Abstract
Many adolescents engage in heavy alcohol use. Limited research in humans indicates that adolescent alcohol use predicts adult tobacco use. The present study investigated whether adolescent intermittent ethanol (AIE) exposure alters nicotine sensitivity in adulthood. Adolescent male Wistar rats (postnatal day 28–53) were exposed to AIE exposure that consisted of 5 g/kg of 25% ethanol three times per day in a 2 days on/2 days off regimen. Control rats received water with the same exposure regimen. In adulthood, separate groups of rats were tested for nicotine intravenous self-administration (IVSA), drug discrimination, and conditioned taste aversion (CTA). The dose-response function for nicotine IVSA under a fixed-ratio schedule of reinforcement was similar in AIE-exposed and control rats. However, AIE-exposed rats self-administered less nicotine at the lowest dose, suggesting that low-dose nicotine was less reinforcing in AIE-exposed, compared with control rats. AIE-exposed rats self-administered less nicotine under a progressive-ratio schedule, suggesting decreased motivation for nicotine after AIE exposure. The discriminative stimulus effects of nicotine were diminished in AIE-exposed rats compared with control rats. No group differences in nicotine CTA were observed, suggesting that AIE exposure had no effect on the aversive properties of nicotine. Altogether, these results demonstrate that AIE exposure decreases sensitivity to the reinforcing, motivational, and discriminative properties of nicotine while leaving the aversive properties of nicotine unaltered in adult rats. These findings suggest that drinking during adolescence may result in decreased sensitivity to nicotine in adult humans, which may in turn contribute to the higher rates of tobacco smoking.
Keywords: Adolescence, Alcohol, Conditioned Taste Aversion, Drug discrimination, Nicotine, Self-administration
Introduction
Drug use often begins in adolescence, and alcohol is the most widely used drug by adolescents. Nearly half of American twelfth grade students report having consumed at least one alcoholic beverage in the last 30 days, with nearly a quarter of students reporting at least one binge drinking episode in the last 2 weeks (Johnston et al., 2013). Alcohol use during adolescence may increase tobacco use in adulthood (Dierker et al., 2013; Paavola et al., 2004). Work in rodents has demonstrated that exposure to nicotine or alcohol during adolescence increased self-administration of the same drug in adulthood (Adriani et al., 2003; Alaux-Cantin et al., 2013), suggesting that adolescence is a time of vulnerability to the development of substance use disorders.
Exposure to high doses of alcohol during adolescence may interfere with the development of corticolimbic brain circuits involved in reward processes (Chambers et al., 2003) and thus may increase drug use in adulthood. The psychoactive effects of nicotine result primarily from the activation of nicotinic acetylcholine receptors located on dopaminergic neurons in the ventral tegmental area (De Biasi and Dani, 2011). Decreases in cortical dopamine and forebrain cholinergic activity have been reported in adulthood after adolescent alcohol exposure (Boutros et al., 2015; Coleman et al., 2011; Ehlers et al., 2011). Based on alterations induced by adolescent alcohol exposure in the cholinergic and dopaminergic systems, we hypothesized that adolescent alcohol exposure will alter adult sensitivity to nicotine.
The present study investigated the long-term effects of adolescent intermittent ethanol (AIE) exposure on nicotine sensitivity in adult rats. The primary reinforcing and motivational effects of nicotine were assessed in the nicotine intravenous self-administration (IVSA) procedure using fixed-ratio (FR) and progressive-ratio (PR) schedules of reinforcement, respectively (Markou et al., 1993). The discriminative stimulus effects of nicotine were assessed in a nicotine discrimination procedure. The aversive properties of nicotine were assessed using a two-bottle conditioned taste aversion (CTA) procedure. Based on our previous work, we used an AIE exposure regimen that resulted in high blood ethanol concentrations, modeling the extreme binge drinking engaged in by 5–10 % of American adolescents (Patrick et al., 2013; Schuckit et al 2014). This AIE exposure regimen has been shown to lead to altered alcohol reward (Boutros et al., 2014), increased impulsive choice (Mejia-Toiber et al., 2014), and increased risky decision making, together with decreased immunohistochemical markers for cholinergic and dopaminergic neurons (Boutros et al., 2015) in adulthood. Similar AIE exposure regimens have resulted in increased alcohol self-administration (Alaux-Cantin et al., 2013; Pascual et al., 2009) and enduring neural changes (Vetreno and Crews, 2012) in adulthood.
Materials and Methods
Subjects
Timed-pregnant female Wistar rats (Charles River, Raleigh, NC, USA) arrived in the vivarium on gestational day 13. Male pups were weaned on postnatal day 21 (PND 21) and pair-housed in a humidity- and temperature-controlled vivarium on a 12 h/12 h reverse light/dark cycle. Food was available ad libitum from weaning and throughout the AIE exposure period. Independent groups of rats were tested in the IVSA (Control: n = 15; AIE-exposed: n = 17), drug discrimination (Control: n = 6, AIE-exposed: n = 6), and CTA (Control: n = 16, AIE-exposed: n = 16) procedures. The rats that were tested in the nicotine IVSA and nicotine discrimination procedures were food-restricted throughout testing. Each rat received 20 g of rat chow per day, in addition to the food pellets obtained during testing. The rats that were tested in the CTA procedure had ad libitum access to food throughout and were restricted to 1 h of water access per day. All of the procedures were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Drugs
Ethanol
Ethanol was administered at a concentration of 25% (v/v) in tap water, with the volume determined by individual body weight. Control rats were administered a volume of water equivalent to the highest ethanol dose (5 g/kg).
Nicotine
Nicotine hydrogen tartrate (Sigma, St. Louis, MO) was dissolved in saline, and the pH was adjusted to 7.4 with sodium hydroxide. Nicotine doses are reported as base concentrations. For IVSA, nicotine was delivered at doses ranging from 0.005 to 0.06 mg/kg/infusion in a volume 0.1 ml/infusion, with the nicotine dose adjusted according to individual body weight. For subcutaneous injections, nicotine was administered at doses of 0.4 or 0.8 mg/kg in a volume of 1 ml/100 g.
Adolescent intermittent ethanol exposure
A timeline of all experimental events, including the ages of the rats during AIE exposure and behavioral testing, is presented in Table 1. From PND 28 to PND 53, the rats were administered 5 g/kg of 25% (v/v) ethanol or an equivalent volume of water (control rats) intragastrically via oral gavage three times per day at 8:00 AM, 12:00 PM, and 4:00 PM in a 2 days on/2 days off pattern. The rats were observed for behavioral intoxication scores (BISs) before each ethanol administration, and the doses were adjusted according to BISs as previously described (Boutros et al., 2014; Mejia-Toiber et al., 2014). Blood samples (200 μl) were taken from the tip of the tail for the analysis of blood ethanol concentrations (BECs) 60–90 min after the final ethanol administration on the second day of binge 2 (PND 33) and binge 6 (PND 49). Blood samples were immediately centrifuged at 1500 rotations per minute for 15 min. Plasma was extracted and stored at −80°C until further analysis. Plasma samples (5 μl) were analyzed using an Analox AM 1 analyzer (Analox Instruments LTD, Lunenberg, MA, USA).
Table 1.
The sequence of training and testing in the three behavioral tasks across the lifespan of the rats.
| Intravenous Self-Administration (n = 32) | Nicotine Discrimination (n = 12) | Conditioned Taste Aversion (n = 24) | |||
|---|---|---|---|---|---|
|
| |||||
| Phase | PND | Phase | PND | Phase | PND |
| Food training and Surgery | 59–80 | Response training | 82–102 | Water restriction | 60–66 |
| FR Acquisition | 81–105 | Discrimination training | 103–167 | Flavor conditioning | 67–74 |
| FR Dose-response | 108–140 | Dose-response | 168–187 | Choice test | 75–76 |
| PR Acquisition | 143–154 | ||||
| PR Dose-response | 157–175 | ||||
Rats from all experiments were exposed to the same AIE regimen on PND 28–53 (see text for details). PND, postnatal day; FR, fixed ratio; PR, progressive ratio.
Apparati
Intravenous nicotine self-administration
IVSA was conducted in 24 standard two-lever operant testing chambers (24 cm × 30 cm × 28 cm; Med Associates, St. Albans, VT, USA) enclosed within sound-attenuated boxes. Each chamber was equipped with a ventilator fan to provide air circulation and ambient low-level noise. One wall was equipped with two metal retractable levers (each 3 × 1.8 cm) mounted 6.5 cm above the metal grid floor of the chamber. One lever was designated as the active lever, and the other lever was designated as the inactive lever. Intravenous infusions through the catheter were delivered by an infusion pump (Razel Scientific Instruments, Stamford, CT, USA) through tubing protected by a spring lead connected to a swivel. All of the experimental events were recorded by an adjacent computer that ran Med-PC software.
Nicotine discrimination
Nicotine discrimination training and testing were conducted in 12 identical standard nine-hole operant chambers (25.5 cm × 28.4 cm × 28.7 cm; Med Associates, St. Albans, VT, USA) enclosed in sound-attenuating boxes. Each chamber was equipped with a ventilator fan to provide air circulation and ambient low-level noise. The rear wall of each chamber was curved with nine nosepoke holes equipped with photobeams. Only the two outermost holes were open. A magazine connected to a food dispenser was located on the opposite wall. All of the experimental events were recorded by an adjacent computer that ran Med-PC software.
Behavioral procedures
Intravenous nicotine self-administration
Methodological details of catheter construction, surgery, and the acquisition of nicotine-maintained responding have been described elsewhere (Liechti et al., 2007). Briefly, the rats were food-restricted and trained to respond for food, progressing from an FR1 timeout 1 s (TO1 s) to FR5 TO20 s schedule. The rats were then prepared with intravenous catheters in the right jugular vein under isoflurane/oxygen vapor (1–1.5% isoflurane) anesthesia and were allowed to self-administer nicotine (0.03 mg/kg/inf). Active lever responses (previously paired with food) resulted in nicotine delivery and the presentation of a 20-s cue light above the active lever, during which time active-lever responses had no consequences (i.e., timeout). Responding on the inactive lever (introduced during the first self-administration session) had no consequences. In the nicotine IVSA procedure with FR schedules of reinforcement, training and testing sessions were 1 h in duration and conducted 5 days per week.
Fixed-ratio schedule of reinforcement
When nicotine-maintained responding stabilized for all rats (< 20% variability in the number of infusions per session over five consecutive sessions; approximately 21 training sessions total), the nicotine dose per infusion (0.01, 0.03, and 0.06 mg/kg/infusion) was varied according to a within-subjects Latin-square experimental design. Each dose was administered for 5 days. The lowest nicotine dose (0.005 mg/kg/infusion) was tested after completion of the Latin square.
Progressive-ratio schedule of reinforcement
After completion of the dose-response function under the FR schedule, PR schedule training commenced using a 0.03 mg/kg/infusion dose. In the PR schedule, the response requirement for each successive nicotine reward increased according to the following formula: {5e[(pellet#+2)/4]}-6. The progression of required lever presses to earn one pellet was 5, 8, 11, 16, 23, 31, 41, 55, 72, 94, 123, etc. Sessions ended after 1 h with no responses or after 6 h total. All animals reached breaking points before the end of the 6 h period. On average, the rats completed testing and breaking points were reached within 3 h. The breakpoint was defined as the highest ratio completed. When responding stabilized for all rats (< 20% variability in the number of infusions per session over 5 consecutive sessions) the nicotine dose (0.005, 0.01, and 0.06 mg/kg/infusion) was varied according to a within-subjects Latin-square experimental design.
Nicotine discrimination
All of the rats were first trained to make nosepoke responses in both response holes according to an FR10 schedule of food reinforcement. In the nicotine-saline discrimination sessions, responses to only one of the two alternatives was reinforced each session. The reinforcement for one nosepoke hole was associated with saline, whereas the reinforcement for the other nosepoke hole was associated with nicotine (0.4 mg/kg, subcutaneously, 5 min before the session). The daily order of sessions was according to the following sequence: Nicotine, Saline, Nicotine, Saline, Nicotine, Nicotine, Saline, Saline. A response to the incorrect alternative reset the FR counter on the correct alternative. Training was conducted until the following criteria were met during four consecutive sessions (two saline, two nicotine): (1) first completed FR10 was to the nosepoke hole reinforced that session, (2) the percentage of correct responses was ≥ 80% for the entire session, (3) at least 50 rewards were earned per session. When all of the rats met the above criteria, generalization probes were conducted. Generalization probes were identical to training sessions, with the exception that responses to either alternative were reinforced according to an FR10 schedule. During 30-min test probes, saline and nicotine (0.05, 0.1, 0.2, 0.3, and 0.4 mg/kg, 5 min before the session) were administered in a random order. Test sessions were conducted once per week with training sessions conducted on the other days of the week.
Conditioned taste aversion
Conditioned taste aversion training and testing were conducted in chambers that were identical to the home cages as previously described (D’Souza and Markou, 2014). Briefly, during conditioning (8 days), bottles that contained water that was flavored with grape or cherry Kool-aid (both unsweetened) were alternated daily. The rats had access to the flavored solution for 15 min. Nicotine was paired with one flavor, and saline was paired with the other flavor (counterbalanced across rats within each group). Half of the rats in each group received high-dose nicotine (0.8 mg/kg), and half received low-dose nicotine (0.4 mg/kg). During the two 20 min test sessions, the rats had access to both flavors with the side position of the bottles reversed in the second test session.
Statistical analyses
All of the group data were subjected to univariate analysis of variance (ANOVA) using SPSS 18 software (SPSS, Chicago, IL, USA). In the IVSA study, the dependent variable was the number of nicotine infusions. In the FR dose-response function, the lowest nicotine dose (0.005 mg/kg/infusion) was administered after the Latin square and analyzed separately using a t-test. For this lowest nicotine dose, the effect size (Cohen’s d value) was calculated. In the nicotine discrimination study, the percentage of correct responses was calculated for each training session as the following: Correct responses/(Correct responses + Incorrect responses). Separate ANOVAs were conducted for sessions when nicotine was administered and when saline was administered. In the generalization probes, the percentage of nicotine responses was calculated. For the CTA study, the mean of the volume of saline-paired and nicotine-paired solution (in milliliters) that was consumed during the two test sessions was measured. For AIE exposure, we compared the BECs on PND 33 and PND 49 using a paired-subjects t-test. We also analyzed the total ethanol dose received on each binge day and the average daily BIS using a repeated-measures ANOVA, with Binge number and Binge day as the factors.
For all of the analyses, AIE exposure was used as a between-subjects factor. For response acquisition in the IVSA and nicotine discrimination experiments, Training session was included as a within-subjects factor. For evaluation of the dose-response functions in the IVSA and drug discrimination experiments, nicotine Dose was used as a within-subjects factor. In the CTA experiment, Dose was a between-subjects factor. The level of significance was 0.05. Significant main and interaction effects were followed by t-tests using a Šidák adjustment for multiple comparisons. For repeated-measures analyses, Mauchly’s test of sphericity of the covariance matrix was applied. When the sphericity assumption was violated, the degrees of freedom for any term that involved that factor were adjusted to more conservative values by applying the Huynh-Feldt correction. We report the uncorrected degrees of freedom.
Results
Adolescent intermittent ethanol exposure
Blood ethanol concentrations were significantly higher on PND 49 than on PND 33 (238.7 ± 2.7 and 204.5 ± 7.7, respectively; t40 = 4.42, p < .001). The mean daily BIS was measured immediately before the second and third daily injections (4 h after the previous ethanol administration). The total ethanol dose administered on each binge day and BISs are presented in Table 2. For the BIS, there were significant main effects of Binge Number (F6,240 = 58.22, p < .001) and Binge Day (F1,40 = 310.03, p < .001) and a Binge Number × Binge Day interaction (F6,240 = 17.09, p < .001). The post hoc tests revealed that the BIS was significantly higher on Binge Day 2 during Binges 1, 2, 3, 4, and 6. For the total ethanol dose administered, there were significant main effects of Binge Number (F6,246 = 36.66, p < .001) and Binge Day (F1,41 = 117.47, p < .001) and a significant Binge Number × Binge Day interaction (F6,246 = 19.63, p < .001). The ethanol dose administered was significantly smaller on the second day of the 2-day binge for Binge 1, Binge 2, Binge 4, and Binge 6.
Table 2.
Behavioral intoxication score (BIS) and total daily ethanol dose (g/kg) administered on binge days 1 and 2 of each of the seven 2-day binges of the AIE exposure period.
| Binge Number | Binge Day 1 | Binge Day 2 | ||
|---|---|---|---|---|
| BIS | Daily Dose | BIS | Daily Dose | |
| 1 | 1.0 ± 0.1 | 14.4 ± 0.2 | 1.7 ± 0.1 | 11.8 ± 0.2 |
| 2 | 0.5 ± 0.0 | 15.0 ± 0.0 | 1.2 ± 0.1 | 13.1 ± 0.2 |
| 3 | 0.4 ± 0.0 | 14.8 ± 0.1 | 0.7 ± 0.0 | 14.6 ± 0.1 |
| 4 | 0.6 ± 0.0 | 14.5 ± 0.1 | 0.9 ± 0.1 | 14.0 ± 0.1 |
| 5 | 0.8 ± 0.1 | 14.3 ± 0.2 | 0.9 ± 0.1 | 14.0 ± 0.1 |
| 6 | 0.7 ± 0.1 | 14.5 ± 0.1 | 1.3 ± 0.0 | 13.7 ± 0.2 |
| 7 | 1.3 ± 0.0 | 13.5 ± 0.2 | 1.3 ± 0.1 | 13.5 ± 0.2 |
The data are expressed as mean ± SEM.
Nicotine intravenous self-administration
Fixed-ratio schedule of reinforcement
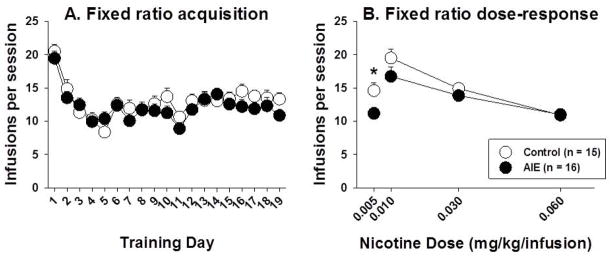
There were no differences between AIE-exposed and control rats during training for a food reinforcer (data not shown). When nicotine was substituted for food, there was an initial drop in response rates from training day 1 to day 2 in all rats (Figure 1A). During nicotine IVSA acquisition, both AIE-exposed and control rats acquired nicotine self-administration at similar rates (significant main effect of Training session, F18,540 = 18.02, p < .001; no main effect of AIE exposure; no AIE exposure × Training session interaction; Figure 1A). For the dose-response function (including doses of 0.01, 0.03, and 0.06 mg/kg/infusion), there was a significant main effect of Dose (F4,116 = 28.74, p < .001) but no main effect of AIE exposure and no Dose × AIE exposure interaction (Figure 1B). At the lowest dose (0.005 mg/kg/infusion), control rats obtained significantly more nicotine infusions (14.60 ± 4.42) than AIE-exposed rats (11.17 ± 3.59; t29 = 2.38, p < .05; d=0.23; Figure 1B).
Figure 1.
Nicotine infusions self-administered under a fixed-ratio (FR) schedule of reinforcement (FR5 TO20 s) during acquisition (A) and across the dose-response function (B) in AIE-exposed and control rats. The asterisk denotes a significant difference between AIE-exposed and control rats at the lowest nicotine dose (independent-samples t-test, p < .05). The data are expressed as mean ± SEM.
Progressive-ratio schedule of reinforcement
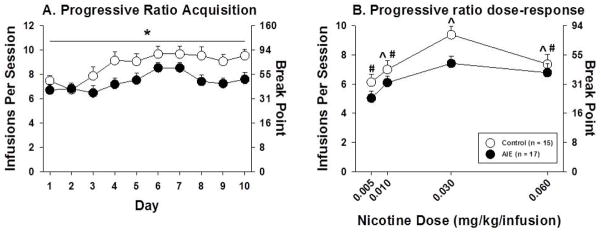
During acquisition, AIE-exposed rats acquired nicotine self-administration at lower rates (Figure 2A). There were significant main effects of Training session (F9,270 = 9.05, p < .001) and AIE exposure (F1,30 = 4.97, p < .05) but no AIE exposure × Training session interaction (Figure 2A). In the dose-response function, there was a significant main effect of Dose (F3,90 = 19.57, p < .001). Post hoc tests comparing responding of all rats at each dose indicated significantly more responses per session and a higher breakpoint when nicotine was available at the training dose (0.03 mg/kg/infusion) compared to all other doses. In addition, there were significantly fewer responses per session and a lower breakpoint when nicotine was available at the lowest dose tested (0.005 mg/kg/infusion) compared to all higher doses. There was also a nearly significant main effect of AIE exposure (F1,30 = 3.19, p = .084), but no significant AIE exposure × Dose interaction (Figure 2B).
Figure 2.
Nicotine infusions self-administered under a PR schedule of reinforcement (left axis) and breakpoint (right axis) during acquisition (A) and across the dose-response function (B) in AIE-exposed and control rats. The asterisk denotes a significant main effect of AIE exposure in the ANOVA (p < .05). The carot denotes a significant difference from 0.005 mg/kg/infusion and the hash-mark denotes a significant difference from 0.030 mg/kg/infusion. The data are expressed as mean ± SEM.
Nicotine discrimination
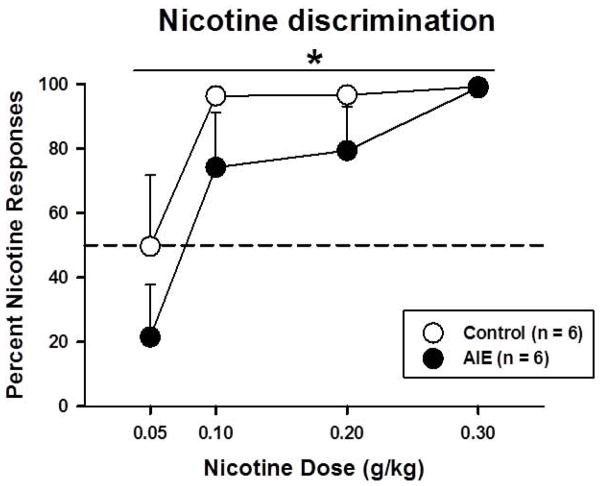
AIE-exposed and control rats were trained to discriminate between saline and nicotine (0.4 mg/kg, s.c.) during 23 training sessions. There was a significant main effect of Training session during sessions when nicotine (F22,220 = 2.38, p < .005) and saline (F22,220 = 4.01, p < .001) were administered, with no main effect of AIE exposure and no interactions (data not shown). During nicotine generalization probes, there were significant main effects of Nicotine dose (F3,30 = 9.48, p < .001) and AIE exposure (F1,10 = 4.90, p = 0.05). Control rats were more likely to make a nicotine-appropriate response at doses that were lower than the training dose (Figure 3). No interactions were observed.
Figure 3.
Percentage of nicotine responses in AIE-exposed and control rats in the nicotine discrimination task. The asterisk denotes a significant main effect of AIE exposure in the ANOVA (p < .05).
Conditioned taste aversion
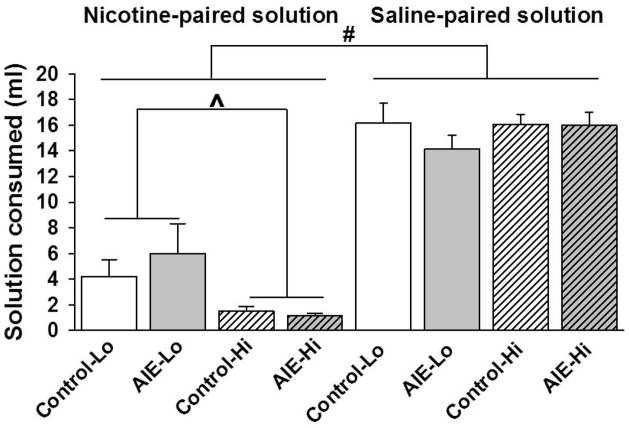
All of the rats consumed more of the saline-paired solution (15.60 ± 0.56 ml) relative to the nicotine-paired solution (3.20 ± 0.68; main effect of Nicotine, F1,28 = 133.11, p < .001; Figure 4). There was no effect of either AIE exposure or Nicotine dose on the amount of saline-paired solution consumed. The ANOVA of the amount of nicotine-paired solution consumed revealed a significant main effect of Nicotine Dose (F1,28 = 7.80, p < .01) but no effect of AIE exposure or Test Day and no interactions. AIE-exposed and control rats that were conditioned with the high nicotine dose (0.8 mg/kg) consumed less of the nicotine-paired solution compared with rats that were conditioned with the low nicotine dose (0.4 mg/kg; Figure 4).
Figure 4.
Nicotine-paired solution (Left) and saline-paired solution (Right) consumed (in milliliters) in AIE-exposed and control rats conditioned with high (Hi) or low (Lo) doses of nicotine in the conditioned taste aversion test. The Hash sign denotes a significant main effect of Nicotine vs. Saline pairing (p < .001). The carrot sign denotes a significant main effect of Nicotine dose in the ANOVA (p < .01) in the nicotine-paired solution only (Left). The data are expressed as mean ± SEM.
Discussion
Our results demonstrated that AIE exposure decreased nicotine sensitivity in adulthood. Decreased nicotine reinforcement and motivation for nicotine after AIE exposure were reflected in decreased nicotine intake in AIE-exposed rats compared with control rats during nicotine IVSA under FR and PR schedules of reinforcement, respectively. In the nicotine discrimination task, AIE-exposed rats were less likely than control rats to make responses to the nicotine-associated alternative, indicating decreased nicotine discrimination. The lack of an effect of AIE exposure on nicotine aversion was reflected in equivalent aversion to the nicotine-associated flavor after nicotine CTA in AIE-exposed and control rats.
AIE exposure decreased the primary reinforcing effects of self-administered nicotine at the lowest dose tested (0.005 mg/kg/inf), although the effect size was small. There were no differences in the sensitivity to nicotine between AIE-exposed and control rats at higher nicotine doses, including the training dose. The pattern of fixed-ratio responding for nicotine at the lowest dose tested throughout the 5-day period was stable (i.e., no initial compensatory increase in responding or gradual decrease in responding during subsequent days) in both control and AIE-exposed rats (data not shown). This finding suggests that decreases in responding were not associated with extinction conditions, and the lowest nicotine dose was reinforcing. Moreover, decreased responses in AIE-exposed rats compared to water-exposed rats reflect a difference in the reinforcing effects of low-dose nicotine rather than a difference in extinction responding. The effects of AIE exposure on the motivational properties of nicotine were more robust. AIE-exposed rats exhibited decreased motivation to self-administer nicotine during acquisition, together with a tendency for decreased nicotine intake and breakpoints throughout the dose-response function under a PR schedule of reinforcement. However, considering that motivation for nicotine is maintained by the conditioned rewarding effects of nicotine, as well as the weak reinforcing effects of nicotine (Caggiula et al., 2001), it is possible that AIE exposure may result in increased motivation for nicotine after a period of abstinence when subjects are re-exposed to nicotine-associated cues. More research in this area is warranted.
Consistent with the decreased self-administration of low-dose nicotine after AIE exposure, the AIE-exposed rats made fewer nicotine-paired responses when tested with nicotine doses that were lower than the dose used during acquisition in the nicotine discrimination task. Thus, for AIE-exposed rats but not control rats, the interoceptive properties of lower nicotine doses were more similar to saline than to the interoceptive properties of nicotine at the training dose, suggesting that AIE exposure decreased the subjective effects of nicotine at the low dose.
The aversive effects of nicotine at high doses (e.g., nausea and gastrointestinal distress) may play a role in nicotine intake. In choice tests after CTA, all of the rats consumed less of the solution that was previously paired with nicotine compared with the solution that was paired with saline, with no effect of AIE exposure. Moreover, rats that were conditioned with the high nicotine dose exhibited greater aversion to the nicotine-paired flavor compared with rats that were conditioned with low-dose nicotine, again independent of AIE-exposure. These results suggest that the decreased sensitivity to the reinforcing, motivational, and discriminative effects of nicotine was not attributable to nicotine aversion. Alternatively, nicotine-induced CTA may also suggest positive conditioned suppression related to the rewarding properties of nicotine (Grigson, 1997; Stolerman and Dmello, 1981). The lack of effects of AIE exposure on nicotine-induced CTA may suggest that AIE exposure may not affect the conditioned rewarding effects of nicotine, but this hypothesis needs further examination.
Previous studies have demonstrated that exposure to ethanol results in cross-tolerance to some of the effects of nicotine when tested shortly after ethanol exposure (Collins et al., 1988). In the present studies, the decreased nicotine sensitivity was observed long after the final ethanol administration, suggesting that mechanisms other than ethanol-nicotine cross-tolerance may account for the present pattern of results.
The decreased sensitivity to nicotine after AIE exposure may have resulted from a loss of cholinergic neurons in the basal forebrain. Previous studies have reported that AIE exposures similar or identical to that used in the present study resulted in decreased cholinergic immunoreactivity in adulthood (Boutros et al., 2015; Coleman et al., 2011; Ehlers et al., 2011). Interestingly, intermittent ethanol exposure during adulthood did not affect cholinergic neurons (Vetreno et al., 2014), indicating that this effect was specific to AIE exposure. Although the effects of adult alcohol exposure on nicotine sensitivity have not been evaluated in the present work, it is possible that the decreased nicotine sensitivity following AIE exposure may be specific to adolescent ethanol exposure, though this hypothesis needs further investigation. Consistent with our findings, fetal alcohol exposure resulted in decreased cholinergic neurons (Swanson et al., 1995) and decreased sensitivity to some of the effects of nicotine (Nagahara and Handa, 1999a, 1999b).
The reinforcing, motivational, and discriminative effects of nicotine are mediated by nicotinic acetylcholine receptors (nAChRs) that contain the α4β2 subunit (De Biasi and Dani, 2011; Smith et al., 2007; Stolerman et al., 1997; Zaniewska et al., 2006). Prolonged exposure to alcohol self-administration decreased the availability of β2 subunit-containing nAChRs in monkeys (Cosgrove et al., 2010), and numerous effects of alcohol are mediated by β2 nAChRs (Dawson et al., 2013). Notably, mice that lack the β2 subunit showed flat generalization curves after training in a nicotine discrimination task but showed only partial attenuation of nicotine CTA (Shoaib et al., 2002). Our results showed that nicotine self-administration and discrimination were attenuated by AIE exposure, whereas nicotine CTA was unaffected, suggesting a decrease in α4β2 nAChR availability after AIE exposure. Currently unknown is whether increased tobacco use in people with a history of drinking during adolescence (Dierker et al., 2013; Paavola et al., 2004) may be related to low levels of β2 nAChRs. However, increased smoking rates have been linked to the decreased availability of β2 nAChRs in people with schizophrenia (D’Souza et al., 2012). Thus, more research in this area is warranted.
The rewarding effects of nicotine arise partly from the activation of β2 nAChR-containing neurons that project from the ventral tegmental area to nucleus accumbens, resulting in dopamine release (Nisell et al., 1996; Picciotto et al., 1998). Our recent work demonstrated lower levels of tyrosine hydroxylase, a marker for dopamine (and norepinephrine), in the prelimbic cortex after AIE exposure (Boutros et al., 2015) Moreover, in vitro studies demonstrated that cells that were exposed to alcohol showed decreased nicotine-induced dopamine release (Dohrman and Reiter, 2003). These studies suggest that AIE-exposed rats may have decreased nicotine-induced dopaminergic activity in the corticolimbic reward pathway compared with control rats, leading to decreased sensitivity to the positive effects of nicotine. Alternatively, glutamatergic neurotransmission is also involved in nicotine reward (D’Souza and Markou, 2011; Markou, 2008). Decreased sensitivity to nicotine reward may be attributable to decreased glutamate levels in the nucleus accumbens after adolescent alcohol exposure (Lallemand et al., 2009).
In summary, our results demonstrated that adolescent alcohol exposure decreased sensitivity to the rewarding, motivational, and discriminative effects of nicotine, together with no changes in nicotine-induced CTA in adulthood. Longitudinal studies in humans have reported increased tobacco use in adulthood after heavy adolescent alcohol use (Dierker et al., 2013; Paavola et al., 2004). Notably, nicotine-associated cues play a more important role in the maintenance of nicotine dependence than the weak reinforcing effects of nicotine (Caggiula et al., 2001). Thus, it is possible that AIE exposure may lead to increased conditioned rewarding effects of nicotine together with decreased sensitivity to the primary reinforcing effects of nicotine because these phenomena are mediated by different neurobiological mechanisms (Markou and Paterson, 2009). Consistent with this hypothesis, a recent study showed that decreased sensitivity to the locomotor activity stimulating effects of nicotine predicted increased conditioned rewarding effects of nicotine (Pastor et al 2013). Further, studies in humans have revealed that low sensitivity to alcohol predicted increased alcohol intake and the development of alcohol use disorders later in life (King et al., 2011; Schuckit, 1994). One intriguing possibility may be that a similar phenomenon exists for nicotine dependence. Namely, the selective decrease in the positive effects of low dose nicotine after adolescent alcohol exposure may result in a more rapid acceleration of tobacco use in order to achieve the desired psychoactive effects of nicotine. More research is needed to reveal the experimental conditions that model the increased tobacco use reported in humans with histories of heavy adolescent alcohol use.
Acknowledgments
This work was supported by NIH grant: U01-AA019970-NADIA (AM). AM has received contract research support from Bristol-Myers Squibb, Forest Laboratories, and Astra-Zeneca and honoraria/consulting fees from AbbVie during the past 3 years. The authors would like to thank Mr. Michael Arends for editorial assistance and members of the Markou research group for assistance and input.
Footnotes
Authors contributions
NB, SS and AM designed the experiments, analyzed the data and wrote the manuscript. NB conducted the experiments and collected the data.
The remaining authors report no financial conflicts of interest.
References
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FTC, Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int J Neuropsychopharmacol. 2015;18:1–9. doi: 10.1093/ijnp/pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Markou A. Adolescent intermittent ethanol exposure diminishes anhedonia during ethanol withdrawal in adulthood. Eur Neuropsychopharmacol. 2014;24:856–864. doi: 10.1016/j.euroneuro.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Be. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Burch JB, de Fiebre CM, Marks MJ. Tolerance to and cross tolerance between ethanol and nicotine. Pharmacol Biochem Be. 1988;29:365–373. doi: 10.1016/0091-3057(88)90170-0. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Kloczynski T, Bois F, Pittman B, Tamagnan G, Seibyl JP, Krystal JH, Staley JK. Decreased Beta2*-nicotinic acetylcholine receptor availability after chronic ethanol exposure in nonhuman primates. Synapse. 2010;64:729–732. doi: 10.1002/syn.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Miles MF, Damaj MI. The β2 nicotinic acetylcholine receptor subunit differentially influences ethanol behavioral effects in the mouse. Alcohol. 2013;47:85–94. doi: 10.1016/j.alcohol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Selya A, Piasecki T, Rose J, Mermelstein R. Alcohol problems as a signal for sensitivity to nicotine dependence and future smoking. Drug Alcohol Depend. 2013;132:688–693. doi: 10.1016/j.drugalcdep.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrman DP, Reiter CK. Chronic ethanol reduces nicotine-induced dopamine release in PC12 cells. Alcohol Clin Exp Res. 2003;27:1846–1851. doi: 10.1097/01.ALC.0000095923.41707.C8. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Differential role of N-methyl-D-aspartate receptor-mediated glutamate transmission in the nucleus accumbens shell and core in nicotine seeking in rats. Eur J Neurosci. 2014;39:1314–1322. doi: 10.1111/ejn.12491. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6:4–16. [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, Pittman B, Ranganathan M, Cosgrove K, Staley J. Lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–334. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–345. doi: 10.1016/j.neuroscience.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci. 1997;111:129–136. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Backman JG, Schulenberg JE. Overview of Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, University of Michigan; 2013. Monitoring the Future: National Results on Adolescent Drug Use: 2012. [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand F, Ward RJ, De Witte P. The influence of chronic nicotine administration on behavioural and neurochemical parameters in male and female rats after repeated binge drinking exposure. Alcohol. 2009;44:535–546. doi: 10.1093/alcalc/agp047. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE. Multiple forces contribute to nicotine dependence. In: Bevins RA, Caggiula AR, editors. Nebraska symposium on motivation: The motivational impact of nicotine and its role in tobacco use. Springer; New York: 2009. pp. 65–89. [Google Scholar]
- Markou A, Weiss F, Gold L, Caine S, Schulteis G, Koob G. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res. 2014;266:19–28. doi: 10.1016/j.bbr.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Fetal alcohol-exposed fats exhibit differential response to cholinergic drugs on a delay-dependent memory task. Neurobiol Learn Mem. 1999a;72:230–243. doi: 10.1006/nlme.1999.3909. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Loss of nicotine-induced effects on locomotor activity in fetal alcohol-exposed rats. Neurotoxicol Teratol. 1999b;21:647–652. doi: 10.1016/s0892-0362(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svenssom TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pastor V, Andrés ME, Bernabeu RO. The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology. 2013;226:551–560. doi: 10.1007/s00213-012-2928-1. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD. Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr. 2013;167:1019–1025. doi: 10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola M, Vartiainen E, Haukkala A. Smoking, alcohol use, and physical activity: a 13-year longitudinal study ranging from adolescence into adulthood. J Adolesc Health. 2004;35:238–244. doi: 10.1016/j.jadohealth.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Schuckit M. Low-level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Bucholz KK, Agrawal A, Dick DM, Nurnberger JI, Kramer J, Hesselbrock M, Saunders G, Hesselbrock V. Predictors of subgroups based on maximum drinks per occasion over six years for 833 adolescents and young adults in COGA. J Stud Alcohol Drugs. 2014;75:24–34. doi: 10.15288/jsad.2014.75.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Stolerman I, D’Mello G. Role of training conditions in discrimination of central nervous system stimulants by rats. Psychopharmacology (Berl) 1981;73:295–303. doi: 10.1007/BF00422421. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-β-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Swanson D, King M, Walker D, Heaton M. Chronic prenatal ethanol exposure alters the normal ontogeny of choline acetyltransferase activity in the rat septohippocampal system. Alcohol Clin Exp Res. 1995;19:1252–1260. doi: 10.1111/j.1530-0277.1995.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS ONE. 2014;9(11):e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegalinski E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]