Abstract
Purpose
To review the current literature describing cases of fungal keratitis and endophthalmitis following Boston Keratoprosthesis (KPro) implantation, and characterize the antifungal activity of 0.01% hypochlorous acid against medically relevant fungi.
Methods
A literature review of fungal keratitis or endophthalmitis in KPro patients from January 2001 to April 2015, and an in vitro time kill assay characterizing the fungicidal activity of 0.01% hypochlorous acid against fungi causing ocular infections.
Results
Fifteen publications, predominantly retrospective case series, were identified. Infection rates following KPro implantation ranged from 0.009–0.02 fungal infections per patient-year of follow-up. The largest single surgeon series reported an incidence of 2.4% for fungal endophthalmitis during a 10-year period. Causative organisms included both yeasts and molds. Outcomes were favorable if infections were caught early and treated appropriately; less favorable outcomes were reported in developing countries where fungal species are endemic and resources limited.
0.01% hypochlorous acid is rapidly fungicidal, reducing the number of viable yeast cells or mold conidia by at least 99.99% within 60 seconds. The antifungal activity extended to all molds (Acremonium kiliense, Aspergillus flavus, Aspergillus fumigatus, Fusarium solani, Mucor indicus) and yeast species (Candida albicans, Candida parapsilosis) tested.
Conclusions
Fungal infections remain a lifelong concern in patients following KPro implantation. There is a growing need for a standard antifungal prophylaxis regimen, especially in the developing world. The rapid broad-spectrum in vitro fungicidal activity of 0.01% hypochlorous acid against all fungi tested makes it an attractive candidate as an antifungal prophylaxis in KPro patients.
INTRODUCTION
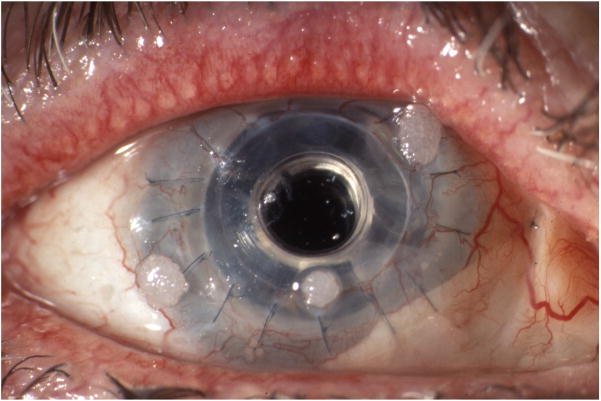
The Boston Keratoprosthesis (B-KPro) has been used in the management of patients with severe corneal disease where success with traditional penetrating keratoplasty is unlikely.1 Prophylactic use of broad-spectrum antibiotics, such as the combination of polymyxin B and trimethroprim or a fluoroquinolone, with the addition of vancomycin (14 mg/ml with 0.005% benzalkonium chloride) in high risk patients, has dramatically reduced the incidence of bacterial endophthalmitis.2 However, it has also increased the risk of fungal colonization and infections.3 Fungal colonization of the soft contact lens presents as small, white, mulberry shaped deposits (Fig. 1A). Culture and replacement of the lens is advisable in addition to a course of topical antifungal, for example, amphotericin B 0.15% twice daily for several weeks. When an active fungal keratitis (white sheen around optic stem, Fig. 1B) or endophthalmitis is identified clinically or confirmed with corneal or vitreous cultures, a combination of topical and systemic/intravitreal antifungal is indicated and adjusted according to fungal Gram staining and culture results.
Figure 1.
Figure 1A. Fungal colonization: white, mulberry shaped deposits on the soft contact lens.
Figure 1B. Active fungal keratitis: white sheen around the optic stem.
Despite the lifelong risk of fungal infections in KPro patients, an optimal fungal prophylaxis agent and regime has not yet been defined. Long-term success of the KPro depends on both the choice of antimicrobial prophylaxis as well as patient compliance. An ideal agent should be widely available, inexpensive, and highly efficacious while minimizing ocular surface irritation and toxicity. Fungal prophylaxis is not routinely administered but may benefit at-risk patients, such as those living in endemic areas, agricultural workers, or patients with a history of fungal infection or recurrent culture proven colonization of their soft contact lens. Current recommendations for fungal prophylaxis include brief periodic bursts of topical antifungal agents although these are not evidence based. Amphotericin B 0.15%, or natamycin 5%, twice daily for 1–2 weeks every 3 months are options, although high cost and lack of availability remain barriers to patient compliance, especially in developing countries where the need for prevention of fungal infections is most pressing.4 Monthly administration of 5% povidone-iodine at each clinic visit or during contact lens exchange to prevent fungal colonization has also been described, but corneal toxicity and patient discomfort with more frequent administration limits its long term use.5–6 There is an unmet need for novel antimicrobial prophylaxis agents to prevent fungal colonization and infections in KPro patients.
In this study, we review the literature to summarize reported cases of fungal infections (keratitis and endophthalmitis) in KPro patients and characterize infection rates, causative organisms, treatments and outcomes. We also report on the in vitro antifungal activity of 0.01% hypochlorous acid against representative mold and yeast species known to cause ocular infections.
MATERIALS AND METHODS
Literature review
A review of the literature from January 2001 to April 2015 was performed using PubMed and the search terms “Boston Keratoprosthesis” as well as “fungal”, “bacterial”, or “infectious” in combination with “keratitis”, or “endophthalmitis”. Cases of fungal keratitis or endophthalmitis following KPro surgery were identified by review of title and abstracts of search results. Infection rates were recorded as fungal infections per patient-year of follow-up if cumulative follow-up months were reported.
In vitro antifungal activity of 0.01% hypochlorous acid
A time kill assay based on the Clinical and Laboratory Standards Institute method for testing fungal species was performed at the Massachusetts Eye and Ear Infirmary microbiology laboratory.7 Fungal species tested included 5 mold (Acremonium kiliense Strain ATCC 14491, Aspergillus flavus Strain ATCC 204304, Aspergillus fumigatus Strain ATCC MYA-3626, Fusarium solani Strain ATCC MYA-3636, Mucor indicus Strain ATCC MYA-4678) and 2 yeast species (Candida albicans Strain ATCC 24433, Candida parapsilosis Strain ATCC 22019).
Fungal inocula were prepared as follows: fungi were grown on Sabouraud Dextrose Agar (SDA) plates for 48–72 hours at 30°C. Plates growing molds were flooded with 2 ml normal saline (0.9% NaCl in water) and a suspension of conidia was prepared by scrubbing the plate with an inoculation loop. Hyphae and other large fragments were allowed to settle, and conidia were transferred to a new tube and serial-diluted in normal saline to obtain a final concentration of 5 × 106 – 5 × 107 CFU/ml. For yeasts, a loop-full of cells was suspended in 0.5 ml normal saline and serial-diluted as described above. All work with yeasts and molds was performed in a class II biosafety cabinet.
The in vitro time kill assay was performed by exposing 2 μl of the conidia or cell suspension to 18 μl of normal saline (negative control) or 18 μl of 0.01% hypochlorous acid (Avenova™, NovaBay Pharmaceuticals, Emeryville, CA). After 1 minute, 180 μl of Dey-Engley neutralizing broth (D/E broth, Fluka) was added to inactivate the hypochlorous acid. Twenty microliters of sample was 10-fold serially diluted in 180 μl of D/E broth up to a final dilution of 1:10,000,000. Plates were incubated at 30°C for 2–4 days, depending on the growth rate of the organism. Each species was tested in triplicate by using three independent cell or conidia suspensions. Tests with hypochlorous acid were performed in duplicate (technical duplicates). After 2–4 days at 30°C, each well of the microtiter dish was scored for the presence of fungal growth. D/E broth without fungal growth was clear and purple due to the presence of a pH indicator dye. Wells were scored as positive for fungal growth if they showed visual turbidity or fungal mycelia. Depending on the species, a change in media color from purple to yellow was indicative of metabolic activity and aided in scoring. Samples that were exposed to saline served as a negative control and were used to calculate the concentration of cells or conidia in the initial inoculum. A reduction in viable cells or conidia by four log10 units was reported as a 99.99% kill rate.
RESULTS
Literature review
Fifteen publications, consisting predominantly of retrospective case series, were identified. Table 1 summarizes cases of published fungal keratitis or endophthalmitis in KPro patients between January 2001 and April 2015.3,5,8–20 Rates of infection vary from 0.009 to 0.02 fungal infections per patient-year of follow-up.3,15 In the largest single surgeon series reported (291 eyes), the incidence of fungal endophthalmitis was 2.4% during a 10-year period.19 Fungal organisms are responsible for approximately 10% of all reported KPro endophthalmitis cases in the literature.17 Reports originating from developing countries were scarce and consisted mostly of case series from Brazil and India.13–14, 20 Causative organisms included both yeasts (Candida albicans, Candida parapsilosis, Candida glabrata, Candida famata) and molds (Fusarium spp., Aspergillus spp., Alternaria spp.). Reported antibiotic prophylaxis at the time of diagnosis included predominantly 4th generation fluoroquinolone or vancomycin mono-prophylaxis, or a combination of fluoroquinolone and vancomycin. Vancomycin use and contact lens wear were identified as significant risk factors for development of fungal infections in some series 3 but not in others.15 Treatment regimes included stopping steroids, increasing antibiotic frequency, and the addition of topical antifungal agents, amphotericin B, natamycin, or voriconazole, for fungal keratitis. An advancing fungal keratitis or endophthalmitis was supplemented with intravitreal and/or systemic antifungals including oral voriconazole, itraconazole, and fluconazole. KPro exchange or explantation was required in progressive cases despite maximal medical therapy.3,14–15 If caught early and treated appropriately, the majority of patients retained good vision following cases of fungal infection.3,15 This was not the case with reports from developing countries, including India and Brazil, where outcomes were poor, often resulting in phthisis with no useful vision or evisceration.14, 20
Table 1.
Cases of Published Fungal Keratitis or Endophthalmitis in Boston Keratoprosthesis Patients Between January 2001 and April 2015.
| Author, Year Location Type of Study |
Eyes (Patients) | Study Period | Rate of Fungal Infection* or Positive Surveillance Culture (SC) | Causative Organisms | Baseline Diagnosis | Antibiotic Prophylaxis | Treatment† | Outcome/Comment |
|---|---|---|---|---|---|---|---|---|
|
Nouri8 2001 USA PSC |
30 (28) | 1990–2000 | 3 SC/30 = 10% | Yeast, species NR |
NR | Gentamicin, ofloxacin, or polymyxin B-trimethoprim | NR | No patients with positive surveillance cultures developed infection. |
|
Zerbe9 2006 USA (multicentered) P case series |
136 (133) | 2003 – 2005 | 2 K/136 = 1.5% | NR | NR | NR | Topical amphotericin B | NR |
|
Barnes3 2007 USA R case series/PSC |
202 (182)** SC: 36 (35) |
1990–2004 | 0.009 fungal K+E infections per patient-year 5 K+E /202 = 2.5% 4 SC/36 = 11% |
C. glabrata (1E) C. parapsilosis (1E) C. albicans (1E) Alternaria (1K) Fusarium (1E) SC: Candida spp. (4) |
OCP (2) Graft failure (3) |
Ofloxacin 0.3%, vancomycin 14 mg/ml (since 1999) | Topical/IV amphotericin B, oral fluconazole, itraconazole and voriconazole | Vancomycin and CL wear are risk factors for fungal infections. HM, CF at 5 ft, phthisis/ NLP, 20/60-20/70, 20/40 post repeat KPro. |
|
Khan10 2007 USA R case series |
17 (14) | NR | 1 K/17 = 5.9% | Fusarium spp. (1) | HSV keratitis | Moxifloxacin BID | “complete infection control,” antifungal treatment NR | Post repeat KPro: 20/60 at 14 months |
|
Aldave11 2009 USA R case series |
50 (49) | 2004 – 2008 | 2 K/50 = 4% | C. parapsilosis (2) | Repeat graft failure (1) NR (1) |
Gatifloxacin QID | Topical antifungal with resolution (1) Repeat KPro (1) |
PEDs are risk factors for fungal infection. NR (1), 20/80 at 13 months post repeat KPro (1) |
|
Greiner12 2011 USA CS |
40 (35) | 2004 – 2010 | 1 E/40 = 2.5% | C. parapsilosis (1) | NR | Vancomycin (50 mg/ml) | NR | NR |
|
Nascimento13 2011 Brazil R case series |
1 (1) | NR | 1 K | Aerobasidium pullalans (1) | Limbal stem cell failure secondary to dry eye | Moxifloxacin 0.5% | Amphotericin B 0.15% QID | 2 weeks prior to keratitis, Aerobasidium pullalans was cultured from CL. |
|
Jain14 2012 India R case reports |
2 (2) | NR | 2 E |
Aspergillus fumigatus (CC + VC) (1) “septate fungal mold” (1) |
Graft rejection (1) PBK/graft failure (1) |
Ofloxacin 0.3% BID or moxifloxacin 0.5% QD | Topical natamycin 5% or amphotericin B 0.15%, IV or IC amphotericin B, oral itraconazole | Lost to FU/eviscerated (1) KPro removal, graft with phthisis and “no useful vision” at 6 months (1) |
|
Chan15 2012 USA R review |
126 (105) | 2004 – 2010 | 0.02 fungal K infections per patient-year 5 K/126 = 4 % |
Fusarium spp. (1) Candida spp. (1) C. parapsilosis (1) Candida spp. (1) Dactylaria constricta (1) |
Failed PK for: Aniridia (1) Acid injury (2) OCP (2) |
Moxifloxacin BID, vancomycin (14 mg/ml) | Topical amphotericin B 0.15%, voriconazole 1%, natamycin; oral fluconazole or voriconazole | Vancomycin and CL wear are not risk factors for fungal infections. 20/300, 20/60 (after KPro exchange), CF, 20/40, 20/60 |
|
Chan16 2012 USA R review |
126 (105) | 2004 – 2010 | 0.009 fungal E infections per patient-year 2 E/126 = 1.6% |
C. parapsilosis (1) C. albicans (1) |
Failed PK for: SJS (1) KPro melt from SJS (1) |
Moxifloxacin BID, vancomycin (14 mg/ml) | Topical amphotericin B, oral fluconazole (1) Oral fluconazole, IV amphotericin B (1) |
20/60, LP |
|
Robert17 2012 Canada Review of literature |
NR | 2000 – 2011 | 5 E/53 = 9.4% of all reviewed cases of endophthalmitis are fungal*** |
C. parapsilosis (2) C. glabrata (1) Fusarium (1) Alternaria (1) |
NR | NR | NR | NR |
|
Kim18 2013 USA R case series |
110 (105) | 2004 – 2012 | 0.015 fungal K infections per eye-year 4 K/110 = 3.6% |
C. parapsilosis (3) Acremonium spp. (1) |
SJS (1) Graft failure (3) |
4-FQ QID indefinitely, vancomycin (25 mg/ml) QID for 4 months | Topical amphotericin B 0.5%, natamycin 5% or voriconazole 1 % QID +/voriconazole 200 mg PO BID | Vancomycin and PEDs are risk factors for fungal infections. |
|
Magalhaes5 2013 Brazil PSC |
10 (10) | NR | 1 SC/10 = 10% | Aerobasidium pullalans (1) | Graft failure (6) Chemical burn (8) |
Moxifloxacin 0.5 % QD | 5% monthly PI, moxifloxacin 0.5% | Culture positive patient did not develop infection, became culture negative after 5% monthly PI |
|
Behlau19 2014 International SR + SS + SSS |
291 (NR) | 1990 – 2010 | 7 E/291 = 2.4% | NR | 50% of patients were “non-autoimmune, non-burn” | 4-FQ, vancomycin (14 mg/ml) | NR | 4/7 cases cured with “minimal visual loss” |
|
Chhablani20 2015 India R case series |
45 (NR) | 2009– 2012 | 2 E/45 = 4.4% |
C. glabrata (VC)/C. famata (CL) (1) Aspergillus tereus (1) |
Failed graft for microbial keratitis (1) Congenital glaucoma and HSV (1) |
Topical antibiotic, name NR | IV voriconazole, KPro explantation, new PK (1) KPro explantation, new PK (1) |
Both eyes phthisical and NLP |
Rates of fungal infection are reported as a percentage (number of patients developing fungal keratitis or endophthalmitis divided by total patients) and additionally as fungal infections per patient-year of follow-up if specified.
Includes KProI (n=148) and KPro2 (n=54)
Drug, route, frequency and/or dosing reported if specified in study.
BID, twice daily; CC, corneal culture; CF, count fingers; CL, contact lens; CS, cohort study; E, endophthalmitis; FU, follow-up; HM, hand motion; HSV, herpes simplex virus; IC, intracameral; IV, intravitreal; K, keratitis; KPro, Keratoprosthesis; LP, light perception; NLP, no light perception; NR, not reported; OCP, ocular cicatricial pemphigoid; P, prospective; PBK, pseudophakic bullous keratopathy; PEDs, persistent epithelial defects; PI, 5% povidone iodine; PK, penetrating keratoplasty; PO, by mouth, PSC, prospective surveillance cultures; QD, once daily; QID, four times daily; R, retrospective; SC, surveillance cultures; SJS, Stephen-Johnson Syndrome; spp., species; SR, systematic review; SS, surveillance survey; SSS, single surgeon series; VC, vitreous culture; 4-FQ, 4th generation fluoroquinolone
A search for a potential novel antimicrobial agent to prevent fungal infections in KPro patients identified hypochlorous acid (HOCl), whose application in wound treatment was described 100 years ago.21 While HOCl’s antibacterial spectrum has been well characterized previously, its activity against fungal species frequently isolated from ocular infections remains to be determined.
In vitro antifungal activity of 0.01% hypochlorous acid
Preliminary results showed that 0.01% hypochlorous acid reduced the number of viable conidia of Acremonium kiliense, Aspergillus flavus, Aspergillus fumigatus, Fusarium solani, and Mucor indicus by at least 99% within 15 seconds. After a 1 minute incubation period, antifungal activity was 99.9% or better, which did not change when the exposure time was extended to 4 minutes. Therefore, all fungal isolates were tested for 1 minute in the presence of 0.01% hypochlorous acid.
Table 2 shows the kill rate or percent reduction of viable cells or conidia (spores) of mold and yeast species tested after exposure to 0.01% hypochlorous acid for 1 minute. At an inoculum concentration of 5 × 106 – 5 × 107 CFU/ml, the minimal fold reductions in viable cells or conidia was 99.99% or better for all mold and yeast species tested. In some species, kill rates of ≥99.999% were observed. These results highlight the substantial fungicidal activity of 0.01% hypochlorous acid that takes place within 60 seconds. When 0.01% hypochlorous acid was tested at a cell or conidia density exceeding the recommended concentration, the reduction in viable cells ranged from 90% to 99.9999% (data not shown).
Table 2.
Kill Rate after Exposure of Select Fungal Species to 0.01% Hypochlorous Acid for 1 Minute Using a 96-well Microtiter Time Kill Assay.
| Fungal Species | Kill rate* | |
|---|---|---|
| Acremonium kiliense | Mold | ≥ 99.999% |
| Aspergillus flavus | Mold | ≥ 99.99% |
| Aspergillus fumigatus | Mold | ≥ 99.999% |
| Fusarium solani | Mold | ≥ 99.99% |
| Mucor indicus | Mold | ≥ 99.99% |
| Candida albicans | Yeast | ≥ 99.999% |
| Candida parapsilosis | Yeast | ≥ 99.99% |
A reduction in viable cells or conidia by four log10 units is reported as a 99.99% kill rate. Results represent the median value of three independent experiments.
DISCUSSION
While bacterial endophthalmitis has dramatically declined since the introduction of vancomycin to the prophylactic antibiotic regimen in 1999, the rate of fungal colonization and infections in KPro patients have subsequently increased. The prolonged use of broad-spectrum antibiotics, steroids, and therapeutic contact lenses has led to increased rates of fungal infections. Treatment and prophylaxis of fungal infections in KPro patients is complicated by the fact that fungal colonization fluctuates over time, positive cultures cannot predict infection, and there is no consensus on long-term antifungal prophylaxis.3
From our review of the literature, we found that the proportion of eyes developing fungal infections varies greatly among published reports and is difficult to compare across studies given inconsistent reporting of follow-up periods. The best estimate is obtained from a single surgeon series spanning over 20 years, where fungal endophthalmitis increased from 0% between 1990–1999 to 2.4% from 2000–2010.19 This shift was coincident with the introduction of vancomycin and therapeutic soft contact lens wear. Although there are few case reports14, 20 originating from developing countries, given the endemic nature of fungal organisms in these tropical, humid environments, we surmise the true incidence of fungal infections would be higher than those reported in the United States. Reported outcomes for KPro patients in the Western hemisphere are good, as early recognition and treatment of indolent fungal infections can limit visual loss. However, a subset of these patients will have carrier corneal tissue that remains prone to melting and cure often involves months of antifungal treatment.15 In contrast, for patients in the developing world outcomes are uniformly poor and often related to limited medical and economic resources coupled with patient non-compliance. An effective, safe, and inexpensive antifungal prophylaxis is needed in this setting.
In our study, we addressed this need by testing the in vitro activity of hypochlorous acid against mold and yeast species known to cause ocular infections and which also pose a threat to KPro patients as identified in our literature review. Avenova™ (NovaBay® Pharmaceuticals) is a solution of 0.01% hypochlorous acid (HOCl) in unbuffered saline that has been FDA cleared as an adjunct treatment for blepharitis. In an in vitro time kill assay, the solution was rapidly fungicidal and sporicidal within 60 seconds for all mold and yeast species tested. In temperate climates, yeasts predominate, while in hot and humid environments, such as India, molds are more common. This pattern was reflected in the fungal species isolated from KPro patients with Candida spp., including C. parapsilosis, being among the most common causative organisms responsible for fungal infections in the Western hemisphere (Table 1). C. parapsilosis characteristically adheres to prosthetic materials with a particular propensity to cause infections in KPro patients. In our in vitro assay, hypochlorous acid reduced all viable cells of C. parapsilosis by more than 4 log10 units (>99.99% kill rate) in 60 seconds. Mold species, such as Aspergillus and Fusarium, were also frequent causes of ocular infections, especially in tropical climates, and hypochlorous acid was shown to reduce viable conidia by ≥99.99% as well.
An ideal antifungal prophylaxis agent would be rapidly fungicidal and sporicidal, broad spectrum, and relatively inexpensive with proven in vitro and in vivo efficacy and a favourable side effect profile. In our study, hypochlorous acid, 0.01%, effectively eliminated all molds (including Fusarium and Aspergillus) and yeasts (Candida spp.) tested, with fungicidal activity observed in as little as 15 seconds. This rapid fungicidal and sporicidal activity is a significant advantage over traditional antifungal treatments that require hours or even days to show activity and may not be active against conidia (spores). Unlike some antifungal agents that are active against certain species but not others, hypochlorous acid showed rapid and potent activity against all species tested, including the genera Acremonium, Aspergillus, Fusarium, Mucor, and Candida. This broad spectrum of activity makes hypochlorous acid an attractive candidate for a global antifungal prophylaxis agent, especially in developing countries. However, in order to confirm the candidacy of hypochlorous acid as a topical prophylactic antifungal, concerns regarding its ocular surface and potential intraocular toxicity must be addressed. In addition, a subset of KPro patients, such as those with Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TENS), mucous membrane pemphigoid (MMP) and chemical burn patients have already compromised ocular surfaces with increased risk of corneal melting; therefore, we must ensure that topical agents cannot diffuse into the eye around the KPro stem.22 Hypochlorous acid is a naturally occurring antimicrobial produced by neutrophils and monocytes as part of our body’s innate immune response. In vitro testing of HOCl against mouse dermal fibroblasts showed that a single 10-fold dilution was comparable to the cytotoxicity observed in unexposed control fibroblasts.23 In addition, its topical use as a wound cleanser in humans has been reported without adverse events or systemic toxicity.24 Because the active ingredient in HOCl is reactive, not persistent, and even further diluted by the tear film, we believe the potential for ocular surface and intraocular toxicity are low. To address these questions, we are currently planning a clinical trial in KPro patients to monitor the effects of daily application of 0.01% hypochlorous acid to the eyelid margin on both the ocular surface and intraocular environment. In the long run, prophylactic therapy should be tailored with both patient and regional factors in mind. Precisely which patients should receive chronic prophylaxis, as well as prophylaxis dosing and duration will require further evaluation.
Footnotes
Conflict of Interest: All authors are full time employees of Massachusetts Eye and Ear Infirmary (a not-for-profit organization) which is the manufacturer of the Boston Keratoprosthesis.
References
- 1.Ma JJ, Graney JM, Dohlman CH. Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin. 2005;45:49–59. doi: 10.1097/01.iio.0000176365.71016.28. [DOI] [PubMed] [Google Scholar]
- 2.Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009;28:896–901. doi: 10.1097/ICO.0b013e3181983982. [DOI] [PubMed] [Google Scholar]
- 3.Barnes SD, Dohlman CH, Durand ML. Fungal colonization and infection in Boston keratoprosthesis. Cornea. 2007;26:9–15. doi: 10.1097/01.ico.0000224650.19837.25. [DOI] [PubMed] [Google Scholar]
- 4.Walcott-Harris R, Chodosh J, Dohlman CH. Antimicrobial prophylaxis for life: as important as ever. [Accessed May 5, 2015];Boston KPro News [serial online] 2011 8:1–3. Available from: http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0CCMQFjAA&url=http%3A%2F%2Fwww.masseyeandear.org%2Fgedownload!%2F2011%2520KPro%2520newsletter.pdf%3Fitem_id%3D70213024&ei=AkFJVae3PLHlsASiwICIDg&usg=AFQjCNFTcYP_j7ayZsSLAgiUyTqAiWg61A&sig2=2RZJkSkD3AYSkXeXxBiWPA. [Google Scholar]
- 5.Magalhães FP, do Nascimento HM, Ecker DJ, et al. Microbiota evaluation of patients with a Boston type I keratoprosthesis treated with topical 0. 5% moxifloxacin and 5% povidone-iodine. Cornea. 2013;32:407–411. doi: 10.1097/ICO.0b013e31824a8b9b. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Wu M, Shen T. The toxic effect of different concentrations of povidone iodine on the rabbit’s cornea. Cutan Ocul Toxicol. 2009;28:119–124. doi: 10.1080/15569520903080511. [DOI] [PubMed] [Google Scholar]
- 7.Rex JH, Alexander BD, Andes D, et al. M38-A2 Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. 2. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Test Procedures; pp. 5–11. [Google Scholar]
- 8.Nouri M, Terada H, Alfonso EC, et al. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119:484–489. doi: 10.1001/archopht.119.4.484. [DOI] [PubMed] [Google Scholar]
- 9.Zerbe BL, Belin MW, Ciolino JB. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779–1784. doi: 10.1016/j.ophtha.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Khan BF, Harissi-Dagher M, Pavan-Langston D, et al. The Boston keratoprosthesis in herpetic keratitis. Arch Ophthalmol. 2007;125:745–749. doi: 10.1001/archopht.125.6.745. [DOI] [PubMed] [Google Scholar]
- 11.Aldave AJ, Kamal KM, Vo RC, et al. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116:640–651. doi: 10.1016/j.ophtha.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118:1543–1550. doi: 10.1016/j.ophtha.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento HM, Oliveira LA, Höfling-Lima AL. Infectious keratitis in patients undergoing Boston Type 1 keratoprosthesis (Boston KPro) procedure: case series. Arq Bras Oftalmol. 2011;74:127–129. doi: 10.1590/s0004-27492011000200012. [DOI] [PubMed] [Google Scholar]
- 14.Jain V, Mhatre K, Shome D, et al. Fungal keratitis with the type 1 Boston keratoprosthesis: early Indian experience. Cornea. 2012;31:841–843. doi: 10.1097/ICO.0b013e3182068614. [DOI] [PubMed] [Google Scholar]
- 15.Chan CC, Holland EJ. Infectious keratitis after Boston type 1 keratoprosthesis implantation. Cornea. 2012;31:1128–1134. doi: 10.1097/ICO.0b013e318245c02a. [DOI] [PubMed] [Google Scholar]
- 16.Chan CC, Holland EJ. Infectious endophthalmitis after Boston type 1 keratoprosthesis implantation. Cornea. 2012;31:346–349. doi: 10.1097/ICO.0b013e31821eea2f. [DOI] [PubMed] [Google Scholar]
- 17.Robert MC, Moussally K, Harissi-Dagher M. Review of endophthalmitis following Boston keratoprosthesis type 1. Br J Ophthalmol. 2012;96:776–780. doi: 10.1136/bjophthalmol-2011-301263. [DOI] [PubMed] [Google Scholar]
- 18.Kim MJ, Yu F, Aldave AJ. Microbial keratitis after Boston type I keratoprosthesis implantation: incidence, organisms, risk factors, and outcomes. Ophthalmology. 2013;120:2209–2216. doi: 10.1016/j.ophtha.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Behlau I, Martin KV, Martin JN, et al. Infectious endophthalmitis in Boston keratoprosthesis: incidence and prevention. Acta Ophthalmol. 2014;92:546–555. doi: 10.1111/aos.12309. [DOI] [PubMed] [Google Scholar]
- 20.Chhablani J, Panchal B, Das T, et al. Erratum to: Endophthalmitis in Boston keratoprosthesis: case series and review of literature. Int Ophthalmol. 2015;35:149–154. doi: 10.1007/s10792-014-0033-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith JL, Drennan AM, Rettie T, et al. Experimental observations on the antiseptic action of hypochlorous acid and its application to wound treatment. Br Med J. 1915;2:129–136. doi: 10.1136/bmj.2.2847.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert MC, Dohlman CH. A review of corneal melting after Boston Keratoprosthesis. Semin Ophthalmol. 2014;29:349–357. doi: 10.3109/08820538.2014.959186. [DOI] [PubMed] [Google Scholar]
- 23.Rani SA, Hoon R, Najafi RR, et al. The in vitro antimicrobial activity of wound and skin cleansers at nontoxic concentrations. Adv Skin Wound Care. 2014;27:65–69. doi: 10.1097/01.ASW.0000443255.73875.a3. [DOI] [PubMed] [Google Scholar]
- 24.Crew J, Varilla R, Rocas TA, et al. Neutrophase ® in chronic non-healing wounds. Int J Burns Trauma. 2012;2:126–134. [PMC free article] [PubMed] [Google Scholar]