Abstract
Purpose
Given reportedly high clustering but limited validity of retrospectively-reported time-to-pregnancy (TTP), we assessed within-woman clustering for retrospectively-reported TTPs alone and including gold-standard prospectively observed TTPs among women with ≥2 retrospectively-reported and ≥1 prospectively observed TTPs. We further investigated whether past trying times inform future trying time among women with ≥1 retrospectively-reported and ≥1 prospectively observed TTPs.
Methods
501 couples attempting pregnancy were prospectively observed until hCG pregnancy or 12 months of trying. Women reported TTP for past planned pregnancies. Clustering as measured by the frailty variance was estimated using discrete Cox frailty models, adjusted for age, BMI, smoking at each attempt. Utility of past attempts to inform future attempts was assessed with discrete Cox models and relative risk regression, adjusted for baseline age, BMI, smoking.
Results
75 women with ≥2 prior pregnancies contributed 180 retrospective and 91 prospective TTPs for frailty modeling. Retrospectively-reported TTP clustering was high (frailty variance=0.89) but substantially lower when including prospectively observed TTPs (frailty variance=0.42). Among 202 women with ≥1 prior pregnancies, past trying times did not inform future trying time.
Conclusions
TTP recall rather than TTP may account for clustering. Past trying times may not inform future trying times.
Keywords: time-to-pregnancy, fecundity, conception, conception delay
Introduction
Several reproductive outcomes cluster within women, including pregnancy loss, preterm birth, preeclampsia, and gestational diabetes (1–6), and women’s prior outcomes are often used to inform likelihood of future occurrence. Time-to-pregnancy (TTP), defined as the number of cycles or months of unprotected sexual intercourse required to achieve pregnancy, also reportedly clusters within women (7, 8). Two previous studies using retrospectively-reported TTP among fertile women in Europe and the US reported high TTP clustering (7, 8). In contrast, using data from a US preconception cohort with prospective TTP measurement, clustering was low among women experiencing pregnancy loss (9).
Given these reported differences in TTP clustering by method of TTP ascertainment, we evaluated TTP clustering among a unique cohort of women with information on both retrospectively-reported and prospectively observed TTPs. We investigated the extent of TTP clustering within women for retrospectively-reported TTPs alone and including gold-standard prospectively observed TTPs for women with ≥2 retrospectively-reported and ≥1 prospectively observed TTPs. We further investigated whether retrospectively-reported trying times informed prospectively observed trying time for women with ≥1 retrospectively-reported and ≥1 prospectively observed TTPs.
Material and methods
Study population
The Longitudinal Investigation of Fertility and the Environment (LIFE) Study is a population-based, preconception cohort of 501 couples recruited upon discontinuing contraception to try for pregnancy and followed for 12 months of trying (10). Couples experiencing pregnancy loss were able to continue in the study allowing for measurement of subsequent TTPs; couples not pregnant after 12 months were censored. The inclusive study design only excluded couples with clinically diagnosed infertility/sterility. Inclusion criteria comprised couples in a committed relationship, intending to begin pregnancy attempts or off contraception for ≤2 months, partners communicate in English or Spanish, men aged ≥18-years-old and women aged 18-40-years-old, menstrual cycle lengths between 21–42 days, and no past year use of injectable contraceptives. Institutional Review Board approval was obtained from all participating institutions; informed consent was obtained from all participants prior to data collection.
At enrollment, women were queried on their medical, social, and reproductive histories. Particularly, women were asked about each previous pregnancy including age at pregnancy, whether pregnancy was planned, TTP for planned pregnancies, and pregnancy outcome(s). Women were asked if they currently smoked and ages they started and stopped smoking cigarettes, if applicable. They provided their weight for 5-year intervals from 15-years-old until baseline. Height and weight were measured upon enrollment, and women were given and instructed in the use of the urine-based ClearBlue ™ Easy digital fertility monitor. These monitors provide valid measures of ovulation (11) to help couples time intercourse relative to impending ovulation. Women were provided with highly sensitive (25 IU/L) urine-based ClearBlue™ Easy digital home pregnancy tests to facilitate ascertainment of pregnancies. A single positive pregnancy test on day of expected menstruation denoted an hCG pregnancy.
Measures
Prospectively observed menstrual cycles were used to measure TTP during the study as retrospectively-reported TTP has limited validity relative to prospective measurement (12). At enrollment, women were administered a home pregnancy test to ensure they were not pregnant. Time couples were off contraception prior to study entry (7% one and 15% two months) was accounted for in analysis of TTP. For these analyses, we assume that months and cycles are equivalent. Median observed cycle length was 30 days (interquartile range (IQR)=27–35). Conception delay was defined as TTP>6.
Other factors relevant to TTP that may change between pregnancy attempts include maternal age, body mass index (BMI), and smoking status (13–16); therefore, these were included as covariates in modeling. For attempts during the study, age, measured BMI, and self-reported smoking status at enrollment were used. For pregnancies occurring before study entry, reported age at pregnancy was used. If a woman’s reported age at pregnancy fell in the interval during which she reported smoking cigarettes, she was considered a smoker for that attempt. A woman’s height at enrollment was considered fixed for all pregnancies and her self-reported weight in the 5-year interval corresponding to the age at which she reported her pregnancy was used to calculate BMI. Pregnancy loss included losses reported during the baseline interview (i.e., miscarriage, stillbirth, or ectopic).
Statistical analysis
Summary statistics of the sample were conducted using differences in TTP between first prospectively observed attempt and the mean of all retrospectively-reported TTPs and computing sensitivity, specificity, positive and negative predictive values (PPV, NPV) of past conception delay for prospective conception delay.
Discrete Cox frailty models with lognormal frailty distribution were used to estimate TTP clustering as measured by the frailty variance (8) for women with ≥2 retrospectively-reported TTP (n=75). These models incorporate a frailty variable, which is akin to a random effect, to quantify the degree of within-woman dependency in TTP due to unobserved factors (e.g. after adjustment for covariates) and yield standard errors (SE) for the frailty variance. Higher frailty variance indicates higher within-woman TTP clustering. Separate models were constructed for retrospectively-reported TTP only and retrospectively-reported with prospectively observed TTP; all adjusted for age, BMI, and smoking at each attempt. When including both retrospectively-reported and prospectively observed TTPs, models included a strata statement for TTP type and a TTP type*BMI interaction term (17). A bootstrap approach was used to test difference in frailty variances between models based on overlapping subgroups of women. 500 bootstrap samples of 75 women each were resampled from the original data and frailty models were run for each of these samples and a percentile based 95% confidence interval for the difference in the frailty variance for each pair of models was calculated (18).
To determine if estimates varied by recall period, we ran frailty models restricted to all prospective attempts and retrospective pregnancies within 3, 6, and 10 years of enrollment. These cutoffs reflect 40%, 80%, and 90% of past pregnancies for women with ≥2 retrospectively-reported TTP. We also assessed clustering of all retrospective attempts with only the first prospective attempt as only women with observed losses had multiple prospective attempts.
For women with ≥1 retrospectively-reported TTP (n=202), we assessed whether retrospectively-reported TTP or conception delay may inform prospectively observed TTP or conception delay, respectively, adjusted for maternal age, BMI, and smoking at study enrollment. The risk of prospectively observed conception delay was modeled with an indicator of any past conception delay (19), where risk ratio (RR)>1 indicates greater risk of prospective conception delay if retrospective delay was reported. Using discrete survival models with robust variances, we computed fecundability odds ratios (FOR), the odds of achieving a pregnancy in a cycle given no pregnancy in the previous cycle, for the first prospective attempt using the mean of all retrospectively-reported TTP, where FOR<1 indicates a longer TTP in the first prospective attempt for longer retrospectively-reported TTP. Analyses were conducted in SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
75 women with ≥2 prior pregnancies contributed 180 retrospectively-reported and 91 prospectively observed TTPs to frailty models. RR and FOR models included 307 retrospectively-reported and 202 prospectively observed TTPs from 202 women. Only their first prospective attempt was included as the interest was whether retrospective reported trying times informed the very next prospectively observed trying time to mimic a preconception counseling office visit. Characteristics of these two samples were largely similar (Table 1a) with the only notable difference that 75% of women with ≥2 retrospectively-reported TTP experienced a prior pregnancy loss compared with 42% of women with ≥1 retrospectively-reported TTP.
Table 1.
| a. Demographic Characteristics Among Women with ≥2 and ≥1 Retrospectively Reported Time-to-Pregnancies | ||
|---|---|---|
| Women with ≥2 Retrospectively Reported TTP (n=75) |
Women with ≥1 Retrospectively Reported TTP (n=202) |
|
| Median (IQRa) | Median (IQR) | |
| Age at study enrollment | 33 (29, 36) | 32 (29, 34) |
| Body mass index at study enrollment | 27.2 (22.5, 31.7) | 26.2 (22.7, 31.0) |
| n (%) | n (%) | |
| Smoked at study enrollment | 8 (11) | 19 (9) |
| Nulliparous at study enrollment | 7 (9) | 20 (10) |
| b. Fecundity for Retrospectively Reported and Prospectively Observed Pregnancy Attempts Among Women with ≥2 and ≥1 Retrospectively Reported Time-to-Pregnancies | ||
|---|---|---|
| Women with ≥2 Retrospectively Reported TTP (n=75) |
Women with ≥1 Retrospectively Reported TTP (n=202) |
|
| Fecundity History at Enrollment | Median (IQR) | Median (IQR) |
| TTPb for past pregnancies | 2 (1, 5) | 2 (1, 6) |
| Recall period for all past pregnanciesc | 4 (3, 6) | 4 (3, 6) |
| Recall period for first past pregnancy | 5 (4, 8) | 4 (3, 6) |
| n (%) | n (%) | |
| Conception delayd in past pregnancy | 15 (20) | 47 (23) |
| Pregnancy loss in past pregnancy | 56 (75) | 85 (42) |
| Prospectively Observed Fecundity Outcomes | Median (IQR) | Median (IQR) |
| TTP in LIFE Study | 4 (2, 6) | 4 (2, 6) |
| n (%) | n (%) | |
| Conception delay in LIFE Study | 20 (22) | 50 (25) |
| Pregnancy loss in LIFE Study | 22 (29) | 49 (31) |
Interquartile range
Time-to-pregnancy (in months for retrospective report, in cycles for prospective observation)
Recall period is time since pregnancy, including past year (minimum of 1 year recall)
Conception delay is TTP>6 cycles or months
Reported fecundity history and observed fecundity outcomes during the study were similar between groups (Table 1b). Median retrospectively-reported TTP was 2 months in both groups; conception delay preceding a prior pregnancy was reported by 20% and 23% of women with ≥2 and ≥1 retrospectively-reported TTPs, respectively. Median prospectively observed TTP was 4 cycles in both groups with conception delay in 22% and 25% of pregnancy attempts for women with ≥2 and ≥1 retrospectively-reported TTPs, respectively.
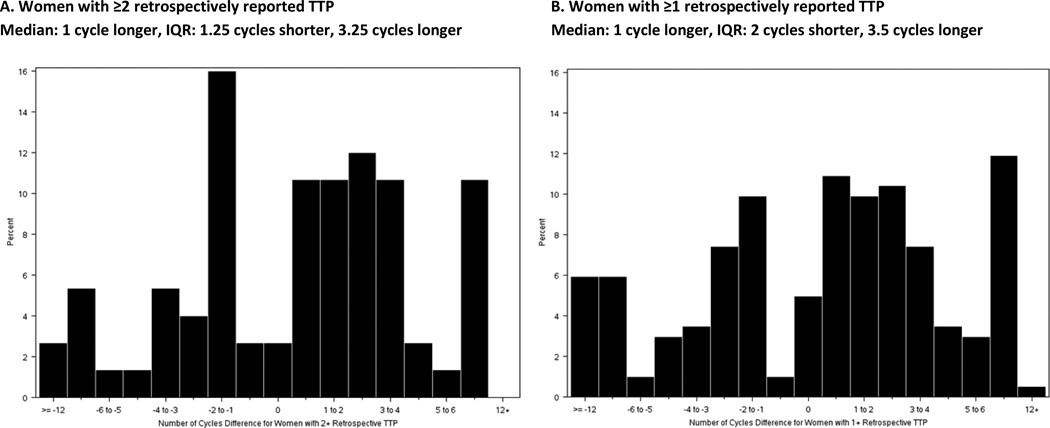
Comparing retrospectively-reported conception delay to the gold-standard of prospectively observed conception delay, sensitivity was 25% and 26% and specificity was 82% and 78% for women with ≥2 and ≥1 retrospective reported TTPs, respectively. When using retrospectively-reported conception delay as the test for prospectively observed conception delay, PPV was 33% and 28% and NPV was 75% and 76% for women with ≥2 and ≥1 retrospective reported TTPs, respectively. Differences in TTP between the first prospectively observed attempt and the mean of all retrospectively-reported TTP show prospectively observed TTP was longer for the majority of women (median=1 cycle longer) in both groups, though shorter prospectively observed TTP was also observed in >25% of women in both groups (Figure 1).
Figure 1.
Difference in TTP between first prospectively observed attempt and mean of all retrospectively reported attempts with positive integers indicating a longer and negative integers a shorter prospectively observed TTP.
Clustering of retrospectively-reported TTP was high in unadjusted (frailty variance=0.79, SE=0.24) and adjusted (frailty variance=0.89, SE=0.27) models (Table 2a). However, when prospectively observed TTPs were included with retrospectively-reported TTPs, clustering was lower (unadjusted frailty variance=0.32, SE=0.12, adjusted frailty variance=0.42, SE=0.14). Using bootstrap samples, 95% CI for the difference in frailty variance was 0.008 to 1.136 and −0.004 to 1.233 in unadjusted and adjusted models, indicating significant difference in clustering between unadjusted models and substantial difference in adjusted models. Results were similar when only the first prospective attempt was considered with all past attempts (frailty variance=0.48, SE=0.16). When restricting to shorter recall periods, clustering for all prospective attempts and past attempts within ≤3 years (frailty variance=0.81, SE=0.27) and ≤6 years (frailty variance=0.52, SE=0.18) was still lower than for retrospectively-reported TTPs alone. Mean retrospectively-reported TTP was not associated with TTP in the first prospectively observed attempt (FOR=1.00, 95% CI=0.99–1.01, Table 2b), nor was past conception delay associated with prospectively observed conception delay in the first attempt (RR=1.07, 95% CI=0.57–2.01, Table 2c). The above models were assessed for heterogeneity by pregnancy loss; none were statistically significant.
Table 2.
| a. Frailty Models for Time-to-Pregnancy (TTP) | |||
|---|---|---|---|
| Model | Number of TTPsa | Frailty varianceb | Standard error |
| All retrospective pregnancies, unadjusted | 180 | 0.79 | 0.24 |
| All retrospective pregnancies and prospective attempts, unadjusted | 271 | 0.32 | 0.12 |
| All retrospective pregnancies and first prospective attempt, unadjusted | 255 | 0.38 | 0.14 |
| All retrospective pregnancies, adjustedc | 180 | 0.89 | 0.27 |
| All retrospective pregnancies and prospective attempts, adjustedc | 271 | 0.42 | 0.14 |
| All retrospective pregnancies and first prospective attempt, adjustedc | 255 | 0.48 | 0.16 |
| All prospective attempts and retrospective pregnancies within past 3 yearsc | 165 | 0.81 | 0.28 |
| All prospective attempts and retrospective pregnancies within past 6 yearsc | 238 | 0.52 | 0.18 |
| All prospective attempts and retrospective pregnancies within past 10 yearsc | 261 | 0.37 | 0.14 |
| b. Model for Discrete TTP in First Prospective Attempt (n=202) | ||||
|---|---|---|---|---|
| Unadjusted | Adjustede | |||
| FORd | 95% CI | FOR | 95% CI | |
| Mean discrete TTP in past attempts | 0.99 | 0.98, 1.01 | 1.00 | 0.99, 1.01 |
| Age at study enrollment | 0.92 | 0.88, 0.96 | 0.92 | 0.88, 0.96 |
| Body mass index at study enrollment | 0.97 | 0.94, 1.00 | 0.98 | 0.95, 1.01 |
| Smoker at study enrollment | 0.52 | 0.30, 0.89 | 0.49 | 0.28, 0.86 |
| c. Model for Conception Delay in First Prospective Attempt (n=202) | ||||
|---|---|---|---|---|
| Unadjusted | Adjustede | |||
| RRf | 95% CI | RR | 95% CI | |
| Conception delay in past attempt | 1.16 | 0.62, 2.18 | 1.07 | 0.57, 2.01 |
| Age at study enrollment | 1.09 | 1.01, 1.17 | 1.08 | 1.00, 1.16 |
| Body mass index at study enrollment | 1.04 | 1.00, 1.07 | 1.03 | 1.00, 1.07 |
| Smoker at study enrollment | 1.31 | 0.56, 3.08 | 1.21 | 0.51, 2.85 |
Number of TTPs included in the frailty model
Discrete Cox frailty model with lognormal frailty distribution
Adjusted for age, BMI, and smoking status at each pregnancy attempt
Fecundability odds ratio
Adjusted for all covariates listed in table
Risk ratio
Discussion
To our knowledge, this is the first assessment of TTP clustering across multiple pregnancy attempts where at least one attempt has been measured by the gold-standard, prospective measurement. We find retrospectively-reported TTP clustering is high, though notably lower when incorporating prospectively observed TTP. We found past trying times, measured by TTP or conception delay, did not inform prospective trying times. Collectively, these results suggest the high clustering for retrospectively-reported TTP may reflect reliability of recalled TTP (20) but not the actual TTP. Recall period may be important as clustering is higher when retrospective report is restricted to ≤3 years compared with reports ≤6 or ≤10 years.
Our findings are consistent with two previous studies reporting high clustering of retrospectively-reported TTP (7, 8). They also corroborate the low clustering of prospectively observed TTP in women with pregnancy losses (9). A possible explanation for the differences in clustering may reflect the limited validity of retrospectively-reported TTP relative to the gold-standard of prospectively observed TTP; validity has been shown to be good for short (<20 months) (21) but not long term (10 years) (12) recall. Our data show that even when restricted to a recall period of ≈6 years, within-woman clustering is low when prospectively observed TTP is considered along with retrospectively-reported TTP.
As previous work on clustering of prospectively observed TTP was conducted among women with fecundity impairment, we considered pregnancy loss status in our analysis. We did not observe differences in the extent of clustering by prior pregnancy loss; retrospectively-reported TTP is apparently not influenced by prior loss. This finding may suggest that clustering of prospectively observed TTPs in general is low, perhaps reflecting changes in underlying couple fecundity over time, other exposures, or even change in partnerships. Due to the LIFE Study design, we only have information on multiple prospectively observed attempts for women with observed losses and cannot evaluate prospectively observed TTP clustering by loss status; however, clustering was only slightly higher when only the first prospective attempt was considered alongside retrospectively-reported TTPs.
These findings suggest past trying times as measured by self-reported TTP or its related impairment (conception delay) are not highly informative about future prospectively observed trying attempts. If corroborated, these data may reassure couples concerned about repeating prolonged trying times. Further, if TTP as measured by the gold-standard is informative for pregnancy outcomes or later adult health (22–25), efforts to enhance couple’s accurate counting and report seem warranted.
Acknowledgements
Funding: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts N01-HD-3-3355, N01-HD-3-3356, and NOH-HD-3-3358). The study funders had no role in the study design; collection, analysis or interpretation of data; writing of the report; or the decision to submit the article for publication.
List of abbreviations
- TTP
time-to-pregnancy
- hCG
human chorionic gonadotropin
- BMI
body mass index
- RR
risk ratio
- IQR
interquartile range
- SE
standard error
- FOR
fecundability odds ratio
- PPV
positive predictive value
- NPV
negative predictive value
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bramham K, Briley AL, Seed P, Poston L, Shennan AH, Chappell LC. Adverse maternal and perinatal outcomes in women with previous preeclampsia: a prospective study. American journal of obstetrics and gynecology. 2011;204(6):512, e1–e9. doi: 10.1016/j.ajog.2011.02.014. Epub 2011/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heuser C, Dalton J, Macpherson C, Branch DW, Porter TF, Silver RM. Idiopathic recurrent pregnancy loss recurs at similar gestational ages. American journal of obstetrics and gynecology. 2010;203(4):343, e1–e5. doi: 10.1016/j.ajog.2010.05.010. Epub 2010/06/29. [DOI] [PubMed] [Google Scholar]
- 3.Khambalia AZ, Ford JB, Nassar N, Shand AW, McElduff A, Roberts CL. Occurrence and recurrence of diabetes in pregnancy. Diabetic medicine : a journal of the British Diabetic Association. 2013;30(4):452–456. doi: 10.1111/dme.12124. Epub 2013/01/18. [DOI] [PubMed] [Google Scholar]
- 4.Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. American journal of obstetrics and gynecology. 2014;210(2):131, e1–e8. doi: 10.1016/j.ajog.2013.09.014. Epub 2013/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melamed N, Hadar E, Peled Y, Hod M, Wiznitzer A, Yogev Y. Risk for recurrence of preeclampsia and outcome of subsequent pregnancy in women with preeclampsia in their first pregnancy. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(11):2248–2251. doi: 10.3109/14767058.2012.684174. Epub 2012/04/25. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen SE, Lyon JL, Stanford JB, Porucznik CA, Esplin MS, Varner MW. Risk factors for recurrent preterm birth in multiparous Utah women: a historical cohort study. BJOG : an international journal of obstetrics and gynaecology. 2013;120(7):863–872. doi: 10.1111/1471-0528.12182. Epub 2013/02/20. [DOI] [PubMed] [Google Scholar]
- 7.Basso O, Olsen J, Bisanti L, Bolumar F, Kuppers-Chinnow M The European Study Group on Infertility and Subfecundity. Repeating episodes of low fecundability. A multicentre European study. Hum Reprod. 1997;12(7):1448–1453. doi: 10.1093/humrep/12.7.1448. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 8.McLain AC, Sundaram R, Cooney MA, Gollenberg AL, Buck Louis GM. Clustering of fecundability within women. Paediatric and perinatal epidemiology. 2011;25(5):460–465. doi: 10.1111/j.1365-3016.2011.01219.x. Epub 2011/08/09. [DOI] [PubMed] [Google Scholar]
- 9.Sapra KJ, McLain AC, Maisog JM, Sundaram R, Buck Louis GM. Successive time to pregnancy among women experiencing pregnancy loss. Hum Reprod. 2014;29(11):2553–2559. doi: 10.1093/humrep/deu216. Epub 2014/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development--the LIFE Study. Paediatric and perinatal epidemiology. 2011;25(5):413–424. doi: 10.1111/j.1365-3016.2011.01205.x. Epub 2011/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15(12):2478–2482. doi: 10.1093/humrep/15.12.2478. Epub 2000/12/01. [DOI] [PubMed] [Google Scholar]
- 12.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20(1):56–59. doi: 10.1097/EDE.0b013e31818ef47e. Epub 2008/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, et al. Heavy metals and couple fecundity, the LIFE Study. Chemosphere. 2012;87(11):1201–1207. doi: 10.1016/j.chemosphere.2012.01.017. Epub 2012/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Hum Reprod. 2013;28(10):2856–2864. doi: 10.1093/humrep/det333. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford JH, MacCormac L, Hiller J. PALS (pregnancy and lifestyle study): association between occupational and environmental exposure to chemicals and reproductive outcome. Mutation research. 1994;313(2–3):153–164. doi: 10.1016/0165-1161(94)90045-0. Epub 1994/10/01. [DOI] [PubMed] [Google Scholar]
- 16.Buck Louis GM, Dmochowski J, Lynch C, Kostyniak P, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod. 2009;24(2):451–458. doi: 10.1093/humrep/den373. Epub 2008/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole SR, Chu H, Allison PD, Gange SJ. Combined analysis of retrospective and prospective occurrences in cohort studies: HIV-1 serostatus and incident pneumonia. International journal of epidemiology. 2006;35(6):1442–1446. doi: 10.1093/ije/dyl176. Epub 2006/08/29. [DOI] [PubMed] [Google Scholar]
- 18.Efron BaT R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 19.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.Joffe M, Villard L, Li Z, Plowman R, Vessey M. A time to pregnancy questionnaire designed for long term recall: validity in Oxford, England. Journal of epidemiology and community health. 1995;49(3):314–319. doi: 10.1136/jech.49.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. International journal of epidemiology. 1992;21(6):1151–1156. doi: 10.1093/ije/21.6.1151. Epub 1992/12/01. [DOI] [PubMed] [Google Scholar]
- 22.Bajuk Studen K, Jensterle Sever M, Pfeifer M. Cardiovascular risk and subclinical cardiovascular disease in polycystic ovary syndrome. Frontiers of hormone research. 2013;40:64–82. doi: 10.1159/000341838. Epub 2013/09/05. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, et al. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29(7):1567–1574. doi: 10.1093/humrep/deu106. Epub 2014/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. American journal of epidemiology. 2009;170(5):559–565. doi: 10.1093/aje/kwp168. Epub 2009/07/29. [DOI] [PubMed] [Google Scholar]
- 25.Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: a prospective population-based cohort study (EPIC-Heidelberg) Heart. 2011;97(1):49–54. doi: 10.1136/hrt.2010.202226. Epub 2010/12/03. [DOI] [PubMed] [Google Scholar]