Abstract
Rationale
GDF11 (Growth Differentiation Factor 11) is a member of the transforming growth factor β (TGFβ) super family of secreted factors. A recent study showed that reduced GDF11 blood levels with aging was associated with pathological cardiac hypertrophy (PCH), and restoring GDF11 to normal levels in old mice rescued PCH.
Objective
To determine if and by what mechanism GDF11 rescues aging dependent PCH.
Methods and Results
24-month-old C57BL/6 mice were given a daily injection of either recombinant (r) GDF11 at 0.1mg/kg or vehicle for 28 days. rGDF11 bioactivity was confirmed in-vitro. After treatment, rGDF11 levels were significantly increased but there was no significant effect on either heart weight (HW) or body weight (BW). HW/BW ratios of old mice were not different from 8 or 12 week-old animals, and the PCH marker ANP was not different in young versus old mice. Ejection fraction, internal ventricular dimension, and septal wall thickness were not significantly different between rGDF11 and vehicle treated animals at baseline and remained unchanged at 1, 2 and 4 weeks of treatment. There was no difference in myocyte cross-sectional area rGDF11 versus vehicle-treated old animals. In vitro studies using phenylephrine-treated neonatal rat ventricular myocytes (NRVM), to explore the putative anti-hypertrophic effects of GDF11, showed that GDF11 did not reduce NRVM hypertrophy, but instead induced hypertrophy.
Conclusions
Our studies show that there is no age-related PCH in disease free 24-month-old C57BL/6 mice and that restoring GDF11 in old mice has no effect on cardiac structure or function.
Keywords: Myokines, pathological hypertrophy, aging, cardiac function
INTRODUCTION
Cardiovascular function can decline in old age, and the disease independent factors that induce these changes are not well known. A recent study suggests that disease-free aging induces pathological cardiac hypertrophy (PCH) in 24-month-old mice and that this results in large part from an age-related reduction in the circulating blood levels of Growth Differentiation Factor 11 (GDF11), a member of the Transforming Growth Factor β (TGFβ) super family of cytokines1. GDF11 and related family members generally reduce skeletal muscle protein synthesis and repair and enhance protein degradation, which leads to muscle atrophy in adults2, 3. Loss of these factors, particularly myostatin (also called GDF8)4, 5 is primarily associated with skeletal muscle hypertrophy but with limited effects on the heart6. Recent work suggests that circulating levels of GDF11 decrease with aging, and restoring a “youthful” circulation containing normal levels of GDF11 to old mice via parabiosis reversed age-dependent pathological cardiac hypertrophy1. A major finding of this study was that restoring youthful levels of GDF11 by injecting recombinant (r) GDF11 into old animals restored normal myocyte size and gene expression in the old mouse heart. Related studies suggest that restoring GDF11 can also have beneficial effects on skeletal muscle7 and brain function8, suggesting that restoring GDF11 to levels seen in young animals could reverse critical aspects of age-related brain, skeletal muscle and cardiac dysfunction. These studies suggest that restoring normal GDF11 levels in old age can reverse aging effects on critical organ systems.
The idea that a member of the TGF super family of cytokines, GDF11, is singularly responsible for aging related organ dysfunction is not supported by some recently published reports9. A study to reexamine the idea that reduced GDF11 in aging is responsible for defective skeletal muscle repair could not confirm most aspects of the studies related to GDF11-induced rescue of skeletal muscle wasting with aging9. This newest report9 showed that aging involves skeletal muscle wasting and that increased rather than decreased levels of myostatin and GDF11 are involved. These data suggest that inhibition rather than stimulation of myostatin/GDF11 signaling in aging could blunt the associated skeletal muscle dysfunction.
The goals of the present study were to reexamine the idea that restoring youthful levels of GDF11 in old mice, by injection of rGDF11, reverses pathological cardiac hypertrophy and imparts a “youthful” phenotype to the old heart1. If these findings could be confirmed, we then planned to explore what aspects of pathological myocyte function were rescued by rGDF11 treatment.
We performed a blinded study in which we treated 24-month-old C57BL/6 mice with rGDF11 for 28 days, following the protocol used previously1. We measured cardiac structure and function before and after rGDF11 treatment and then measured heart and myocyte size and changes in molecular remodeling after treatment. Our studies suggest that while hearts of older mice are larger there is no pathological hypertrophy present. We also found that daily injection of rGDF111 significantly raised blood levels of rGDF11 in old mice. However, we did not observe any reduction in heart or myocyte size, nor did we observe any changes in cardiac performance. We also showed that rGDF11 induced hypertrophy in neonatal myocytes and did not block phenylephrine-induced neonatal myocyte hypertrophy1.
METHODS
For detailed methods refer to online supplemental material section.
Briefly, all animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Temple University School of Medicine and conducted in accordance with the Guide for the Use and Care of Laboratory Animals. Aged (24 months) C57BL/6 male mice were provided by Boehringer Ingelheim Pharmaceuticals. Functional characterization, analysis of antibody selectivity and dosing of rGDF11 is described in detail in online supplemental material. Cardiac Function before and after rGDF11 treatment was measured by echocardiography using the Vevo2100 ultrasound system and hemodynamic parameters were measured using ADInstruments Powerlab 16/30 as described in online supplemental material. Tissue processing, histology, heart weight to body weight ratio (HW/BW), myocardial fibrosis, and hypertrophy were measured in vivo and in in-vitro as described in the online supplemental materials.
RESULTS
Recombinant GDF11 is functional and can be selectively and reliably detected
Using Western analysis, we first tested the specificity of the reagents used in the previous study (Abcam anti-GDF11) to document reduced GDF11 with aging and reductions in cardiac hypertrophy after rGDF11 injections1. We found, as reported recently by others,9 that the Abcam GDF11 antibody readily detected both GDF11 and myostatin (Online Figure IA), suggesting that this is not an appropriate reagent to define GDF11 changes with aging or after rGDF11 injection. The Abcam antibody also did not readily detect the non-reduced forms of either GDF11 or myostatin. We then tested the specificity of an R&D systems GDF11 antibody (Online Figure IB). This antibody had high specificity for GDF11 versus myostatin and was able to detect both reduced and non-reduced forms of GDF11. This antibody was used to detect GDF11 in the present experiments.
We documented the bioactivity of recombinant proteins before injecting them into old mice. The rGDF11 protein was analyzed for its ability to induce Smad2/3 (its known signaling pathway) activity using HepG2 Smad2/3 luciferase reporter cells (Online Figure IC). rGDF11 induced Smad2/3 activity with an EC50 and EC90 of 1.9 nM and 8.6 nM respectively (Online Figure IC), documenting that rGDF11 binds to its native receptor with high affinity.
GDF11 blood levels increase after injection of rGDF11
We next performed studies to determine if a daily intraperitoneal injection of rGDF11 (0.1 mg/kg for 28 days) into 24-month-old male mice reverses any existing pathological cardiac hypertrophy. We measured circulating levels of rGDF11 in the plasma of old mice, either 1–3 hours after rGDF11 injection (for peak levels) or 24 hours after injection (for trough levels) (Online table I). These studies showed that rGDF11 rises to a detectable peak within a few hours and then falls to low levels within 24 hours. Importantly, the native GDF11 levels in old mice were below the quantification level (0.1ng/ml) of this assay. Therefore, we were unable to determine if GDF11 levels in the blood decreased with age in C57BL/6 mice, similar to what has been reported recently9.
GDF11 has no effect on cardiac hypertrophy
Pathological cardiac hypertrophy was assessed using heart weight to tibia length (HW/TL) and heart weight to body weight (HW/BW) ratios. Heart size was also determined with echocardiographic measures. In addition, immunohistochemistry and qRT-PCR were used to examine cardiac fibrosis and the presence of common markers of PCH. rGDF11-treated 24-month-old male animals were compared to vehicle-treated animals. We also studied 8 or12 week old young mice to define the magnitude of age dependent pathological cardiac hypertrophy.
Our studies showed that rGDF11 had no effect on the heart weight or body weight of old mice (Figure 1A). Heart weight to body weight (HW/BW) and heart weight to tibia length (HW/TL) ratios were not significantly different between rGDF11 and vehicle treated animals. In addition, the HW/BW ratio of 24-month-old animals was not significantly different from 8 or 12 week old mice (Figure 1A). These results show that while the heart weight (and body weight) of 24-month-old animals is greater than that of young animals, there is no pathological hypertrophy, but rather normal growth associated with changes in body mass10–12.
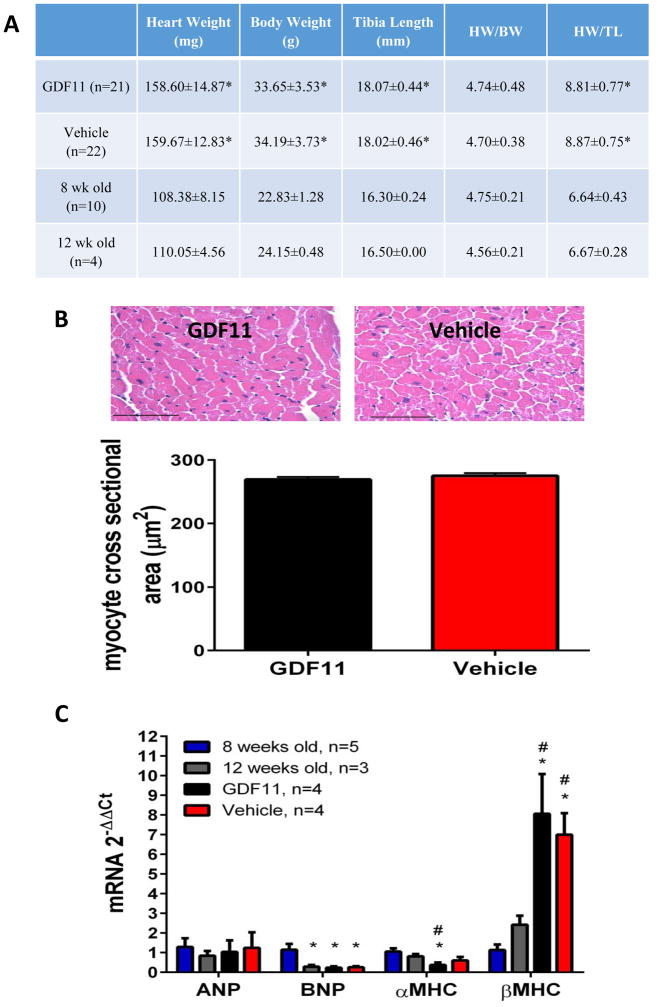
Figure 1. Assessment of hypertrophy in vivo.
A: Heart weight, body weight, and tibia length was measured at time of sacrifice 28 days post initial injection of GDF11. No significant differences were found between GDF11 and vehicle treated animals. *=p≤0.05 compared to 8 week old mice. B: There was no difference in myocyte cross sectional area between rGDF11 and vehicle treated animals. 100–175 myocytes with nuclei from 6 animals were analyzed per group Scale bar= 50 microns. C: There was no difference in ANP levels between young and aged mice. There was no significant difference between GDF11 and vehicle treated animals. *p<0.05 vs 8 week old mice. #p<0.05 vs 12 week old mice.
Myocyte cross-sectional area was measured in vehicle and rGDF11 treated 24-month-old mouse hearts by performing morphometric analysis of cardiac histological sections. No differences in myocyte cross-sectional area between rGDF11 and vehicle treated 24-month-old animals were observed (Figure 1B).
mRNA levels of the hypertrophic markers ANP, BNP, αMHC, and βMHC were also measured in vehicle and rGDF11 treated 24-month-old mice. We also compared young and old mice to explore age related changes in these parameters. There were no significant differences in ANP, BNP, αMHC, or βMHC mRNA expression between rGDF11 and vehicle treated animals (Figure 1C). While there were no differences in ANP mRNA levels between young and aged mice, BNP was greater in 8-week-old animals versus other ages. In addition, there appear to be age related reductions in αMHC mRNA and increases in βMHC. These likely represent maturational changes in these molecules13.
Cardiac fibrosis was measured in vehicle and rGDF11 treated 24-month-old mice by measuring the percentage of collagen in cardiac histological sections using Masson’s trichrome staining. There was no significant difference in fibrosis between rGDF11 and vehicle treated animals (Online figure IIA). We also tested the ability of rGDF11 to stimulate fibroblast activation in-vitro using primary cultures of normal human dermal fibroblasts. rGDF11 stimulated fibroblast activation with an EC50 of 176pM (Online figure IIB). This was similar to the effects of myostatin, which had an EC50 of 83pM.
GDF11 had no effect on cardiac function
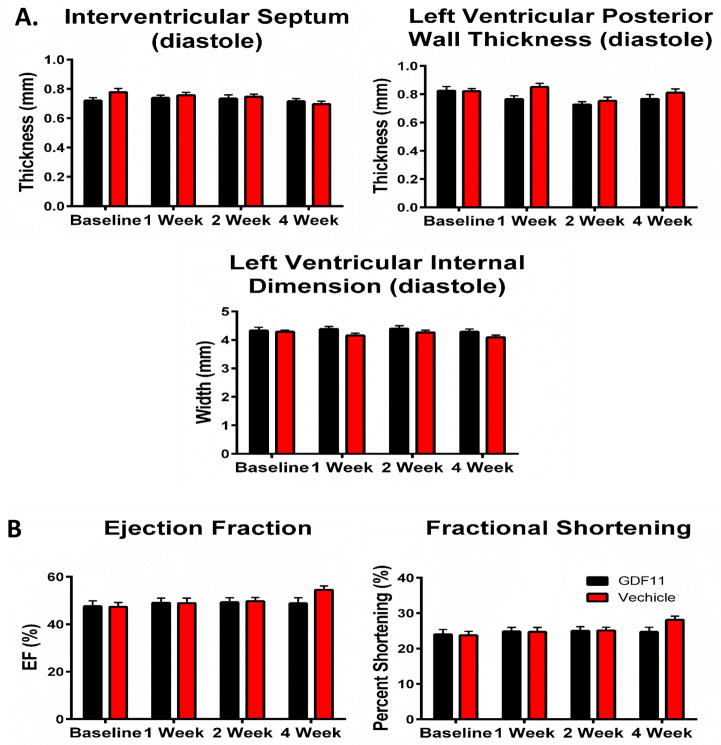
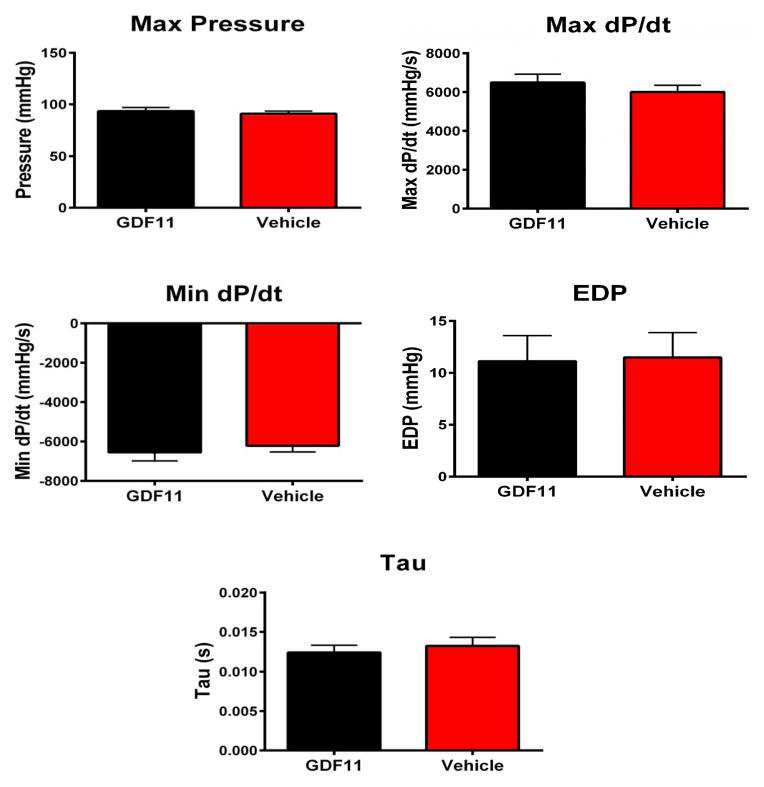
Cardiac structure and function remained unchanged at 1, 2, and 4 weeks of rGDF11 treatment as measured by echocardiography (Figure 2A–B.) In terminal hemodynamic studies, there was no difference in max pressure, max dP/dT, min dP/dt, EDP, or Tau between rGDF11 and vehicle treated animals (Figure 3). Collectively these experiments show that rGDF11 had no effects on cardiac structure or function.
Figure 2. Cardiac structure and function measured by echocardiography.
Mice received echocardiography at baseline, 1, 2, and 4 weeks after the start of injections. rGDF11 did not affect any A: structural or B: functional parameters measured. rGDF11 (n=21) or vehicle (n=22)
Figure 3. Intra-left ventricular pressures.
In vivo intra-LV pressures were measured at time of sacrifice. There was no difference in max pressure, max dP/dT, min dP/dt, EDP, or Tau between rGDF11 (n=21) and vehicle (n=22) treated animals.
GDF11 did not prevent phenylephrine-induced hypertrophy in vitro
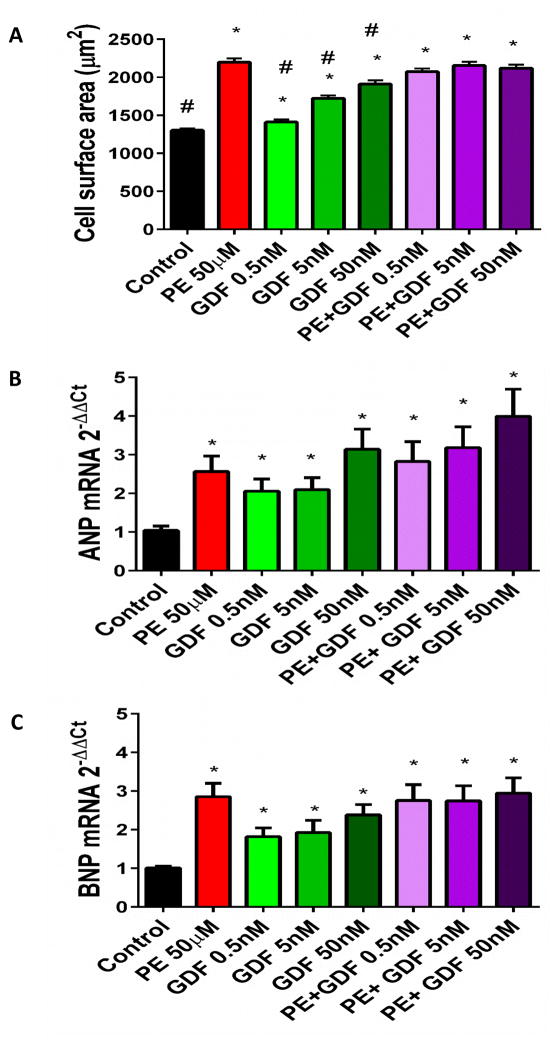
Finally, we tested the effects of rGDF11 treatment on phenylephrine-induced hypertrophy in cultured neonatal rat ventricular myocytes (NRVMs)1. NRVMs were treated with rGDF11 at three concentrations: 0.5nM, 5nM, and 50nM with and without simultaneous phenylephrine treatment. rGDF11 treatment failed to inhibit phenylephrine-induced increases in myocyte surface area, but instead caused a dose dependent increase in myocyte size (Figure 4A). rGDF11 failed to inhibit phenylephrine-induced increases in ANP and BNP mRNA expression, while by itself it induced a dose-related increase in ANP and BNP mRNA compared to controls (Figure 4B–C).
Figure 4. GDF11 induces hypertrophy in NRVMs.
A: 150–300 myocytes were analyzed from at least 20 fields of view per condition B–C: GDF11 treatment increased expression of ANP and BNP mRNA expression (n=3) *p<0.05 vs control. #p<0.05 vs PE 50μM.
DISCUSSION
Aging related cardiomyopathy is often secondary to the accumulation of cardiovascular disease (CVD) and disease-induced changes in cardiac structure and function lead to cardiomyopathy14–16. True age dependent changes in cardiac structure and function in CVD-free individuals are not well understood and if defined could provide novel clues for protection from aging-specific cardiac functional decline.
Others have searched for aging-specific changes in factors that circulate in the blood that if corrected could prevent or reverse age dependent decline in the function of critical organ systems17, 18. Indeed, a recent study, which has generated a significant amount of attention and collateral reporting (http://hsci.harvard.edu/aging-and-gdf11-what-we-know), (http://www.nature.com/cr/journal/v24/n12/full/cr2014107a.html), suggests that the blood levels of the myostatin-related protein, growth differentiation factor 11 (GDF11), decreases with age1. These myokine proteins are members of the transforming growth factor β (TGFβ) family of proteins. Myostatin can potently inhibit skeletal muscle growth and differentiation19, with smaller effects on cardiac muscle20. Reductions or loss of function of myostatin causes increases in skeletal muscle mass, with little or no effect on the heart4–6.
A recent study reported that the circulating level of GDF11 decreases with aging, and this reduction correlated with the development of pathological cardiac hypertrophy in old mice1. Pathological cardiac hypertrophy was rescued in these old mice by either restoring a “youthful” circulation with parabiosis or by injection of rGDF11 to restore youthful GDF11 levels1. Related studies suggest that restoring youthful GDF11 levels rejuvenates skeletal muscle7 and brain8 structure and function. These findings suggest that restoring youthful levels of GDF11 can reverse aging dependent decline of critical organ systems and they were highly touted as the discovery of the long sought after tissue rejuvenation factor (http://hsci.harvard.edu/aging-and-gdf11-what-we-know). However, the skeletal muscle findings have recently been challenged9 and, considering our results in the old heart, it does not appear that GDF11 is an anti-aging factor (http://stemcellassays.com/2015/05/gdf11-contoversy-antibody-better/) (http://pipeline.corante.com/archives/2015/05/20/a_young_blood_controversy.php). (http://www.nature.com/news/young-blood-anti-ageing-mechanism-called-into-question-1.17583). A recent editorial has also highlighted that GDF-11 is not the long-sought rejuvenation factor21.
The objectives of our experiments were to reexamine the idea that rGDF11 is a critical cardiac anti-aging factor in the mouse1. Our goal was to define the mechanisms underlying the ability of rGDF11 to reverse aging dependent cardiac pathological structural and functional remodeling.
We first set out to develop assays with appropriate sensitivity and specificity for GDF11 detection. We first examined the antibody used in previous work1 and found, similar to results in another recent report9, that this antibody also identified the highly homologous family member, myostatin (Online figure IA). We then characterized an antibody (R&D systems) that detected GDF11 and not myostatin (Online Figure IB) and we used this antibody to determine that treatment increases blood levels of rGDF11 in old mice. We found that endogenous levels of GDF11 were below our detection limits in both young and old mice. Therefore, we could not determine if GDF11 levels fell with age similarly to what has been reported recently in a study of skeletal muscle9. Our results show that the reagents used in previous work 1 are inadequate to determine if GDF11 levels fall in old mice or increase after rGDF11 treatment.
Our studies show that treatment of old mice with rGDF11 had no effect on heart or myocyte size, overall cardiac structure and cardiac pump function (Figures 1, 2, 3). Indeed, hearts and their myocytes are larger in old versus young mice22, 23. However, old mice have greater body weights than young mice and 24-month-old mice have the same HW/BW ratio as 8 or 12 week old mice, which is inconsistent with the presence of pathological hypertrophy. In addition, we could not find evidence for activation of pathological hypertrophy signaling in old animals. Collectively our results suggest that the increase in heart size with aging is what is expected in healthy animals that have increases in their body mass. There are numerous reports showing that heart size changes with increases or decreases in body size in mature adult animals24, 25. Our results suggest that HW/TL cannot discriminate between pathological hypertrophy and physiological growth in mature animals where tibia length does not change.
The suggestion of an anti-hypertrophic effect of a youthful circulation in old mice came from parabiosis experiments in which old and young mice had a shared circulation1. In these studies, heart size was clearly reduced in old mice sharing a circulation with young mice1. However, the old mice lost 25–30% of their body weight during the month of parabiosis with the young mice and the HW/BW ratios in these old mice appear to have been unchanged. The unexplained data in this previous report1, upon which the conclusions in the parabiosis experiments rest, is that there was no change in the HW of old mice sharing a circulation with other old mice, even though these old mice also lost 25% of their body weight. The observation that mice can reduce their BW by 25% with no corresponding change in HW1 does not fit with a large body of existing work11, 24, 25.
We also studied the effects of GDF11 on phenylephrine-induced hypertrophy of neonatal rat ventricular myocytes. We found that rGDF11, by itself, activated pathological hypertrophy signaling and increased myocyte size, but it did not exacerbate or block the effects of phenylephrine reported previously1.
In summary, our studies show that daily injections of biologically active rGDF11 raised the blood levels of rGDF11 in old mice, but had no effect on heart and myocyte size; overall cardiac structure and cardiac pump function. We also did not find evidence for the existence of pathological hypertrophy in 24-month-old disease-free C57BL/6 mice. These results do not support the idea that GDF11 should be part of an “anti-aging” elixir.
Supplementary Material
Novelty and Significance.
What Is Known?
Cardiovascular structure and function can become abnormal with aging, and these changes are often accompanied by the accumulation of cardiovascular disease risk factors such as hypertension.
Aging could also lead to cardiac structural and functional defects that are largely independent of cardiovascular disease, but the basis of these changes are not well understood.
A recent study showed that restoring “youthful” levels of Growth Differentiation Factor 11 (GDF11) by sharing the circulation of a young animal (parabiosis) or by injection of recombinant GDF11 (rGDF11) into old mice restored youthful levels of GDF11 and revered pathological hypertrophy.
What New Information Does This Article Contribute?
We found no evidence for the existence of pathological cardiac hypertrophy in 24-month-old, disease free C57BL/6 mice: the HW/BW ratio of these old mice was identical to that of young animals and there were no molecular markers of pathological hypertrophy signaling.
Daily injection of GDF11 into old mice increased blood levels into a range of biological activity, but the rGDF11 injections did not affect the heart or myocyte size, cardiac fibrosis, or cardiac function.
rGDF11 did not reduce phenylephrine induced neonatal myocyte hypertrophy, but had pro-hypertrophic effects when tested alone.
Pathological hypertrophy and myocyte dysfunction can occur in response to cardiovascular diseases. GDF11 has been shown to reverse pathological cardiac hypertrophy; however, we were unable to confirm these findings. Additionally, the reagents used in previous studies could not reliably detect GDF11, so the idea that GDF11 falls with aging and was increased in old animals after parabiosis with young animals or after injection of rGDF11 is not based on trustworthy data. Collectively our results do not support the idea that GDF11 has any significant effect on the size, structure or function of the old mouse heart.
Acknowledgments
SOURCES OF FUNDING
The study was supported by Boehringer Ingelheim, NIH grants to SRH and an AHA SDG to SM.
Nonstandard Abbreviations and Acronyms
- GDF11
Growth differentiation factor 11
- NRVM
Neonatal rat ventricular myocyte
- HW
Heart weight
- BW
Body weight
- TGFβ
Transforming Growth Factor β
- ANP
Atrial natriuretic peptide
- BNP
Brain natriuretic peptide
- αMHC
α-myosin heavy chain
- βMHC
β-myosin heavy chain
- EDP
End diastolic pressure
Footnotes
DISCLOSURES
None.
References
- 1.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–39. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohsawa Y, Okada T, Nishimatsu S, Ishizaki M, Suga T, Fujino M, Murakami T, Uchino M, Tsuchida K, Noji S, Hinohara A, Shimizu T, Shimizu K, Sunada Y. An inhibitor of transforming growth factor beta type I receptor ameliorates muscle atrophy in a mouse model of caveolin 3-deficient muscular dystrophy. Lab Invest. 2012;92:1100–14. doi: 10.1038/labinvest.2012.78. [DOI] [PubMed] [Google Scholar]
- 3.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol (1985) 2008;104:579–87. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 4.Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–85. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3:e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–25. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–4. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiper C, Grimes B, Van Zant G, Satin J. Mouse strain determines cardiac growth potential. PLoS One. 2013;8:e70512. doi: 10.1371/journal.pone.0070512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savabi F, Kirsch A. Diabetic type of cardiomyopathy in food-restricted rats. Can J Physiol Pharmacol. 1992;70:1040–7. doi: 10.1139/y92-143. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GL, Harris MM, Ghidoni JJ, Page A, Cantu TL, Young E. Analysis of myocardial response to significant weight loss in obese rats. Am J Clin Nutr. 1994;59:566–71. doi: 10.1093/ajcn/59.3.566. [DOI] [PubMed] [Google Scholar]
- 13.Pandya K, Kim HS, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci U S A. 2006;103:16864–9. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch LA, Cappola TP, Harrison RW, Crone JK, Rodriguez ER, Burnett AL, Hare JM. Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mol Cell Cardiol. 2003;35:637–44. doi: 10.1016/s0022-2828(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 15.Waller BF. The old-age heart: normal aging changes which can produce or mimic cardiac disease. Clin Cardiol. 1988;11:513–7. doi: 10.1002/clc.4960110802. [DOI] [PubMed] [Google Scholar]
- 16.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–92. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 19.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 20.Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord. 2007;17:290–6. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brun CE, Rudnicki MA. GDF11 and the Mythical Fountain of Youth. Cell Metab. 2015;22:54–6. doi: 10.1016/j.cmet.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Helms SA, Azhar G, Zuo C, Theus SA, Bartke A, Wei JY. Smaller cardiac cell size and reduced extra-cellular collagen might be beneficial for hearts of Ames dwarf mice. Int J Biol Sci. 2010;6:475–90. doi: 10.7150/ijbs.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Azhar G, Furr MC, Zhong Y, Wei JY. Model of functional cardiac aging: young adult mice with mild overexpression of serum response factor. Am J Physiol Regul Integr Comp Physiol. 2003;285:R552–60. doi: 10.1152/ajpregu.00631.2002. [DOI] [PubMed] [Google Scholar]
- 24.Lim HW, De Windt LJ, Steinberg L, Taigen T, Witt SA, Kimball TR, Molkentin JD. Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation. 2000;101:2431–7. doi: 10.1161/01.cir.101.20.2431. [DOI] [PubMed] [Google Scholar]
- 25.Ernsberger P, Koletsky RJ, Baskin JS, Collins LA. Consequences of weight cycling in obese spontaneously hypertensive rats. Am J Physiol. 1996;270:R864–72. doi: 10.1152/ajpregu.1996.270.4.R864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.