Abstract
Purpose
To report the intraoperative use of microscope-integrated optical coherence tomography (MIOCT) to enable visualization for Descemet’s stripping automated endothelial keratoplasty (DSAEK) in 2 patients with advanced bullous keratopathy.
Methods
Patient 1 was an 83-year-old female and Patient 2 was a 28-year-old male both with limited vision and significant pain from bullous keratopathy that underwent palliative DSAEK. Due to the severity and chronicity of the corneal decompensation in both patients, the view past the anterior cornea was negligible using standard microscope illumination techniques. We used spectral-domain (Patient 1) and swept-source (Patient 2) MIOCT, both of which rely on infrared illumination, to visualize key parts of the DSAEK procedure.
Results
Graft insertion, unfolding, tamponade, and attachment could be dynamically visualized intraoperatively despite the nearly opaque nature of the host corneas. Postoperatively, the grafts remained attached with significant corneal clearing, improvement in visual acuity, and pain relief for both patients.
Conclusions
MIOCT is a valuable tool for the corneal surgeon, allowing for DSAEK to be successfully performed even when the surgical microscope view is limited from severe corneal edema, as is often the case in patients with advanced bullous keratopathy. By using MIOCT, these patients can benefit from the advantages of DSAEK despite a clinically opaque cornea, which would otherwise be treated with a penetrating keratoplasty.
Keywords: OCT, DSAEK, bullous keratopathy
INTRODUCTION
Descemet’s stripping automated endothelial keratoplasty (DSAEK) is an alternative to penetrating keratoplasty (PK) for the treatment of endothelial corneal disease. Compared to PK, DSAEK has less morbidity with better uncorrected visual acuity, best-corrected visual acuity, and contrast acuity along with less surgically induced astigmatism and higher order aberrations.1 Furthermore, the frequency of graft rejection episodes is lower with DSAEK.2,3 Studies observing the long-term results of DSAEK have demonstrated excellent graft clarity and visual acuity results.4,5
However, in advanced cases of bullous keratopathy that fail medical treatment, severe longstanding corneal edema can limit visualization of the anterior chamber (AC) with standard microscope illumination. This limited visualization may preclude the use of DSAEK in these advanced cases because of the inability to see and manipulate the inserted endothelial graft intraoperatively. Several techniques have been used to try to improve visualization in these situations, including chandelier illumination, epithelium stripping, anterior lamellar corneal dissection, AC air injection, and trypan blue staining.6–8
Microscope-integrated optical coherence tomography (MIOCT) is a recently developed technology that adds OCT visualization to standard ophthalmic surgical microscopes.9,10 OCT is particularly helpful for visualization in the presence of corneal edema because the infrared illumination used by OCT scatters less than the visible light used by standard ophthalmic microscopes. Also, because MIOCT only adds visualization and is integrated into the surgical microscope, no alteration of the surgeon’s usual techniques is necessary. Here we report the use of MIOCT to enable visualization for DSAEK in 2 patients with advanced bullous keratopathy with nearly opaque corneas under standard illumination.
MATERIALS AND METHODS
Informed consent to use MIOCT was obtained from both patients under a Duke University Health System Institutional Review Board approved protocol. Due to advances in technology, a faster 3D capable swept-source (SS) MIOCT system was used for the second patient, while a spectral-domain (SD) MIOCT system capable of live 2D B-scan imaging was used for the first patient.
Our MIOCT consisted of a Leica M844 ophthalmic surgical microscope (Leica Microsystems; Buffalo Grive, IL) with a customized mechanical attachment allowing for seamless integration of OCT into the optical path of the microscope. This ensured shared focus between the two systems, enabling simultaneous surgery and OCT imaging. Technical details of this research (investigational device) MIOCT system developed at Duke have been described previously.9 Specifically for these cases, the SD system used a source with λ0 = 865 nm, A-scan rate = 17 kHz, A/B-scan ratio = 1000, B-scan rate = 17 Hz, resolution = 7.3 x 5 μm [x,z], and sensitivity = 103 dB. The SS system used a longer wavelength and faster source with λ0 = 1040 nm, A-scan rate = 100 kHz, A/B-scan ratio = 500, volume rate = 2 Hz, resolution = 14 x 14 x 7.8 μm [x,y,z], and sensitivity = 102 dB.
RESULTS
Case Reports
Patient 1
The first patient was an 86-year-old woman with an extensive ocular history in the left eye including posterior chamber intraocular lens (PCIOL), aqueous misdirection with iris-cornea touch, and severe end stage glaucoma which required surgical intervention with goniosynechialysis, laser peripheral iridotomy, Nd:YAG laser of the hyaloid face, and a superior glaucoma tube. Though her pressure regularized and her chamber deepened after these treatments, she subsequently had progressive corneal decompensation resulting in symptomatic, painful bullae twice a week despite frequent use of topical hypertonic NaCl drops. Her vision in the left eye on presentation to the cornea service was hand motions with an intraocular pressure of 19 mmHg. Slit-lamp examination of the left eye showed an overtly edematous and hazy 10.3 mm diameter cornea with epithelial bullae, prominent Descemet’s folds, and no visible detail past the cornea (pre-incision image in Fig. 1A). Corneal thickness was thicker than the ultrasonic pachymeter could measure. Given her advanced age, prior history of aqueous misdirection, and questionable visual potential in this eye, a DSAEK was offered to the patient instead of a PK, taking care to discuss with her that palliation of her pain from the bullous keratopathy was the primary goal.
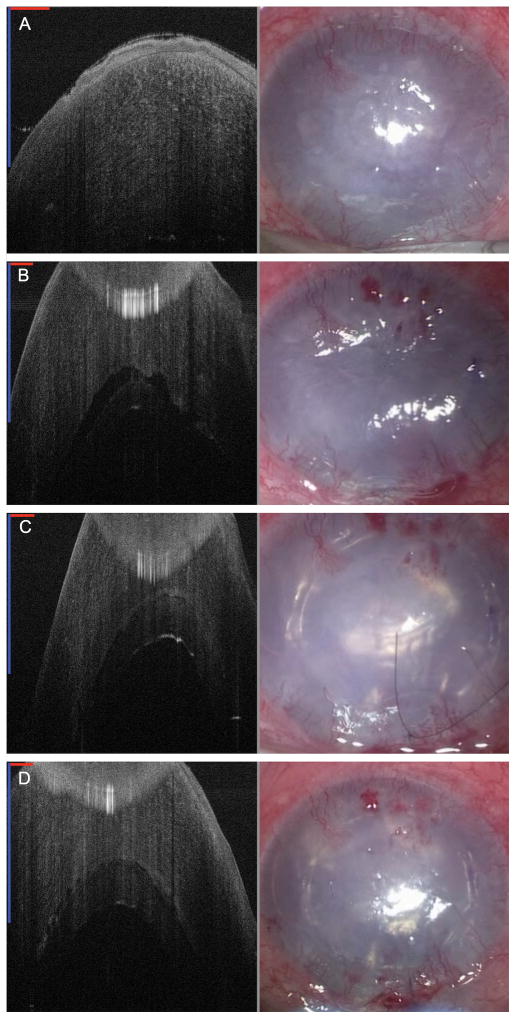
FIGURE 1. Spectral-domain microscope-integrated optical coherence tomography (SD-MIOCT) 2D B-scan images (left) and surgical microscope images (right) during DSAEK for severe bullous keratopathy in Patient 1.
Visualization of the anterior chamber (AC) is difficult in all of the surgical microscope images on the right due to severe corneal edema. A, Preoperative images showing severely edematous cornea. In the MIOCT image on the left, the edematous cornea nearly fills the entire 1.5 mm depth range of this system. B, Images following graft insertion showing a large fluid interface between the graft and host cornea in the MIOCT image. Note that the cornea is artifactually folded over at the top of the image because the depth range was exceeded. The inserted graft is not visible in the microscope image on the right. C, Air tamponade images showing minimal fluid interface between the graft and host cornea on MIOCT. Notice the air reflection under the graft in the SD-MIOCT image. With the presence of the air tamponade, the graft edges are now visible in the microscope view as well. D, Conclusion of the case confirming minimal fluid interface between the graft and host cornea. (In all SD-MIOCT images, the red scale bar measures 1 mm laterally and the blue scale bar measures 1 mm axially.)
Under topical anesthesia, Descemet’s membrane (DM) was stripped using a Gorovoy spatula (Harvey Precision Instruments; Rotunda, CA) under Healon (Abbott Medical Optics; Abbott Park, IL) through a superior clear corneal incision. MIOCT was used to confirm that there were no free fragments of DM from the posterior cornea after the stripping. Automated irrigation-aspiration was used to remove all Healon from the AC. Due to the known presence of an AC glaucoma tube, a smaller diameter endothelial graft was chosen to try to limit any graft interaction with the tube. The donor cornea was trephined to 6.75 mm, and the donor endothelial graft was delivered stromal side up into the AC using an EndoSerter (Ocular Systems Inc; Winston-Salem, NC). Using MIOCT, the graft was confirmed to be in the AC, and a fluid interface was visible between the graft and host cornea (Fig. 1B). Air was instilled into the AC underneath the graft to tamponade the graft to the host. At this point, MIOCT showed minimal fluid interface between the graft and host cornea (Fig. 1C). An anterior corneal venting incision confirmed the absence of any significant interface fluid, and the graft position remained unchanged with SD-MIOCT (Fig. 1D). With the air tamponade, lateral centration of the graft could also be visually confirmed using the surgical microscope.
At follow-up the next day, the vision remained hand motions. The graft appeared centered without obvious fluid interface on slit-lamp examination and was confirmed using clinical spectral-domain OCT. By 4 months post-operatively, her vision had improved to 20/200, which was consistent with her vision following treatment for aqueous misdirection 3 years prior. The graft remained centered and attached with a clear view into the AC on slit-lamp examination, and most importantly, the patient was pain free.
Patient 2
A 28-year-old male Somali refugee was referred to us for a PK or Gundersen flap to relieve severe pain he was experiencing in his left eye despite use of hypertonic NaCl drops and ointment. He had a complex and unclear ocular history of his left eye including cataract extraction for unknown pathology approximately 15 years earlier in Ethiopia followed subsequently by either placement or replacement of intraocular lens overseas in the last 5 years; no records from the overseas facilities were available to further elucidate the history or any details. The patient stated that vision in his left eye had been poor dating back to the initial procedure 15 years ago. His vision in the left eye on presentation to the cornea service was hand motions with an intraocular pressure of 13 mmHg. Slit lamp examination of the left eye showed a decompensated 11.6 mm diameter cornea with ruptured and intact bullae along with nasal neovascularization of the cornea (pre-incision image in Fig. 2A). The severity of the corneal edema limited the view into the AC. Using anterior segment OCT (Zeiss Visante; Dublin, CA), the central corneal thickness was measured to be 710 μm, a PCIOL was present but decentered inferiorly, and a reflective substance that was thought to be vitreous was present superior to the decentered PCIOL. B-scan ultrasound demonstrated a grossly normal posterior eye. Given his questionable visual potential, ocular history, and intent to work as a manual laborer, the less invasive DSAEK was offered to him as an initial option, taking care to discuss with him that palliation of his pain from the bullous keratopathy was the primary goal.
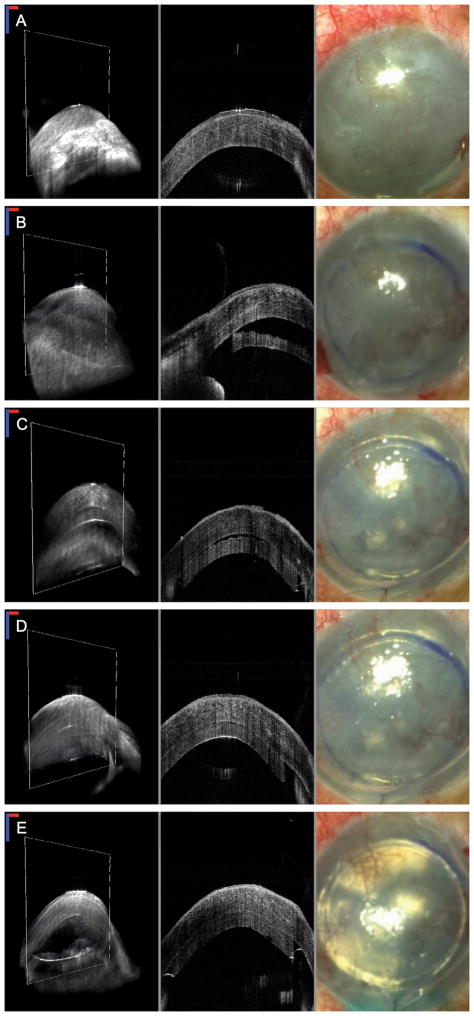
FIGURE 2. Swept-source microscope-integrated optical coherence tomography (SS-MIOCT) 3D volume images (left) with white boxes marking the corresponding 2D B-scan images (center) and surgical microscope images (right) during DSAEK for advanced bullous keratopathy in Patient 2.
Visualization of the anterior chamber (AC) is difficult in all of the surgical microscope images on the right due to advanced corneal edema. A, Preoperative images showing advanced corneal edema with epithelial irregularity. B, Images after graft insertion showing the relative position of the unfolded graft to the host cornea in the MIOCT images. The presence of the graft is not visible in the microscope view on the right. C, Images following air tamponade showing residual fluid interface between the graft and host cornea in the MIOCT images. In the standard microscope view, the graft edges are now visible with the air tamponade but no information about the presence of the interface fluid is available in this view. D, Images following an anterior corneal venting incision showing a reduction in the fluid interface between the graft and host cornea. E, Images following partial BSS-air exchange confirming good graft-host apposition at the conclusion of the case. (In all SS-MIOCT images, the red scale bar measures 1 mm laterally and the blue scale bar measures 1 mm axially.)
Under retrobulbar block, DM was stripped using a Gorovoy spatula. No free tags of DM from the cornea were seen on MIOCT following the stripping. The donor cornea was trephined to 7.5 mm. The donor endothelial graft was then placed on an EndoSerter and delivered stromal side up into the AC. Using MIOCT, the unfolded graft was confirmed to be in the AC, and a fluid interface was visible between the graft and host cornea (Fig. 2B). Air was instilled into the AC underneath the graft to tamponade the graft to the host. At this point, MIOCT showed the continued presence of a fluid interface between the graft and host cornea (Fig. 2C). An anterior corneal venting incision was placed inferior to the location of the fluid pocket to relieve the interface fluid (Fig. 2D). A partial BSS-air exchange was subsequently carried out, and MIOCT confirmed graft-host apposition at the conclusion of the case (Fig. 2E).
At follow-up the next day, his vision was stable at hand motions. The graft appeared centered without obvious fluid interface on slit-lamp examination or clinical OCT. At 1-month postoperatively, his vision had improved to 20/400 with a significant reduction in pain
DISCUSSION
Although DSAEK has clear advantages over PK for the treatment of corneal endothelial dysfunction, the need to manipulate the endothelial graft in the anterior chamber requires that the surgeon be able to visualize the AC. In cases of severe bullous keratopathy as in the cases presented here, the chronic significant corneal edema can compromise the necessary intraoperative views using standard microscope illumination.
By using MIOCT, we were able to visualize key graft-host interactions even in the presence of significant corneal edema. Specifically, MIOCT confirmed the presence of the graft in the AC, its configuration (edges folded/unfolded), its relative location to the host cornea, and the presence or absence of interface fluid to ensure adequate graft-host adherence. In these cases, because we knew from the MIOCT images that the graft was fairly anterior after delivery, we were able to merely point the air cannula posteriorly to tamponade the graft after delivery. OCT uses longer wavelength infrared light for illumination and scatters less than the visible light used in the surgical microscope. Hence, the host and graft tissues were readily visible in the OCT images as presented. Further, no alteration in surgical technique was required because the OCT was integrated into the surgical microscope, providing the necessary complementary visualizations where the surgeon was already working.
There are limitations to the use of MIOCT. In our experience, MIOCT does not reveal graft polarity; to maximize the probability that the graft stroma would be correctly anteriorly oriented with minimum AC graft manipulation, we utilized a non-folding insertion technique and reduced our graft diameters. However, given what we have learned from these cases regarding the degree to which MIOCT can improve visualization even in severely edematous corneas, we realize now that we can use larger diameter grafts in the future. Larger diameter grafts would ideally survive longer than smaller diameter ones. Depending on the system used, there may also be limitations in the depth and lateral extent of the OCT scans. To overcome these limits in field of view, the OCT scan should be moved to the specific areas of interest such as the central or peripheral cornea. Evolution of this technology should result in improvements in the scan ranges, as can already be seen in the progression from SD to SS systems from Patients 1 to 2. The increased imaging range and 3D volumetric information provided by the newer SS system also makes attempts at advanced image guided maneuvers such as tube trimming possible, a task which would have been difficult with the limited range 2D SD MIOCT system used in the first case. Our research system as currently designed also presented the OCT information on an external display. Integration of the OCT information into the surgical oculars – as has already been done in some commercial systems – will allow greater surgical interactivity with the OCT information.10 Though the MIOCT systems we used in this report were research systems, we would expect that the overall experience of improved visualization in edematous corneas would be similar for commercially available MIOCT systems.
In conclusion, the ability of MIOCT to visualize the donor graft even in the presence of a markedly edematous host cornea allowed us to successfully perform DSAEK for cases of severe bullous keratopathy without notable alteration of standard intraocular DSAEK techniques. Though PK can still be a good and appropriate choice for patients with severe bullous keratopathy, being able to successfully perform DSAEK in these patients with visibly opaque corneas allows them to experience the benefits of lower morbidity associated with the less invasive DSAEK technique.
Acknowledgments
This work was supported by the National Institutes of Health Bioengineering Research Partnership Grant R01-EY023039, the National Institutes of Health R01-EY024312, and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Duke University School of Medicine. The ClinicalTrials.gov identifier is NCT 01588041.
Footnotes
Conflicts of Interest
At the time of this work, Dr. Izatt was Chairman and Chief Scientific Advisor for Bioptigen, Inc., and had corporate, equity, and intellectual property interests (including royalties) in this company.
Dr. Toth receives financial support from Alcon, Bioptigen, and Genentech, is a consultant to Thrombogenics, and has an intraoperative imaging patent with Duke University. Dr. Kuo has an imaging algorithm patent licensed by Duke to Bioptigen. For the remaining authors no conflicts were declared.
References
- 1.Bahar I, Kaiserman I, Levinger E, et al. Retrospective contralateral study comparing descemet stripping automated endothelial keratoplasty with penetrating keratoplasty. Cornea. 2009;28:485–8. doi: 10.1097/ICO.0b013e3181901df4. [DOI] [PubMed] [Google Scholar]
- 2.Hjortdal J, Pedersen IB, Bak-Nielsen S, et al. Graft rejection and graft failure after penetrating keratoplasty or posterior lamellar keratoplasty for fuchs endothelial dystrophy. Cornea. 2013;32:e60–3. doi: 10.1097/ICO.0b013e3182687ff3. [DOI] [PubMed] [Google Scholar]
- 3.Price MO, Gorovoy M, Price FW, Jr, et al. Descemet’s stripping automated endothelial keratoplasty: three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology. 2013;120:246–51. doi: 10.1016/j.ophtha.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JY, Terry MA, Goshe J, et al. Three-year visual acuity outcomes after Descemet’s stripping automated endothelial keratoplasty. Ophthalmology. 2012;119:1126–9. doi: 10.1016/j.ophtha.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Ratanasit A, Gorovoy MS. Long-term results of Descemet stripping automated endothelial keratoplasty. Cornea. 2011;30:1414–8. doi: 10.1097/ICO.0b013e31820ca34b. [DOI] [PubMed] [Google Scholar]
- 6.Inoue T, Oshima Y, Hori Y, et al. Chandelier illumination for use during descemet stripping automated endothelial keratoplasty in patients with advanced bullous keratopathy. Cornea. 2011;30 (Suppl 1):S50–3. doi: 10.1097/ICO.0b013e3182281538. [DOI] [PubMed] [Google Scholar]
- 7.Ardjomand N, Fellner P, Moray M, et al. Lamellar corneal dissection for visualization of the anterior chamber before triple procedure. Eye (Lond) 2007;21:1151–4. doi: 10.1038/sj.eye.6702409. [DOI] [PubMed] [Google Scholar]
- 8.Mehta JS, Hantera MM, Tan DT. Modified air-assisted descemetorhexis for Descemet-stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2008;34:889–91. doi: 10.1016/j.jcrs.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Hahn P, Migacz J, O’Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33:1328–37. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98:1329–32. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]