Abstract
Objective
To evaluate cardiac troponin T (cTnT) as a predictor of end-stage renal disease (ESRD) and death in a cohort of African-American and white community dwelling adults with hypertensive families.
Patients & Methods
3,050 participants (whites from Rochester, Minnesota; African-Americans from Jackson, Mississippi) of the Genetic Epidemiology Network of Arteriopathy study were followed from baseline exam (June 1996–August 2000) through January 22, 2010. Cox regression models were used to examine the association of cTnT with ESRD and death adjusting for traditional risk factors.
Results
Cohort demographics and measurements included: whites (46%), hypertensive (71%), eGFR<60 mL/min/1.73m2 (32%), high-sensitivity C-reactive protein>3 mg/L (52%), and abnormal cTnT (≥0.01 ng/mL) (2%). At 10 years, 27% with abnormal cTnT developed ESRD compared to 1% with normal cTnT. Similarly, at 10 years, 47% with an abnormal cTnT had died compared to 7% with a normal cTnT. Abnormal cTnT was strongly associated with ESRD and death. This effect was attenuated but was still highly significant after adjustment for demographics, eGFR, and traditional risk factors for ESRD (unadjusted hazard ratio [HR] 23.91 (95% CI 12.9, 44.2); adjusted HR 2.81 (CI 1.3, 5.9) and death (unadjusted HR 8.43 [CI 6.0, 11.9]); adjusted HR 3.46 (CI 2.3, 5.1).
Conclusion
cTnT makes an independent contribution beyond traditional risk markers to the prediction of ESRD and all-cause death in community-dwelling individuals. Further studies may be needed to determine if cTnT screening among individuals with hypertension or within a subset of hypertensives would help identify those at risk for ESRD and all-cause death.
Keywords: blood pressure, coronary artery disease, diabetes mellitus, nephrology, renal disease
INTRODUCTION
Hypertension remains a priority health condition,1–3 and by 2030 it is estimated that greater than 40% of United States (US) adults will have one or more forms of cardiovascular disease, including hypertension, leading to significant economic cost.4 Hypertensive adults have up to a 4-fold increase in cardiovascular disease-associated death with disproportionate rates seen in racial and ethnic minorities.5–7 Hypertension-associated morbidity extends to kidney disease, comprising one of the most common causes of end-stage renal disease (ESRD), a condition affecting approximately 600,000 people and costing nearly $50 billion in public and private funds in the US.8,9
Persons with hypertension represent a high-risk population for the development of ESRD and death, likely due to the interplay of other cardiovascular diseases and associated morbidity. Recently published guidelines by the American College of Physicians10 suggest that screening for chronic kidney disease stages 1 to 3 is not clearly beneficial in the general population despite the increased risk for adverse cardiovascular and renal outcomes in these patients.11 Instead, it may be more beneficial to identify the higher-risk patients who need close monitoring for ESRD and other comorbid events. Identification of risk markers that improve risk assessment for death and the prediction of ESRD may be more cost effective, improve medical decision-making, and help target interventions in those who will clearly benefit.
Cardiac troponin T (cTnT) is a sensitive and specific biomarker for myocyte injury in the setting of acute coronary syndromes.12 In the general population, minimally increased cTnT as measured by the standard, readily-available assay is rare among subjects without chronic conditions such as heart failure, left ventricular hypertrophy, chronic kidney disease, or diabetes mellitus.13–15 However, minimally elevated cTnT levels in asymptomatic, older individuals and in patients with chronic kidney failure are associated with all-cause death.14,16–23 Whether cTnT can distinguish those at greatest risk of ESRD in addition to death is less clear. To address this question, we used the Genetic Epidemiology Network of Arteriopathy (GENOA) multi-ethnic cohort study of hypertensive families sampled from the community. We tested the hypothesis that cTnT would predict death and ESRD independent of traditional cardiovascular risk factors and kidney function.
SUBJECTS AND METHODS
Design Overview
The study design is a prospective cohort study of individuals from hypertensive families. The association of baseline cTnT with subsequent ESRD or all-cause death was assessed.
Setting and Participants
GENOA Cohort
Study participants were members of sibships enrolled in the GENOA study designed to identify genetic determinants of hypertension in multiple racial groups.24,25 The GENOA sibships were ascertained based on two or more members of the sibships having primary hypertension diagnosed prior to age 60 years. In Rochester Minnesota, a Mayo Clinic diagnostic index was used to identify all non-Hispanic white residents of Olmsted County with a diagnosis of essential hypertension made before the age of 60, who were then recruited for enrollment. In Jackson, Mississippi, subjects were recruited through hypertensive probands from the Atherosclerosis Risk in Communities cohort, a probability sample of 45–64 year-old, non- Hispanic black or African American residents of that community.26 All siblings of recruited subjects from these cohorts were invited to participate in the GENOA study. Sibships in which the index sibling was known to have a cause of secondary hypertension (e.g., renal artery stenosis) including severely impaired kidney function (e.g., serum creatinine ≥2.0 mg/dL) were not recruited. All available members of the recruited sibships, including normotensive siblings, were invited to the initial (baseline) study visit conducted between June 1996 and August 2000. Participants were excluded if they lacked stored serum samples at the baseline visit or had kidney failure at their baseline visit. This study was approved by the Mayo Clinic and University of Mississippi Medical Center Institutional Review Boards.
Cohort Baseline Assessment
The initial GENOA study visit consisted of a questionnaire regarding personal medical history, comorbid conditions, family history; blood pressure (BP) measurement; and blood draw for measurements of serum glucose, lipids (total cholesterol, high density lipoprotein, triglycerides), and creatinine. Hypertension was confirmed if a prior diagnosis and prescription antihypertensive medication were reported, or if the average systolic blood pressure (SBP) or diastolic blood pressures (DBP) was ≥140 mm Hg or ≥90 mm Hg, respectively. Diabetes was diagnosed if the subject reported treatment with insulin or oral hypoglycemic agents or the fasting serum glucose concentration was ≥126 mg/dL. Serum creatinine measurements were obtained with a standardized enzymatic assay.27 Estimated glomerular filtration rate (eGFR) at the baseline exam was calculated using the Modification of Diet in Renal Disease (MDRD) study equation.28,29 eGFR was also calculated using the CKD–EPI 2009 equation30 but this led to no meaningful difference in the study findings compared to using the MDRD study equation and was thus not reported. cTnT and high-sensitivity C-reactive protein (hsCRP) were measured in stored serum samples from the baseline exam (June 1996–August 2000) in Rochester, MN. cTnT was measured using immunoassay methods on Roche® e-modulers using an electrochemiluminescent immunoassay (Roche Diagnostics, Indianapolis, IN) in the Central Clinical Laboratory at Mayo Clinic in Rochester, MN. The limit of detection for this assay is <0.01 ng/mL, which is also the 99th percentile of the upper reference range.31,32 The 10% coefficient of variation for this assay is 0.035 ng/mL. In our laboratory, at the limit of detection the coefficient of variation is 18%. hsCRP was measured using the Roche Cobas® 6000, latex-enhanced immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN).
Outcomes and Follow-up
The primary outcome measures were all-cause death and ESRD. Vital status and death date were queried using Accurint (www.accurint.com). For study participants who were deceased as of January 22, 2010, death certificates were obtained for death verification and to determine primary cause of death. Primary causes of death were grouped into the following categories: malignancy, cardiac, sepsis/infection, pulmonary, cerebrovascular, trauma, renal, and unknown/other. ESRD events (i.e., initiation of maintenance dialysis therapy or kidney transplantation) as of May 2008 were determined via query conducted in 2010 of the United States Renal Data System (USRDS), a comprehensive national database of ESRD in the United States (www.usrds.org).
Statistical Analysis
Because 98% of cTnT values were undetectable, cTnT was stratified into normal (undetectable; <0.01 ng/mL) and abnormal (detectable; ≥0.01 ng/mL) values.13,21,22,33 Both hsCRP and triglycerides were assessed as continuous variables, and measurements were log-transformed due to skewness. hsCRP was also analyzed as a categorical variable with two categories: low-average risk (≤3 mg/L) and high-risk (>3 mg/L).34,35 Demographic and clinical variables were compared between abnormal and normal cTnT groups using the 2-sample rank-sum test for quantitative variables and the Chi square test for categorical variables. Cumulative rates of all-cause death and kidney failure (ESRD) events were estimated using the Kaplan-Meier method, and log rank tests were used for group comparisons. Proportional hazards regression (Cox) models were used to examine the association between cTnT and all-cause death (or ESRD) in univariable and multivariable models. Multi-variable regression models were used to adjust for potential confounding variables. Age, sex, race, diabetes, hypertension, low density lipoprotein cholesterol, cigarette smoking, history of myocardial infarction, hsCRP, and eGFR were evaluated as covariates in multivariable models.36,37 Due to the limited number of ESRD events, the number of covariates used in the multivariable models for predicting ESRD was restricted. Event times were defined as the time elapsed from study entry to the time of death or ESRD. Subjects free of death event were censored at time of death survey, January 22, 2010. Subjects free of ESRD events were censored at death or time of most recent USRDS ESRD survey data availability (two year reporting delay), May 2008. For both outcomes, the presence of an interaction between eGFR and cTnT was tested using a likelihood ratio test comparing nested Cox models (with vs. without interaction). The C-statistic was used to assess the impact of cTnT in different models. Results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). A secondary analysis for ESRD was conducted using the regression method of Fine and Gray to obtain the subdistribution HR for ESRD.38 This method accounts for subjects who died before occurrence of ESRD (competing risk). Subjects lost to follow-up before ESRD or death were censored. Subjects who died before ESRD remained in the risk set with an adjusted weight. All analyses were done in Statistical Analysis System (SAS version 9.3; SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
Of 3,431 GENOA participants, the final study cohort consisted of 3,050 due to exclusions for the following: lack of stored serum samples available for both cTnT and hsCRP measurements (n=361); kidney failure based on documented ESRD prior to baseline exam (n=8) or estimated GFR <15 mL/min/1.73m2 (n=6); and missing data (n=6). Baseline characteristics are presented in Table 1 for participants with abnormal cTnT (≥0.01 ng/mL) and normal cTnT (<0.01 ng/mL) levels. Overall, cTnT was abnormal or detectable in 66 (2.1%) with measurements ranging from 0.01–0.63 ng/mL. Participants with abnormal cTnT were older, more likely to be male and have comorbidities including hypertension, diabetes, myocardial infarction, and stroke compared to those with normal cTnT. However, no significant racial difference was observed. eGFR was lower while the cardiovascular biomarker, hsCRP, was higher in those with abnormal cTnT.
Table 1.
Comparison of Variablesa between Subjects with Abnormal cTnT versus Normal cTnT Concentrations.
| Abnormal cTnT (N=66) |
Normal cTnT (N=2984) |
P valueb | |
|---|---|---|---|
| Demographic and Cardiovascular Risk Factors: | |||
| Age, years | 67 (59,72) | 57 (49, 64) | <.0011 |
| African American | 41 (62.1%) | 1614 (54.1%) | .22 |
| Male | 41 (62.1%) | 1067 (35.8%) | <.0012 |
| Education | .14 | ||
| Precollege | 46 (69.7%) | 1615 (55.3%) | |
| University | 13 (19.7%) | 846 (28.4%) | |
| Graduate | 4 (6.1%) | 242 (8.1%) | |
| Trade | 3 (4.6%) | 245 (8.2%) | |
| BMI, kg/m2 | 29 (27, 35) | 30 (26,34) | .651 |
| Hypertension | 62 (93.9%) | 2112 (70.8%) | <.0012 |
| Diabetes | 34 (51.5%) | 493 (16.5%) | <.0012 |
| Myocardial Infarction | 19 (28.8%) | 131 (4.4%) | <.0012 |
| Stroke | 12 (18.2%) | 97 (3.3%) | <.0012 |
| Medications: | |||
| Hypertension medication | 58 (87.9%) | 1832 (61.4%) | <.0012 |
| RAAS agent | 31 (47.0%) | 673 (22.6%) | <.0012 |
| Lipid Lowering medication | 13 (19.7%) | 328 (11.0%) | .032 |
| HMG Co-A reductase inhibitor | 13 (19.7%) | 286 (9.6%) | .0062 |
| Exam Measurements: | |||
| Systolic BP, mmHg | 143 (126,166) | 132 (120, 146) | <.0011 |
| Systolic BP > 150 mmHg | 26 (39.4%) | 607 (20.3%) | <.012 |
| Diastolic BP, mmHg | 76 (67, 87) | 78 (71, 85) | .641 |
| Pulse, beats per minute | 70 (62, 80) | 68 (62, 76) | .341 |
| Laboratory measurements: | |||
| Serum Creatinine, mg/dL | 1.4 (1.2, 1.9) | 1.1 (1.0, 1.3) | <.0011 |
| Estimated GFR, ml/min/1.73m2 | 52 (41, 63) | 66 (57, 74) | <.0011 |
| Estimated GFR | <.0012 | ||
| >=60 | 21 (31.8%) | 2037 (68.3%) | |
| 45–59 | 22 (33.3%) | 815 (27.3%) | |
| 30–44 | 13 (19.7%) | 122 (4.1%) | |
| 15–29 | 10 (15.2%) | 10 (0.3%) | |
| Glucose, mg/dL | 103 (93,157) | 93 (86, 105) | <.0011 |
| Total cholesterol, mg/dL | 195 (159, 229) | 204 (178,231) | .071 |
| Low density lipoprotein, mg/dL | 115 (85, 145) | 124 (98,150) | .091 |
| High density lipoprotein, mg/dL | 49 (37,60) | 52 (42,63) | .041 |
| Triglycerides, mg/dL | 171 (121–217) | 140 (105–196) | .021 |
| Log triglycerides | 7.4 (6.9,7.8) | 7.1 (6.7,7.6) | .011 |
| hsCRP, mg/L | 3.9 (1.9, 10.6) | 3.2 (1.4, 7.0) | <.0011 |
| hsCRP > 3 mg/L | 40 (60.6%) | 1534 (51.4%) | .142 |
| Log hsCRP | 1.9 (0.9,3.3) | 1.7 (0.5,2.8) | .031 |
Abbreviations: BMI, body mass index; BP: blood pressure; GFR: glomerular filtration rate (calculated using the Modification of Diet in Renal Disease equation); HMG Co-A reductase inhibitor, 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor; hsCRP: high-sensitivity C-reactive protein; RAAS: renin angiotensin aldosterone system blockade agent
Values are median (25th, 75th) or n (percent) as appropriate.
P value from (1) rank sum test or (2) chi-square test as appropriate.
Outcome: All-cause death
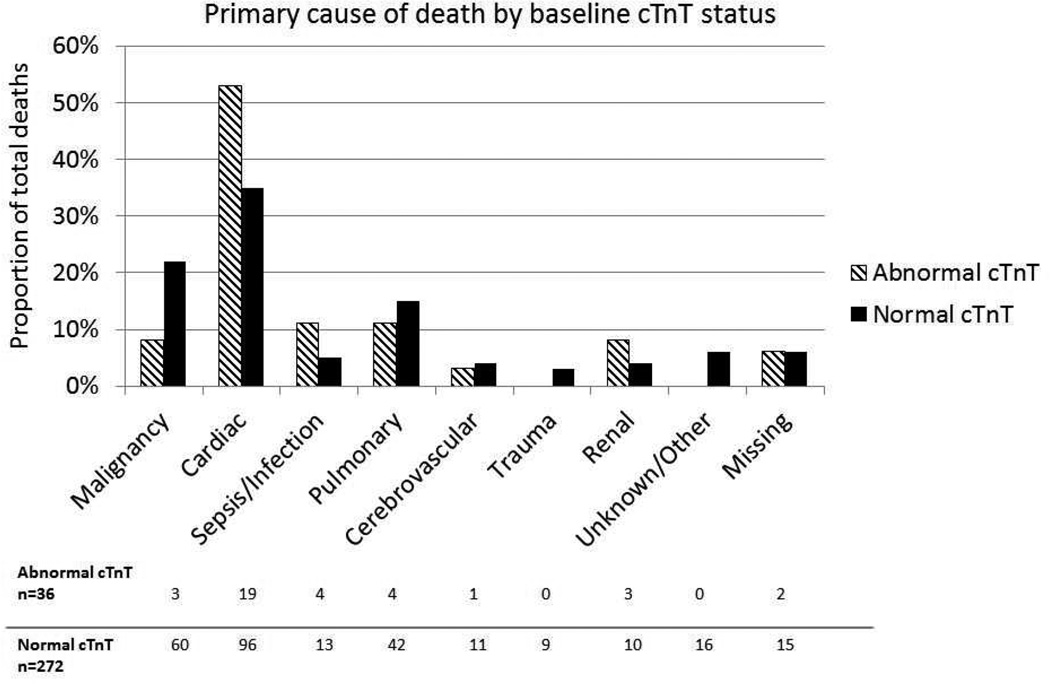
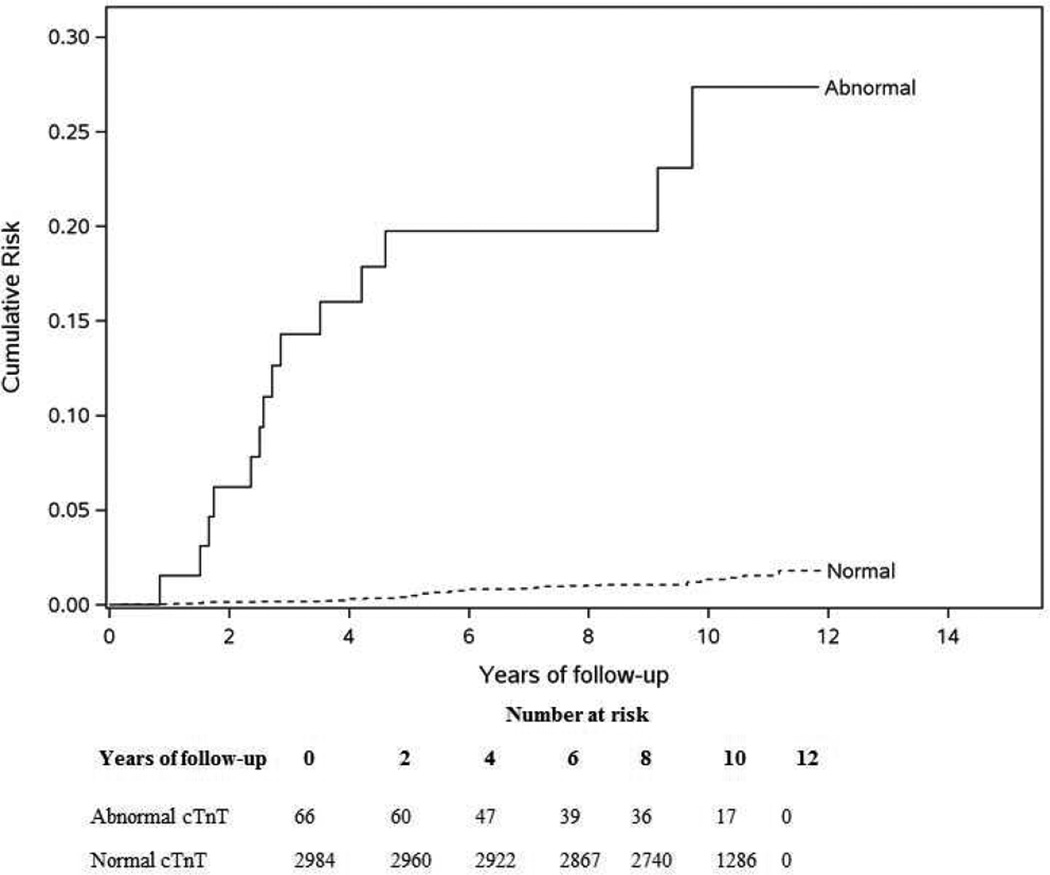
During a median follow-up period of 11.5 years (25th, 75th percentile: 10.6, 12.4 years), 308 participants died (incidence density 0.90/100 person-years). An abnormal cTnT was significantly associated with risk of all-cause death (Log rank P<.001, Figure 1 and Table 2). At 10 years, 47% (95% CI 33.5%, 55.8%) with an abnormal cTnT died compared to 7.3% (95% CI 6.3%, 8.2%) with a normal cTnT. The leading cause of death was cardiac among those with and without an abnormal cTnT at baseline (52.8% vs. 35.3%, respectively), Figure 2. There was an increased risk of all-cause death with an abnormal cTnT that remained significant even after multivariable adjustment (Table 2, HR 3.46; 95% CI 2.3, 5.1). The final model for mortality without cTnT had a C-statistic of 0.779 (95% CI 0.75, 0.8) that increased to 0.784 (95% CI 0.76, 0.81) with cTnT. A forest plot showing the effect of cTnT within subgroups made no suggestion that the effect of cTnT differed between other subgroups (Supplemental Figure 1). While the death risk with an abnormal cTnT was higher with eGFR≥60 mL/min/1.73 m2 than with eGFR<60 mL/min/1.73 m2 (HR 10.47 vs. 6.35), the interaction was not statistically significant (P=.2) even when eGFR was modeled as continuous (P=.7).
Figure 1.
All-cause death risk by Abnormal versus Normal cTnT
Table 2.
Risk of all-cause death with abnormal cTnT
| Model | cTnT Hazard Ratio |
95% Confidence Interval |
P-value | C-statistic |
|---|---|---|---|---|
| 1. Unadjusted | 8.43 | 5.95, 11.94 | <.001 | 0.552 |
| 2. Adjusted for demographic factors (age, sex, race) | 4.49 | 3.13, 6.43 | <.001 | 0.768 |
| 3. Adjusted for demographic factors and traditional risk factorsa | 3.75 | 2.57, 5.47 | <.001 | 0.783 |
| 4. Adjusted for demographic factors, traditional risk factorsa, and eGFR | 3.52 | 2.40, 5.18 | <.001 | 0.783 |
| 5. Adjusted for demographic factors, traditional risk factorsa, eGFR, and Log hsCRP | 3.46 | 2.35, 5.09 | <.001 | 0.784 |
Abbreviations: eGFR: estimated glomerular filtration rate; hsCRP: high-sensitivity C-reactive protein;
Hypertension, diabetes, myocardial infarction, low-density lipoprotein cholesterol, smoking
Figure 2.
Primary cause of death by baseline cTnT status
Outcome: ESRD
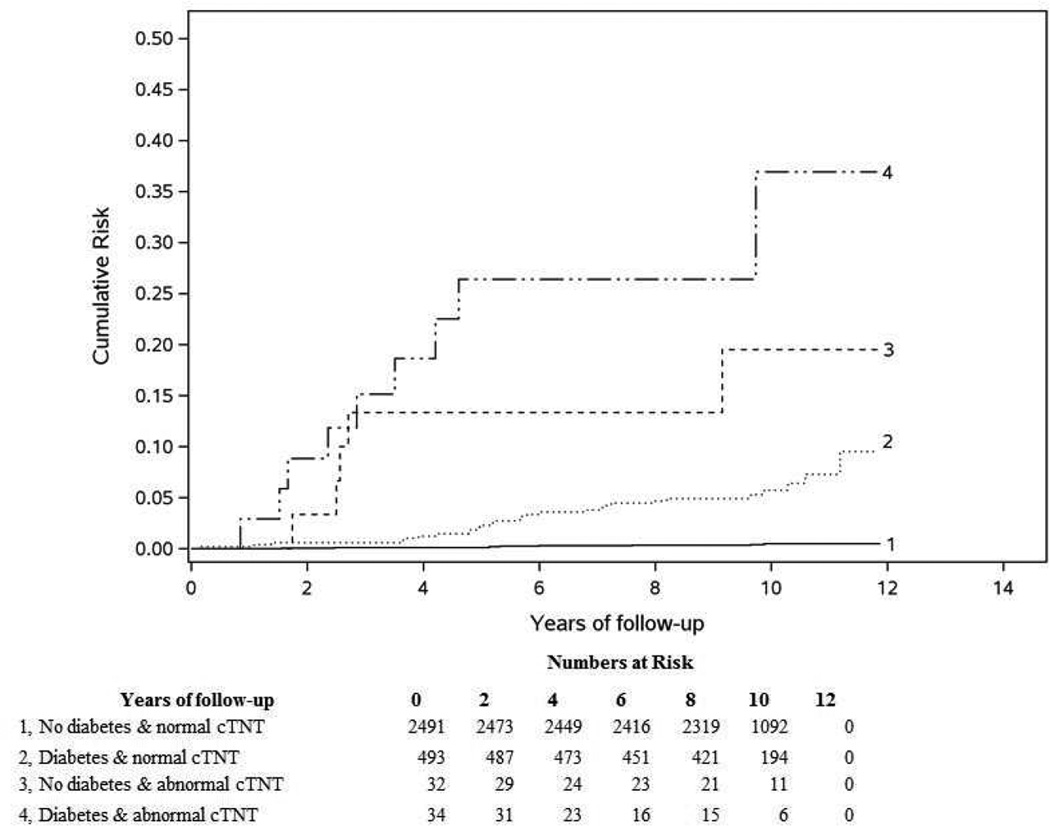
During a median follow-up period of 9.8 years (25th, 75th percentile: 8.9, 10.8 years), 52 participants developed ESRD (incidence density 0.18/100 person-years). An abnormal cTnT was significantly associated with risk of ESRD (Log rank P<.001, Figure 3 and Table 3). At 10 years, 27.4% (12.3, 39.8 %) with abnormal cTnT developed ESRD compared to 1.3% (0.9, 1.8%) with normal cTnT. The primary cause of ESRD was attributed to diabetes in 59.6%, and other causes were due to glomerulonephritis, multiple myeloma, unspecified, or unknown. There was an increased risk of ESRD with an abnormal cTnT that remained significant even after adjusting for demographics, hypertension, diabetes, and eGFR (HR 2.81; 95% CI 1.34, 5.90). This adjusted risk remained significant with death modeled as a competing risk in the fully adjusted model (HR 2.37; 95% CI 1.02, 5.53). The final model for ESRD without cTnT had a C-statistic of 0.901 (95% CI 0.85, 0.95) that increased to 0.909 (95% CI 0.86, 0.96) with cTnT. A forest plot showing the effect of cTnT within subgroups suggested that the effect of cTnT differed between those with and without diabetes (Supplemental Figure 2). The likelihood ratio test suggested evidence of an interaction between cTnT and diabetes (P=.008). Inspection of the interaction between diabetes and cTnT (Figure 4) revealed that subjects with normal cTnT and no diabetes were at very low risk for ESRD.
Figure 3.
End-stage renal disease risk by Abnormal versus Normal cTnT
Table 3.
Risk of end-stage renal disease with abnormal cTnT
| Model | cTnT Hazard Ratio |
95% Confidence Interval |
P value | C-statistic |
|---|---|---|---|---|
| 1. Unadjusted | 23.91 | 12.92, 44.24 | <.001 | 0.635 |
| 2. Adjusted for demographic factors (age, sex, race) | 20.84 | 10.58, 41.05 | <.001 | 0.757 |
| 3. Adjus ted for demographic factors, hypertension, and diabetes | 11.95 | 6.05, 23.60 | <.001 | 0.853 |
| 4. Adjusted for demographic factors, hypertension, diabetes, and eGFR | 2.81 | 1.34, 5.90 | .01 | 0.909 |
Abbreviation: eGFR: estimated glomerular filtration rate
Figure 4.
End-stage renal disease risk by Diabetes and cTnT status
DISCUSSION
In this study of community-dwelling individuals sampled for hypertension, abnormal cTnT concentration (≥0.01 ng/mL) independently predicted death and ESRD events. Our GENOA study participants with abnormal cTnT were generally older with comorbid conditions and reduced kidney function. However, abnormal cTnT provided prognostic information independent of traditional cardiovascular disease or ESRD risk factors. Findings in our predominantly hypertensive population are similar to other reports wherein a mildly-increased or detectable cTnT level in patients with heart failure, kidney disease, or even healthy older individuals portends worsened prognosis.19,39 However, our study is the first to demonstrate the ability of cTnT to predict ESRD beyond traditional risk markers and kidney function.
Despite the known relationship between hypertension, kidney function, and cTnT, cTnT has not previously been studied as a risk marker for ESRD. Recently, Bansal et al. found that cTnT, measured by high-sensitivity assays not yet clinically available in the US, and N-terminal pro-B-type natriuretic peptide were associated with rapid decline of kidney function and incident chronic kidney disease in elderly patients free of heart failure.39 Our investigations to date are plausible given the well-described biologic interaction between cardiac and kidney function.40–45 Dysfunction of the heart often leads to dysfunction of the kidney, and vice versa. In patients with chronic kidney disease, the high cardiovascular morbidity and all-cause death rates observed are believed to be multifactorial in etiology from accelerated atherosclerotic vascular disease, congestive heart failure, left ventricular hypertrophy, and toxicity from the circulating uremic milieu.43,46 Hence, cTnT serves as a marker of this cardiorenal interplay allowing for selection of a higher risk group for ESRD.
Race also plays an important role in the all-cause death risk, with African Americans having life expectancy 3–8 years less than white counterparts.47–49 One advantage of our cohort was the balanced racial sampling, which allowed testing for racial differences in the cTnT relationships. Most prior investigations of cTnT had few African American participants. Wallace et al. assessed the prevalence and determinants of cTnT elevations with conventional cTnT assays in 3,557 subjects of the Dallas Heart study, a multi-ethnic study of subclinical cardiovascular disease, which oversampled African Americans (~52%).13 They found that African American race was associated with detectable cTnT levels on univariate analysis, but in multivariable analyses, only diabetes, left ventricular hypertrophy, chronic kidney disease, and congestive heart failure were associated with detectable cTnT. Similarly, we did not find race to be a determinant of abnormal cTnT levels in our GENOA cohort. Taken together, these studies suggest that cTnT, as a composite marker of myocardial or endothelial damage resulting from comorbid conditions, identifies a high-risk group regardless of race, albeit this is generally more common in African Americans.
The prevalence (2.1%) of an abnormal cTnT may appear low in this study. However, in healthy populations previously studied, the prevalence of a detectable or abnormal cTnT is rare, ranging 0.7% to 4%.13,17 The prevalence of cTnT increases with age and with multimorbidity, hence illustrating why we intentionally examined a higher-risk, community-based cohort. Despite the small number of participants with an abnormal cTnT, cTnT proved to be an independent predictor of all-cause death and ESRD in our study population. Recently, a more sensitive assay for cTnT not yet clinically available in the United States has been associated with death and cardiovascular morbidity in community-based samples.50–52 In a study of 4,221 community-dwelling adults aged 65 years or older from the Cardiovascular Health Study (51% hypertensives and 16% African Americans), very low levels of cTnT measured with a highly-sensitive assay were significantly associated with cardiovascular death and incident heart failure independent of other biomarkers, including C-reactive protein and N-terminal pro-type B natriuretic peptide. Other investigations revealed that variations of levels in this marker over time were associated with concordant changes in the risk of heart failure and cardiovascular death.50,52 The additive information from high-sensitivity assays for cTnT has allowed for identification of individuals at high risk for death who have both increased high-sensitivity cTnT and left ventricular hypertrophy.53,54 Given these observations, it is possible that slight elevations in high-sensitivity cTnT levels previously undetected by our conventional cTnT assay may predict a significant proportion of deaths in the remaining participants from our study who died having had undetectable levels at baseline.
Our study had some limitations. The aim of the GENOA baseline exam was not to identify genetic predictors of target organ damage. Hence, we lacked baseline measurements of urine protein excretion rates in addition to electrocardiogram and echocardiogram studies, which may have provided insight into the presence of left ventricular hypertrophy, a condition previously associated with abnormal cTnT. The limitation from lack of urine samples is important, particularly given the findings in the Prevention of Renal and Vascular Endstage Disease study that albuminuria added significantly to the identification of individuals at risk of cardiovascular morbidity and all-cause death,55 development of progressive albuminuria,56 and its association with high-sensitivity cTnT assays.57 Second, we were unable to determine the impact of changes in medical therapy, cardiac interventions, and subsequent cardiac or renal events leading to adverse outcomes over time. Third, our cohort consists of individuals belonging to hypertension-enriched families and therefore may limit applicability to the general population. Fourth, multiple factors influence cTnT, and a single measurement may not appropriately represent a subject’s true baseline.
In conclusion, we report that cTnT provides prognostic information with respect to all-cause death and ESRD independent of traditional risk factors and baseline kidney function in community-dwelling individuals at potentially higher risk by virtue of ascertainment through hypertension. As patient populations grow older with increasing multimorbidity,58 identifying those at highest risk for death or ESRD could improve patient management strategies. Unfortunately, abnormal cTnT, measured with the standard assay, is relatively uncommon and thus does not improve risk prediction enough to support routine use. Further study is needed to determine if there is a particular patient group in which cTnT screening would meaningfully improve discrimination between the low- and high-risk patients for these sequelae.
Supplementary Material
Acknowledgments
We would like to acknowledge the contribution of Jodie van de Rostyne for laboratory support, Daniel Crusan, Cindy Crowson, and Dr. Eric Bergstralh for statistical support, and Drs. Fernando Cosio, Mira Keddis, and Ziad el-Zoghby for critical review of manuscript and study design.
Support: Mary Kathryn and Michael B. Panitch Career Development Award and funds from the Mayo Foundation (L.J.H); Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic (L.J.H.); NIH grants R01 HL054464 and R01 DK073537 (S.T.T), U01 HL054463-10 (T. Mosley), K23 DK078229 (A.D.R), and UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abstract Abbreviations
- cTnT
Cardiac troponin T
- CI
Confidence Interval
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Data for these analyses were provided by United States Renal Data System (USRDS), but the analysis and conclusions are those of the authors and do not represent the USRDS or National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Financial disclosure: A.S.J. has or presently consults with most of the major diagnostic companies (troponin).
REFERENCES
- 1.Heart Disease and Stroke 2020 Objectives: Hypertension. U.S. Department of Health and Human Services; 2014. Healthy People 2020. [Google Scholar]
- 2.Agency for Healthcare Research and Quality. Hypertension Care Strategies: Closing the Quality Gap. Rockville, MD: [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement From the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Gu Q, Burt VL, Paulose-Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among U.S. adults: the third National Health and Nutrition Examination Survey mortality follow-up study. Ann Epidemiol. 2008;18(4):302–309. doi: 10.1016/j.annepidem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122(7):672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S, Claggett B, Correia AW, et al. Temporal trends in the population attributable risk for cardiovascular disease: the atherosclerosis risk in communities study. Circulation. 2014;130(10):820–828. doi: 10.1161/CIRCULATIONAHA.113.008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302(16):1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 9.System URD. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Vol. 2014. Bethesda, MD: 2013. 2013 Annual Data Report. [Google Scholar]
- 10.Qaseem A, Hopkins RH, Jr, Sweet DE, Starkey M, Shekelle P. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(12):835–847. doi: 10.7326/0003-4819-159-12-201312170-00726. [DOI] [PubMed] [Google Scholar]
- 11.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT. Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn356. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe AS. Troponin--past, present, and future. Curr Probl Cardiol. 2012;37(6):209–228. doi: 10.1016/j.cpcardiol.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113(16):1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 14.Michos ED, Wilson LM, Yeh HC, et al. Prognostic Value of Cardiac Troponin in Patients With Chronic Kidney Disease Without Suspected Acute Coronary Syndrome: A Systematic Review. Ann Intern Med. 2014 doi: 10.7326/M14-0743. [DOI] [PubMed] [Google Scholar]
- 15.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142(9):786–791. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 16.Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106(23):2941–2945. doi: 10.1161/01.cir.0000041254.30637.34. [DOI] [PubMed] [Google Scholar]
- 17.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra RK, Li Y, DeFilippi C, et al. Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis. 2013;61(5):701–709. doi: 10.1053/j.ajkd.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goicoechea M, Garca de Vinuesa S, Gomez-Campdera F, et al. Clinical significance of cardiac troponin T levels in chronic kidney disease patients: predictive value for cardiovascular risk. Am J Kidney Dis. 2004;43(5):846–853. doi: 10.1053/j.ajkd.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Hickson LJ, Cosio FG, El-Zoghby ZM, et al. Survival of patients on the kidney transplant wait list: relationship to cardiac troponin T. Am J Transplant. 2008;8(11):2352–2359. doi: 10.1111/j.1600-6143.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- 22.Hickson LJ, El-Zoghby ZM, Lorenz EC, Stegall MD, Jaffe AS, Cosio FG. Patient survival after kidney transplantation: relationship to pretransplant cardiac troponin T levels. Am J Transplant. 2009;9(6):1354–1361. doi: 10.1111/j.1600-6143.2009.02636.x. [DOI] [PubMed] [Google Scholar]
- 23.Keddis MT, El-Zoghby ZM, El Ters M, et al. Cardiac troponin T before and after kidney transplantation: determinants and implications for posttransplant survival. Am J Transplant. 2013;13(2):406–414. doi: 10.1111/j.1600-6143.2012.04317.x. [DOI] [PubMed] [Google Scholar]
- 24.Daniels PR, Kardia SL, Hanis CL, et al. Familial aggregation of hypertension treatment and control in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Am J Med. 2004;116(10):676–681. doi: 10.1016/j.amjmed.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 25.Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39(1):3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 26.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 27.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. 2013;83(6):1169–1176. doi: 10.1038/ki.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol. 2011;6(8):1963–1972. doi: 10.2215/CJN.02300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apple FS, Parvin CA, Buechler KF, Christenson RH, Wu AH, Jaffe AS. Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. Clin Chem. 2005;51(11):2198–2200. doi: 10.1373/clinchem.2005.052886. [DOI] [PubMed] [Google Scholar]
- 32.Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem. 2003;49(8):1331–1336. doi: 10.1373/49.8.1331. [DOI] [PubMed] [Google Scholar]
- 33.Hamm CW, Giannitsis E, Katus HA. Cardiac troponin elevations in patients without acute coronary syndrome. Circulation. 2002;106(23):2871–2872. doi: 10.1161/01.cir.0000044342.50593.63. [DOI] [PubMed] [Google Scholar]
- 34.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349(9050):462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 35.Rebuzzi AG, Quaranta G, Liuzzo G, et al. Incremental prognostic value of serum levels of troponin T and C-reactive protein on admission in patients with unstable angina pectoris. Am J Cardiol. 1998;82(6):715–719. doi: 10.1016/s0002-9149(98)00458-5. [DOI] [PubMed] [Google Scholar]
- 36.Pencina MJ, D'Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119(24):3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 55(1):31–41. doi: 10.1053/j.ajkd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Fine JP, Gray JG. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Statist Assoc. 1999;94(446):496–509. [Google Scholar]
- 39.Bansal N, Katz R, Dalrymple L, et al. NT-ProBNP and Troponin T and Risk of Rapid Kidney Function Decline and Incident CKD in Elderly Adults. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.04910514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: 'Guyton revisited'. Eur Heart J. 2005;26(1):11–17. doi: 10.1093/eurheartj/ehi020. [DOI] [PubMed] [Google Scholar]
- 41.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Ronco C, Di Lullo L. Cardiorenal syndrome. Heart Fail Clin. 2014;10(2):251–280. doi: 10.1016/j.hfc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 43.McCullough PA, Li S, Jurkovitz CT, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008;156(2):277–283. doi: 10.1016/j.ahj.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre CW, Harrison LE, Eldehni MT, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breidthardt T, Burton JO, Odudu A, Eldehni MT, Jefferies HJ, McIntyre CW. Troponin T for the detection of dialysis-induced myocardial stunning in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(8):1285–1292. doi: 10.2215/CJN.00460112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 47.Kochanek KD, Arias E, Anderson RN. How did cause of death contribute to racial differences in life expectancy in the United States in 2010? NCHS Data Brief. 2013(125):1–8. [PubMed] [Google Scholar]
- 48.Xu J, Kochanek KD, Murphy SL, Arias E. Mortality in the United States, 2012. NCHS Data Brief. 2014;(168):1–8. [PubMed] [Google Scholar]
- 49.Keppel KG, Pearcy JN, Wagener DK. Trends in racial and ethnic-specific rates for the health status indicators: United States, 1990–98. Healthy People 2000 Stat Notes. 2002;(23):1–16. [PubMed] [Google Scholar]
- 50.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304(22):2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chin CW, Shah AS, McAllister DA, et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35(34):2312–2321. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neeland IJ, Drazner MH, Berry JD, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61(2):187–195. doi: 10.1016/j.jacc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smink PA, Lambers Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis. 2012;60(5):804–811. doi: 10.1053/j.ajkd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Scheven L, Halbesma N, de Jong PE, de Zeeuw D, Bakker SJ, Gansevoort RT. Predictors of progression in albuminuria in the general population: results from the PREVEND cohort. PLoS One. 2013;8(5):e61119. doi: 10.1371/journal.pone.0061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hellemons ME, Lambers Heerspink HJ, Gansevoort RT, de Zeeuw D, Bakker SJ. High-sensitivity troponin T predicts worsening of albuminuria in hypertension; results of a nested case-control study with confirmation in diabetes. J Hypertens. 2013;31(4):805–812. doi: 10.1097/HJH.0b013e32835eb5e8. [DOI] [PubMed] [Google Scholar]
- 58.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10E61 doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.