1992), thus acting as an anti-inflammatory agent as well as a potent antioxidant. Vitamin E on the other hand has been shown to be cardioprotective against tobacco smoke-induced peroxidative damage (Al-Malki and Moselhy, 2013; Gumustekin, Taysi, Alp, Aktas, Oztasan, Akcay, Suleyman, Akar, Dane, and Gul, 2010; Koul, Singh, and Sandhir, 2003) and can be a beneficial adjuvant in the treatment of seizures, diabetes and in the reduction of post-ischemic damages (Arato, Kurthy, Sinay, Kasza, Menyhei, Hardi, Masoud, Ripp, Szilagyi, Takacs, Miklos, Bator, Lantos, Kollar, Roth, and Jancso, 2010; dos Santos, Costa, Tome, Saldanha, de Souza, Feng, and de Freitas, 2011; Gupta, Sharma, Kaushik, and Shekhawat, 2011; Venditti, Napolitano, Di, Agnisola, and Di, 2011). Recently published in vitro studies by our group have clearly shown that both vitamin C and E can effectively protect the BBB against CSE-generated oxidative damage (Hossain, Mazzone, Tierney, and Cucullo, 2011b). In this current study, we assessed in vitro the protective effects against CSE toxicity of a host of OTC (Coenzyme Q10, Melatonin, Glutathione, Lipoic acid and Resveratrol) which have been marketed as effective antioxidant remedies. Coenzyme Q10; in contrast to other antioxidants Co-Q10 inhibits both the initiation and the propagation of lipid and protein oxidation and regenerates vitamin E, thereby further inhibiting the propagation step of oxidative damage to cellular components. In addition, Co-Q10 is thought to prevent the oxidation of mitochondrial DNA and LDL. Further, Co-Q10 has been shown to possesses some anti-angiogenic, anti-inflammatory and anti-nociceptive activity and from a clinical stand point, a number of pathological disorders such as Parkinson's and Huntington's diseases seem to be responsive to Co-Q10 treatment (Jung, Park, and Lim, 2009; Quinzii, Lopez, Gilkerson, Dorado, Coku, Naini, Lagier-Tourenne, Schuelke, Salviati, Carrozzo, Santorelli, Rahman, Tazir, Koenig, Dimauro, and Hirano, 2010); 2) Melatonin (N-acetyl-5-methoxytryptamine) is an endogenous neurohormone derived from tryptophan. Melatonin is a potent antioxidant and an effective scavenger of ROS such as hydroxyl and peroxyl radicals. Melatonin crosses all morphophysiological barriers (being distributed throughout all cells thereby reducing oxidative damage in both the lipid and aqueous environments) and also has a powerful capacity to scavenge free radicals thus preventing tissue damage such as in ischemic-reperfusion injuries (Bonnefont-Rousselot and Collin, 2010; Gurpinar, Ekerbicer, Uysal, Barut, Tarakci, and Tuglu, 2012; Pei, Pang, and Cheung, 2003); 3) Glutathione; a major endogenous antioxidant produced by all living cells, participating directly in the neutralization of free radicals and reactive oxygen compounds, as well as maintaining exogenous antioxidants such as vitamin C and E in their reduced (active) forms. Glutathione also plays a central role in the regulation of the nitric oxide cycle as well as DNA synthesis and repair, protein synthesis, and enzyme activation (Aquilano, Baldelli, and Ciriolo, 2011; Mytilineou, Kramer, and Yabut, 2002; Schulz, Lindenau, Seyfried, and Dichgans, 2000). A recent study has shown that GSH counteracts nicotine-induced oxidative stress (Dey and Roy, 2010); 4) Lipoic acid: Current studies support its use in the adjuvant treatment of a variety of pathologies including cancer, diabetes, cardiovascular, neurodegenerative, and autoimmune diseases. Furthermore, lipoic acid plays a major role in boosting the antioxidant defense network by acting as regenerative substrates for other antioxidant vitamins (e.g., vitamin C and E) and has been shown to preserve BBB integrity (Ersahin, Toklu, Cetinel, Yuksel, Erzik, Berkman, Yegen, and Sener, 2010; Goraca, Huk-Kolega, Piechota, Kleniewska, Ciejka, and Skibska, 2011; Wang, Yu, Ji, Liang, Zhang, and Hai, 2011); 5) Resveratrol is a plant-derived polyphenol that exerts diverse effects currently considered a hot topic in numerous animal and human studies. Anticancer, anti-inflammatory, anti-aging, anti-diabetic and beneficial cardiovascular effects of resveratrol have been previously reported (Brasnyo, Molnar, Mohas, Marko, Laczy, Cseh, Mikolas, Szijarto, Merei, Halmai, Meszaros, Sumegi, and Wittmann, 2011; Li, Xia, and Forstermann, 2012; Tang, Xu, Qu, Peng, Xin, Yang, Ying, Sun, and Hao, 2012). Furthermore, resveratrol has been shown to have a protective effect on the BBB during ischemia (Ren, Fan, Chen, Huang, and Yang, 2011; Simao, Matte, Matte, Soares, Wyse, Netto, and Salbego, 2011). Given these premises we tested the antioxidant protective effect of these compounds against CSE exposure on BBB endothelial cells. For this purpose we used hCMEC/D3 cells, a well-established BBB endothelial cell line previously used by our lab as well as many other investigators (Cucullo, Couraud, Weksler, Romero, Hossain, Rapp, and Janigro, 2008; Daniels, Cruz-Orengo, Pasieka, Couraud, Romero, Weksler, Cooper, Doering, and Klein, 2013; Lopez-Ramirez, Male, Wang, Sharrack, Wu, and Romero, 2013; Weksler, Romero, and Couraud, 2013). After establishing that these antioxidants do not impair the viability of our endothelial monolayers as clearly shown in Fig. 1B, we proceeded testing/comparing their ability to counteract CSE-induced oxidative stress and inflammatory responses at the BBB endothelium which, as previously shown, can lead to loss of BBB integrity and function.(Hossain, Mazzone, Tierney, and Cucullo, 2011a; Hossain, Sathe, Fazio, Mazzone, Weksler, Janigro, Rapp, and Cucullo, 2009; Naik, Fofaria, Prasad, Sajja, Weksler, Couraud, Romero, and Cucullo, 2014; Naik, Sajja, Prasad, and Cucullo, 2015; Prasad, Sajja, Park, Naik, Kaisar, and Cucullo, 2015). Our results clearly outlined a differential protective efficacy between these substances which could help evaluate their therapeutic feasibility in preventing (or at least reduce) tobacco smoke-induced vascular/tissue damage. All tested compounds reduced cellular oxidative stress induced by CSE exposure to some degree however, quite surprisingly, both the reduced-free and the lipid-soluble ester forms of glutathione (more permeable across cell membrane) largely failed to elicit any protective effect against CSE-induced oxidative stress and consequent cellular inflammatory responses. Although studying the underlying mechanisms of antioxidant action is beyond the scope of this study, the rationale behind testing glutathione mono ethyl ester in addition to the reduced free form was that, due to the hydrophilic nature, this form might not cross the cell membrane and exerts its cytosolic anti-oxidative detoxification. However, the lipophilic ester form also failed to elicit a measurable protective effect against CSE-induced oxidative damage to the BBB endothelial cells. Although additional studies are required to assess whether administration of glutathione mono ethyl ester can affects the level of GSH cellular storage of the BBB endothelium. At this stage, a possible explanation of our results is that the extracellular GSH may undergo catabolic degradation by gamma glutamyltransferase (GGT) enzyme as demonstrated by previous reports (D'Ambrosio, Gargiulo, Della-Morte, Gallucci, Uomo, Rundek, and Abete, 2013; Philbert, Beiswanger, Manson, Green, Novak, Primiano, Reuhl, and Lowndes, 1995). This result suggests that external administration of glutathione may not translate into an increased/readily available storage of its intracellularly produced form.
CS has been reported (Hossain, Sathe, Fazio, Mazzone, Weksler, Janigro, Rapp, and Cucullo, 2009) to promote the initiation of pro-inflammatory cascade in BBB endothelium by the recruiting leukocytes. This process is mediated by cytokine release followed by adherence of leukocytes on the endothelial wall and then infiltration across the BBB. One well accepted but poorly understood mechanism by which smoking can directly disrupt BBB integrity and function is oxidative stress caused by the many highly reactive oxygen species and free radicals of which cigarette smoke is highly enriched (Raij, Demaster, and Jaimes, 2001b; Tsuchiya, Asada, Kasahara, Sato, Shindo, and Inoue, 2002). The results presented herein suggest that resveratrol, lipoic acid and melatonin provide a protective shielding against CSE at the BBB endothelium by neutralizing ROS. The Nrf2 (nuclear factor erythroid 2 related factor 2)–Keap1 (Kelch-like erythroid cell-derived protein 1) signaling pathway is one of the most prominent cell defenses against oxidative stress. Following oxidative insult, Nrf2 dislodged from Keap-1 binding domain and translocates from the cytoplasm into the nucleus binding to the corresponding antioxidant response element (ARE). This ultimately leads to overexpression of detoxifying agents like NQO1. An elevated level of NQO1 is an indication of the activation of a cellular defense mechanism in response to oxidative stimuli such as CSE exposure. Antioxidants capable of acting intracellularly by stimulating ARE pathways assume to augment this antioxidative response, which was most significant in the case of lipoic acid (and to a lesser degree melatonin) as shown in Fig. 3. Existing evidence also suggests that CSE exposure induces up-regulation and release of pro-inflammatory cytokines (IL-6, IL-8) and expression of cell adhesion molecules (PECAM-1, VECAM-1). Inhibition of this pro-inflammatory response has been previously correlated with a reduction of oxidative stress induced by CSE (Hossain, Mazzone, Tierney, and Cucullo, 2011a). Thus, decreased release of cytokines and corresponding suppression of chemotactic recruitment of leukocytes and adherence to the endothelium are indicative of reduced oxidative stress and inflammatory activity elicited by the tested compounds. In these respects, our findings shown in Fig. 4 are in general accordance with the previously published reports highlighting the anti-inflammatory effects of melatonin (Choi, Jin, Lee, Choi, Choi, and Kim, 2011; Clapp-Lilly, Smith, Perry, and Duffy, 2001), resveratrol (Zhong, Cheng, Wang, Guo, Zhu, and Zhang, 1999), lipoic acid (Maczurek, Hager, Kenklies, Sharman, Martins, Engel, Carlson, and Munch, 2008) and Co-Q10 (Lee, Huang, Chen, and Lin, 2012). Activation and adherence of monocytes to the endothelium is a crucial pro-inflammatory event in CS induced vascular damage. Reduced monocyte-endothelial bonding in lipoic acid, resveratrol and melatonin pretreated cultures substantiates their potential anti-inflammatory effect at the BBB against CSE and reflects their antioxidative capability. The relative lack of effectiveness of glutathione to reduce activation of monocytes (cell adhesion) supports the results found in our previous experiments which outline a lack of antioxidative effect under the tested conditions and suggest a low feasibility option as a possible therapeutic approach to ease tobacco smoke oxidative toxicity.
Although antioxidative efficacies of lipoic acid, resveratrol and melatonin are quite comparable to one another, there are other factors that can argue for or against their prophylactic and/or therapeutic use in smokers. VEGF is an angiogenic signal protein which promotes BBB dysregulation and loss of barrier integrity in favor of formation/sprouting of new blood vessels/capillaries promoting oxygen and nutrient distribution in the affected tissue. VEGF expression has been previously shown to be up-regulated by CSE exposure. All the antioxidant compounds tested have been previously reported to have an anti-angiogenic effect by inhibiting VEGF release. Here the observed anti-angiogenic effect could be attributed to both a direct activity on VEGF expression and/or neutralization of CSE pro-oxidative action which per se promotes VEGF release (Schafer, Cramer, Suske, Kemmner, Wiedenmann, and Hocker, 2003). Interestingly, the anti-angiogenic effect of Co-Q10 and Melatonin did not follow a dose dependent pattern. In fact inhibition of VEGF upregulation by CSE vanished when high dose of Co-Q10 and Melatonin were used. Whether this is a peculiar response of the BBB endothelial cell line used or a combinatorial effect between these specific antioxidants and other CSE components outside ROS is presently unknown. Given the intrinsic limitations in any in vitro study, additional in vivo experiments will be necessary to validate our results.
5. Conclusion
Current scientific opinion considers oxidative stress and ROS-mediated pathways to significantly contribute to the pathogenesis of many neurological diseases including ischemic stroke (Cojocaru, Cojocaru, Sapira, and Ionescu, 2013). Research both in vitro and in vivo has also shown that the oxidation and inflammation induced by CS can be reduced by antioxidants (Hossain, Mazzone, Tierney, and Cucullo, 2011b; Willcox, Ash, and Catignani, 2004b). The analysis of pro-inflammatory cytokines release revealed that, resveratrol, melatonin, lipoic acid and Co-Q10 inhibited IL-6 & IL-8 release following CSE exposure. Glutathione was inactive in both low and high doses. Measurement of oxidative stress response indicated resveratrol, lipoic acid and melatonin to be the most effective treatments against CSE-induced cellular oxidative damage. The effect of Co-Q10 was marginal whereas no effect was noted in response to Glutathione. Pretreatment with lipoic acid, melatonin and resveratrol also significantly reduced CSE-induced up-regulation of PECAM-1, VCAM-1 and E-selectin. Co-Q10 exhibited only marginal anti-inflammatory activities whereas no measureable effects were observed for glutathione. In agreement with these results, adhesion of THP-I cells on hCMEC/D3 monolayer following CSE exposure was inhibited by resveratrol, lipoic acid and melatonin (to a lower extent). However, with the exception of lipoic acid and GSH, the other tested compounds did not follow a dose-dependent anti-angiogenic activity. Specifically, there was no statistically significant differences between low and high dose of resveratrol while, high doses of Co-Q10 and melatonin were surprisingly ineffective. This is important to argue against or in favor to use a specific antioxidant since VEGF can impact BBB integrity in the long term and/or promote tumor cell proliferation. Additional in vivo studies will be necessary to confute our in vitro results and also address the benefits associated with prophylactic versus therapeutic (post-injury) antioxidant administration to prevent or reduce post-ischemic brain injury.
6. Experimental Procedure
6.1 Chemicals and Reagents
Sterile culture ware was purchased from Fisher Scientific (Pittsburgh, PA, USA), antioxidants- Co-Q10 (#C9538), Melatonin (#M5250), Glutathione (#PHR1359), Glutathione reduced ethyl ester (#G1404), (R)-(+)-α-Lipoic acid (#04471) and Resveratrol (#R5010) were obtained from Sigma-Aldrich (St. Louis, MO, USA) while 3R4F research cigarettes equivalent to regular cigarettes composed of 9.4 mg tar and 0.726 mg nicotine were purchased from University of Kentucky. Other molecular biology grade chemicals were purchased either from Sigma-Aldrich or Bio-Rad laboratories (Hercules, CA, USA)- e.g. Mini-Protean® TGXTM gels 4–15% (#456–1084) for gel electrophoresis. Antibodies were obtained from the following sources: mouse anti PECAM-1 (#3528s), mouse anti NQO1 (3187s), rabbit anti VCAM-1 (# 12367), goat anti-mouse (#4408S) and anti-rabbit IgG (#4413S) conjugated to Alexa Fluor 488 and 555 respectively from Cell Signaling Technology (Danvers, MA, USA); mouse anti-E-selectin (#S 9555) and mouse anti-β actin (#A5441) were from Sigma-Aldrich.
6.2 CSE Preparation
Cigarette Smoke Extract (CSE) was prepared by a Single Cigarette Smoking Machine (SCSM, CH Technologies Inc., Westwood, NJ, USA) as shown in Fig.-1A. The Federal Trade Commission (FTC) standard smoking protocol was used to produce the CSE. This consists of 35 ml puff volume, 2 second puff duration and 1 puff per minute generating a total of 8 puffs/cigarette which were bubbled directly into phosphate buffered saline (PBS) through an impinger generating our stock solution (Naik, Fofaria, Prasad, Sajja, Weksler, Couraud, Romero, and Cucullo, 2014; Naik, Sajja, Prasad, and Cucullo, 2015; Prasad, Sajja, Park, Naik, Kaisar, and Cucullo, 2015). To minimize variability, 3× concentration (or 300%) of stock solution was first prepared by lighting three cigarettes. Subsequently it was diluted to 5% concentrations in low serum media depending upon the treatment conditions as described later.
6.3 Cell culture
Immortalized hCMEC/D3 (passage no. 28–30) cell line, a well-established brain micro vascular endothelial cells were donated by Dr. Couraud (INSERM, Paris). Cells were cultured in collagen coated (50 µg/mL rat collagen-1 in 0.02 M glacial acetic acid, incubated at 37 °C for at least 1 hour) cell culture flasks or glass chamber slides (seeding density of 25000/cm2) and maintained at 37°C with 5% CO2 exposure. Cell were fed with EBM-2 basal medium (Lonza, Walkersville, MD, USA), supplemented with 5% FBS (Atlanta Biologicals, Lawrenceville, GA, USA), fibroblast growth factor (1ng/mL, Sigma Aldrich Inc), chemically defined lipid concentrate (1:100, Life Technologies, Carlsbad, CA, USA), 1% antibiotic/antimycotic (1:1, Atlanta Biologicals, GA, USA) and HEPES (10mM). The culture medium was changed every other day until the cells became 100% confluent and formed a complete monolayer.
6.4 Treatment
Once an endothelial monolayer is formed (usually takes 4–5 days) by hCMEC/D3 cells in culture flasks, chamber slides and multi-well plates were exposed to low serum (1%) media overnight. The media was replaced by fresh low serum medium on next day and pretreated with antioxidants in two different concentrations (1 and 10 µg/mL) for 2 hours at 37°C. All the antioxidants except glutathione and glutathione ethyl ester were dissolved in appropriate organic solvent and diluted with fresh low serum medium under minimum exposure to light due to their sensitivity. After two hours of incubation, freshly prepared CSE at 5% concentration was added on top of the antioxidants and incubated again at 37°C for 12 hours. Subsequently, cell culture supernatant and cell pellet was collected for further analysis.
6.5 Cell Viability
Release of cytosolic enzyme lactate dehydrogenase (LDH) in the cell culture media is considered a marker of plasma membrane damage. The extracellular LDH levels in the culture medium were estimated after 12 of exposures to various experimental conditions, by a colorimetric enzymatic reaction (Pierce LDH cytotoxicity assay kit, Thermo Scientific, Rockford, IL) according to the manufacturer protocol.
6.6 ELISA
Following CSE exposure for 12 hours pretreatment with antioxidants for 2 hours, cell culture supernatant from flasks were collected, centrifuged and preserved at a −20°C until analyzed by Quantikine ELISA kits (R&D systems, Minneapolis, MN, USA) for quantitative determination of VEGF, IL-6 and IL-8 according to the manufacturer’s protocol.
6.7 Immunofluorescence
For immunofluorescence imaging hCMEC/D3 cells were grown in two-well chamber slides (2×2 inch) and after treatment fixed with 16 %, methanol free formaldehyde (diluted 1 in 4 in 1× PBS; from Polysciences Inc. # 18814) for 10 min at room temperature. After a PBS wash (3 times), cells were permeabilized by 0.2% triton X-100 in PBS and then blocked with 5% goat serum in PBS at room temperature (RT) for 40 min, followed by incubation with rabbit or mouse (1:100–150) primary antibodies overnight at 4°C. Following one cycle of three PBS washings, cells were incubated for 1h at RT with Alexa Fluor 488 or 555-conjugated secondary antibodies, respectively (1:800). Cells were rinsed with PBS (3 more times) and mounted with DAPI in Prolonged Gold Anti-fade reagent (Invitrogen, OR, USA). Slides were dried overnight in the dark and images were captured with an EVOS digital inverted fluorescence microscope at 40× magnitude. All images were captured under identical exposure, contrast and brightness settings of the microscope depending on the target protein of interest and for better visualization of images post capture adjustment of brightness or contrast were done to the same extent in all conditions. Cells incubated with secondary antibodies without prior primary antibody staining served as negative controls.
6.8 Western Blotting
Cells were lysed in ice-cold Urea-Tris buffer containing Phosphatase and Protease Inhibitors (Roche Diagnostics, Indianapolis, IN, USA), sonicated and centrifuged at 14000 rpm, 4°C for 15 min. Amount of total protein was quantified by Pierce BCA Protein Assay Kit (Thermo Scientific, # 23225) and an equal amount (25 µg) of denatured protein was subjected to SDS-PAGE (4–20% graded gel). Bands were electrotransferred to PVDF membranes for 1h at 100V, blocked with 5% non-fat dry milk in TBS containing 0.1% Tween-20 (TTBS) for 2h and incubated with primary antibodies overnight (dilution range: 1:300–1:500) in a blocking buffer. Blots were washed and incubated with HRP-conjugated secondary antibody (1: 8000) for 2h at RT. After washing, protein bands were visualized by enhanced chemiluminescence using LI-COR C-Digit blot scanner and analyzed by Image Studio software with β-actin as a loading control. Band densities were analyzed by LI-COR Image Studio.
6.9 Oxidative Stress
Reactive Oxygen Species (ROS) level was measured qualitatively by CellROX Oxidative Stress Reagent from Life Technologies (#C10444) which is a cell permeable fluorogenic probe and upon oxidation binds to DNA exhibiting strong localized green fluorogenic nuclear signal in live cells. hCMEC/D3 cells were cultured in two-well chamber slides (2×2 inch). After treatment the dye was added at a final concentration of 5 µM and incubated for 30 minute at 37°C. The reagent was removed, washed with PBS and images were captured.
6.10 Monocyte Activation Assay
hCMEC/D3 and THP-1 cells (purchased from American Type Culture Collection –ATCC, #TIB202) were cultured in two-well chamber slides (2×2 inch) and T-75 respectively and exposed to CSE individually for 1 hour at 37°C. Thereafter, THP-1 cells were added (0.5 × 106 cells/mL) on top of hCMEC/D3 cells pretreated with antioxidants and incubated for 3 hours without agitation for adhesion of monocytes. Subsequently, wells underwent gentle shaking for 5 minutes, unattached monocytes were washed away by fresh media and images were captured.
6.11 Statistical Analyses
Data were expressed as mean ± SEM and analyzed by one-way ANOVA followed by post-hoc multiple comparison tests using GraphPad Prism Software Inc. (La Jolla, CA, USA). For statistical significance, P value was set to less than 0.05.
Highlights.
Except for GSH, all tested antioxidants reduced CSE-Induced release of IL-6 & -8
Lipoic acid, melatonin and resveratrol also reduced PECAM-1, VCAM-1 and E-selectin
THP-I cells adhesion on D3 was inhibited by resveratrol, lipoic acid and melatonin
All tested compounds inhibited CSE-induced VEGF release
Acknowledgments
This study was supported by Alternative Research and Development Fund and in part by National Institutes of Health/National Institute on Drug Abuse R01-DA029121-01A1 to Dr. Luca Cucullo. We also thank Dr. Pooja Naik for her guidance in routine and essential laboratory procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: reversibility with oral L-arginine but not vitamin C. J. Am. Coll. Cardiol. 1997;29:491–497. doi: 10.1016/s0735-1097(96)00537-2. [DOI] [PubMed] [Google Scholar]
- 2.Hossain M, Sathe T, Fazio V, Mazzone P, Weksler B, Janigro D, Rapp E, Cucullo L. Tobacco smoke: a critical etiological factor for vascular impairment at the blood-brain barrier. Brain Res. 2009;1287:192–205. doi: 10.1016/j.brainres.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik P, Fofaria N, Prasad S, Sajja RK, Weksler B, Couraud PO, Romero IA, Cucullo L. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC. Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HW, Chien ML, Chaung YH, Lii CK, Wang TS. Extracts from cigarette smoke induce DNA damage and cell adhesion molecule expression through different pathways. Chem. Biol. Interact. 2004;150:233–241. doi: 10.1016/j.cbi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Davis JW. Some acute effects of smoking on endothelial cells and platelets. Adv. Exp. Med. Biol. 1990;273:107–118. doi: 10.1007/978-1-4684-5829-9_11. [DOI] [PubMed] [Google Scholar]
- 6.Nagy J, Demaster EG, Wittmann I, Shultz P, Raij L. Induction of endothelial cell injury by cigarette smoke. Endothelium. 1997;5:251–263. doi: 10.3109/10623329709052590. [DOI] [PubMed] [Google Scholar]
- 7.Noronha-Dutra AA, Epperlein MM, Woolf N. Effect of cigarette smoking on cultured human endothelial cells. Cardiovasc. Res. 1993;27:774–778. doi: 10.1093/cvr/27.5.774. [DOI] [PubMed] [Google Scholar]
- 8.Raij L, Demaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J. Hypertens. 2001a;19:891–897. doi: 10.1097/00004872-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gill JS, Shipley MJ, Tsementzis SA, Hornby R, Gill SK, Hitchcock ER, Beevers DG. Cigarette smoking. A risk factor for hemorrhagic and nonhemorrhagic stroke. Arch. Intern. Med. 1989;149:2053–2057. doi: 10.1001/archinte.149.9.2053. [DOI] [PubMed] [Google Scholar]
- 10.Panda K, Chattopadhyay R, Ghosh MK, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke induced oxidative damage of proteins and increased proteolysis. Free Radic. Biol. Med. 1999;27:1064–1079. doi: 10.1016/s0891-5849(99)00154-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanna ST. Nicotine effect on cardiovascular system and ion channels. J. Cardiovasc. Pharmacol. 2006;47:348–358. doi: 10.1097/01.fjc.0000205984.13395.9e. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J. Cereb. Blood Flow Metab. 1997;17:136–146. doi: 10.1097/00004647-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, Bickel U, Abbruscato TJ. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J. Pharmacol. Exp. Ther. 2010;332:371–379. doi: 10.1124/jpet.109.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batkin S, Rayner MD. Mitotic changes in neuroblastoma transplanted into denervated host tissue. Res. Commun. Chem. Pathol. Pharmacol. 1976;13:145–148. [PubMed] [Google Scholar]
- 15.Catanzaro DF, Zhou Y, Chen R, Yu F, Catanzaro SE, De Lorenzo MS, Subbaramaiah K, Zhou XK, Pratico D, Dannenberg AJ, Weksler BB. Potentially reduced exposure cigarettes accelerate atherosclerosis: evidence for the role of nicotine. Cardiovasc. Toxicol. 2007;7:192–201. doi: 10.1007/s12012-007-0027-z. [DOI] [PubMed] [Google Scholar]
- 16.Das S, Gautam N, Dey SK, Maiti T, Roy S. Oxidative stress in the brain of nicotine-induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl. Physiol Nutr. Metab. 2009;34:124–135. doi: 10.1139/H08-147. [DOI] [PubMed] [Google Scholar]
- 17.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 19.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989;298:789–794. doi: 10.1136/bmj.298.6676.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannami T, Iso H, Baba S, Sasaki S, Okada K, Konishi M, Tsugane S. Cigarette smoking and risk of stroke and its subtypes among middle-aged Japanese men and women: the JPHC Study Cohort I. Stroke. 2004;35:1248–1253. doi: 10.1161/01.STR.0000128794.30660.e8. [DOI] [PubMed] [Google Scholar]
- 21.Miller GJ, Bauer KA, Cooper JA, Rosenberg RD. Activation of the coagulant pathway in cigarette smokers. Thromb. Haemost. 1998;79:549–553. [PubMed] [Google Scholar]
- 22.Mast H, Thompson JL, Lin IF, Hofmeister C, Hartmann A, Marx P, Mohr JP, Sacco RL. Cigarette smoking as a determinant of high-grade carotid artery stenosis in Hispanic, black, and white patients with stroke or transient ischemic attack. Stroke. 1998;29:908–912. doi: 10.1161/01.str.29.5.908. [DOI] [PubMed] [Google Scholar]
- 23.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev. Food Sci. Nutr. 2004a;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 24.Hossain M, Mazzone P, Tierney W, Cucullo L. In vitro assessment of tobacco smoke toxicity at the BBB: do antioxidant supplements have a protective role? BMC. Neurosci. 2011a;12:92. doi: 10.1186/1471-2202-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobczak A, Golka D, Szoltysek-Boldys I. The effects of tobacco smoke on plasma alpha- and gamma-tocopherol levels in passive and active cigarette smokers. Toxicol. Lett. 2004;151:429–437. doi: 10.1016/j.toxlet.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, Packer L. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am. J. Clin. Nutr. 2003;77:160–166. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105:1155–1157. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 28.Ozan E, Sonmez MF, Ozan S, Colakoglu N, Yilmaz S, Kuloglu T. Effects of melatonin and vitamin C on cigarette smoke-induced damage in the kidney. Toxicol. Ind. Health. 2007;23:479–485. doi: 10.1177/0748233708089023. [DOI] [PubMed] [Google Scholar]
- 29.Rodella LF, Filippini F, Bonomini F, Bresciani R, Reiter RJ, Rezzani R. Beneficial effects of melatonin on nicotine-induced vasculopathy. J. Pineal Res. 2010;48:126–132. doi: 10.1111/j.1600-079X.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- 30.Donmez Z, Yigit O, Bilici S, Dursun N, Gul M, Dastan SD, Uzun H. Evaluation of the antioxidant effects of melatonin on the larynx mucosa of rats exposed to environmental tobacco smoke. Clin. Otolaryngol. 2015 doi: 10.1111/coa.12501. [DOI] [PubMed] [Google Scholar]
- 31.Yang GH, Li YC, Wang ZQ, Liu B, Ye W, Ni L, Zeng R, Miao SY, Wang LF, Liu CW. Protective effect of melatonin on cigarette smoke-induced restenosis in rat carotid arteries after balloon injury. J. Pineal Res. 2014;57:451–458. doi: 10.1111/jpi.12185. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Ren J, Chen H, Huang Y, Li H, Zhang Z, Wang J. Resveratrol protects against cigarette smoke-induced oxidative damage and pulmonary inflammation. J. Biochem. Mol. Toxicol. 2014;28:465–471. doi: 10.1002/jbt.21586. [DOI] [PubMed] [Google Scholar]
- 33.Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, Liu J. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Invest Ophthalmol. Vis. Sci. 2007;48:339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik P, Sajja RK, Prasad S, Cucullo L. Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line. BMC. Neurosci. 2015;16:38. doi: 10.1186/s12868-015-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traber MG, van d V, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin. Chest Med. 2000;21:173–87. x. doi: 10.1016/s0272-5231(05)70016-2. [DOI] [PubMed] [Google Scholar]
- 36.Johnston CS, Martin LJ, Cai X. Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis. J. Am. Coll. Nutr. 1992;11:172–176. [PubMed] [Google Scholar]
- 37.Koul A, Singh A, Sandhir R. Effect of alpha-tocopherol on the cardiac antioxidant defense system and atherogenic lipids in cigarette smoke-inhaling mice. Inhal. Toxicol. 2003;15:513–522. doi: 10.1080/08958370304462. [DOI] [PubMed] [Google Scholar]
- 38.Al-Malki AL, Moselhy SS. Protective effect of vitamin E and epicatechin against nicotine-induced oxidative stress in rats. Toxicol. Ind. Health. 2013;29:202–208. doi: 10.1177/0748233711430976. [DOI] [PubMed] [Google Scholar]
- 39.Gumustekin K, Taysi S, Alp HH, Aktas O, Oztasan N, Akcay F, Suleyman H, Akar S, Dane S, Gul M. Vitamin E and Hippophea rhamnoides L. extract reduce nicotine-induced oxidative stress in rat heart. Cell Biochem. Funct. 2010;28:329–333. doi: 10.1002/cbf.1663. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Sharma TK, Kaushik GG, Shekhawat VP. Vitamin E supplementation may ameliorate oxidative stress in type 1 diabetes mellitus patients. Clin. Lab. 2011;57:379–386. [PubMed] [Google Scholar]
- 41.dos Santos PS, Costa JP, Tome AR, Saldanha GB, de Souza GF, Feng D, de Freitas RM. Oxidative stress in rat striatum after pilocarpine-induced seizures is diminished by alpha-tocopherol. Eur. J. Pharmacol. 2011;668:65–71. doi: 10.1016/j.ejphar.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 42.Venditti P, Napolitano G, Di SL, Agnisola C, Di MS. Effect of vitamin E administration on response to ischaemia-reperfusion of hearts from cold-exposed rats. Exp. Physiol. 2011;96:635–646. doi: 10.1113/expphysiol.2011.058289. [DOI] [PubMed] [Google Scholar]
- 43.Arato E, Kurthy M, Sinay L, Kasza G, Menyhei G, Hardi P, Masoud S, Ripp K, Szilagyi K, Takacs I, Miklos Z, Bator A, Lantos J, Kollar L, Roth E, Jancso G. Effect of vitamin E on reperfusion injuries during reconstructive vascular operations on lower limbs. Clin. Hemorheol. Microcirc. 2010;44:125–136. doi: 10.3233/CH-2010-1260. [DOI] [PubMed] [Google Scholar]
- 44.Hossain M, Mazzone P, Tierney W, Cucullo L. In vitro assessment of tobacco smoke toxicity at the BBB: do antioxidant supplements have a protective role? BMC. Neurosci. 2011b;12:92. doi: 10.1186/1471-2202-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinzii CM, Lopez LC, Gilkerson RW, Dorado B, Coku J, Naini AB, Lagier-Tourenne C, Schuelke M, Salviati L, Carrozzo R, Santorelli F, Rahman S, Tazir M, Koenig M, Dimauro S, Hirano M. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010;24:3733–3743. doi: 10.1096/fj.09-152728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung HJ, Park EH, Lim CJ. Evaluation of anti-angiogenic, anti-inflammatory and antinociceptive activity of coenzyme Q(10) in experimental animals. J. Pharm. Pharmacol. 2009;61:1391–1395. doi: 10.1211/jpp/61.10.0017. [DOI] [PubMed] [Google Scholar]
- 47.Gurpinar T, Ekerbicer N, Uysal N, Barut T, Tarakci F, Tuglu MI. The effects of the melatonin treatment on the oxidative stress and apoptosis in diabetic eye and brain. Scientific World Journal. 2012;2012:498489. doi: 10.1100/2012/498489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pei Z, Pang SF, Cheung RT. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34:770–775. doi: 10.1161/01.STR.0000057460.14810.3E. [DOI] [PubMed] [Google Scholar]
- 49.Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology. 2010;278:55–67. doi: 10.1016/j.tox.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 51.Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism. Relat Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 52.Aquilano K, Baldelli S, Ciriolo MR. Glutathione is a crucial guardian of protein integrity in the brain upon nitric oxide imbalance. Commun. Integr. Biol. 2011;4:477–479. doi: 10.4161/cib.4.4.15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dey SK, Roy S. Role of reduced glutathione in the amelioration of nicotine-induced oxidative stress. Bull. Environ. Contam Toxicol. 2010;84:385–389. doi: 10.1007/s00128-010-9948-5. [DOI] [PubMed] [Google Scholar]
- 54.Goraca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol. Rep. 2011;63:849–858. doi: 10.1016/s1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Yu Y, Ji L, Liang X, Zhang T, Hai CX. Alpha-lipoic acid protects against myocardial ischemia/reperfusion injury via multiple target effects. Food Chem. Toxicol. 2011;49:2750–2757. doi: 10.1016/j.fct.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 56.Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Erzik C, Berkman MZ, Yegen BC, Sener G. Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem. Res. 2010;35:418–428. doi: 10.1007/s11064-009-0072-z. [DOI] [PubMed] [Google Scholar]
- 57.Tang Y, Xu J, Qu W, Peng X, Xin P, Yang X, Ying C, Sun X, Hao L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem. 2012 doi: 10.1016/j.jnutbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Li H, Xia N, Forstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric. Oxide. 2012;26:102–110. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 60.Simao F, Matte A, Matte C, Soares FM, Wyse AT, Netto CA, Salbego CG. Resveratrol prevents oxidative stress and inhibition of Na(+)K(+)-ATPase activity induced by transient global cerebral ischemia in rats. J. Nutr. Biochem. 2011;22:921–928. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Ren J, Fan C, Chen N, Huang J, Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011;36:2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 62.Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Ramirez MA, Male DK, Wang C, Sharrack B, Wu D, Romero IA. Cytokine-induced changes in the gene expression profile of a human cerebral microvascular endothelial cell-line, hCMEC/D3. Fluids Barriers. CNS. 2013;10:27. doi: 10.1186/2045-8118-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daniels BP, Cruz-Orengo L, Pasieka TJ, Couraud PO, Romero IA, Weksler B, Cooper JA, Doering TL, Klein RS. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods. 2013;212:173–179. doi: 10.1016/j.jneumeth.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers. CNS. 2013;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prasad S, Sajja RK, Park JH, Naik P, Kaisar MA, Cucullo L. Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells. Fluids Barriers. CNS. 2015;12:18. doi: 10.1186/s12987-015-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'Ambrosio D, Gargiulo G, Della-Morte D, Gallucci F, Uomo G, Rundek T, Abete P. Gamma-glutamyltransferase predicts functional impairment in elderly adults after ischemic stroke. J. Am. Geriatr. Soc. 2013;61:1040–1041. doi: 10.1111/jgs.12299. [DOI] [PubMed] [Google Scholar]
- 68.Philbert MA, Beiswanger CM, Manson MM, Green JA, Novak RF, Primiano T, Reuhl KR, Lowndes HE. Glutathione S-transferases and gamma-glutamyl transpeptidase in the rat nervous systems: a basis for differential susceptibility to neurotoxicants. Neurotoxicology. 1995;16:349–362. [PubMed] [Google Scholar]
- 69.Raij L, Demaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J. Hypertens. 2001b;19:891–897. doi: 10.1097/00004872-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Choi EY, Jin JY, Lee JY, Choi JI, Choi IS, Kim SJ. Melatonin inhibits Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing NF-kappaB and STAT1 activity. J. Pineal Res. 2011;50:197–206. doi: 10.1111/j.1600-079X.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 71.Clapp-Lilly KL, Smith MA, Perry G, Duffy LK. Melatonin reduces interleukin secretion in amyloid-beta stressed mouse brain slices. Chem. Biol. Interact. 2001;134:101–107. doi: 10.1016/s0009-2797(00)00319-7. [DOI] [PubMed] [Google Scholar]
- 72.Zhong M, Cheng GF, Wang WJ, Guo Y, Zhu XY, Zhang JT. Inhibitory effect of resveratrol on interleukin 6 release by stimulated peritoneal macrophages of mice. Phytomedicine. 1999;6:79–84. doi: 10.1016/S0944-7113(99)80039-7. [DOI] [PubMed] [Google Scholar]
- 73.Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Munch G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Adv. Drug Deliv. Rev. 2008;60:1463–1470. doi: 10.1016/j.addr.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Lee BJ, Huang YC, Chen SJ, Lin PT. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012;28:767–772. doi: 10.1016/j.nut.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Schafer G, Cramer T, Suske G, Kemmner W, Wiedenmann B, Hocker M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J. Biol. Chem. 2003;278:8190–8198. doi: 10.1074/jbc.M211999200. [DOI] [PubMed] [Google Scholar]
- 76.Cojocaru IM, Cojocaru M, Sapira V, Ionescu A. Evaluation of oxidative stress in patients with acute ischemic stroke. Rom. J. Intern. Med. 2013;51:97–106. [PubMed] [Google Scholar]
- 77.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev. Food Sci. Nutr. 2004b;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]