Abstract
AMPK is a cellular energy sensor, which is activated when the intracellular ATP production decreases. The activities of AMPK display circadian rhythms in various organs and tissues, indicating that AMPK is involved in the circadian regulation of cellular metabolism. In vertebrate retina, the circadian clocks regulate many aspects of retinal function and physiology, including light/dark adaption, but whether and how AMPK was involved in the retinal circadian rhythm was not known. We hypothesized that the activation of AMPK (measured as phosphorylated AMPK) in the retina was under circadian control, and AMPK might interact with other intracellular signaling molecules to regulate photoreceptor physiology. We combined ATP assays, Western blots, immunostaining, patch-clamp recordings, and pharmacological treatments to decipher the role of AMPK in the circadian regulation of photoreceptor physiology. We found that the overall retinal ATP content displayed a diurnal rhythm that peaked at early night, which was nearly anti-phase to the diurnal and circadian rhythms of AMPK phosphorylation. AMPK was also involved in the circadian phase-dependent regulation of photoreceptor L-type voltage-gated calcium channels (L-VGCCs), the ion channel essential for sustained neurotransmitter release. The activation of AMPK dampened the L-VGCC currents at night with a corresponding decrease in protein expression of the L-VGCCα1 pore-forming subunit, while inhibition of AMPK increased the L-VGCC current during the day. AMPK appeared to be upstream of extracellular-signal-regulated kinase (ERK) and mammalian/mechanistic target of rapamycin complex 1 (mTORC1) but downstream of adenylyl cyclase in regulating the circadian rhythm of L-VGCCs. Hence, as a cellular energy sensor, AMPK integrates into the cell signaling network to regulate the circadian rhythm of photoreceptor physiology.
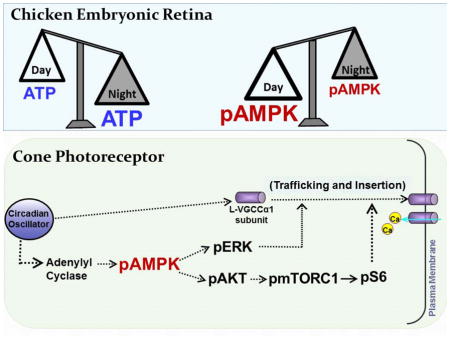
We found that in chicken embryonic retina, the activation of AMP-Activated Protein Kinase (AMPK) is under circadian control and anti-phase to the retinal ATP rhythm. While ATP content is higher at night, phosphorylated AMPK (pAMPK) is higher during the day. AMPK appears to be upstream of extracellular signal-regulated kinase (ERK), protein kinase B (AKT), and mammalian target of rapamycin complex 1 (mTORC1) but downstream of adenylyl cyclase in regulating the circadian rhythm of L-VGCCs. Therefore, as a cellular energy sensor, AMPK integrates into the cell signaling network to regulate the circadian rhythm of photoreceptor physiology.
Keywords: photoreceptor, circadian, metabolism, signaling
Graphical abstract
Introduction
AMP-Activated Protein Kinase (AMPK) is a cellular energy sensor that is responsible for balancing cellular metabolism. When the intracellular AMP to ATP ratio rises, AMPK is activated and promotes catabolic pathways while inhibiting anabolic pathways so that more ATP is generated (Hardie 2007). AMPK is a heterotrimeric protein kinase with a catalytic subunit (α) and two regulatory subunits (β and γ; Hardie 2007). When the intracellular ATP level is low, AMP or ADP binds to the AMPK γ subunit and leads to AMPK activation through the phosphorylation of threonine 172 (Thr172; Oakhill et al. 2011, Xiao et al. 2011). In addition to the binding of AMP or ADP, there are kinases and phosphatases that also modulate AMPK activation by targeting its Thr172, such as live kinase B1, Ca2+/calmodulin-dependent protein kinase kinase β (Hawley et al. 2003, Mihaylova & Shaw 2011, Woods et al. 2005), and protein phosphatase 2A and 2C (Davies et al. 1995, Kudo et al. 1996, Wu et al. 2007).
The activation of AMPK is not only regulated by the cellular energy expenditure and kinases/phosphatases, the activities of AMPK display circadian rhythms in various tissues and organs including the liver, muscles, and heart (Lamia et al. 2009, Um et al. 2011) indicating that either AMPK is under circadian control, or AMPK is part of the circadian regulation for cellular metabolism in these tissues. The endogenous circadian clocks regulate the physiology and behavior in living organisms and allow organisms to anticipate upcoming daily environmental changes, such as daily temperature fluctuations and cycling ambient illumination (Bell-Pedersen et al. 2005). Because the circadian clocks participate in metabolic processes throughout the day, elements of metabolism including metabolites, enzymes, transporters, and receptors all display daily rhythms (Panda et al. 2002). The canonical mechanism of circadian clocks is governed by a specific set of “clock genes” and their protein products, which generate self-regulated transcription-translation feedback loops with a period near 24 hours (Ko & Takahashi 2006). Post-translational modifications such as phosphorylation, ubiquitination, methylation, along with various cellular signaling pathways further contribute to the regulation of circadian oscillations (Gallego & Virshup 2007). Disruption of the clock genes perturbs the energy homeostasis and eventually leads to the development of various metabolic syndromes such as diabetes (Bass & Takahashi 2010, Froy 2010). While the activities of AMPK display circadian rhythms (Lamia et al. 2009, Um et al. 2011), stimulation of AMPK destabilizes cryptochrome, one of the specific clock proteins, and alters the circadian rhythms (Lamia et al. 2009). Genetic disruption of AMPK alters the circadian wheel running behavior and the expression of circadian genes in rodents (Um et al. 2011). Hence, AMPK is not only a cellular energy censor regulated by the circadian clock, it is important in circadian regulation at both cellular and systemic levels.
In the vertebrate retina, the circadian clocks are present in different cell types and involved in many aspects of retinal function and physiology. The circadian clocks in photoreceptors regulate the outer segment disk shedding and renewal (LaVail 1980), gene and protein expressions (Haque et al. 2002, Korenbrot & Fernald 1989, Liu et al. 2012, Pierce et al. 1993), as well as ion channel activities (Ko et al. 2001, Ko et al. 2007). The L-type voltage-gated calcium channels (L-VGCCs) located in the inner segment and synaptic terminals of photoreceptors are essential for cellular metabolism, calcium homeostasis, and neurotransmission (Barnes & Kelly 2002). The L-VGCCs are under circadian control in cone photoreceptors and bipolar cells (Hull et al. 2006, Ko et al. 2007). Both mRNA and protein expressions of L-VGCCα1D display circadian rhythms in cone photoreceptors (Ko et al. 2007). An intricate cell signaling network regulates the trafficking of L-VGCCs from the cytosol to the plasma membrane and is correlated to the circadian rhythms of L-VGCC currents (Huang et al. 2013, Huang et al. 2012, Ko et al. 2009, Ko et al. 2007, Ko et al. 2013).
Since transportation of ion channels to the plasma membrane requires energy, we postulate that AMPK, the energy sensor, might be required in the circadian regulation of L-VGCCs in photoreceptors, and the activation/phosphorylation of AMPK might also display circadian rhythm in the retina. In addition, because the retinal light sensitivities are under circadian regulation (Cameron and Lucas, 2009; Lu et al., 1995; Manglapus et al., 1998; McGoogan and Cassone, 1999), and the retinal energy consumption is light-dependent (Linton et al. 2010, Wei et al. 2012), we hypothesized that the overall retinal energy expenditure and production could be under circadian control. In this report, we examined the possibility of AMPK serving in the circadian regulation of L-VGCCs in the retina, which might shed light on the role of AMPK in modulating retinal light sensitivities.
Materials and Methods
Cell cultures and circadian entrainment
Fertilized eggs (Gallus gallus) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX, USA). Chicken retinas were dissociated at embryonic day 12 (E12) and cultured for 6–7 days as described previously (Ko et al. 2009, Ko et al. 2007). Cultures were prepared in the presence of 20 ng/ml ciliary neurotrophic factor (R&D Systems, Minneapolis, MN, USA), which yields cultures highly enriched with cone photoreceptors (above 70% of total cells; Adler & Hatlee 1989, Adler et al. 1984, Belecky-Adams et al. 1996) and 10% heat-inactivated horse serum. Cell culture incubators (maintained at 39°C and 5% CO2) were equipped with lights and timers for the entrainment of retinal circadian oscillators to 12h: 12h light-dark (LD) cycles in vitro. Zeitgeber time zero (ZT 0) was designated as the time when the lights turned on and ZT 12 was the time when the lights went off. For circadian time (CT) experiments, after LD entrainment chick embryos or cultured cells were kept in constant darkness (DD) for a 24 hour free-run period. Chick embryos were LD entrained for 7 days if whole retinas were used for experiments. For cultured cell experiments, chick embryos (in ovo) were LD entrained for 7–8 days. Retinas were then dissected, cultured, and kept in DD. On the second day of DD, tissues or cultured cells were collected at different CT time points (Ko et al. 2009, Ko et al. 2007). In these two day cultures, photoreceptors nearly reached 50% of total cells. We used chick embryos from E12+6 for in vitro entrainment or E18 for in ovo entrainment because more than 95% of the retinal photoreceptors will express functionally mature VGCC currents by E18 (Gleason et al. 1992). All experiments were approved by the Texas A&M University.
Adenosine 5′-triphosphate (ATP) assay
The ATP assay was carried out using the ATP bioluminescent assay kit (Sigma-Aldrich, St. Louis, MO, USA). Briefly, retinas harvested from E19 embryos at 6 different time points (ZT 1, 5, 9, 13, 17, and 21) were homogenized with a radioimmunoprecipitation assay (RIPA) buffer supplemented with 1mM NaF, 1mM Na3VO4, and a protease inhibitor cocktail (Sigma-Aldrich). The cell lysates were centrifuged at 13,000 rpm for 15 min at 4 °C. The supernatants were diluted (1/5) and processed for the ATP assay in a 96 well optiplate (Perkin-Elmer, Waltham, MA, USA). The intensity of luminescence was detected with a luminometer (Synergy 2 MultiMode Reader; BioTek, Winooski, VT, USA). Relative ATP values were normalized against ZT 1. Each experimental group contained at least 7 different experiments (n=7 for each group).
Immunoblot analysis
Samples were collected and prepared as described previously (Huang et al. 2013, Huang et al. 2012). Briefly, intact retinas were homogenized in Tris lysis buffer including (in mM): 50 Tris,1 EGTA, 150 NaCl, 1% Triton X-100, 1% β-mercaptoethanol, 50 NaF, 1 Na3VO4; pH 7.5. Samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gels by electrophoresis and transferred to nitrocellulose membranes. The primary antibodies used in this study were: anti-di-phospho-ERK (pERK; Sigma-Aldrich), anti-ERK (total ERK, used for loading control; Santa Cruz Biochemicals, Santa Cruz, CA, USA), anti-phospho-AMPK (Thr172, Cell Signaling Technology, Danvers, MA, USA), anti-AMPK (total AMPK, Cell Signaling Technology), anti-phospho-S6 (ser240/244, Cell Signaling Technology), anti-S6 (total S6, Cell Signaling Technology), anti-phospho-AKT (Thr308, Cell Signaling Technology). Blots were visualized using appropriate secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology) and an enhanced chemiluminescence detection system (Pierce, Rockford, IL, USA). Relative expressions for all proteins involved in this study are reported as a ratio to total ERK, which remains constant throughout the day. Band intensities were quantified by densitometry using Scion Image (NIH, Bethesda, MD, USA). Each experimental group contained at least 4 different experiments (n=4 for each group).
Electrophysiology
Whole cell patch-clamp configuration of L-VGCC current recordings were carried out using mechanically ruptured patches. For retinal photoreceptors, the external solution was (in mM): 110 NaCl, 10 BaCl2, 0.4 MgCl2, 5.3 KCl, 20 TEA-Cl, 10 HEPES, and 5.6 glucose, pH 7.35 with NaOH. The pipette solution was (in mM): 135 Cs acetate, 10 CsCl, 1 NaCl, 2 MgCl2, 0.1 CaCl2, 1.1 EGTA, and 10 HEPES, pH 7.3 adjusted with CsOH. Recordings were only made from cells with elongated cell bodies with one or more prominent oil droplets (hallmark of avian cone photoreceptors; Gleason et al. 1992, Ko et al. 2001, Pierce et al. 1993). Currents were recorded at room temperature (RT, 23°C) using an A-M 2400 amplifier (A-M Systems Inc., Carlsborg, WA, USA). Signals were low-pass filtered at 2 kHz and digitized at 5 kHz with Digidata 1440A interface and pCLAMP 10.0 software (Molecular Devices, Sunnyvale, CA, USA). Electrode capacitance was compensated after gigaohm (GΩ) seals were formed. Cells were held at −65 mV, and ramp voltage commands from −80 to +60 mV in 500 ms were used to evoke Ba2+ currents. Current–voltage (I–V) relations were also elicited from a holding potential of −65 mV in 200 ms steps (5 s between steps) to test potentials over a range of −80 to +60 mV in 10 mV increments. The maximal currents were obtained when the steps depolarized to 0 ~ +10 mV. The membrane capacitance, series resistance, and input resistance of the recorded photoreceptors were measured by applying a 5 mV (100 ms) depolarizing voltage step from a holding potential of −65 mV. Cells with an input resistance smaller than 1 GΩ were discarded. The membrane capacitance reading was used as the value for whole cell capacitance. The current densities (pA/pF) were obtained by dividing current amplitudes by membrane capacitances. Leak currents were subtracted manually after data acquisition.
5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and compound C were obtained from EMD Millipore (Billerica, Massachusetts, USA) and Sigma, respectively. AICAR was dissolved in water, while Compound C was dissolved in DMSO (the final concentration of DMSO vehicle was 0.1%). The concentration of AICAR (Yang et al. 2011) and Compound C (Bain et al. 2007) used in this study were based on previous studies using these inhibitors in various neuronal tissue or cell preparations.
Immunocytochemistry
Samples were collected and prepared as described previously (Huang et al. 2013). Briefly, dissociated retinas were cultured on coverslips and entrained under LD cycles. Cells were fixed at CT4 or CT16 with Zamboni fixative then permeabilized in 1% Triton-X phosphate buffer (PB). Samples were blocked in 10% goat serum in 0.1% Triton-X/PB and incubated with VGCCα1D primary antibody (1:100 dilution; Alomone, Jerusalem, Israel) at 4°C overnight. The fluorescent conjugated secondary antibody (1:200 dilution; Alexa Fluor® 488 goat anti-rabbit; Molecular Probes, Carlsbad, CA, USA) was applied on the coverslips at room temperature for 2 h in the dark. Coverslips were then re-washed and mounted with ProLong® Gold antifade reagents with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Eugene, OR, USA) on a glass slide and stored at 4°C for later observation on a Zeiss Stallion microscope (Carl Zeiss AG, Oberkochen, Germany) with epi-fluorescence to determine the localization of VGCCα1D and the nucleus (with DAPI). Green or blue fluorescent images were taken under identical settings including exposure time and magnification. The fluorescence intensity was measured using Adobe Photoshop 12 software (Adobe Systems, San Jose, CA, USA) as described previously (Ko et al. 2007). The fluorescence intensity analyses were done blind. Each experimental group contained at least 4 different experiments (n=4 for each group).
Statistical analysis
All data are presented as mean ± SEM (standard error of mean). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for unbalanced n was used for statistical analyses. Throughout, * p<0.05 was regarded as significant. Any defined rhythmic expression had to exhibit at least a 1.5 fold change in rhythmic amplitude (Karaganis et al. 2008).
Results
Retinal ATP content and AMPK activity are under circadian control
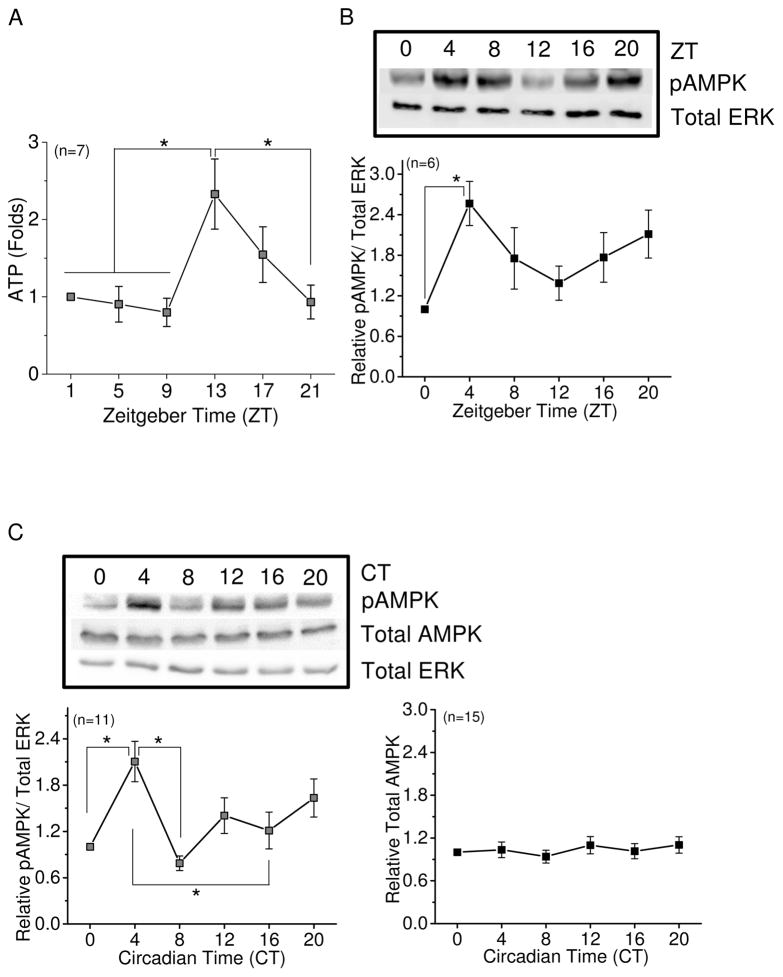
We first examined whether the overall retinal energy levels oscillated daily. Embryonic chicken retinal samples were collected at six different time points throughout a day. The overall retinal ATP content was higher at night with a peak at ZT 13 and lower during the day (Fig. 1A). Since AMPK is the cellular energy sensor, we next examined whether the activation/phosphorylation of AMPK in the retina was under circadian control. Because the AMPKα1 protein, as well as AMPKβ2 and AMPKγ1 mRNAs, display circadian rhythms in the liver (Lamia et al. 2009), we used total ERK as the loading control because retinal total ERK remains constant throughout the day (Ko et al. 2001). We found that phosphorylated AMPK at Thr172 (pAMPK), the major site of AMPK phosphorylation and activation, peaked at ZT 4 when the chicken embryos were entrained under LD cycles (Fig. 1B). The diurnal rhythm of pAMPK was nearly anti-phase with the retinal ATP rhythm. To further confirm that the rhythmicity of AMPK activities (measured as pAMPK) was indeed governed by the retinal circadian clock, retinas were collected (every four hours) on the second day of constant darkness (DD) after embryos were LD entrained. The pAMPK showed a circadian rhythm with a peak during the subjective day at CT 4 (Fig. 1C). Different from the liver (Lamia et al. 2009), the total amount of retinal AMPK (total AMPK) remained constant throughout the day (Fig. 1C). Hence, the retinal ATP content displayed a diurnal rhythm, which revealed the summation of overall ATP production and consumption in the retina. The activity of AMPK was under circadian regulation in the retina, which might reflect the overall retinal energy expenditure throughout a day.
Figure 1.
The retinal ATP content and AMPK activity are under circadian control. The retinas were collected at 6 different time points throughout a day for ATP assays or immunoblotting analyses after entrainment to 12: 12 hr LD cycles for 8 days in ovo. (A) The overall retinal ATP content displays a diurnal rhythm with a peak at ZT 13. * indicates that ZT 13 is significantly different from ZT 1, 5, 9, and 21. (B) The phosphorylation of AMPK at Thr172 (pAMPK) shows a diurnal rhythm with a peak at ZT 4. * indicates that ZT 4 is significantly different from ZT 0. (C) After entrainment to 12: 12 hr LD cycles for 7 days in ovo, the eggs were moved to DD. On the second day of DD, the retinas were collected at 6 circadian time points for immunoblotting analyses. pAMPK exhibits a circadian rhythm with its peak at CT 4 (left panel), while the total amount of AMPK remains constant throughout a day (right panel). * indicates that CT 4 is significantly different from CT 1, 8, and 16 (left panel). * p<0.05.
AMPK is involved in the circadian regulation of L-VGCC currents
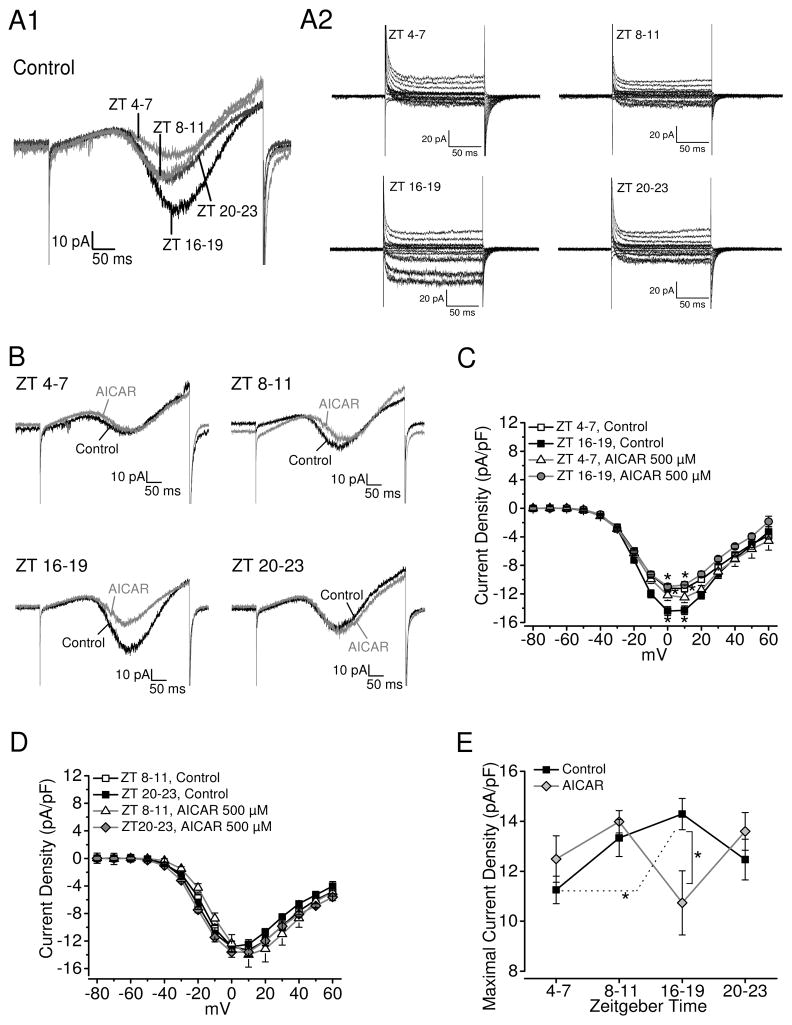
There is a circadian rhythm of L-VGCCs in chick cone photoreceptors, with peak maximal currents elicited at 0 mV in the middle of the night (ZT 16–19) and trough during the middle of the day (ZT 4–7; Fig 2A; Ko et al. 2007). To investigate the role of AMPK in modulating the circadian rhythm of L-VGCCs, we applied the AMPK activator, AICAR, an analog of AMP, to cultured photoreceptors for 2 hr prior to patch-clamp recordings. Treatments with AICAR (500 μM) significantly dampened the L-VGCC currents when cone photoreceptors were recorded in the middle of the night (ZT 16–19; Fig 2B, C, E) but did not have any significant effect on these currents when cells were recorded during the middle of the day (ZT 4–7), late day (ZT 8–11), or late night (ZT 20–23; Fig. 2B–E).
Figure 2.
The AMPK activator AICAR dampens the circadian regulation of L-VGCC currents. The L-VGCC currents were recorded from cultured chick cone photoreceptors on the sixth day of LD entrainment during the day (ZT 4–7 or ZT 8–11) or at night (ZT 16–19 or ZT 20–23). All cells were recorded using a ramp command from −80 to 60 mV in 500 ms, as well as a step command with holding potential at −65 mV and steps from −80 to 60 mV at 10 mV increments. (A) Representative day (4–7 or 8–11) and night (16–19 or 20–23) L-VGCC current traces recorded from the control with (A1) ramp command or (A2) step command. (B) Representative traces of L-VGCC currents from cells that were treated with AICAR (500 μM; gray) for 2 hr prior to recordings compared to control cells (black) during different time periods: ZT 4–7, 8–11, 16–19, and 20–23. (C) The average current-voltage relationships are shown in current density (pA/pF) and step-voltage (mV). * indicates that the current densities of L-VGCCs from the control recorded at night (ZT 16–19; black square) were significantly higher than the control (white square) and AICAR treated cells recorded during the day (ZT 4–7; white triangle). (D) The average current-voltage relationships are shown in current density (pA/pF) and step-voltage (mV) from cells recorded either at ZT 8–11 or ZT 20–23. White square: control cells recorded at ZT 8–11; black square: control cells recorded at ZT 20–23; white triangle: AICAR-treated cells recorded at ZT 8–11; gray diamond: AICAR-treated cells recorded at ZT 20–23. There was no statistical difference between these four groups. (E) The maximal current densities were elicited at 0 mV of the step command at different ZT time periods. * indicates that the current densities recorded at night (control group, ZT 16–19) are significantly larger than during the day (control, ZT 4–7) and AICAR treated cells recorded at night (AICAR, ZT 16–19). Each group had at least 15 cells except the AICAR-treated group at ZT 8–11 (5 cells). *p<0.05.
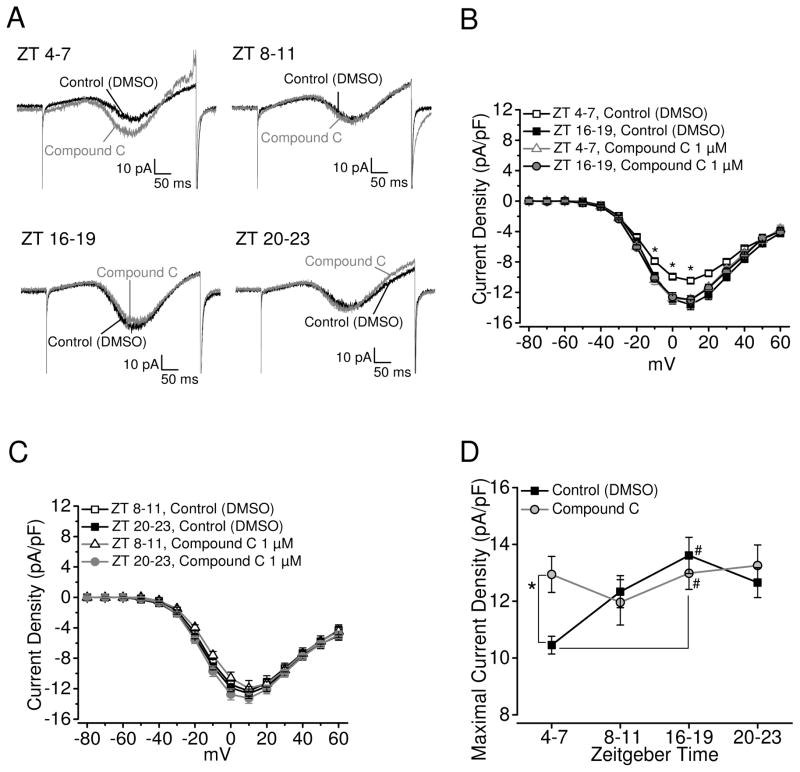
When photoreceptors were treated with Compound C (1μM), an AMPK inhibitor, for 2 hr prior to recordings, the L-VGCC currents were significantly enhanced when the cone photoreceptors were recorded during the middle of the day (ZT 4–7) compared to the control treated with 0.1% DMSO (Fig 3A, B, D). Inhibition of AMPK did not affect L-VGCCs when photoreceptors were recorded during other time periods of the day (Fig 3). These results indicate that AMPK has a circadian phase-specific effect on L-VGCCs in cone photoreceptors: when AMPK is activated by AICAR in the middle of the night (ZT 6–19), the L- VGCCs are significantly decreased, and when AMPK is inhibited by Compound C in the middle of the day (ZT 4–7), the L-VGCCs are significantly enhanced.
Figure 3.
Inhibition of AMPK increases the L-VGCC current densities during the day. (A) Representative L-VGCC current traces from 0.1% DMSO-treated cone photoreceptors (control; black) and 1 μM compound C-treated cells (gray) that were recoded from ZT 4–7, 8–11, 16–19, and 20–23. (B) The average current-voltage relationships are shown in current density (pA/pF) and step-voltage (mV). * indicates that the current densities of L-VGCCs from the control recorded at ZT 4–7 (white square) are significantly lower than the control recorded at night (ZT 16–19; black square) as well as compound C treated cells recorded during the day (ZT 4–7; white triangle) and at night (ZT 16–19; gray circle). (C) The average current-voltage relationships are shown in current density (pA/pF) and step-voltage (mV) from cells recorded from ZT 8–11 or ZT 20–23. White square: control cells recorded at ZT 8–11; black square: control cells recorded at ZT 20–23; white triangle: compound C-treated cells recorded at ZT 8–11; gray circle: compound C-treated cells recorded at ZT 20–23. There was no statistical difference between these four groups. (D) The maximal current densities were elicited at 0 mV of the step command in different ZT phases. * indicates that the current densities of the compound C group are significantly larger than the current densities of the control group recorded during the day (ZT 4–7). # indicates that the current densities of the control group as well as compound C-treated group recorded at night (ZT 16–19) are significantly greater than the control group recorded during the day (ZT 4–7). Each group had at least 15 cells. * p<0.05.
AMPK modulates the protein expression of L-VGCCα1D
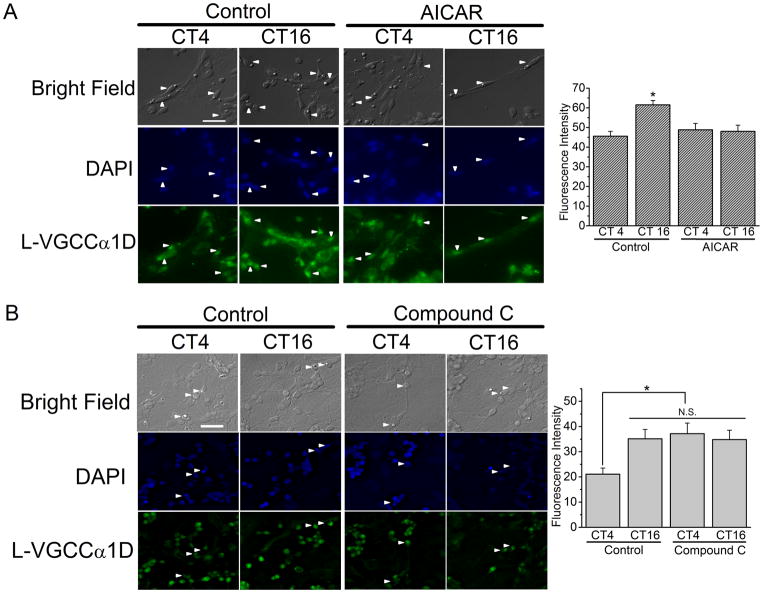
Since AMPK participated in the circadian phase-dependent modulation of L-VGCC currents in cone photoreceptors, we examined whether AMPK affected the protein expression of L-VGCCs. In both mammalian and avian retina, L-VGCCα1D is extensively present in the inner segments, soma, and synaptic terminals of the photoreceptors (Huang et al. 2013, Kersten et al. 2010, Ko et al. 2007). The fluorescence intensity of L-VGCCα1D in cones was significantly higher when cultured cells were fixed at CT 16 compared to cells fixed at CT 4 (Fig 4; Ko, 2007). Treatment with AICAR for 2 hr prevented the increase of L-VGCCα1D fluorescent intensity at night (CT 16) but not during the day (CT 4) in cone photoreceptors (Fig 4A). On the other hand, treatment with Compound C for 2 hr significantly increased the fluorescent intensity of L-VGCCα1D in cone photoreceptors fixed at CT 4 but not at CT 16 (Fig 4B). Hence, activation of AMPK by AICAR reduced the protein expression of L-VGCCα1D, but inhibition of AMPK by Compound C enhanced the protein expression of L-VGCCα1D in cone photoreceptors, which echoed the results from patch-clamp recordings of L-VGCC currents (Fig. 2).
Figure 4.
There is a circadian phase-dependent effect of AMPK activation on the protein expression of L-VGCCs in cone photoreceptors. Representative images from cultured retinal cells are shown. Retinal cells were dissociated and cultured on glass coverslips at E12 and entrained to 12:12 LD cycles for five days in vitro and kept in DD. On the second day of DD, cells were treated with AICAR (A) or Compound C (B) at CT 2 and CT 14 for 2 hr followed by fixation. (A) The left panel shows the fluorescent images from the control and AICAR treated cells. The right panel shows that the fluorescent intensity of L-VGCCα1D in cone photoreceptors is significantly higher at CT 16 of the control compared to the other three groups, as denoted with *. (B) The left panel shows the fluorescent images from the control and Compound C treated cells. The right panel shows that the fluorescent intensity of L-VGCCα1D in cone photoreceptors is significantly lower at CT 4 of the control compared to all other groups. The arrowheads indicate the cone photoreceptors. The scale bar is 20 μm. Each group has at least 20 cells from 4 different trials. * p<0.05.
cAMP-dependent signaling mediates AMPK activities
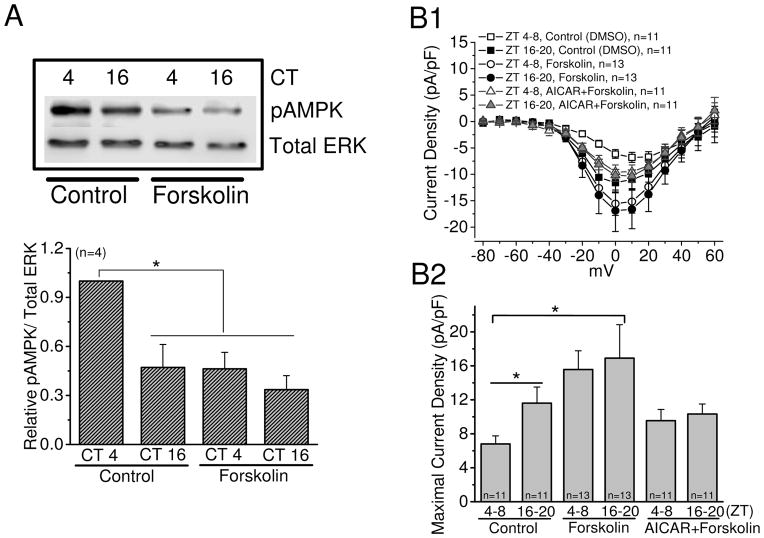
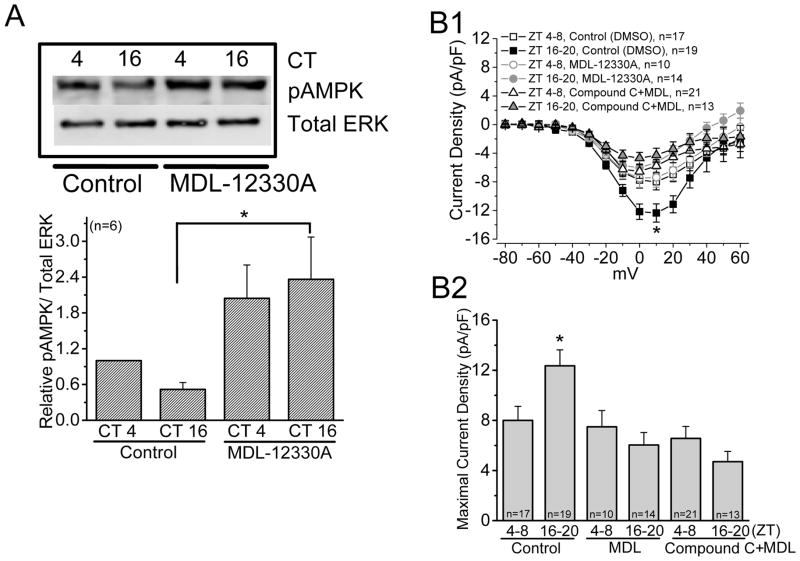
Cyclic AMP is a second messenger that plays important roles in many physiological processes including metabolism (Sutherland & Robison 1969), and its signaling is involved in the regulation of AMPK activities in a tissue-specific manner (Djouder et al. 2010, Hurley et al. 2006, Omar et al. 2009, Yin et al. 2003). We and others previously showed that not only is retinal cAMP under circadian control with its peak at night (Huang et al. 2012, Ivanova & Iuvone 2003, Nikaido & Takahashi 1998), cAMP-signaling is involved in the circadian regulation of cGMP-gated cation channels and L-VGCCs in avian photoreceptors (Huang et al. 2012, Ko et al. 2004). We therefore investigated the relationship between cAMP signaling and AMPK in the retina. Activation of adenylyl cyclase by forskolin (20 μM) resulted in a diminished phosphorylation of AMPK (pAMPK) during the subjective day (Fig 5A) and significantly increased L-VGCC currents when cells were recorded during the day (ZT 4–8; Fig 5B). Application of AICAR in the presence of forskolin was able to dampen the augmentation effect of forskolin on L-VGCC currents (Fig. 5B). In addition, treatment with the adenylyl cyclase inhibitor MDL-12330A (50 μM) significantly increased the AMPK activity during the subjective night (Fig 6A) and significantly decreased L-VGCC currents when cells were recorded at night (ZT 16–20; Fig 6B), but inhibition of AMPK with Compound C was not able to reverse the effect of MDL-12330A (Fig 6B) even though Compound C alone was able to increase L-VGCC currents when cells were recorded during the day (Fig. 3). These data suggest that cAMP signaling was upstream of AMPK in the circadian regulation of cone L-VGCCs and was a negative regulator of AMPK, with the circadian phase of retinal cAMP (Huang et al. 2012, Ivanova & Iuvone 2003, Nikaido & Takahashi 1998) anti-phase to that of pAMPK (Fig. 1).
Figure 5.
Activation of adenylyl cyclase with forskolin inhibits the activity of AMPK. The retinas were excised and cultured in DD after chick embryos were entrained under LD cycles for 7 days. On the second day of DD, the cultured retinas were treated with DMSO (0.1%), forskolin, or forskolin+AICAR for 2 hr prior to harvest for immunoblotting at CT 4 and CT 16. (A) Treatment with forskolin (20 μM) at CT 4 significantly decreased the phosphorylation of AMPK at Thr172 (pAMPK) compared to the control (0.1% DMSO). * indicates that pAMPK is significantly higher in the control at CT 4 than the other three groups. (B1 and B2) Treatment with forskolin alone significantly increases the L-VGCC current densities when cones were recorded during the day (ZT 4–8), and AICAR dampens the augmentation effect of forskolin on L-VGCCs. * p<0.05.
Figure 6.
Adenylyl cyclase inhibitor MDL-12330A enhances the phosphorylation of AMPK. (A) Application of MDL-12330A (50 μM) significantly increases AMPK phosphorylation at CT 16 compared to the control (at CT 16). (B) Treatment with MDL-12330A alone significantly decreases the L-VGCC currents when cones were recorded at night (ZT 16–20). Inhibition of AMPK with Compound C did not reverse the effect of MDL-12330A. * indicates that the control at ZT 16–20 is significantly different from all other groups. * p<0.05.
AMPK regulates the mTORC1 signaling pathway
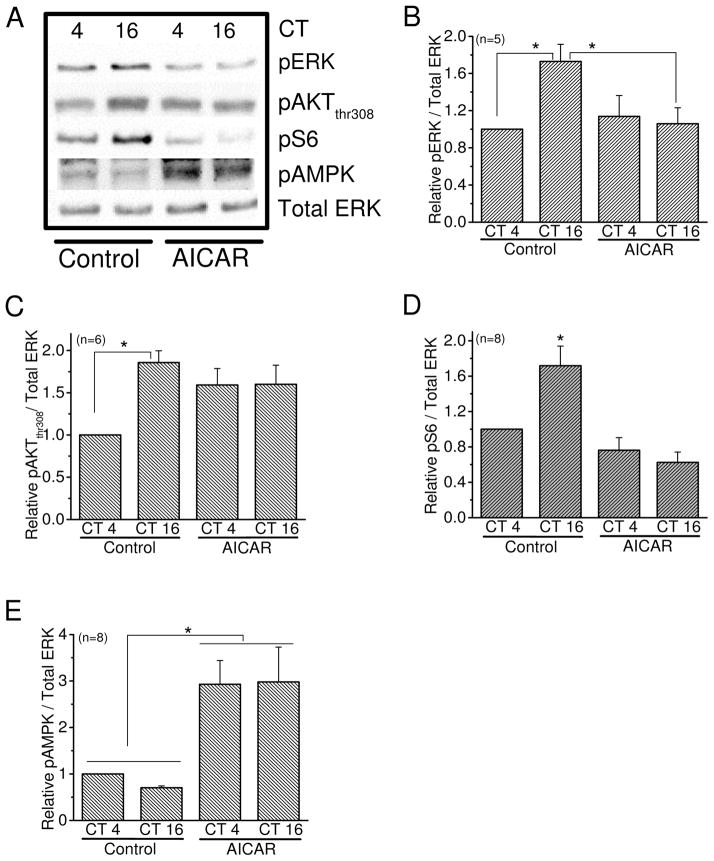
We previously showed that both MAPK-ERK and PI3K-AKT signaling pathways are downstream of cAMP and parallel to each other in the circadian regulation of L-VGCC trafficking (Huang et al. 2013, Huang et al. 2012, Ko et al. 2009, Ko et al. 2007). In addition, mTORC1 signaling is a downstream target of PI3K-AKT in regulating the circadian rhythm of L-VGCCs (Huang et al. 2013). We further examined whether AMPK interacted with these signaling pathways in the circadian regulation of L-VGCCs. We found that activation of AMPK with AICAR decreased the phosphorylation of ERK (pERK; Fig 7A, B) and S6 (pS6), a downstream target of mTORC1 signaling, at night (Fig 6A, D) and dampened the circadian rhythm of pAKT (Fig 7A, C). Hence, AMPK was able to integrate with MAPK-ERK, PI3K-AKT, and mTORC1 as part of the complex signaling network in the circadian output regulation of L-VGCCs.
Figure 7.
AMPK is a negative regulator of mTORC1 signaling. Chick embryos were LD entrained for 7 days. At E17, the retinas were dissected and cultured in DD. On the second day of DD, the cultured retinas were treated with the AMPK activator AICAR for 2 hr. The retinal samples were collected at CT 4 and CT 16 for immunoblotting analyses. (A) The di-phospho-ERK (pERK), phospho-AKT at Thr308 (pAKT), phospho-S6 (pS6), and phospho-AMPK at Thr172 (pAMPK) were detected from samples treated with AICAR (500μM) or control. Since the total amounts of ERK, AKT, and S6 remained constant throughout the day, total ERK was used as loading control. (B) After treatment with AICAR, pERK is decreased at CT 16 compared to the control group at CT 16. * indicates that the control group harvested at CT 16 is significantly higher than CT 4 of control and the AICAR-treated at CT 16. (C) Treatment with AICAR seemed to increase pAKT at CT 4, but it did not reach statistical significance. * indicates a significant difference between CT 4 and CT 16 of the control groups. (D) The level of pS6 is significantly dampened with AICAR treatments compared to the control group at CT 16. * indicates the control group at CT 16 is significantly higher than the other three groups. (E) The phosphorylation of AMPK served as an internal control showing that treatment with AICAR for 2hr increases AMPK activities. * indicates a significant difference between the AICAR groups and control groups at both CT 4 and CT 16, respectively. * p<0.05.
Discussion
In this study, we demonstrated that the overall retinal energy expenditure and production was under circadian control through the measurement of retinal ATP content and AMPK activation (measured as pAMPK), which were nearly anti-phase to each other. Total AMPK in the retina remained constant throughout the course of a day, which was different from a previous report on the circadian oscillations of AMPK (both mRNA and protein expressions) in the liver. Since the liver is the major organ responsible for whole body metabolism, while the retina mainly utilizes glucose as its primary energy source with retinal glucose levels fluctuating following systemic glycemia (Ola et al. 2006, Puchowicz et al. 2004), the difference of AMPK circadian rhythm in the liver versus retina might imply that the AMPK circadian rhythm could be tissue-specific to reflect the functional differences of AMPK in different tissues. Retinal AMPK participated in the circadian regulation of L-VGCCs in the photoreceptors. Activation of AMPK by AICAR led to decreased protein expression and current densities of L-VGCCs at night, while inhibition of AMPK by Compound C resulted in the enhancement of L-VGCC currents during the day in cone photoreceptors. This result also echoes that normally during the day, retinal AMPK activity is higher (Fig. 1) with the current density of L-VGCCs being lower, and at night, retinal AMPK activity is lower with the L-VGCC current density reaching its peak. While AMPK was downstream of cAMP signaling, it integrated into the signaling pathways that regulate L-VGCC trafficking including MAPK-ERK (Ko et al. 2001, Ko et al. 2007), PI3K-AKT (Ko et al. 2009), and mTORC1-S6 (Huang et al. 2013). Even though the complexity of the signaling network that regulates L-VGCCs still requires more thorough investigation, our results showed that AMPK was capable of interacting with multiple signaling pathways, which indicates that AMPK might have multiple functions other than serving as an energy sensor.
In the retinal photoreceptors, energy consumption is highly compartmentalized (Linton et al. 2010, Wei et al. 2012). There is heavy energy expenditure in the outer segments of photoreceptors where phototransduction and protein transport for outer segment renewal are taking place in response to various light intensities (Korenbrot 1995, Koutalos & Yau 1996). In the dark, most of the energy consumption is in the inner segments and synaptic terminals to maintain the dark currents and neurotransmitter release (Wong-Riley 2010). Energy expenditure and production reaches a homeostatic state in any healthy cell. The retinal photoreceptors have a higher metabolic activity in the dark, which means that in one hand, ATP is hydrolyzed in an accelerated rate to support tonic neurotransmitter release. On the other hand, mitochondria will have to produce more ATP to sustain photoreceptor activities. As a result, the mitochondrial enzymes that are responsible for ATP production should be more activated in darkness, which is supported by Huang et al. (2004) where the mitochondrial enzymes cytochrome C oxidase III and adenosine triphosphatase-6 are downregulated by higher light intensity. Thus, the overall ATP production may be lower in the presence of bright light. We found that the overall energy state fluctuated in embryonic chick retina throughout the day (Figure 1A): while the AMPK activity peaked during the mid-day, the ATP content (as the summation of energy expenditure and production) peaked at early night. By measuring the pH changes in the retina, Dmitriev and Mangel (2004) demonstrated that there is a circadian regulation of retinal energy metabolism. Therefore, the retinal energy expenditure and production can be both light intensity-dependent as a reflection of acute light/dark adaptation, as well as circadian clock-regulated as the retina “anticipates” and adapts to the upcoming light changes at dawn and dusk.
Since we measured ATP from the whole retina, the ATP production might not be photoreceptor or retinal neuron specific. ATP receptors are present in all retinal neurons, and ATP is known to serve as a neurotransmitter in the retina and the brain (Ho et al. 2014). ATP and other purines (ADP, AMP, adenosine, adenine, and hypoxanthine) are tonically released in the retina particularly in the dark (Perez et al. 1986), and such release is increased by neuronal activity (Neal & Cunningham 1994). However, Müller glia cells are also able to generate, release, and accumulate extracellular ATP (Loiola & Ventura 2011). Interestingly, in cultured cortical glia cells, extracellular ATP accumulation displays circadian rhythms (Burkeen et al. 2011, Womac et al. 2009). Hence, extracellular ATP could serve as a neurotransmitter to communicate among multiple circadian oscillators existing in the different retinal cell types (Liu et al. 2012, Ruan et al. 2006). In addition, the extracellular ATP can further be converted to adenosine, which could lead to a circadian rhythm of retinal adenosine with a higher concentration at night than during the day (Ribelayga & Mangel 2005). Retinal adenosine is known to regulate circadian rhythms of photoreceptor coupling, as well as retinal light/dark adaptation (Li et al. 2013, Choi et al. 2012, Ribelayga et al. 2008, Ribelayga & Mangel 2007). Therefore, the circadian rhythm of retinal ATP might not only reflect the circadian control of energy status, but it might implicate that ATP and its metabolite adenosine could serve as neuromodulators to coordinate the various circadian oscillators in the retina, so that the overall retinal circadian rhythm is integrated as a way for the retina to adapt to ambient light changes over 12 orders of magnitude over the course of a day (Green & Besharse 2004). It is our great interest to investigate the different sources of retinal ATPs (from retinal neurons versus glia cells) and their contribution to the retinal circadian rhythm and light sensitivities in the future.
The activation of AMPK with AICAR led to attenuation of protein expression and current densities of L-VGCCs at night (Figs 2 and 4). AMPK and its upstream kinase LKB1 are involved in retina synaptic transmission (Samuel et al. 2014). Deletion of either LKB1 or AMPK in young mice reduced ERG a- and b- waves and caused ectopic synapses in the outer retina, where photoreceptors show abnormal axonal retractions, but bipolar and horizontal cells extend their dendrites into the outer nuclear layer (Samuel et al. 2014). These hallmarks caused by deletion of AMPK or LKB1 occur naturally in old mice as part of the aging process, and elevation of AMPK in old mice can rescue these aged-associated synaptic alterations (Samuel et al. 2014). Since L-VGCCs are essential for neurotransmitter release in the synaptic terminals of retinal neurons, the circadian phase-dependent regulation of L-VGCCs by AMPK might be critical for maintaining morphological and functional synapses in the retina, as well as retinal light sensitivities, in addition to AMPK acting as an energy sensor.
As a cellular energy sensor, AMPK regulates many aspects of cellular physiological processes such as the metabolism of glucose, lipids, and proteins, and modulation of ion channels and transporters (Steinberg & Kemp 2009). How AMPK regulates ion channels is diverse and tissue/cell type-specific (Andersen & Rasmussen 2012, Dermaku-Sopjani et al. 2014). For example, constitutively active AMPK slows the inactivation of voltage-gated sodium channels (Nav1.3) and shifts the voltage-activation curve toward more hyperpolarized potentials in rat ventricular myocytes (Light et al. 2003). Co-expression of AMPK with Ca2+-sensitive large conductance potassium channels (BK channel) in Xenopus oocytes enhances the current and protein expression of BK channels in the cell membrane, and BK channel expression in inner ear cells is reduced in AMPK−/− mice (Foller et al. 2012). Meanwhile, AMPK inhibits the current densities of BK channels in rat carotid body type I cells (Ross et al. 2011, Wyatt et al. 2007). Even though AMPK has been found to regulate sodium and potassium channels as well as various transporters, our study is the first to demonstrate the role of AMPK in regulating calcium channels, in which AMPK might be important for the regulation of calcium-dependent synaptic transmission in the nervous system. Since photoreceptors are non-spiking neurons (Barnes & Kelly 2002), calcium influx through L-VGCCs at the synaptic terminals allows for the continuous release of neurotransmitters from the ribbon synapses (Sterling & Matthews 2005). Here, we show that AMPK regulated the L-VGCCs in cone photoreceptors, which has not been reported previously. The L-VGCCs in cone photoreceptors exhibit circadian rhythms with the currents reaching maximum during the middle of the night (Ko et al. 2007). Activation of AMPK decreased the L-VGCC currents in cone photoreceptors in the middle of the night with a corresponding decrease in L-VGCCα1D protein, while inhibition of AMPK caused the opposite effect during the mid-day. These results give the first insight into the role of AMPK in circadian phase-dependent regulation of L-VGCCs, which indicates that AMPK might be important for photoreceptors to respond to various ambient light throughout the course of a day.
The circadian regulation of L-VGCCs is in part through two parallel pathways: Ras-ERK and Ras-PI3K-AKT, and both are downstream of cAMP (Ko et al. 2009, Ko et al. 2007, Woods et al. 2005). Cyclic AMP had a negative action on AMPK, as we discovered that the activation of adenylyl cyclase by forskolin reduced AMPK activity, but inhibition of adenylyl cyclase increased AMPK phosphorylation (Fig 5). While our result was similar to previous studies that showed activation of cAMP-protein kinase A (PKA) signaling decreases the phosphorylation of AMPK in several cultured cell-lines and adipocytes (Djouder et al. 2010, Hurley et al. 2006), cAMP signaling apparently increases AMPK phosphorylation on different amino acid residues in adipocytes (Omar et al. 2009, Yin et al. 2003). The seemly conflicting reports on how cAMP signaling interacts with AMPK indicates that cAMP signaling triggered increase or decrease of AMPK phosphorylation could be “phosphorylation site” and tissue specific. Phosphorylation of AMPK at different amino acids might contribute to different cellular functions, which will be very interesting for future investigation. Further, the activity of AMPK is influenced by other various factors including the intracellular Ca2+ concentration, the cellular AMP/ATP ratio, nitric oxide formation, and other metabolite signals (Mihaylova & Shaw 2011, Viollet et al. 2010). We previously showed that the retinal nitric oxide content and the expression of neuronal nitric oxide synthase display circadian rhythms, and nitric oxide-dependent signaling further modulates L-VGCCs in cone photoreceptors (Ko et al. 2013). Hence, it is possible that Ca2+ influx through L-VGCCs, as well as nitric oxide-signaling might influence the activity of AMPK and subsequently promote AMPK to regulate L-VGCC trafficking in cone photoreceptors.
In our previous study, we found that mTORC1-S6K-S6 signaling is a downstream target of PI3K-AKT to modulate L-VGCCα1D trafficking and translocation (Huang et al. 2013). The stimulation of AMPK significantly diminished the phosphorylation of S6 and moderately decreased the phosphorylation of ERK (Fig 6). The integration of AMPK into various signaling pathways to regulate L-VGCCs demonstrates the complexity of cell-signaling networks in the circadian outputs to regulate physiology and function at the cellular level. It also illustrates that AMPK may have multiple roles other than serving as an energy sensor in a cell. Regulation of ion channels, such as L-VGCCs, is energy consuming, since transporting synthesized channel proteins to the plasma membrane and membrane retention of channel proteins require energy. The circadian fluctuation of photoreceptor AMPK activation might be a way for photoreceptors to be more energy efficient when light intensities change throughout the day.
Taken together, we showed that the activity of AMPK was under circadian control in the retina, with the retinal ATP content displaying a diurnal rhythm. This report is the first to demonstrate the diurnal/circadian rhythm of the overall retina energy status. There was a circadian phase-dependent regulation of L-VGCCs by AMPK, and the action of AMPK was through crosstalk with ERK and mTORC1-S6, which was part of the complex signaling network in the circadian output regulation of photoreceptor physiology. Since L-VGCCs are essential in retinal neurotransmission, these results imply that AMPK plays an important role in the retina in regulating light sensitivities and adaptation to changes in ambient illumination across several orders of magnitude in the course of a day (Green & Besharse 2004).
Acknowledgments
We are very grateful for the comments from Drs. Louise Abbott and Evelyn Tiffany-Castiglioni. This study was supported by NIHR01EY017452 and NIHR21EY023339 from the National Eye Institute of the National Institutes of Health to G.K.
Abbreviations
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- AKT
protein kinase B
- AMPK
AMP-Activated Protein Kinase
- CT
circadian time
- DAPI
4′,6-diamidino-2-phenylindole
- DD
constant darkness
- ERK
extracellular signal-regulated kinase
- L-VGCC
L-type voltage-gated calcium channel
- LD
light-dark
- MAPK
mitogen-activated protein kinase
- mTORC1
mammalian target of rapamycin complex 1
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- ZT
Zeitgeber time
Footnotes
The authors have no conflict of interest to declare.
Conflicts of interest: None
References
- Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243:391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Adler R, Lindsey JD, Elsner CL. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol. 1984;99:1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MN, Rasmussen HB. AMPK: A regulator of ion channels. Commun Integr Biol. 2012;5:480–484. doi: 10.4161/cib.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochemical Journal. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams T, Cook B, Adler R. Correlations between terminal mitosis and differentiated fate of retinal precursor cells in vivo and in vitro: analysis with the “window-labeling” technique. Dev Biol. 1996;178:304–315. doi: 10.1006/dbio.1996.0220. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Ribelayga CP, Mangel SC. Cut-loading: a useful tool for examining the extent of gap junction tracer coupling between retinal neurons. J Vis Exp. 2012 doi: 10.3791/3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PTW, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2A(c) Febs Letters. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Dermaku-Sopjani M, Abazi S, Faggio C, Kolgeci J, Sopjani M. AMPK-sensitive cellular transport. Journal of Biochemistry. 2014;155:147–158. doi: 10.1093/jb/mvu002. [DOI] [PubMed] [Google Scholar]
- Djouder N, Tuerk RD, Suter M, et al. PKA phosphorylates and inactivates AMPK alpha to promote efficient lipolysis. Embo Journal. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. Retinal pH reflects retinal energy metabolism in the day and night. J Neurophysiol. 2004;91:2404–2412. doi: 10.1152/jn.00881.2003. [DOI] [PubMed] [Google Scholar]
- Foller M, Jaumann M, Dettling J, et al. AMP-activated protein kinase in BK-channel regulation and protection against hearing loss following acoustic overstimulation. Faseb Journal. 2012;26:4243–4253. doi: 10.1096/fj.12-208132. [DOI] [PubMed] [Google Scholar]
- Froy O. Metabolism and Circadian Rhythms-Implications for Obesity. Endocrine reviews. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nature reviews Molecular cell biology. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Gleason E, Mobbs P, Nuccitelli R, Wilson M. Development of functional calcium channels in cultured avian photoreceptors. Vis Neurosci. 1992;8:315–327. doi: 10.1017/s0952523800005058. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- Haque R, Chaurasia SS, Wessel JH, 3rd, Iuvone PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13:2247–2251. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Reviews Molecular Cell Biology. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T, Vessey KA, Fletcher EL. Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neuroscience. 2014;277:55–71. doi: 10.1016/j.neuroscience.2014.06.055. [DOI] [PubMed] [Google Scholar]
- Huang CC, Ko ML, Ko GY. A new functional role for mechanistic/mammalian target of rapamycin complex 1 (mTORC1) in the circadian regulation of L-type voltage-gated calcium channels in avian cone photoreceptors. PLoS One. 2013;8:e73315. doi: 10.1371/journal.pone.0073315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Ko ML, Vernikovskaya DI, Ko GY. Calcineurin serves in the circadian output pathway to regulate the daily rhythm of L-type voltage-gated calcium channels in the retina. J Cell Biochem. 2012;113:911–922. doi: 10.1002/jcb.23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Li F, Alvarez RA, Ash JD, Anderson RE. Downregulation of ATP synthase subunit-6, cytochrome c oxidase-III, and NADH dehydrogenase-3 by bright cyclic light in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2489–2496. doi: 10.1167/iovs.03-1081. [DOI] [PubMed] [Google Scholar]
- Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol. 2006;96:2025–2033. doi: 10.1152/jn.00364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. Journal of Biological Chemistry. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Karaganis SP, Kumar V, Beremand PD, Bailey MJ, Thomas TL, Cassone VM. Circadian genomics of the chick pineal gland in vitro. BMC Genomics. 2008;9:206. doi: 10.1186/1471-2164-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten FF, van Wijk E, van Reeuwijk J, et al. Association of whirlin with Cav1.3 (alpha1D) channels in photoreceptors, defining a novel member of the usher protein network. Invest Ophthalmol Vis Sci. 2010;51:2338–2346. doi: 10.1167/iovs.09-4650. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Jian K, Shi L, Ko GY. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J Neurochem. 2009;108:1607–1620. doi: 10.1111/j.1471-4159.2009.05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Shi L, Huang CC, Grushin K, Park SY, Ko GY. Circadian phase-dependent effect of nitric oxide on L-type voltage-gated calcium channels in avian cone photoreceptors. J Neurochem. 2013;127:314–328. doi: 10.1111/jnc.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI. Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates. Cell Calcium. 1995;18:285–300. doi: 10.1016/0143-4160(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochimica Et Biophysica Acta-Lipids and Lipid Metabolism. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Wallace CHR, Dyck JRB. Constitutively active adenosine monophosphate - Activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- Linton JD, Holzhausen LC, Babai N, et al. Flow of energy in the outer retina in darkness and in light. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8599–8604. doi: 10.1073/pnas.1002471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP. Heterogeneous Expression of the Core Circadian Clock Proteins among Neuronal Cell Types in Mouse Retina. Plos One. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiola EC, Ventura AL. Release of ATP from avian Muller glia cells in culture. Neurochem Int. 2011;58:414–422. doi: 10.1016/j.neuint.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M, Cunningham J. Modulation by endogenous ATP of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol. 1994;113:1085–1087. doi: 10.1111/j.1476-5381.1994.tb17106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido SS, Takahashi JS. Day/night differences in the stimulation of adenylate cyclase activity by calcium/calmodulin in chick pineal cell cultures: evidence for circadian regulation of cyclic AMP. J Biol Rhythms. 1998;13:479–493. doi: 10.1177/074873098129000318. [DOI] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- Ola MS, Berkich DA, Xu Y, King MT, Gardner TW, Simpson I, LaNoue KF. Analysis of glucose metabolism in diabetic rat retinas. Am J Physiol Endocrinol Metab. 2006;290:E1057–1067. doi: 10.1152/ajpendo.00323.2005. [DOI] [PubMed] [Google Scholar]
- Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: Roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cellular Signalling. 2009;21:760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Perez MT, Ehinger BE, Lindstrom K, Fredholm BB. Release of endogenous and radioactive purines from the rabbit retina. Brain Res. 1986;398:106–112. doi: 10.1016/0006-8993(86)91255-2. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Puchowicz MA, Xu K, Magness D, Miller C, Lust WD, Kern TS, LaManna JC. Comparison of glucose influx and blood flow in retina and brain of diabetic rats. J Cereb Blood Flow Metab. 2004;24:449–457. doi: 10.1097/00004647-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–222. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. Tracer coupling between fish rod horizontal cells: modulation by light and dopamine but not the retinal circadian clock. Vis Neurosci. 2007;24:333–344. doi: 10.1017/S0952523807070319. [DOI] [PubMed] [Google Scholar]
- Ross FA, Rafferty JN, Dallas ML, et al. Selective Expression in Carotid Body Type I Cells of a Single Splice Variant of the Large Conductance Calcium- and Voltage-activated Potassium Channel Confers Regulation by AMP-activated Protein Kinase. Journal of Biological Chemistry. 2011:286. doi: 10.1074/jbc.M110.189779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci U S A. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Voinescu PE, Lilley BN, et al. LKB1 and AMPK regulate synaptic remodeling in old age. Nat Neurosci. 2014;17:1190–1197. doi: 10.1038/nn.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiological reviews. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Robison GA. The role of cyclic AMP in the control of carbohydrate metabolism. Diabetes. 1969;18:797–819. doi: 10.2337/diab.18.12.797. [DOI] [PubMed] [Google Scholar]
- Um JH, Pendergast JS, Springer DA, Foretz M, Viollet B, Brown A, Kim MK, Yamazaki S, Chung JH. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. Plos One. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Critical Reviews in Biochemistry and Molecular Biology. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Schubert T, Paquet-Durand F, et al. Light-driven calcium signals in mouse cone photoreceptors. J Neurosci. 2012;32:6981–6994. doi: 10.1523/JNEUROSCI.6432-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metabolism. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. Journal of Biological Chemistry. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. Journal of Biological Chemistry. 2007;282:8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Atasoy D, Su HH, Sternson SM. Hunger States Switch a Flip-Flop Memory Circuit via a Synaptic AMPK-Dependent Positive Feedback Loop. Cell. 2011;146:991–1002. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. Journal of Biological Chemistry. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]