Abstract
Opioid receptors can display spontaneous agonist-independent G-protein signaling (basal signaling/constitutive activity). While constitutive κ-opioid receptor (KOR) activity has been documented in vitro, it is unknown if KORs are constitutively active in native systems. Using [35S] GTPγS coupling assay that measures receptor functional state, we identified the presence of medial prefrontal cortex (mPFC) KOR constitutive activity in young rats that declined with age. Furthermore, basal signaling showed an age-related decline and was insensitive to neutral opioid antagonist challenge. Collectively, the present data are first to demonstrate age-dependent alterations in the mPFC KOR constitutive activity in rats and changes in the constitutive activity of KORs can differentially impact KOR ligand efficacy. These data provide novel insights into the functional properties of the KOR system and warrant further consideration of KOR constitutive activity in normal and pathophysiological behavior.
Keywords: Constitutive activity, kappa-opioid receptors, negative-efficacy antagonist, nor-binaltorphimine, dynorphin, age
INTRODUCTION
G protein-coupled receptors (GPCRs) constitutive activity is well documented in receptor pharmacology. First demonstrated in delta-opioid receptors (DORs), constitutive activity is thought to occur in most GPCRs systems (Costa and Herz 1989;Costa and Cotecchia 2005;Bond and Ijzerman 2006). Antagonists block agonist-induced effects; however, it has been shown that antagonists can range from zero efficacy to negative efficacy depending on the ligands ability to alter receptor constitutive activity (Strange 2002;Kenakin 2004;Bond and Ijzerman 2006). Neutral antagonists (zero efficacy antagonists) inhibit agonist-induced effects without affecting basal signaling, whereas negative efficacy antagonists (possessing inverse agonist properties) attenuate receptor constitutive activity (Strange 2002;Kenakin 2004). Interestingly, ~ 85% of antagonists for known neurotransmitter systems can function as negative-efficacy antagonists (Milligan 2003;de Ligt et al. 2000;Greasley and Clapham 2006). It is prudent to mention that in addition to efficacy of a given ligand, the state and physiological environment of the receptor system (e.g., receptor basal signaling state, certain receptor mutations, availability of G-proteins, intracellular ionic milieu, etc.) are all critical determinants of the magnitude and direction of the actual response elicited by a given ligand (Kenakin 2002;Perez and Karnik 2005;Sirohi et al. 2007;Sirohi et al. 2009;Navani et al. 2011). In the absence of constitutively active receptor system, negative efficacy antagonists and neutral antagonists are indistinguishable.
Like many GPCRs, constitutive activity has been documented for opioid receptors. Following the seminal discovery of constitutively active DORs (Costa and Herz 1989), studies have reported basal mu-opioid receptor (MOR) signaling activity (Wang et al. 2001). Basal kappa-opioid receptor (KOR) signaling has been documented in vitro (Wang et al. 2007); however, it is not yet known if KORs are constitutively active in native systems. As identified above, only negative efficacy antagonists can assess constitutive activity and one extensively studied KOR antagonist (nor-binaltorphimine; nor-BNI) has shown evidence of inverse agonist properties in vitro (Wang et al. 2007) and given our previous experience with the compound, nor-BNI was selected for use in the present investigations as a putative negative efficacy antagonist. We previously demonstrated a KOR specific effect of nor-BNI in the GTPγS assay (Kissler and Walker, 2015) and initiated research on KOR involvement in executive function that implicated the KOR as being an important regulator of impulse control specific to stopping an already-initiated action (Walker and Kissler, 2013). Converging lines of evidence suggested that KORs in the mPFC could be important regulators of executive function: 1) The mPFC has been implicated as a substrate for stop-signal reaction time performance (e.g., Bari et al. 2011), 2) KOR-mediated regulation of neurotransmitters within the mPFC could promote maladaptive behavioral regulation (Tejeda et al. 2013;Tejeda et al. 2015), 3) dynorphin (DYN; an endogenous KOR ligand) and KOR mRNA are upregulated in the PFC of deceased human alcoholics (Bazov et al. 2013), a report that nicely parallels our published data showing a `one-two punch of alcoholism' due to increases in both dynorphin (DYN; an endogenous KOR ligand) A-like immunoreactivity and KOR function in the central amygdala. Such dysregulation could be characteristic of dynorphin (DYN; an endogenous KOR ligand) / KOR dysregulation in distinct brain nuclei that mediate executive function and contribute to normal goal-directed behavior.
Given our established and sustained interest in identifying the nature and extent of DYN / KOR dysregulation that could contribute to alcohol dependence-induced cognitive impairment, and considering observations of age-dependent KOR agonist effects in rodents (e.g., Goodwin and Barr 2005;Anderson et al. 2014), we evaluated the hypothesis that ontogenic alterations in mPFC KOR function are sensitive to maturation in a manner consistent with age-dependent KOR agonist effects observed in the literature. To this end, we evaluated the functional state of KORs in mPFC tissue obtained from 60-150 day old rats.
MATERIAL AND METHODS
Animals
Male Wistar rats 60-150 days were obtained from our breeding colony at WSU that is semi–annually restocked with breeders from Charles River Laboratory (Hollister, CA) and housed in an environmentally controlled vivarium on a reverse light cycle (lights off at 6 a.m.). Food and water was available ad libitum. All work adhered to National Research Council's Guide for the Care and Use of Laboratory Animals (2011) and Institutional Animal Care and Use Committee guidelines.
General Procedure
Brain tissue was collected from 60-150 days old rats and the mPFC microdissected. First, the effect of nor-BNI (negative-efficacy KOR antagonist) and CTOP (neutral mu-opioid antagonist) on basal signaling was determined in the presence or absence of DYN A (endogenous KOR agonist) and DAMGO (exogenous MOR agonist), respectively using 60 days old rat brain tissue. Next, in order to examine the impact of age on the nor-BNI negative efficacy, mPFC brain tissue obtained from 60-150 days old rats was incubated with nor-BNI in the GTPγS signaling assay.
[35S]GTPγS Signaling Assay
The assay was conducted as described previously (Kissler et al. 2014). It is a functional assay that measures the guanine nucleotide exchange at G proteins as a result of ligand binding to G protein-coupled receptors (GPCRs), by monitoring the binding of a radiolabeled, [35S]GTPγS, in the presence of unlabeled GDP and is described in detail elsewhere (Harrison and Traynor 2003). In short, an agonist will increase GTPγS binding; whereas, a negative efficacy antagonist will reduce GTPγS binding, if receptors are constitutively active (Bond and Ijzerman 2006). A neutral antagonist has no efficacy and thus does not alter GTPγS binding. Therefore, a negative efficacy antagonist can serve as a tool to detect constitutive receptor activity.
Rat mPFC tissue was micro-dissected and homogenized (45 strokes; on ice) in 1.5ml of membrane buffer (pH 7.4, 50.0mM Tris–HCl, 3.0mM MgCl2, and 1.0mM EGTA). The homogenate was centrifuged (21004g, 4°C for 30 min), re-suspended in 1.5ml of membrane buffer, homogenized (12-15 strokes) and centrifuged again. The pellet was re-homogenized (12-15 strokes) in 1.5ml of assay buffer (pH 7.4, 50.0mM Tris–HCl, 3.0mM MgCl2, 0.2mM EGTA, 100.0mM NaCl). Protein estimation was conducted using a BCA protein assay (Pierce). Samples were homogenized (12-15 strokes) prior to the addition of the protein homogenate (3μg). DYN A (0.0-1.0 μM), nor-BNI (0.0-10.0μM), CTOP (0.0-10.0μM) or DAMGO (0.0-1μM), were incubated in assay buffer in triplicates (90 min; 25°C) with 10μM GDP and 0.05nM [35S] GTPγS in 1.0ml total volume. The reaction was terminated by filtration using a cell harvester, and washed 3x in ice cold phosphate buffer (pH 7.2). Bound radioactivity on the filter discs was quantified by liquid scintillation spectrophotometry.
Data Analysis
One-way repeated-measure of ANOVA was utilized to examine the impact of nor-BNI or CTOP on basal or agonist-stimulated GTPγS coupling. Data from the GTPγS coupling assay was analyzed using a mixed-model two-way ANOVA to compare antagonist-mediated changes in GTPγS signaling for the 60-150 days old rats. The within-subject variable was nor-BNI concentration and the between-groups variable was age. If a main effect was identified, one way repeated measure of ANOVA was done.
RESULTS
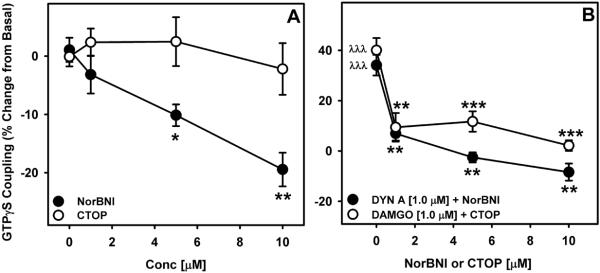
First the effect of nor-BNI (a negative efficacy antagonist) and CTOP (a neutral antagonist) on basal and agonist-stimulated GTPγS coupling was examined in the 60 days old rat mPFC. Nor-BNI concentration-dependently decreased (F3,15=10.60, p=0.001, power=0.992) basal GTPγS coupling, whereas CTOP was ineffective in altering the basal GTPγS coupling (Figure 1a). Nor-BNI and CTOP concentration dependently blocked DYN [1.0μM] and DAMGO [1.0 μM]-stimulated GTPγS coupling, respectively (Figure 1b).
Figure 1. Effect of nor-BNI and CTOP on basal and agonist-stimulated GTPγS coupling in the rat mPFC.
A) Nor-BNI (negative efficacy opioid receptor antagonist) decreased (F3,15=10.60, p=0.001, power=0.992) basal GTPγS coupling in a concentration dependent manner, whereas a neutral opioid receptor antagonist (CTOP) failed to alter basal GTPγS coupling (*p<0.05, **p<0.01 compared to zero concentration). B) As an internal control for assay responsivity, opioid receptor agonists (i.e., DYN A and DAMGO) significantly stimulated GTPγS coupling that was blocked by nor-BNI and CTOP, respectively, λλλp<0.001 when compared to basal signaling. **p<0.01, ***p<0.001 compared to the respective agonist-stimulated GTPγS coupling. Mean (±SEM) is plotted from six experiments ran in triplicates.
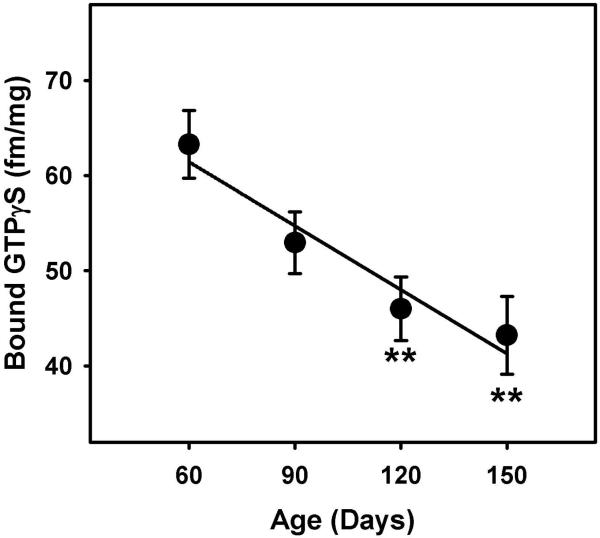
Next, we examined age-dependent differences in the basal signaling. The one-way ANOVA revealed a main effect of age on basal signaling (F3, 80= 6.248, p=0.001, power=0.957) showing a decline in the basal signaling activity with age. Post-hoc analyses revealed significant differences in basal signaling of 120 (p=0.005) and 150 (p=0.001) days group compared to 60 days old rat mPFC tissue (Figure 2).
Figure 2. Maturational changes in the basal GTPγS coupling in the rat mPFC.
A oneway ANOVA revealed an age-dependent decline in the basal GTPγS signaling. **p<0.01 (F3, 80=6.248, p=0.001, power =0.957), **p<0.01 compared to 60 days old group. Mean (±SEM) is plotted from seven experiments ran in triplicates.
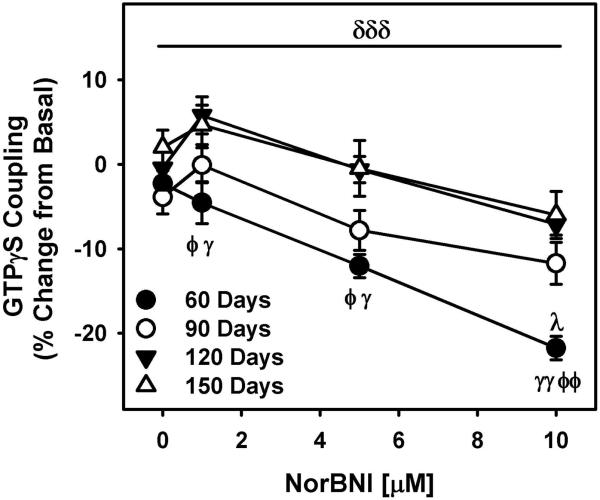
In order to examine age-dependent changes in nor-BNI negative efficacy, mPFC tissue from 60-150 days old rat brain was incubated with nor-BNI [0–10 μM] in the GTPγS coupling assay. Nor-BNI concentration-dependently decreased (F2.7,215=36.27, p=0.000, power=1.0) basal GTPγS coupling in all age groups. A main effect of age was observed for norBNI-induced decrease in basal GTPγS coupling (F3, 80=11.11, p=0.000, power=1.0) with greatest decrease observed in 60 days old rat mPFC (Figure 3).
Figure 3. Maturational changes in nor-BNI negative efficacy in the rat mPFC.
Nor-BNI concentration-dependently decreased (F2.7,215=36.27, p=0.000, power=1.0) basal GTPγS coupling as a function of age with greatest decrease observed in 60 days old rat mPFC. This effect was attenuated in an age-dependent manner. δδδ Main effect of age (F3,80=11.11, p=0.000, power=1.0), λp<0.05 when compared with same nor-BNI concentration of 90 days old group, φp<0.05, φφp<0.01 with 120 days old group and γp<0.05, γγp<0.01 with 150 days old group. Mean (±SEM) is plotted from seven experiments conducted in triplicates.
DISCUSSION
Negative efficacy antagonists are critical for evaluating receptor constitutive activity and studies have reported this phenomenon for many GPCRs, including opioid receptors (Costa and Herz 1989;Wang et al. 2004;Wang et al. 2007). While basal signaling activity at MOR and DOR have been documented repeatedly (Liu and Prather 2001;Liu and Prather 2002;Wang et al. 1994;Wang et al. 2004;Costa and Herz 1989), constitutive activity at KORs has remained relatively undocumented, at least in vivo (Becker et al. 1999;Wang et al. 2007). Nor-BNI, a classical KOR-selective antagonist with no detectable affinity (Ki≥10uM) for most non-opioid receptors and transporters (Munro et al. 2013;Metcalf and Coop 2005), has been shown to act as a negative-efficacy antagonist in vitro (Wang et al. 2007). Here we show that nor-BNI reduced basal signaling in rat mPFC brain tissue and this effect declines with age.
The GTPγS coupling assay has been successfully utilized for in vitro and in vivo assessment of constitutive opioid receptors activity (Wang et al. 2004;Bilsky et al. 2010;Barreda-Gomez et al. 2010). Membranes for the GTPγS assay were prepared from the rat brain tissue by utilizing an approach similar to that used previously to measure constitutive activity of opioid receptors (Raehal et al. 2005;Wang et al. 2004). By definition, both neutral and negative-efficacy antagonists can block agonist-stimulated receptor activity but only a negative-efficacy antagonist is capable of reducing agonist-independent receptor signaling. To ensure that altered basal signaling in response to nor-BNI was mediated by negative efficacy and not the result of competitive displacement of an agonist, it was imperative that the effects of the neutral antagonist were tested under identical experimental conditions. As neutral antagonists for KORs are not well characterized, we tested the effect of CTOP (a neutral mu-opioid antagonist; Hawkins et al. 1989;Meye et al. 2012) on basal signaling. Using this assay, first we examined the effect of nor-BNI on basal signaling in 60 day old rat mPFC tissue. Overall, nor-BNI and CTOP blocked DYN and DAMGO-stimulated GTPyS coupling, respectively (Figure 1b). However, only nor-BNI significantly decreased basal binding (Figure 1a) which supports the concept of agonist-independent KOR constitutive activity. As shown in Figure 1a, nor-BNI concentration dependently decreased basal signaling with highest concentration decreasing basal signaling by ~20%. nor-BNI has been previously shown to reduce basal signaling in the cell line expressing KORs (Wang et al. 2007), however this is the first demonstration of KOR constitutive activity in the native rat brain tissue. CTOP was ineffective in altering basal signaling under identical assay conditions. As such, these data support the hypothesis that KORs display constitutive activity in the rat mPFC.
Nor-BNI's reduction in basal signaling was specific to 60 days old rat brain tissue and this effect was absent in older rat brain region (unpublished observations). An age-dependent decline in KOR density in the rat brain (Bhargava et al. 1994) suggests that KOR constitutive activity could decline as a function of age-dependent changes in KOR density with support provided by the demonstration that relatively high expression of the receptors is critical for observations of constitutive activity in vitro (Costa and Cotecchia 2005;Parra and Bond 2007;Bond and Ijzerman 2006;Greasley and Clapham 2006;Herrick-Davis et al. 1999). Therefore, we evaluated the effect of nor-BNI in the mPFC tissue collected from 60-150 day old rats. Basal signaling declined with the age (Figure 2) and importantly, there was an age-dependent decline in nor-BNI alteration of basal signaling (Figure 3). These data suggest high KOR constitutive activity in the mPFC of young rats decreases with age and explains the age-dependent decline in the nor-BNI negative efficacy. An age-dependent decline in DYN concentration could account for the observed differences in nor-BNI efficacy and norBNI-induced effects merely reflect blockade of endogenous agonist-stimulated signaling. However, age-dependent increases in the DYN peptide content have been reported in the rat mPFC brain region which negates this concern (Nguyen et al. 2005). It is also important to note that the negative efficacy profile of nor-BNI was relatively abolished in 150 day old rat brain tissue which further speaks against endogenous agonist contamination in our assay conditions.
One characteristic of nor-BNI is an unusually long duration of action (Endoh et al. 1992) related, in part, to its interactions with intracellular signaling cascades that raises the interesting question of whether reduced nor-BNI negative efficacy in the older animals of the current study is representative of some cascade-mediated generalized lack of effect for nor-BNI in older animals. To be sure, that is not the case as studies have confirmed nor-BNI efficacy in older rodents using techniques that range from gene to behavior (e.g., Kissler et al. 2014;Menard et al. 2014). The two competing hypotheses to explain the long-term effects of nor-BNI relate to 1) abnormal c-Jun N-terminal kinase (JNK) activation by nor-BNI (Bruchas et al. 2007;Bruchas and Chavkin 2010;Melief et al. 2010) that is required for long-term effects in a manner unrelated to non-competitive antagonism and 2) nor-BNI's pharmacokinetic properties prolong elimination and allow for continued competitive antagonism (Patkar et al. 2013). Neither theory is exclusionary of the other nor do they lack support for their claims which suggests that there is a combined contribution to the long-term effects of nor-BNI.
Receptor basal signaling has been best characterized in artificially transfected systems in vitro through mutagenesis or pharmacology, but observing constitutive activity in vivo has traditionally been difficult (Costa and Cotecchia 2005;Parra and Bond 2007;Bond and Ijzerman 2006;Greasley and Clapham 2006;Herrick-Davis et al. 1999;Lam et al. 2011). The present data are first to demonstrate that KORs are constitutively active in the rat mPFC in an age-dependent manner providing additional mechanisms whereby dysregulation of the DYN / KOR system could occur to promote maladaptive behavioral regulation.
The physiological importance of GPCRs constitutive activity is increasingly becoming apparent and antagonists with negative efficacy could have clinical utility (de Ligt et al. 2000;Parra and Bond 2007). These current results indicate the presence of KOR constitutive activity in one brain substrate mediating impulse control and strategic behavior (Kim and Lee 2011;Bechara and Van Der Linden 2005;Boes et al. 2009). We have recently reported KOR activation-induce impulsive phenotypes in rats (Walker and Kissler 2013) and, well documented in both human and animal models, younger organisms engage in more impulsive and risky behaviors than their adult counterparts (Green et al. 1994;Doremus-Fitzwater et al. 2012;Whelan R et al. 2012). Considering the possibility that age-related declines in impulsivity are a function of maturational changes in KOR constitutive activity, dysregulated constitutive activity under pathological conditions such as alcohol dependence and withdrawal could contribute to symptoms such as heightened impulsivity and impaired executive control.
In conclusion, for the first time, KORs constitutive activity was identified to be age-dependent in the rat mPFC. Maturational changes in KOR constitutive activity could differentially impact KORs ligand efficacy. Moreover, dysregulation of constitutive activity in addictive and neuropsychiatric disorders such as alcohol dependence could underlie many of the symptoms that contribute to maladaptive behavioral regulation.
ACKNOWLEDGEMENTS
Support for this research was provided by R01AA020394 awarded to BMW from the National Institute on Alcohol Abuse and Alcoholism and the WSU Alcohol and Drug Abuse Research Program. BMW is a consultant for H. Lundbeck A/S (Copenhagen, Denmark). The authors have no financial, personal or organizational conflicts of interest to report in relation to this manuscript.
ABBREVIATIONS
- GTPγS
(guanosine 5'-O-[gamma-thio] triphosphate)
- GPCR
(G-protein-coupled receptor)
- Nor-BNI
(nor-binaltorphimine)
- DAMGO
([D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin)
- KOR
(κ-opioid receptor)
- DOR
(delta-opioid receptor)
- MOR
(mu-opioid receptor)
- CTOP
(D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2)
Footnotes
ARRIVE guidelines have been followed:
Yes
=> if No, skip complete sentence
=> if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
Conflicts of interest: none
=> if `none', insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already included
REFERENCES
- Anderson RI, Morales M, Spear LP, Varlinskaya EI. Pharmacological activation of kappa opioid receptors: aversive effects in adolescent and adult male rats. Psychopharmacology (Berl) 2014;231:1687–1693. doi: 10.1007/s00213-013-3095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J. Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreda-Gomez G, Teresa GM, Rodriguez-Puertas R. Methods to measure g-protein-coupled receptor activity for the identification of inverse agonists. Methods Enzymol. 2010;485:261–273. doi: 10.1016/B978-0-12-381296-4.00015-4. [DOI] [PubMed] [Google Scholar]
- Bazov I, Kononenko O, Watanabe H, Kuntic V, Sarkisyan D, Taqi MM, Hussain MZ, Nyberg F, Yakovleva T, Bakalkin G. The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict. Biol. 2013;18:161–169. doi: 10.1111/j.1369-1600.2011.00366.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr. Opin. Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Becker JA, Wallace A, Garzon A, Ingallinella P, Bianchi E, Cortese R, Simonin F, Kieffer BL, Pessi A. Ligands for kappa-opioid and ORL1 receptors identified from a conformationally constrained peptide combinatorial library. J. Biol. Chem. 1999;274:27513–27522. doi: 10.1074/jbc.274.39.27513. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Matwyshyn GA, Reddy PL, Veeranna Brain and spinal cord kappa opiate receptors and pharmacological responses to U-50,488H in rats of differing ages. Pharmacol. Biochem. Behav. 1994;48:87–91. doi: 10.1016/0091-3057(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Giuvelis D, Osborn MD, Dersch CM, Xu H, Rothman RB. In vitro and in vivo assessment of mu opioid receptor constitutive activity. Methods Enzymol. 2010;484:413–443. doi: 10.1016/B978-0-12-381298-8.00021-6. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc. Cogn Affect. Neurosci. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond RA, Ijzerman AP. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol. Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan S. C., Li S., Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J. Biol. Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Cotecchia S. Historical review: Negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol. Sci. 2005;26:618–624. doi: 10.1016/j.tips.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ligt RA, Kourounakis AP, Ijzerman AP. Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery. Br. J. Pharmacol. 2000;130:1–12. doi: 10.1038/sj.bjp.0703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behav. Neurosci. 2012;126:735–741. doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Goodwin GA, Barr GA. Developmental changes in the behavioral and autonomic effects of kappa opioid receptor stimulation of the midbrain periaqueductal gray. Dev. Psychobiol. 2005;46:47–56. doi: 10.1002/dev.20039. [DOI] [PubMed] [Google Scholar]
- Greasley PJ, Clapham JC. Inverse agonism or neutral antagonism at G-protein coupled receptors: a medicinal chemistry challenge worth pursuing? Eur. J. Pharmacol. 2006;553:1–9. doi: 10.1016/j.ejphar.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of Delayed Rewards: A Life-Span Comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Harrison C, Traynor JR. The [35S]GTPgammaS binding assay: approaches and applications in pharmacology. Life Sci. 2003;74:489–508. doi: 10.1016/j.lfs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hawkins KN, Knapp RJ, Lui GK, Gulya K, Kazmierski W, Wan YP, Pelton JT, Hruby VJ, Yamamura HI. [3H]-[H-D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2] ([3H]CTOP), a potent and highly selective peptide for mu opioid receptors in rat brain. J. Pharmacol. Exp. Ther. 1989;248:73–80. [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Niswender CM. Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J. Neurochem. 1999;73:1711–1717. doi: 10.1046/j.1471-4159.1999.731711.x. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol. Pharmacol. 2004;65:2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biol. Psychiatry. 2011;69:1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry. 2014;75:774–782. doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Walker BM. Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala kappa-Opioid Receptors. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.183. 10.1038/npp.2015.183[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, Walwyn W. Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking beta-arrestin 2. Mol. Pain. 2011;7:24. doi: 10.1186/1744-8069-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol. Pharmacol. 2001;60:53–62. doi: 10.1124/mol.60.1.53. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic agonist treatment converts antagonists into inverse agonists at delta-opioid receptors. J. Pharmacol. Exp. Ther. 2002;302:1070–1079. doi: 10.1124/jpet.102.035964. [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Herzog H, Schwarzer C, Quirion R. Possible role of dynorphins in Alzheimer's disease and age-related cognitive deficits. Neurodegener. Dis. 2014;13:82–85. doi: 10.1159/000353848. [DOI] [PubMed] [Google Scholar]
- Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS. J. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, van ZR, Smidt MP, Adan RA, Ramakers GM. Morphine withdrawal enhances constitutive mu-opioid receptor activity in the ventral tegmental area. J. Neurosci. 2012;32:16120–16128. doi: 10.1523/JNEUROSCI.1572-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol. Pharmacol. 2003;64:1271–1276. doi: 10.1124/mol.64.6.1271. [DOI] [PubMed] [Google Scholar]
- Munro TA, Huang XP, Inglese C, Perrone MG, Van't Veer A, Carroll FI, Beguin C, Carlezon WA, Jr., Colabufo NA, Cohen BM, Roth BL. Selective kappa opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters. PLoS. One. 2013;8:e70701. doi: 10.1371/journal.pone.0070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Institute for Laboratory Animal Research and National Academies Press . Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C.: 2011. [Google Scholar]
- Navani DM, Sirohi S, Madia PA, Yoburn BC. The role of opioid antagonist efficacy and constitutive opioid receptor activity in the opioid withdrawal syndrome in mice. Pharmacol. Biochem. Behav. 2011;99:671–675. doi: 10.1016/j.pbb.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, Choi DY, Foster TC, Usynin I, Bakalkin G, Bing G. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav. Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Parra S, Bond RA. Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr. Opin. Pharmacol. 2007;7:146–150. doi: 10.1016/j.coph.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, Toll L, McLaughlin JP. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J. Pharmacol. Exp. Ther. 2013;346:545–554. doi: 10.1124/jpet.113.206086. [DOI] [PubMed] [Google Scholar]
- Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol. Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadee W, Bilsky EJ. In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J. Pharmacol. Exp. Ther. 2005;313:1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. The Journal of pharmacology and experimental therapeutics. 2009;330:513–519. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Kumar P, Yoburn BC. Mu-opioid receptor up-regulation and functional supersensitivity are independent of antagonist efficacy. The Journal of pharmacology and experimental therapeutics. 2007;323:701–707. doi: 10.1124/jpet.107.127019. [DOI] [PubMed] [Google Scholar]
- Strange PG. Mechanisms of inverse agonism at G-protein-coupled receptors. Trends Pharmacol. Sci. 2002;23:89–95. doi: 10.1016/s0165-6147(02)01993-4. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O'Donnell P. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology. 2013;38:1770–1779. doi: 10.1038/npp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O'Donnell P. Prefrontal Cortical Kappa Opioid Receptors Attenuate Responses to Amygdala Inputs. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.138. 10.1038/npp.2015.138 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Kissler JL. Dissociable Effects of Kappa-Opioid Receptor Activation on Impulsive Phenotypes in Wistar Rats. Neuropsychopharmacology. 2013;38:2278–2285. doi: 10.1038/npp.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadee W. Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J. Neurochem. 2001;77:1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadee W. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J. Pharmacol. Exp. Ther. 2004;308:512–520. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J. Pharmacol. Exp. Ther. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Porreca F, Sadee W. Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:L339–L350. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Buchel C, Byrne M, Cummins TD, Fauth-Buhler M, Flor H, Gallinat J, Heinz A, Lttermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature neuroscience. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]