Abstract
The remarkable plasticity of CD4+ T cells allows individuals to respond to environmental stimuli in a context-dependent manner. A balance of CD4+ T cell subsets is critical to mount responses against pathogen challenges to prevent inappropriate activation, to maintain tolerance, and to participate in antitumor immune responses. Specification of subsets is a process beginning in intrathymic development and continuing within the circulation. It is highly flexible to adapt to differences in nutrient availability and the tissue microenvironment. CD4+ T cell subsets have significant cross talk, with the ability to “dedifferentiate” given appropriate environmental signals. This ability is dependent on the metabolic status of the cell, with mTOR acting as the rheostat. Autoimmune and antitumor immune responses are regulated by the balance between regulatory T cells and Th17 cells. When a homeostatic balance of subsets is not maintained, immunopathology can result. CD4+ T cells carry complex roles within tumor microenvironments, with context-dependent immune responses influenced by oncogenic drivers and the presence of inflammation. Here, we examine the signals involved in CD4+ T cell specification towards each subset, interconnectedness of cytokine networks, impact of mTOR signaling, and cellular metabolism in lineage specification and provide a supplement describing techniques to study these processes.
1. An Introduction to CD4+ T Cell Diversity
Production of a diverse repertoire of antigen-specific CD4+ T lymphocytes is essential for a host to respond to emerging microbial threats to create memory for heightened secondary responses to previously encountered pathogens and to suppress immune responses after microbial clearance to avoid tissue damage resulting from excessive or protracted inflammation [1]. Plasticity of CD4+ T cells is required to maintain immunocompetence after the thymic involution in adulthood [2]. Varying functional CD4+ T cell clones are also required to operate immune responses in different tissues as well as to produce high-affinity, class-switched immunoglobulin [3].
It is hypothesized that CD4+ T cells undergo subset specification but not lineage determination [3]. CD4+ T cells mature to form subsets with specified phenotypes and differences in cytokine production but fall short of terminal differentiation. Specification is a reversible maturation process that allows CD4+ T cells to undergo alternate fates, depending on environmental signals received. Signals contributing to subset specification include the prevailing cytokine environment, cytokine receptor expression profiles, transcription factor expression, and differential chromatin remodeling of loci that regulate production of effector cytokines [4].
Naïve CD4+ T cells undergo specification by many innate immune signals, including cytokines, chemokines, and inflammasome activation, which result in activation of signal transducers and activators of transcription, subsequent activation of lineage-specific transcription factors, cytokine production, and epigenetic adjustments at the cytokine loci to result in commitment to a given lineage.
Once a naïve T cell is primed by signals received from an antigen-presenting cell, proliferation occurs before lineage specification begins. If differentiation of CD4+ T cells occurred early after priming, peripheral CD4+ T cells would be restricted with binary options, being able to turn on or repress production of only a restricted subset of cytokines [5]. Subset determination occurring after clonal proliferation is consistent with an activated CD4+ naïve T cell producing many diverse progeny with pleiotropic, distinct fates, producing a highly flexible, dynamic, and context-driven CD4+ T cell repertoire [5].
Surprisingly, CD4+ T cell that has undergone lineage specification is capable of adopting alternate fates when innate immune signals change. The molecular basis for cytokine memory involves imprinting gene loci encoding cytokines by demethylation of DNA or histone acetylation as cells progress through S phase, so stable patterns of gene expression occur with an increasing number of cell divisions [6]. Yet, later chromatin remodeling occurs within CD4+ T cells to turn on new cytokine production profiles [5].
In this review, we will first examine functional differences between CD4+ T cell subsets and their lineage specification. A focus on the interconnectedness among pathways of maturation will follow with a presentation of experimental evidence supporting the hypothesis that CD4+ T cells maintain plasticity. The role of mTOR and cellular metabolism in T cell differentiation and function will be discussed. Finally, the impact of CD4+ T cell subsets in immunopathology and in antitumor immune responses will be considered.
2. T Cell Subsets and Lineage Specification
2.1. CD4+ T Cell Diversity Begins during Development
Diversity of the CD4+ T cell repertoire begins during intrathymic development. Thymocyte differentiation produces a diversity of CD4+ T cells with varying antigen specificities through β-selection, followed by α-chain rearrangement to form diverse αβ TCR specificities [27]. CD4 lineage selection is mediated through interaction of the T cell receptor (TCR) with class II MHC ligands. CD4+ T cell development is promoted by high TCR signal strength and signaling downstream of the TCR contributes to CD4 lineage commitment through association of Lck with the CD4 coreceptor and MAP kinase signaling to favor maintenance of CD4 expression with concurrent downregulation of CD8 [28]. CD4 commitment is mediated through induction of the transcription factor, T helper-inducing POZ/Kruppel-like factor (Th-POK), by GATA3 which represses Runx3 to release activity of the CD4 silencer [10, 29].
The lineage decision of commitment to the CD4+ or CD8+ T cell lineage was thought to be committed and inflexible, although it is now understood that there is a high degree of latitude in the CD4+ T cell compartment [3]. Lineage commitment is regulated not only through positive and negative selection but also through additional mechanisms. Helper-deficient (HD) mice, which lack the ability to produce CD4+ T cells, have spontaneous redirection of MHC class II-restricted T cells to the CD8+ lineage. The factor identified to be responsible for the redirection of the MHCII-restricted T cells to the CD8+ lineage was a mutation within the transcription factor, ThPOK. Wild-type ThPOK suppresses the cytolytic gene expression profiles in CD4+ T cells to induce lineage maturation and is both required and sufficient for maturation of the CD4+ T cell lineage. It was recently identified that antigen-experienced CD4+ T cells can turn off ThPOK to reactivate genes of the CD8 lineage, showing that this maturation step in intrathymic development is not fixed [30].
Early after priming by the innate immune response, CD4+ T cells are able to undergo conversion to an alternate subset through cytokine and chemokine receptor signaling, which induces changes in transcription factor expression [3]. T cell subset specification is influenced by interactions with dendritic cells (DCs) or peritoneal macrophages, the dose and form of presented antigen, the affinity of peptide-TCR interaction, cytokines, and costimulatory interactions [4, 31]. Asymmetric cell division during the DP to SP transition in intrathymic development also influences CD4+ T cell lineage decisions as daughter cells may “inherit” unequal shares of signaling molecules due to altered positioning across autocrine or paracrine chemokine gradients, influenced in part by Notch signaling [10]. Notch binding DLL 1 and 4 ligands promote lineage commitment to the Th1 subset, while Notch-Jagged interactions result in Th2 specification [7].
During at least the first several rounds of cell division under polarizing conditions, Th subset populations are heterogeneous, have low frequencies of cytokine producing cells, and have reversible phenotypes and effector cytokine production [4]. CD4+ T cells are plastic at this stage and beyond and are capable of switching their phenotypes to produce cytokines based on their activation status, environment, and metabolism. Reversibility is possible because the lineage-specific transcription factors that act as master regulators for subset specification are not fully repressed in other lineages but carry both permissive and repressive epigenetic marks or bivalency [3]. Bivalent epigenetic marks allow for rapid transition between active transcription and repressed transcription [32]. CD4+ T cells maintain flexibility in expression of genes encoding transcription factors that regulate cytokine loci, allowing adaptation to altered programs of cytokine expression in a potentially damaging inflammatory milieu [12].
2.2. CD4+ T Cell Subsets
Identification and characterization of CD4+ T cell lineage subsets began nearly three decades ago with the landmark papers of Mosmann et al., which described and identified two CD4+ T cell subsets, Th1 and Th2 [8, 33–35]. Subsets of CD4+ T cells were identified based on production of cytokines in specific responses to antigen or generalized stimulation with Con A [33, 34]. It was identified that Th1 cells produce IL-2 and IFNγ, while Th2 cells produced IL-4, IL-5, preproenkephalin, and p600 [34]. Both clones produced IL-3, GM-CSF, and TNF. They further defined the role of Th1 cells in mediating antigen-specific, MHC restricted, delayed type hypersensitivity reactions for a variety of antigens, while this ability was absent in Th2 cells [8]. Additionally, it was shown that Th2 cells produce a “cytokine synthesis inhibitory factor” capable of inhibiting Th1 cytokine production without a change in viability of the Th1 clones [35]. These discoveries first identified that CD4+ T cells were functionally and phenotypically heterogeneous and capable of cross talk.
One mechanism in which CD4+ T cells undergo subset specialization is through responding to cytokine signals produced in the innate immune response, inducing activation of lineage-specific transcription factors that result in production of a set of effector cytokines [4]. The initial priming cytokines are those produced by antigen presenting cells (APCs) [5]. Activated APCs deliver three types of signals required for the clonal expansion and maturation of CD4+ T cells [31]. The first signal is mediated by the peptide-MHC interaction with the TCR. The second involves costimulatory interactions between the APC and the T cell. The third signal directs differentiation of naïve T cells to effector T cell subsets through cytokines, Ras-MAPK signaling, and Notch ligand interactions [31, 36]. Pathogen recognition by macrophages and dendritic cells of the innate immune response initiates a signaling program that stimulates T lymphocytes and initiates adaptive responses [37]. The fate of a naïve T cell to undergo subset differentiation depends upon cytokine signaling and activation of proteins of the signal transducer and activation of transcription family (STATs). STAT activation is mediated by Janus kinases (JAKs) that are induced during the initial priming period. JAK-STAT triggering leads to activation of lineage-specific transcription factors, which results in expression of effector cytokines [4].
The differential function of CD4+ T cells is determined through which specific cytokines they produce [38]. Cytokines responsible for induction of CD4+ T cell differentiation, lineage specific transcription factors activated during subset specification, effector cytokines produced, and general functions of T cell subsets are summarized in Table 1.
Table 1.
Characterization of CD4+ T cell subsets.
| Th subset | Factors inducing lineage | STAT activated | Lineage-specifying transcription factor | Effector cytokines produced | Functions |
|---|---|---|---|---|---|
| Th1 | IL-12 IL-27 |
STAT4, STAT1 | T-bet | IFNγ, lymphotoxin, TNFα | Cell-mediated immunity, delayed-type hypersensitivity responses, clearance of intracellular pathogens and tumor cells, opsonizing Ab production by B cell class-switching to IgG2a [3, 4, 7–9] |
|
| |||||
| Th2 | IL-4 Indoleamine 2,3-dioxygenase |
STAT6 | Gata-3 c-MAF |
IL-4, IL-5, IL-13, IL-10 | Humoral immunity, clearance of extracellular bacteria and worms, B cell class-switching to IgE, allergic responses [3, 4, 7, 10, 11] |
|
| |||||
| Th9 | IL-4 TGFβ |
STAT6 | BATF | IL-9, IL-10 | Protection against parasitic worms/helminth infections [12, 13] |
|
| |||||
| Th17 | IL-6 MyD88 Low TGFβ IL-23 |
STAT3 | RORγT, RORα | IL-17, IL-17F, IL-6, IL-22, TNFα, IL-10 | Protection of mucosal surfaces, recruitment of neutrophils, clearance of Mycobacterium tuberculosis and Klebsiella pneumonia [3, 14–17] |
|
| |||||
| Th22 | IL-10Rβ
IL-6 TNFα |
STAT3 | Aryl hydrocarbon receptor | IL-22, IL-13, FGF, CCL15, CCL17, TNFα | Mucosal immunity, prevention of microbial translocation across epithelial surfaces, promotes wound repair. [18–21] |
|
| |||||
| Th25 | IL-4, IL-25 | Unknown | Act1 | IL-25, IL-4, IL-5, IL-13 | Mucosal immunity, stimulates nonlymphoid cells to produce IL-4, limits Th1 and Th17 induced inflammation, CD4+ T cell memory (mouse) [12, 22] |
|
| |||||
| TFH | Strong TCR signal, IL-12, CXCR5, IL-21, IL-4 | STAT3 | MAF (IL-21 transactivator) | IL-21, OX40, ICOS | Helps B cells produce high affinity, class-switched antibodies, guides migration into germinal centers [23, 24] |
|
| |||||
| Treg
(includes Tr1 and Th3 cells) |
High TGFβ
mTOR |
STAT5 | Foxp3 | IL-10, TGFβ | Suppression of existing immune responses, maintains tolerance/protection against autoimmunity [15, 16, 25, 26] |
Known CD4+ T cell subsets include Th1, Th2, Th17, Th9, Th25, T follicular helper cells (TFH), and regulatory T cells (Treg). Th1 cells are produced in response to intracellular pathogens (including parasites, viruses, and intracellular bacteria) and mediate cell-mediated immunity and delayed-type hypersensitivity reactions [8]. The Th1 program is induced by IFNγ produced by natural killer (NK) and dendritic cells, which activates STAT1, resulting in activation of the lineage-specific transcription factor T-bet [9]. IL-27, a cytokine of the IL-12 family, also contributes to STAT1 phosphorylation and T-bet activation. T-bet expression increases production of the IL-12 receptor, which activates STAT4, leading to activation of IFNγ transcription and subsequent IFNγ production [7]. This serves as positive feedback, stimulating more naïve T cell clones to undergo Th1 specification to polarize the immune response towards fighting an intracellular pathogen. Th1 cells also produce TNFα and lymphotoxin, cytokines which trigger neutrophil chemotaxis and macrophage activation to potentiate innate immune reactions [38, 39]. Th1 cells also help B cells in antibody class-switching to produce high-affinity IgG for opsonization of an offending pathogen [40].
Th2 specification is required for B cell help in humoral immunity and elimination of extracellular microbes and intestinal helminthes [5, 40]. Th2 cells are involved in antibody class-switching to produce IgE which can provoke or sustain allergic reactions [8, 36, 39]. Differentiation of the Th2 subset requires IL-4 produced by Notch ligand activation of dendritic cells which, in turn, induces STAT6, which activates the lineage-specifying transcription factor, GATA-3 [36]. GATA-3 activates transcription of the Th2 cytokine cluster leading to IL-4, IL-5, and IL-13 production. Th2 cytokines provide positive feedback for maturation of naïve T cells to the Th2 lineage and inhibit Th1 development [39] by the homeostatic cytokine IL-10. Th2 cells also heighten the innate immune response through activation of macrophages by induction of IL-4 and macrophage activating factor (MAF) [5].
Th17 cells provide protection against bacteria and fungi at mucosal surfaces and confer coverage of some microbes that are not targeted in Th1 or Th2 responses, including, but not limited to, Mycobacterium tuberculosis, Bacteroides fragilis, and Klebsiella pneumoniae [15]. Induction of the Th17 lineage occurs when IL-6, IL-23, and TGFβ are present in the inflammatory milieu without IL-4 or IL-12 (which promote Th2 or Th1 responses, resp.) [41, 42]. Toll-like receptor signaling, leading to MyD88 signaling, is another innate immune signal fostering Th17 differentiation [43]. IL-6 promotes STAT3, which induces retinoic orphan receptor (ROR) transcription factors, RORα and RORγT, leading to production of Th17 cytokines IL-17, IL-17F, and IL-22 [44, 45].
Mucosal immunity is provided through Th9, Th22, and IL-25 producing cells [46]. Th9 cells provide protection against intestinal helminth infections [13]. IL-9 producing cells are proinflammatory as they stimulate proliferation and inhibit apoptosis of hematopoietic cells and also activate Th17 cells [47]. This is due to stimulation of Jak1 by IL-9, resulting in activation of STATs 1, 3, and 5. Th9 cells undergo a maturation program similar to Th2 cells, with IL-4 inducing STAT6 activation and produce the Th2 cytokines IL-9 and IL-10, but, unlike Th2 cells, they require TGFβ for maturation [13, 48]. The lineage-specific transcription factor for Th9 development may be the activator protein 1 family transcription factor, BATF, leading to a transcriptional program which results in increased IL-9 and IL-10 production [13, 49]. Th22 cells are CD4+ T cells that are phenotypically and functionally related to Th17 cells that participate in wound repair and in protection against bacterial, viral, and fungal infections at epithelial surfaces, including the skin and GI tract [21]. They prevent translocation of microbes across epithelia, which limit the extent of infection [18]. Th22 specification is promoted by IL-6 and TNF-α, which induces STAT3, and expression of the aryl hydrocarbon receptor [19]. This parallels Th17 maturation, and numerous phenotypic markers are expressed in common between Th17 and Th22 cells, including CCR6, CCR4, dipeptidyl peptidase IV, CD26, and CD90 [20]. CCR10 is also expressed on Th22 cells, distinct from Th17 [20]. Th22 cells produce IL-22, IL-13, fibroblast growth factor, CCL15, CCL17, and TNFα at epithelial surfaces. IL-22, an IL-10 family cytokine, production is not unique to Th22 cells but is also produced by Th1 and Th17 cells; however, Th22 cells can produce IL-22 in the absence of IFNγ or IL-17 [20]. IL-25-producing cells may represent a new subset, Th25 cells, which stimulate nonlymphoid cells to produce effector cytokines in response to extracellular pathogens [22]. They are induced by the transcription factor Act1, but can be derived from the Th2 lineage [12, 46]. IL-25-producing cells and the Th2 subset may be linked as IL-4 is required for production of both cell types and IL-25 enhances production of Th2 cytokines, inducing IL-4, IL-5, and IL-13 secretion [12, 50].
T follicular helper cells (TFH) improve B cell class-switching for immunoglobulin production and guide B cells into germinal centers by chemotaxis mediated by CXCR5 signaling [23]. TFH cells require a strong TCR signal for induction, which is also required for Treg responses [24, 51]. TFH specification requires activation of the inducible costimulator (ICOS), a CD28-related costimulatory signal provided by activated dendritic cells or B cells, which initiates transcription of the transcription factor MAF that transactivates IL-21 [24]. OX-40/CD134 ligation is another required costimulatory signal, which downregulates CTLA-4, a dominant suppressor of T cell activation [24]. IL-6 and STAT3 are required for TFH development similar to Th17 cells, yet TFH cells can be generated in the absence of Th17 cytokines, IL-17, IL-17F, or TGFβ [23].
Suppression of immune responses and maintenance of peripheral tolerance is provided by Tregs [25]. Tregs are a heterogeneous population which includes thymic-derived natural Tregs (nTregs), adaptive regulatory T cells involved in maintaining oral tolerance (Th3 cells), and T regulatory type 1 cells (Tr1 cells), induced by IFNα secreted by neighboring plasmacytoid dendritic cells (pDCs). nTregs require a strong TCR signal, which is potentially self-reactive, for development [52]. They are generated with minimal costimulation, for T cell recognition of antigen without a strong second signal from a CD28 family member can provide induction of tolerance [52]. Differentiation of induced Tregs, Th3 cells, and Tr1 cells occurs in the periphery and requires high concentrations of TGFβ, with the absence of proinflammatory cytokines [15]. Cell-cell contact and IL-10 secretion is required for suppressor function, mediated through STAT5-induced activation of the lineage-specific transcription factor Foxp3, with concurrent downregulation of the Th17 transcription factor RORγT [16]. Suppressor function of Tregs requires Foxp3 expression [53]. Reduced Treg numbers and effector function occur in autoimmune diseases and complete deficiency of this subset results in a severe autoimmune disease, immune dysregulation, polyendocrinopathy, and enteropathy with X-linked inheritance (IPEX) syndrome [52].
A population of non-Treg Foxp3-expressing CD4+ T cells has been identified, which is known as the “exFoxp3” T cell [54]. exFoxp3 cells have transient Foxp3 expression in an activated state, and these cells can accumulate at sites of inflammation. These represent effector T cells that gain Foxp3 expression and not conversion to the Treg lineage. A small population of Tregs with loss of Foxp3 expression while maintaining commitment to the Treg lineage also exists. This is through demethylation at the TSDR locus, which retains memory of its suppressor phenotype [54].
Central memory CD4+ T cells, created through initial priming and restimulation, consist of a heterogeneous population that is not lineage-committed, for memory responses are subject to manipulation under cytokine-polarizing conditions to adapt to new antigenic stimuli [55]. Effector memory CD4+ T cells are thought to have undergone lineage determination. Plasticity of the central memory population is essential for maintenance of specific CD4+ T cells after pathogen clearance, since 90–99 percent of Th1 or Th2 effector cells will undergo apoptosis after antigenic challenge [56].
3. Cross Talk and Flexibility in T Cell Subset Lineage Specification
Effector cytokines produced by CD4+ T cells provide positive feedback to increase further differentiation of naïve T cells to that lineage while inhibiting differentiation of opposing subsets [57]. Signal transduction pathways induced by cytokines and chemokines influence lineage commitment events through activation or repression of a subset-specific transcriptional program [58].
Th 1 and Th 2 Subsets. Commitment to the Th1 lineage inhibits Th2 development, and Th2 commitment inhibits Th1 responses [39]. IFNγ production by Th1 cells inhibits production of Th2 cytokines [39]. Likewise, IL-4 produced during Th2 specification inhibits production of IFNγ and IL-12, preventing differentiation of naïve CD4+ T cells to the Th1 lineage [39]. GATA3 expression by Th2 cells leads to upregulation of sphingosine kinase I expression and downregulation of STAT4, which inhibit Th1 development [59].
Plasticity occurs between the Th1 and Th2 lineages, and early after naïve CD4+ T cell activation, production of IFNγ and IL-4 can occur simultaneously [55]. Decreased expression of intracellular osteopontin by APCs with increased soluble osteopontin produced by T cells (increased soluble-to-intracellular osteopontin ratio) stimulates IL-12 production to promote Th1 lineage commitment [60]. With culture of CD4+ T cells in a Th1-promoting environment (containing IL-12 and anti-IL-4 antibody), the population of cells will polarize to produce IFNγ. Removal of the polarized CD4+ T cells into IL-4 containing medium promotes Th2 cytokine production, displaying the capacity of converting between the two phenotypes. In addition, forced overexpression of GATA3 in Th1 polarized cells results in conversion to a Th2 phenotype, while T-bet overexpression in Th2 polarized cells results in a Th1 phenotype [61]. Flexibility between Th1 and Th2 cytokine production is lost, however, with repeated stimulation and multiple rounds of cell division [6]. This is thought to be due to chromatin remodeling at cytokine loci to increase efficiency of effector cytokine production and inhibit opposing cytokine programs [62].
Intrachromosomal interactions through modifications of chromatin structure are also responsible for repression of the alternate lineage program [32]. Stimulation under Th1 or Th2 polarizing conditions results in altered chromatin accessibility after 4 to 6 cell divisions. In naïve T cells, the IFNγ locus is bivalent, poised for enhancing gene expression or transcriptional silencing, depending on which signals are received. Under a Th2 polarizing cytokine environment, permissive histone modifications are lost at the IFNγ locus by DNA methylation. Similarly, repressive methylation at the Th2 locus occurs during Th1 polarization [63]. In addition to intrachromosomal modifications, interchromosomal interactions exist between the IFNγ and Th2 cytokine clusters for negative regulation of the opposite lineage. Direct interaction between the IFNγ promoter and regulatory regions of the Th2 cytokine cluster cross-regulate one another [64]. Naïve T cells have the ability to express both Th1 and Th2 cytokines within hours of T cell activation due to the interaction of these two loci creating a chromatin hub configuration between the IFNγ promoter and the Th2 locus control region [64].
Expressions of Th1 and Th2 cytokines from a single cell, as well as environments rich in both Th1 and Th2 cytokines, further show flexibility in subset specification. When CD4+ T cells are stimulated in vitro with IL-12, they produce both IFNγ and IL-4 [65]. Yet, repeated stimulation will reduce the percentage of cells expressing this phenotype, suggesting that the double-positive cells represent a transition state of differentiation.
In vivo polarization experiments using model pathogens have also demonstrated interconversion between Th1 and Th2 cells. CD4+ T cells exposed to Leishmania major infection differentiate into the Th1 lineage and produce IFNγ while maintaining the capacity of interchanging into a Th2 phenotype when exposed to IL-2 and IL-4 ex vivo [65]. There is the possibility, however, that reversal from a Th1 to Th2 phenotype may simply reflect the outgrowth of a population of uncommitted cells rather than dedifferentiation from a Th subset [65].
T reg and Th 17 Subsets. Th17 and Treg subsets are in a homeostatic balance and are derived from a common precursor. CD4+ T cells with dual expression of Foxp3 and RORγT exist during early Th17 cell development and in naïve T cells after stimulation with TGFβ. These Foxp3+RORγT+ expressing cells may occur as an intermediate during commitment to an effector lineage, Treg or Th17 cells [66]. They mature into either a Treg or Th17 cell depending on the cytokine profile in the environment [16]. IL-6, IL-21, IL-23, and low levels of TGFβ support induction of RORγT and Th17 development. High levels of TGFβ, retinoic acid, and IL-2 support Treg commitment through activation of Foxp3 [15, 66]. Th17 cells can be converted from induced Treg cell populations in the presence of the cytokines IL-6 and IL-1. When TGFβ expression is high and IL-6 is present, a population of Foxp3+IL-17+ cells results. STAT3 phosphorylation in cells committing to a Th17 lineage inhibits TGFβ-induced Foxp3 expression [32]. Additionally, RORγT directly interacts with exon 2 of the Foxp3 gene to suppress Treg development and activates transcription of Th17 cytokines. Similarly, Foxp3 can bind RORγT to suppress IL-17 production [66]. Other coexpressed transcription factors influence the Treg v. Th17 lineage branch point. These include interferon regulatory factor-4 and Runx1, which promote Th17 differentiation through interaction with the Foxp3 locus [29]. Both thymic-derived and peripherally induced Tregs express Helios [67, 68], which could be used to identify whether a Th17 cell was derived from a Treg versus being induced from a naïve T cell precursor. Low levels of Helios expression can indicate T cell activation, while high expression suggests Treg origin [69]. Proinflammatory cytokine production by Th17 cells inhibits generation of Treg cells, and Treg production of IL-10 suppresses Th1 and Th17 generation [14, 25]. Tregs antagonize Th17 function and reduce IL-17 production when it is no longer required for pathogen clearance to avoid tissue injury [16].
Treg and Th17 cells are the predominant CD4+ T cells within tumor microenvironments [70]. Tregs suppress antitumor immune responses through promoting tolerance to the tumor by IL-10 production. In the presence of IL-6, TGFβ, type I interferons, IL-12, and intact toll-like receptor signaling via MyD88, Th17 cell specification is induced from Treg cells [70, 71]. Overall, the number of Tregs is increased in many cancers and has been shown in gastric adenocarcinoma [72], esophageal adenocarcinoma [72], squamous cell carcinoma (head and neck) [73–75], breast carcinoma [76], and non-small cell lung carcinoma [76]. Reduced numbers of intratumoral Th17 cells have been associated with poor prognosis in several tumor models [70]. Yet, in a recent lung adenocarcinoma model, K-rasG12D expression promoted recruitment of Th17 cells to the tumor and increased tumor growth, with IL-17 blockade reducing tumor burden [77]. Whether an excess of Tregs or Th17 cells is pathogenic within a tumor could be context-dependent, based on the type of tumor, its oncogenic drivers, the microenvironment, and the immunocompetence (versus compromise) of the host.
Proinflammatory cytokine production by Th17 cells inhibits generation of Treg cells, and Treg production of IL-10 suppresses Th1 and Th17 generation [14, 25]. Tregs antagonize Th17 function and reduce IL-17 production when it is no longer required for pathogen clearance to avoid tissue injury [16]. The Treg lineage may not be fixed, as Tregs have been identified to differentiate into Th17 or TFH cells. Foxp3+ T cells in the thymus develop into Th17 cells and produce IL-17 when taken ex vivo and put into IL-6-containing medium. Additionally, over one-fourth of Th17 cells along the small intestine mucosa are thought to be derived from Foxp3+ iTregs [15]. However, it is possible that differentiation into effector CD4+ T cell subsets could represent a population of activated T cells with aberrant Foxp3 expression (exFoxp3 cells) rather than Tregs themselves.
Whether plasticity of Tregs exists is under debate; however, there is known heterogeneity within Treg populations. Three Treg subsets have been identified, which have varying functions, defined by expression of CD45RA, CD25 levels, and Foxp3 [78]. Activated Tregs are CD45RA−CD25hiFoxp3hi and show suppressor function; CD45RA+CD25moderateFoxp3lo subset represents resting regulatory T cells without suppressor function; and CD45RA−CD25moderateFoxp3lo cells are non-Treg effector T cells that are capable of cytokine production, making IL-2, IL-17, or IFNγ [78]. In autoimmune conditions, including systemic lupus erythematosus and sarcoidosis, there is an increased ratio CD45RA−CD25+Foxp3hi cells compared to CD45RA−CD25+Foxp3lo cells, although the absolute number of Treg cells is overall reduced. This ratio was found to be reversed in cancer [79].
Th 17 Compared to Th 1 or Th 2. Th17 cells have bivalent expression of T-bet and GATA-3, allowing them to reprogram into either Th1 or Th2 cells [32, 80]. Th17 cells generated by TGFβ and IL-6 in vitro can convert into IL-12-producing Th1 or IL-4-producing Th2 cells when ongoing stimulation with proinflammatory cytokines is not sustained [1]. Th1 cells differentiate from Th17 cells in vitro when IL-12 is present in medium in the absence of IL-6. IL-17+IFNγ +-producing cells may represent an intermediate state during Th1 development from Th17 precursors. T-bet and RORγT are coexpressed in this transition state, allowing maturation of either the Th1 or Th17 lineage [32].
A distinct population of Th1/Th17 cells has been identified, which are CD4+ T cells capable of producing IFNγ, GM-CSF, and IL-17 [81]. Th1/Th17 cells express both T-bet and RORC2 concurrently to allow for bivalent cytokine production. In an inflammatory environment, they can further become polarized to become Th1/exTh17 cells through loss of active transcription from the RORC2 locus. Th1/ex-Th17 cells produce IFNγ and GM-CSF but lost the ability to produce IL-17 [81]. Although Tregs are capable of transforming into IFNγ and IL-17 producing cells, Th1/Th17 cells are a separate entity, as expression of the transcription factor Helios (present in thymic-derived Tregs and to a lesser extent in peripherally induced Tregs) is absent or low in this subset.
Th1/Th17 cells have been associated with promoting autoimmune target organ damage in β-cells of human and animal models of type I diabetes mellitus [82] within synovial tissue of children with juvenile arthritis [83] and within the gastrointestinal tract of patients with Crohn's disease [84]. Blockade of IFNγ with monoclonal antibodies reduced pancreatic β-cell destruction in an animal model, supporting that Th17 cells that express a Th1-like phenotype are pathogenic [81].
T FH Compared to T reg , Th 17 , or Th 1. TFH cells can be differentiated from Tregs, which requires loss of Foxp3 expression by T-B cell interaction with costimulation provided by CD40-CD40L interaction [24]. Tregs differentiate into TFH cells in Peyer's patches to promote IgA production and mucosal immunity [1]. TFH cells mature from TGFβ-induced Treg precursors in response to IL-21 and chemotaxis through CXCR5 signaling, which homes CD4+ T cells to follicles in secondary lymphoid tissue [23].
Th17 and TFH cells both require STAT3 activation and costimulation mediated by ICOS, which increases activation of the transcription factor, c-MAF, involved in both Th17 and TFH maturation [85]. In ICOS knockout mice, decreased c-MAF activation prevents development of TFH cells and prevents defects in Th17 cells, which produce less IL-17 in response to stimulation [85]. IL-12 is required during early dendritic cell-mediated priming of both Th1 and TFH cells, suggesting that TFH and Th1 cells are derived under common innate immune signals [86].
Th 2 Compared to T FH or Th 9. The same priming cytokine, IL-4, is required for the development of Th2, TFH, and Th9 subsets. Th2 cells can become TFH cells through upregulation of CXCR5 expression for homing to germinal center follicles will continue to produce IL-4 but lose the ability to produce other Th2 cytokines, including IL-5 and IL-13 [13, 48]. TFH cells do not have to progress through a Th2 stage in their development, as they still develop in GATA3 knockout mice [23].
Th9 cells can be derived from Th2 cells when TGFβ is added to cells polarized with IL-4 in culture medium [13]. Continued activation of the Th2 cytokine, IL-4, or the Th9 cytokine, IL-9, in transgenic mice leads to the same phenotype, with asthma and bronchial hyperresponsiveness due to mucosal inflammation, also suggesting interconnectedness in their maturation [87].
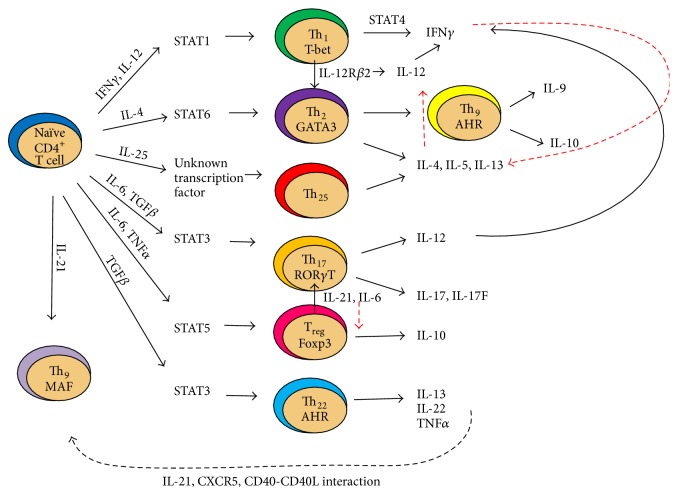
A schematic diagram of interactions involved in CD4+ T cell lineage specification is provided in Figure 1.
Figure 1.
Cross talk between CD4+ T cell subsets mediated by effector cytokines.
Naïve CD4+ T cells undergo subset specification based on predominant cytokines and chemokines present within the environment. In the presence of IFNγ and IL-12, naïve CD4+ T cells upregulate STAT1 which induces specification to the Th1 lineage through T-bet expression [9, 58]. Th1 cells then produce IFNγ through STAT4 expression [88]. A positive feedback loop exists to further promote Th1 specification [89]. Conversely, Th1 cells inhibit Th2 specification through IFNγ production, as well as through T-bet expression. Th2 cells develop when IL-4 is present in the environment and require STAT6 upregulation to induce GATA-3 expression within the nucleus [90]. Th2-specified cells produce IL-4, IL-5, and IL-13. When TGFβ is also present in the cytokine microenvironment, Th9 specification can occur [91]. As Th1 cells inhibit Th2 specification, Th2 cells also provide negative feedback to Th1 differentiation through suppressing IL-12 expression [89]. Th25 cells, induced by IL-25, represent a separate mechanism of GATA-3 induction and production of Th2-cytokines, IL-4, IL-5, and IL-13, which has been linked to reactive airway inflammation [46]. Th25 specification requires expression of the E3 ubiquitin ligase Act1 and the cognate receptor IL-17RB [46], related to the Th17 subset. The Th17 and Treg lineages are interrelated, and both can be induced in the presence of TGFβ, with Th17 specification preferred when IL-6 is also present. Th17 cells express lineage-specific transcription factor RORγT as well as other retinoic orphan receptors, which leads to production of Th17 cytokines, IL-12, IL-17, and IL-17F [92]. IL-12 produced by Th17 cells can provide feedback to promote Th1 specification [1]. In the presence of IL-6 or IL-21, Treg cells can become Th17 cells [16]. IL-6 and TNFα can induce STAT3 activation and induce Th22 specification, a process that is dependent on the aryl hydrocarbon receptor [19]. In the presence of IL-21, CXCR5, and costimulation by CD40-CD40 ligand interaction, Tregs can undergo T follicular helper (TFH) specification [23].
3.1. Epigenetic Modifications of Cytokine Loci Determine Lineage Specification
Flexibility of CD4+ T cells to switch effector cytokine function in response to environmental signals depends on permissiveness of chromatin to transcription factor binding at loci encoding cytokines.
CD4+ T cells undergo multiple cell divisions before producing subset-specific effector cytokines, since maturation of CD4+ T cells into subsets requires continued stimulation under cytokine polarizing conditions, resulting in stable patterns of gene expression due to chromatin remodeling of loci encoding cytokine genes [1].
Epigenetic influences bring alterations of cytokine production profiles through mechanisms including alterations of chromatin structure through histone acetylation or DNA demethylation and modifications of microRNA activity [1]. Histone acetylation or demethylation of DNA brings decondensation (“opening”) of chromatin (or conversion of heterochromatin to euchromatin), resulting in increased access for transcription factor binding [4]. Modifications that occur when signals within the extracellular environment change allow a CD4+ T cell to redirect its differentiation program to provide flexibility to shift cytokine production for the optimal clearance of offending pathogens [1].
Genome-wide chromatin immunoprecipitation studies (CHIP) have characterized histone modifications which accompany changes in gene expression within CD4+ T cells. Trimethylation of lysine 4 at histone 3 (H3K4me3) occurs at promoter and enhancer elements of actively expressed genes. Trimethylation of lysine 27 of histone 3 (H3K27me3) represses a locus, and this repression is reversed by histone demethylases [3]. Presence of both of these histone modifications (bivalency of histone 3, or coexpression of H3K4me3 and H3K27me3) allows a gene promoter to become activated or silenced, depending on the signal received [1]. In addition, chromatin looping can bring regulatory elements to proximity with promoters of target genes enabling regulation of gene expression, a process mediated by CCCTC-binding factor, an insulator protein that binds proximal elements of a gene to prevent it from interacting with surrounding chromatin [32]. Looping allows transcriptional regulatory elements to reposition in the nucleus during T cell maturation to promote or repress transcription [7]. Chromatin looping allows the Th2 cytokine locus control region (LCR) to form a complex with the promoters that induce IL-4, IL-5, and IL-13 cytokine expression for activation of the Th2 transcriptional program [64].
In addition to changes in chromatin structure, interchromosomal associations allow for regulation of effector cytokine expression. Chromosome conformation capture studies (3C technique) have identified an interchromosomal interaction between the IFNγ promoter of human chromosome 10 and the LCR of the Th2 cytokine locus on chromosome 11 [64]. A chromatin hub configuration between Th1 and Th2 cytokine loci primes a naïve CD4+ T cell to produce either Th1 or Th2 cytokines 1 hour after TCR activation. After this early wave of cytokine production activation of STAT proteins is required for maintenance of the signal [62].
The lineage-specific transcription factors that direct Th1, Th2, and Th17 commitment (Tbx21, GATA3, and RORγT, resp.) carry bivalent epigenetic marks, signifying the possibility of subset specification reversibility. However, Foxp3 expression in Treg cells is univalent, suggesting subset differentiation is possible but not reversible [32, 93].
3.2. mTOR as a Regulator of CD4+ Differentiation
The serine-threonine kinase, mammalian target of rapamycin (mTOR), is a candidate gene as a master regulator of CD4+ T cell differentiation and metabolism [94], which are interconnected. mTOR activation in CD4+ T cells has diverse roles in regulation of cell growth and proliferation, mRNA turnover and transcription, translation, regulation of vesicular traffic, autophagy and amino acid recycling, cytoskeletal reorganization, and control of cell size [95]. It exerts its function through phosphorylation of its target substrates, such as p70 S6 kinase and 4E-BP1 (which regulate translation), and DAP1 (which inhibits induction of autophagy). mTOR is positively regulated by the GTPase Rheb and negatively regulated by the tuberous sclerosis complex (TSC) [96].
mTOR activation results in increased maturation of CD4+ T cells into effector cells, with reduction of Foxp3 expression and Treg generation [97]. Absence of mTOR at the DP phase in T cell development abrogates the ability to produce Th1, Th2, or Th17 cells and results in a high proportion of thymocytes maturing as Tregs [26]. This maturation defect is associated with decreased activation of STAT 4, 6, and 3, resulting in failure to upregulate lineage-specific transcription factors T-bet, GATA3, and RORγT [26]. Consistently, treatment with rapamycin (an mTOR inhibitor) results in thymic involution, decreased egress of T cells, and blockade of the DN to DP transition of T cell development [94]. Conversely, in effector CD4+ T cells, activation of the sphingosine-1-phosphate (S1P1) signaling pathway results in increased mTOR-Akt activation, which inhibits intrathymic generation and suppressor function of Tregs [98, 99]. Therefore, mTOR overexpression renders effector CD4+ T cells resistant to Treg suppressor activity [94].
During effector CD4+ T cell activation, the PI3K-Akt-mTOR pathway is activated and regulates the effector T cell/Treg cell fate decision [99]. mTOR activation transmits signaling through the IL-2 receptor, and IL-2 binding to the IL-2 receptor prevents T cell anergy, required for maintenance of effector T cells [25]. Treg cells do not rely on mTOR activation for IL-2 receptor signaling but instead use an alternate PIM2-dependent pathway for IL-2 signaling, as STAT5 increases IL-2 through activation of PIM2. The activity of mTORC1 in comparison to mTORC2 determines whether naïve T cells differentiate into the Th1/Th17 lineage versus Th2 cells [100]. In the absence of Rheb, a kinase required for the function of mTORC1, Th1 and Th17 cells are not produced, as mTORC1 is required for Th1/Th17 specification [100]. In the absence of rictor, a component of mTORC2, Th1, and Th17 cells is generated but not Th2 cells. Therefore, mTORC1 activity is required to generate Th1 cells, while mTORC2 activity is required for Th2 specification [100].
Lineage determination by mTOR is through induction of changes in cellular metabolism through activation of its substrates (hypoxia-inducible factor, sterol regulatory element binding proteins 1 and 2), through regulation of mitochondrial function, and through negative regulation of autophagy [101]. mTOR activation results in a stimulation of glycolysis, pentose phosphate shunt pathway activity (oxidative branch), and lipid biosynthesis with a concurrent reduction in fatty acid oxidation [101]. Since mTOR stimulates glycolytic metabolism, it promotes Th1, Th2, and Th17 specification, as these subsets have high metabolic requirements [102]. As such, Th1, Th2, and Th17 cells also show high expression of the Glut1 receptor to facilitate increased glucose transport [102]. As mTORC1 supports an anabolic state with lipid biosynthesis over its utilization/oxidation, this results in reduced AMPK activity [103]. AMPK activity is critical to Treg metabolism, supporting effector over Treg cell specification when expression is low [103]. mTOR additionally impacts aerobic metabolism through control over mitochondrial function through regulation of mitochondrial number [104], transmembrane potential [105], oxygen consumption [105, 106], and autophagy [107–109].
A model of CD4+ T cell specification has been proposed based on differential activation of mTOR. In this model, the generation of effector CD4+ T cells from naïve CD4+ T cell precursors is dependent on mTOR-mediated induction of metabolic programs within CD4+ T cells [110]. mTORhi and mTORlo naïve CD4+ T cells were found to have different fates [111]. mTORhi CD4+ T cells will become effector cells, while mTORlo CD4+ T cells represent a long-lived CD4+ T cell population, with expression of Bcl-2, CD62L, and CD25 and a higher propensity to develop into Tregs [111]. Interestingly, the mTORlo and mTORhi naïve T cell populations can be separated based on their size, with the mTORhi population having increased cell size [111].
Although mTOR activity is critical to the regulation of CD4+ T cell development, specification, and metabolism, its hyperactivation is pathogenic. mTOR complex I is overexpressed in autoimmune diseases, genetic cancer syndromes, and obesity which correlates with a reduction in suppressor Tregs [112]. mTOR stimulates aerobic glycolysis, which promotes the Warburg effect within tumors [112]. mTORC1 activity increases Th17 cell number and reduces Tregs in systemic lupus erythematosus (SLE) [113, 114]. Overexpression of mTOR results in defects in macroautophagy [105, 115], which is pathogenic through mitochondrial dysfunction, ATP depletion, and increased oxidative stress [116]. mTOR inhibitors, such as rapamycin, are therefore therapeutic through inhibiting aberrant mTOR activity in the treatment of autoimmunity and malignancies [117–119].
4. Conclusion
As mentioned, naïve CD4+ T cells mature into Th1, Th2, Th9, TFH, Th17, or Treg subsets in response to innate immune signals, costimulatory interactions with APCs, paracrine cytokine signals, and through mTOR-mediated changes in energy metabolism [31, 120]. The resulting CD4+ T cell subsets are highly plastic with numerous transitory populations identified that are capable of heterogeneous cytokine production as well as the ability to cross talk with other naïve, effector, memory, and regulatory CD4+ T cells. With continued stimulation, CD4+ T cells develop patterns of stable cytokine expression, yet chromatin remodeling alters cytokine programs in subsets containing bivalent chromatin modifications at loci encoding lineage-specific transcription factors to maintain the capability of shifting their phenotype in response to environmental alterations [32]. mTOR was recently identified as a possible master regulator of CD4+ T cell differentiation [26] and exerts CD4+ T cell specification through alterations in cellular metabolism. An improved understanding of how to modulate CD4+ T cell pools through inducing phenotypic shifts could provide wide health benefits from limiting autoimmune responses to optimizing antitumor immune responses and represents an exciting area of investigation.
Supplementary Material
Supplemental figure 1: Simplified diagram of CD4+ T cell lineage commitment. Numbers over arrows correspond to types of experiments described in Table 2, which are used to identify the effector molecule following the arrow.
Supplemental table 1: Experiments used to study CD4+ T cell subset specification.
Conflict of Interests
The authors declare that there is no conflict of interests regarding publication of this paper.
References
- 1.Zhou L., Chong M. M. W., Littman D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Hakim F. T., Memon S. A., Cepeda R., et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. Journal of Clinical Investigation. 2005;115(4):930–939. doi: 10.1172/JCI200522492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone J. A., MacKay C. R., O'Shea J. J., Stockinger B. The functional plasticity of T cell subsets. Nature Reviews Immunology. 2009;9(11):811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Garra A., Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends in Cell Biology. 2000;10(12):542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 5.Murphy K. M., Reiner S. L. The lineage decisions of helper T cells. Nature Reviews Immunology. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 6.Richter A., Löhning M., Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. The Journal of Experimental Medicine. 1999;190(10):1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amsen D., Spilianakis C. G., Flavell R. A. How are TH1 and TH2 effector cells made? Current Opinion in Immunology. 2009;21(2):153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. The Journal of Immunology. 1987;138(11):3688–3694. [PubMed] [Google Scholar]
- 9.Szabo S. J., Kim S. T., Costa G. L., Zhang X., Fathman C. G., Glimcher L. H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 10.Reiner S. L. Decision making during the conception and career of CD4+ T cells. Nature Reviews Immunology. 2009;9(2):81–82. doi: 10.1038/nri2490. [DOI] [PubMed] [Google Scholar]
- 11.Xu H., Zhang G.-X., Ciric B., Rostami A. IDO: a double-edged sword for TH1/TH2 regulation. Immunology Letters. 2008;121(1):1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locksley R. M. Nine lives: plasticity among T helper cell subsets. Journal of Experimental Medicine. 2009;206(8):1643–1646. doi: 10.1084/jem.20091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldhoen M., Uyttenhove C., van Snick J., et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunology. 2008;9(12):1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Laurence A., O'Shea J. J. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Seminars in Immunology. 2007;19(6):400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver C. T., Harrington L. E., Mangan P. R., Gavrieli M., Murphy K. M. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y. K., Mukasa R., Hatton R. D., Weaver C. T. Developmental plasticity of Th17 and Treg cells. Current Opinion in Immunology. 2009;21(3):274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Harrington L. E., Mangan P. R., Weaver C. T. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Current Opinion in Immunology. 2006;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Kim C. J., Nazli A., Rojas O. L., et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunology. 2012;5(6):670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 19.Pickert G., Neufert C., Leppkes M., et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. The Journal of Experimental Medicine. 2009;206(7):1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trifari S., Kaplan C. D., Tran E. H., Crellin N. K., Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nature Immunology. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 21.Eyerich S., Eyerich K., Pennino D., et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. Journal of Clinical Investigation. 2009;119(12):3573–3585. doi: 10.1172/jci40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallon P. G., Ballantyne S. J., Mangan N. E., et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of Experimental Medicine. 2006;203(4):1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nurieva R. I., Chung Y., Hwang D., et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King C. New insights into the differentiation and function of T follicular helper cells. Nature Reviews Immunology. 2009;9(11):757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 25.Josefowicz S. Z., Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgoffe G. M., Kole T. P., Zheng Y., et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore T. A., Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995;86(5):1850–1860. [PubMed] [Google Scholar]
- 28.Germain R. N. T-cell development and the CD4-CD8 lineage decision. Nature Reviews Immunology. 2002;2(5):309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 29.Collins A., Littman D. R., Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nature Reviews Immunology. 2009;9(2):106–115. doi: 10.1038/nri2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucida D., Husain M. M., Muroi S., et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nature Immunology. 2013;14(3):281–289. doi: 10.1038/ni2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moser M., Murphy K. M. Dendritic cell regulation of TH1-TH2 development. Nature Immunology. 2000;1(3):199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 32.Wilson C. B., Rowell E., Sekimata M. Epigenetic control of T-helper-cell differentiation. Nature Reviews Immunology. 2009;9(2):91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann T. R., Cherwinski H., Bond M. W. Two types of murine helper T cell clone. I. definition according to profiles of lymphokine activities and secreted proteins. The Journal of Immunology. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 34.Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 an Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. The Journal of Experimental Medicine. 1987;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. Journal of Experimental Medicine. 1989;170(6):2081–20095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amsen D., Antov A., Flavell R. A. The different faces of Notch in T-helper-cell differentiation. Nature Reviews Immunology. 2009;9(2):116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 37.Kaiko G. E., Horvat J. C., Beagley K. W., Hansbro P. M. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. The Journal of Immunology. 2005;175(1):5–14. [PubMed] [Google Scholar]
- 39.Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of Experimental Medicine. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith K. M., Pottage L., Thomas E. R., et al. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. Journal of Immunology. 2000;165(6):3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 41.Brucklacher-Waldert V., Steinbach K., Lioznov M., Kolster M., Hölscher C., Tolosa E. Phenotypical characterization of human Th17 cells unambiguously identified by surface IL-17A expression. Journal of Immunology. 2009;183(9):5494–5501. doi: 10.4049/jimmunol.0901000. [DOI] [PubMed] [Google Scholar]
- 42.Korn T., Bettelli E., Oukka M., Kuchroo V. K. IL-17 and Th17 cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 43.Davila E., Kolls J. A ‘toll’ for Th17 cell expansion. Journal of Leukocyte Biology. 2010;88(1):5–7. doi: 10.1189/jlb.0110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romano M., Sironi M., Toniatti C., et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 45.Yang X. O., Pappu B. P., Nurieva R., et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ . Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaidani S., Bulek K., Kang Z., et al. T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation. The Journal of Immunology. 2011;187(6):3155–3164. doi: 10.4049/jimmunol.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elyaman W., Bradshaw E. M., Uyttenhove C., et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dardalhon V., Awasthi A., Kwon H., et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nature Immunology. 2008;9(12):1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabeen R., Goswami R., Awe O., et al. Th9 cell development requires a BATF-regulated transcriptional network. Journal of Clinical Investigation. 2013;123(11):4641–4653. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tato C. M., Laurence A., O'Shea J. J. Helper T cell differentiation enters a new era: le roi est mort; vive le roi! The Journal of Experimental Medicine. 2006;203(4):809–812. doi: 10.1084/jem.20060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogquist K. A. Signal strength in thymic selection and lineage commitment. Current Opinion in Immunology. 2001;13(2):225–231. doi: 10.1016/S0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X., Bailey-Bucktrout S., Jeker L. T., Bluestone J. A. Plasticity of CD4+ FoxP3+ T cells. Current Opinion in Immunology. 2009;21(3):281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X., Jeker L. T., Fife B. T., et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. Journal of Experimental Medicine. 2008;205(9):1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyao T., Floess S., Setoguchi R., et al. Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36(2):262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Krawczyk C. M., Shen H., Pearce E. J. Functional plasticity in memory T helper cell responses. Journal of Immunology. 2007;178(7):4080–4088. doi: 10.4049/jimmunol.178.7.4080. [DOI] [PubMed] [Google Scholar]
- 56.Goldrath A. W., Bevan M. J. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402(6759):255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 57.Rao A., Avni O. Molecular aspects of T-cell differentiation. British Medical Bulletin. 2000;56(4):969–984. doi: 10.1258/0007142001903634. [DOI] [PubMed] [Google Scholar]
- 58.Rieger M. A., Hoppe P. S., Smejkal B. M., Eitelhuber A. C., Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325(5937):217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 59.Ho I.-C., Tai T.-S., Pai S.-Y. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nature Reviews Immunology. 2009;9(2):125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantor H., Shinohara M. L. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nature Reviews Immunology. 2009;9(2):137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sundrud M. S., Grill S. M., Ni D., et al. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. Journal of Immunology. 2003;171(7):3542–3549. doi: 10.4049/jimmunol.171.7.3542. [DOI] [PubMed] [Google Scholar]
- 62.Grogan J. L., Mohrs M., Harmon B., Lacy D. A., Sedat J. W., Locksley R. M. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14(3):205–215. doi: 10.1016/S1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 63.Lee G. R., Kim S. T., Spilianakis C. G., Fields P. E., Flavell R. A. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 2006;24(4):369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Spilianakis C. G., Lalioti M. D., Town T., Lee G. R., Flavell R. A. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435(7042):637–645. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- 65.Murphy E., Shibuya K., Hosken N., et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. Journal of Experimental Medicine. 1996;183(3):901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huehn J., Polansky J. K., Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nature Reviews Immunology. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 67.Thornton A. M., Korty P. E., Tran D. Q., et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of Immunology. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottschalk R. A., Corse E., Allison J. P. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. The Journal of Immunology. 2012;188(3):976–980. doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 69.Akimova T., Beier U. H., Wang L., Levine M. H., Hancock W. W. Helios expression is a marker of T cell activation and proliferation. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0024226.e24226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart C. A., Metheny H., Iida N., et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. The Journal of Clinical Investigation. 2013;123(11):4859–4874. doi: 10.1172/jci65180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gnerlich J. L., Mitchem J. B., Weir J. S., et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. Journal of Immunology. 2010;185(7):4063–4071. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kono K., Kawaida H., Takahashi A., et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunology, Immunotherapy. 2006;55(9):1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mumm J. B., Emmerich J., Zhang X., et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20(6):781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Strauss L., Bergmann C., Gooding W., Johnson J. T., Whiteside T. L. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clinical Cancer Research. 2007;13(21):6301–6311. doi: 10.1158/1078-0432.ccr-07-1403. [DOI] [PubMed] [Google Scholar]
- 75.Bergmann C., Strauss L., Wang Y., et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clinical Cancer Research. 2008;14(12):3706–3715. doi: 10.1158/1078-0432.ccr-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okita R., Saeki T., Takashima S., Yamaguchi Y., Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncology Reports. 2005;14(5):1269–1273. [PubMed] [Google Scholar]
- 77.Chang S. H., Mirabolfathinejad S. G., Katta H., et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(15):5664–5669. doi: 10.1073/pnas.1319051111/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyara M., Yoshioka Y., Kitoh A., et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 79.Sun W., Li W.-J., Wu C.-Y., Zhong H., Wen W.-P. CD45RA−Foxp3high but not CD45RA+Foxp3low suppressive T regulatory cells increased in the peripheral circulation of patients with head and neck squamous cell carcinoma and correlated with tumor progression. Journal of Experimental & Clinical Cancer Research. 2014;33(1, article 35):10. doi: 10.1186/1756-9966-33-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janson P. C. J., Winerdal M. E., Winqvist O. At the crossroads of T helper lineage commitment-epigenetics points the way. Biochimica et Biophysica Acta: General Subjects. 2009;1790(9):906–919. doi: 10.1016/j.bbagen.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Bending D., De La Peña H., Veldhoen M., et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. Journal of Clinical Investigation. 2009;119(3):565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reinert-Hartwall L., Honkanen J., Salo H. M., et al. Th1/Th17 plasticity is a marker of advanced B cell autoimmunity and impaired glucose tolerance in humans. The Journal of Immunology. 2014;194(1):68–75. doi: 10.4049/jimmunol.1401653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nistala K., Adams S., Cambrook H., et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Globig A., Hennecke N., Martin B., et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflammatory Bowel Diseases. 2014;20(12):2321–2329. doi: 10.1097/mib.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 85.Bauquet A. T., Jin H., Paterson A. M., et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH -17 cells. Nature Immunology. 2009;10(2):167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma C. S., Suryani S., Avery D. T., et al. Early commitment of naïve human CD4+ T cells to the T follicular helper (TFH) cell lineage is induced by IL-12. Immunology and Cell Biology. 2009;87(8):590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 87.Soroosh P., Doherty T. A. Th9 and allergic disease. Immunology. 2009;127(4):450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lawless V. A., Zhang S., Ozes O. N., et al. Stat4 regulates multiple components of IFN-γ-inducing signaling pathways. Journal of Immunology. 2000;165(12):6803–6808. doi: 10.4049/jimmunol.165.12.6803. [DOI] [PubMed] [Google Scholar]
- 89.Ria F., Penna G., Adorini L. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. European Journal of Immunology. 1998;28(6):2003–2016. doi: 10.1002/(SICI)1521-4141(199806)28:06<2003::AID-IMMU2003>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 90.Ouyang W., Löhning M., Gao Z., et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12(1):27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 91.Kaplan M. H., Hufford M. M., Olson M. R. The development and in vivo function of T helper 9 cells. Nature Reviews Immunology. 2015;15(5):295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harrington L. E., Hatton R. D., Mangan P. R., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 93.Lee C.-G., Sahoo A., Im S.-H. Epigenetic regulation of cytokine gene expression in T lymphocytes. Yonsei Medical Journal. 2009;50(3):322–330. doi: 10.3349/ymj.2009.50.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haxhinasto S., Mathis D., Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. The Journal of Experimental Medicine. 2008;205(3):565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomson A. W., Turnquist H. R., Raimondi G. Immunoregulatory functions of mTOR inhibition. Nature Reviews Immunology. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoki K., Li Y., Xu T., Guan K.-L. Rheb GTpase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes and Development. 2003;17(15):1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Battaglia M., Stabilini A., Roncarolo M.-G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 98.Liu G., Burns S., Huang G., et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nature Immunology. 2009;10(7):769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sauer S., Bruno L., Hertweck A., et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(22):7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delgoffe G. M., Pollizzi K. N., Waickman A. T., et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature Immunology. 2011;12(4):295–304. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Düvel K., Yecies J. L., Menon S., et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Michalek R. D., Gerriets V. A., Jacobs S. R., et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of Immunology. 2011;186(6):3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hahn-Windgassen A., Nogueira V., Chen C.-C., Skeen J. E., Sonenberg N., Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. The Journal of Biological Chemistry. 2005;280(37):32081–32089. doi: 10.1074/jbc.m502876200. [DOI] [PubMed] [Google Scholar]
- 104.Koyanagi M., Asahara S.-I., Matsuda T., et al. Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023238.e23238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramanathan A., Schreiber S. L. Direct control of mitochondrial function by mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22229–22232. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schieke S. M., Phillips D., McCoy J. P., Jr., et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. The Journal of Biological Chemistry. 2006;281(37):27643–27652. doi: 10.1074/jbc.m603536200. [DOI] [PubMed] [Google Scholar]
- 107.Koren I., Reem E., Kimchi A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Current Biology. 2010;20(12):1093–1098. doi: 10.1016/j.cub.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 108.Kim J., Kundu M., Viollet B., Guan K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flinn R. J., Yan Y., Goswami S., Parker P. J., Backer J. M. The late endosome is essential for mTORC1 signaling. Molecular Biology of the Cell. 2010;21(5):833–841. doi: 10.1091/mbc.e09-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Powell J. D., Heikamp E. B., Pollizzi K. N., Waickman A. T. A modified model of T-cell differentiation based on mTOR activity and metabolism. Cold Spring Harbor Symposia on Quantitative Biology. 2013;78:125–130. doi: 10.1101/sqb.2013.78.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pollizzi K. N., Waickman A. T., Patel C. H., Sun I. H., Powell J. D., Kim C. H. Cellular size as a means of tracking mTOR activity and cell fate of CD4+ T cells upon antigen recognition. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0121710.e0121710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Annals of the New York Academy of Sciences. 2015 doi: 10.1111/nyas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kato H., Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+CD4−CD8− double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. Journal of Immunology. 2014;192(9):4134–4144. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai Z.-W., Hanczko R., Bonilla E., et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis & Rheumatism. 2012;64(9):2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pua H. H., Guo J., Komatsu M., He Y.-W. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. The Journal of Immunology. 2009;182(7):4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 116.Gergely P., Jr., Grossman C., Niland B., et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 2002;46(1):175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernandez D., Bonilla E., Mirza N., Niland B., Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2006;54(9):2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Motzer R. J., Escudier B., Oudard S., et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. The Lancet. 2008;372(9637):449–456. doi: 10.1016/s0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 119.Rao R. D., Buckner J. C., Sarkaria J. N. Mammalian target of rapamycin (mTOR) inhibitors as anti-cancer agents. Current Cancer Drug Targets. 2004;4(8):621–635. doi: 10.2174/1568009043332718. [DOI] [PubMed] [Google Scholar]
- 120.Thauland T. J., Koguchi Y., Wetzel S. A., Dustin M. L., Parker D. C. Th1 and Th2 cells form morphologically distinct immunological synapses. Journal of Immunology. 2008;181(1):393–399. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Simplified diagram of CD4+ T cell lineage commitment. Numbers over arrows correspond to types of experiments described in Table 2, which are used to identify the effector molecule following the arrow.
Supplemental table 1: Experiments used to study CD4+ T cell subset specification.