Abstract
Exosomes, microvesicles, and other extracellular vesicles are released by many cell types, including cancer cells and cancer-related immune cells. Extracellular vesicles can directly or indirectly facilitate the transfer of bioinformation to recipient cells or to the extracellular environment. In cancer, exosomes have been implicated in tumor initiation, proliferation, and metastasis. Extracellular vesicles can transmit proteins and nucleic acids that participate in DNA methylation, histone modification, and posttranscriptional regulation of RNA. Factors transmitted by extracellular vesicles reflect the donor cell status, and extracellular vesicles derived from tumor cells may be also responsible for altering expression of tumor promoting and tumor suppressing genes in recipient cells. Thus, circulating extracellular vesicles may act as biomarkers of cancer, and detection of these biomarkers may be applied to diagnosis or assessment of prognosis in patients with cancer.
1. Introduction
Extracellular vesicles include a variety of nanoscale membranous vesicles [1] released by many cell types into the intercellular microenvironment [2, 3]. Subtle changes in the cellular microenvironment may stimulate malignant transformation of cells, and cellular microenvironment has been implicated in tumor initiation, proliferation, and metastasis. Transformed cancer cells may disseminate bioinformation in autocrine and paracrine manners, to help the cancer proliferate and metastasize. This cell-cell or cell-microenvironment communication may be achieved by direct contact or over longer distances by secreted molecules and secreted membranous vesicles. A growing body of evidence suggests that cancer cells release more extracellular vesicles than healthy cells [4–6], partly due to activation of certain oncogenes, including ras [7].
Whether individual extracellular vesicles participate in normal physiological regulation or promotion of pathological processes is dependent on what they contain [8–10]. Exosomes, microvesicles and other extracellular vesicles differ in properties such as size, morphology, buoyant density, and protein composition [11]. Exosomes range in size from 40 to 1000 nm, and microvesicles are >1000 nm. Microvesicles bud directly from the plasma membrane, whereas exosomes are derived from endosomes and are released from cells by fusion of the multivesicular endosome with the plasma membrane [2]. Due to their endosome origin, exosomes contain endosome-associated proteins. Exosomes, microvesicles, and other extracellular vesicles can contain proteins, RNA, DNA, and lipids [12], and thus can deliver these factors to the intercellular environment or recipient cells. Extracellular vesicles are transported in the blood, urine, ascites, and cerebrospinal fluid [13–16] and thus may deliver their contents to either neighboring or distant recipient cells and produce corresponding physiological or pathologic effects. For example, melanoma-derived exosomes can deliver the receptor tyrosine kinase MET oncoprotein to bone marrow progenitor cells, which directs their development toward a prometastatic phenotype [17].
The content of extracellular vesicles, therefore, may be clinically relevant to disease progression, and detection of extracellular vesicles may be useful in diagnosis of cancer and assessment of prognosis. Circulating microvesicles and exosomes have been detected in the blood samples of patients with glioblastoma [18], colorectal cancer [19], and ovarian cancer [20]. These microvesicles and exosomes contain bioinformation reflecting primary tumor mutations and can act as early indicators of drug efficacy.
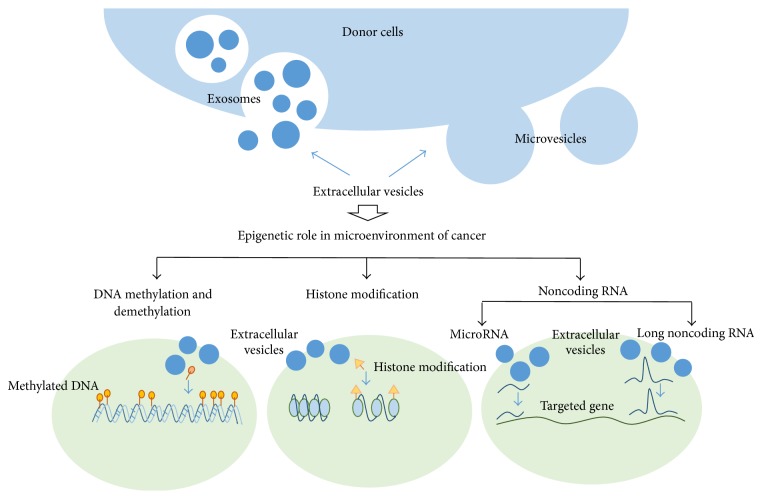
Epigenetic regulation involves processes causing functionally relevant changes to the genome which do not alter the nucleotide sequence but do alter gene expression. The genome plays a significant role in the tumor microenvironment, and the microenvironment influences cancer initiation, proliferation, and metastasis [21]. Important mechanisms of epigenetic regulation such as DNA methylation, histone modification and microRNA (miRNA), or long noncoding RNA (lncRNA) regulation are hot topics in cancer research. Recent research has implicated extracellular vesicles in epigenetic regulation of cancer progression [22, 23]. Gene ontology (GO) analysis has indicated that many mRNAs and proteins contained in extracellular vesicles are involved in epigenetic regulation [24], and exosome-mediated transfer of miRNAs is considered to be an important mechanism of genetic exchange between cells [25]. Thus extracellular vesicles regulate epigenetic processes including DNA methylation, histone modification, and miRNA or lncRNA regulation, and the resultant epigenetic modifications are responsible for changes in the expression of tumor promoting genes and tumor suppressing genes. Recent research indicated that the detection of epigenetic biomarkers, such as miRNAs, in extracellular vesicles could be exploited for diagnosis of cancer or assessment of cancer prognosis [26]. In this review we discuss the role of extracellular vesicles in transmission of factors responsible for three forms of epigenetic regulation: DNA, histone, and noncoding RNA modification.
2. The Role of Extracellular Vesicles in DNA Methylation and Demethylation in the Cancer Microenvironment
Epigenetic DNA modification of oncogenes or antioncogenes is crucially important for the initiation, proliferation, and metastasis of many tumors [27–29]. Dynamic variation in DNA methylation is one of the most universal factors influencing transcription of oncogenes and antioncogenes. DNA methyltransferase 1 (DNMT1), DNA methyltransferase 3a (DNMT3a), and DNA methyltransferase 3b (DNMT3b) add methyl groups to specific cytosines in the CpG islands of regulatory sequences, thus silencing certain genes [30]. The level of oncogene or antioncogene transcription fluctuates according to promoter methylation status, thus dynamically affecting tumor progression. Enzymes that demethylate DNA, such as activation-induced cytidine deaminase (AICDA) [31] and thymine DNA glycosylase (TDG) [32], protect unmethylated regions of mammalian genomes from de novo methylation.
Due to their complex bioactive cargo, extracellular vesicles can cause malignant transformation of normal cells. The protein, DNA, or RNA contained in extracellular vesicles could induce epigenetic changes in recipient cells by affecting the methylation status of their genome. Microvesicles are extracellular vesicles of 100–1000 nm in diameter [2, 33] which bud from the plasma membrane of tumor cells, while exosomes are 30–100 nm in diameter and are derived from the endosome [2, 34]. Microvesicles released from leukemia cells have been demonstrated to increase global DNA methylation levels in recipient cells. Hypermethylation of the promoter regions of tumor-suppressor genes P53 and RIZ1 was observed in cells incubated with leukemia-derived microvesicles and attributed to the increased level of DNMT3a and DNMT3b mRNA and protein [34]. Interestingly, protein and mRNA levels of activation-induced cytidine deaminase (AICDA), a deaminase involved in DNA initiative demethylation, was also increased in recipient cells. These results indicate that genomic instability was promoted in recipient cells, which might further induce leukemic transformation [34]. When microvesicles were treated with RNase, the level of DNMT3a, DNMT3b, and AICDA decreased, indicating that leukemia-derived microvesicles influence the methylation status of recipient cells via transmission of microvesicular RNA. Breakpoint cluster region-Abelson leukemia gene human homolog 1 (BCR-ABL1) has been reported to be the dominant onco-mRNA in microvesicles released by the K562 leukemia cell line [34]. Extracellular vesicles derived from transformed donor cells may thus transmit enzymes involved in both methylation and demethylation to recipient cells, ultimately inducing alteration in expression of tumor-related genes and accelerating tumor initiation, proliferation, and metastasis.
In addition to inducing malignant transformation of recipient cells, extracellular vesicles may represent useful biomarkers of cancer [35]. Detection of extracellular vesicles, and the molecular markers they contain, in blood or other clinical specimens represents a potentially valuable noninvasive approach for the assessment of tumor initiation, proliferation, and metastasis. Exosomes obtained from the serum of patients with pancreatic cancer were reported to carry genomic double-stranded DNAs (dsDNAs), which contained mutated KRAS and p53 genes [36]. Exosome-associated molecular markers of gastric cancers have been detected in gastric washes and even highly acidic gastric juice [37–39]. Exosomes were purified from the gastric juice of gastric cancer patients, and the content of cancer-related methylated LINE1 and methylated SOX17 DNA was analyzed by bisulfite pyrosequencing [37]. The methylation status of LINE1 and SOX17 DNA in gastric juice-derived exosomes was found to accurately reflect the methylation status of nuclear DNA in the corresponding tumors, indicating a role in the noninvasive diagnosis of cancer.
3. The Role of Extracellular Vesicles in Histone Modification in the Cancer Microenvironment
Chromosomal DNA coils around structural histone proteins to form the basic chromatin structure, which is maintained or altered by histone modification. Relaxation of the chromatin structure will expose more transcriptional regions of chromosomal DNA, inducing gene expression [40, 41]. Histone modification and, frequently, concomitant methylation have been implicated in pathogenic expression of tumor-related genes, and thus chromatic remodeling represents another potentially detectable biomarker for cancer [42]. Posttranslational modification of specific residues in the N-terminal tails of core histones, including acetylation, methylation, ubiquitination, or phosphorylation influence the chromatin shape, and thus transcription of associated genes [43–45]. Methylation of H3K4, H3K48, and H3K79 is commonly associated with gene activation, whereas methylation of H3K9 and H3K27 is associated with gene inactivation [46, 47]. Histone acetylation is regulated by histone acetyltransferase (HAT) and histone deacetylase (HDAC). The latter removes the positive charge on histones, thereby relaxing the condensed chromatin structure to promote gene transcription [48, 49]. The cancer microenvironment may thus influence epigenetic regulation of cancer-related gene expression by DNA modification and histone modification.
The role of extracellular vesicles in histone modification is currently controversial. Bioinformatic analysis has indicated a striking overlap between genes relevant to transgenerational epigenetic inheritance and the contents of exosomes released by a variety of cells, including cancer cells [24, 50–52]. By examining the GO biological processes associated with these overlapping mRNAs and proteins, it appears that the exosome content does not affect cellular activities indiscriminately but is focused on a limited network of processes including several processes related to epigenetic modification. Genes relevant to histone acetylation or deacetylation, histone ubiquitination, and other histone modifications, and even chromatin remodeling, represent a remarkably large proportion of those genes determined to be relevant to transgenerational epigenetic inheritance [24]. These findings indicate that exosomal mRNAs and proteins may directly or indirectly participate in both the response to environmental exposure and epigenetic modification, particularly histone modification. Additional analyses of exosomal miRNAs have indicated a similar association between environmental exposure and histone modification [50].
In the cancer microenvironment, these extracellular vesicle-related processes may participate in cancer initiation, proliferation, and metastasis. One cancer cell line, the G26/24 oligodendroglioma cell line, was reported to release extracellular vesicles containing the differentiation-specific linker histone H1°, which is not released by normal astrocytes [53]. The H1 histone family is the most divergent histone family and each H1 protein subtype or variant is associated with specific functions and distributions [54–57]. H1° is mostly associated with terminal differentiation [58, 59]. Although the precise pathophysiological significance of this phenomenon remains to be understood, enrichment of histone H1° in cancer cell-derived extracellular vesicles represents a promising potential molecular marker for oligodendroglioma diagnosis. Furthermore, extracellular vesicles containing histone H1° may subsequently influence recipient cells in the cancer microenvironment.
4. The Role of Extracellular Vesicles in Transmitting Noncoding RNA in the Cancer Microenvironment
Noncoding RNA refers to nonprotein coding transcripts, categorized as lncRNAs and small noncoding RNAs, including miRNAs, according to their length. Circulating tumor-associated miRNAs were first detected in the serum of a patient with diffuse large B-cell lymphoma [60], and the potential for circulating noncoding RNAs to act as noninvasive biomarkers of cancer diagnosis spurred accelerated research in this field. Circulating miRNAs were found to be present in plasma in a stable form that was protected from endogenous RNase activity [61], and recently noncoding RNAs were determined to be packaged in extracellular vesicles secreted by tumor cells, explaining why circulating RNAs are stable and thus detectable in the serum [62]. The capacity of extracellular vesicles containing noncoding RNAs to facilitate cell-to-cell communication and alter the cancer microenvironment remains to be seen; however, the potential for noncoding RNAs in extracellular vesicles to reflect the status of cancer cells or cancer-related immune cells is clinically promising. The lncRNAs and miRNAs contained in extracellular vesicles represent promising molecular markers of cancer diagnosis and prognosis assessment.
4.1. The Role of Extracellular Vesicles in Transmitting MicroRNAs in the Cancer Microenvironment
MiRNAs are small noncoding RNA molecules of about 22–25 nucleotides which act to silence RNA through posttranscriptional epigenetic regulation [63, 64]. Probably owing to their relatively small size, miRNAs are the most abundant RNA species in exosomes, making up over 42.32% of all raw reads and 76.20% of all mappable reads in 14 size-selected sequencing libraries [65]. The loading of miRNAs into exosomes may be controlled by specific proteins involved in the miRNA network. GW182 is a protein marker of P-bodies which can bind the Argonaute2 (AGO2) protein. The presence of AGO2 protein and striking enrichment of GW182 in purified monocyte-derived exosome-like vesicles suggests the specific and selective loading of miRNAs into exosomes [66–69]. Furthermore the ceramide-dependent machinery has been reported to regulate release of miRNAs [69].
Metastatic cancer cells shed particular types of miRNAs in extracellular vesicles. Microvesicles released from metastatic melanoma cells contain high levels of prominin-1, which promotes metastatic progression [70–72]. Micro-RNA profiling revealed 49 species of miRNA present at higher concentrations in these metastatic-melanoma derived microvesicles than in donor cells, including 20 species of cancer-related miRNAs. The invasiveness of bone marrow-derived stromal cells was found to be increased following exposure to prominin-1 expressing exosomes [73]. In metastatic gastric cancer, the let-7 miRNA family is selectively secreted into the extracellular environment via exosomes [74], inducing a prometastatic phenotype in selected host tissues. The exosomes released by the metastatic rat adenocarcinoma BSp73ASML contain higher levels of miR-494 and miR-542-3p than the exosomes of poorly metastatic BSp73ASML CD44v4-v7 knockdown cells [75]. The mRNA and miRNA content of extracellular vesicles derived from cancer stem cells also differ from those derived from differentiated cancer cells. miR-29a, miR-650, and miR-151 are associated with tumor invasion and metastases, and miR-19b, miR-29c, and miR-151 are upregulated in renal carcinomas and stimulate formation of a lung premetastatic niche [76]. Exosomes derived from chronic myelogenous leukemia cells were shuttled into endothelial cells, causing modulation of their motility and adhesion. This process was associated with exosome content of miR-126, which was concentrated in chronic myelogenous leukemia cell exosomes [77]. Brain metastatic cancer cells released miRNA-181c-containing extracellular vesicles which disrupt the blood-brain barrier [78]. Exosome-mediated transfer of cancer-secreted miR-105 disrupts tight junctions to promote metastasis [79]. Exosomal transfer of miRNA-23b from the bone marrow promotes breast cancer cell dormancy in a metastatic niche [80]. miR-210, released by metastatic cancer cells, could be transported to endothelial cells and regulate cancer cell metastasis [81]. MiRNAs in extracellular vesicles also modulate tumor proliferation [82, 83], and several extracellular vesicle miRNAs have been recommended for cancer diagnosis or assessment of cancer prognosis [10, 26, 37].
4.2. The Role of Extracellular Vesicles in Transmitting Long Noncoding RNAs in the Cancer Microenvironment
LncRNAs are nonprotein coding transcripts longer than 200 nucleotides [84] which can participate in epigenetic transcriptional or posttranscriptional regulation. Extracellular vesicle lncRNAs have received less attention than miRNAs, but in the previously described sequence analysis of 14 size-selected sequencing libraries, lncRNAs were found to be the most abundant exosomal RNA species after miRNAs and ribosomal RNA, representing 3.36% of all mappable, countable RNAs [65].
As previously described for miRNAs, the lncRNA content of exosomes differs from that of donor cells, indicating selective secretion of lncRNAs [85]. In two cancer cell lines, HeLa and MCF-7, the difference between the exosome and donor cell content of six lncRNAs (MALAT1, HOTAIR, lincRNA-p21, GAS5, TUG1, and CCND1-ncRNA) was assessed in donor cells under DNA damage stress [86]. Whilst MALAT1 is prevalent in donor cells, the MALAT1 level in exosomes turns out to be relatively low; in contrast lincRNA-p21 is enormously enriched in exosomes. Under cellular stress, the cellular content and selective loading of lncRNAs to extracellular vesicles was altered, indicating the packaging of lncRNAs in extracellular vesicles changes in response to the cancer microenvironment, potentially facilitating adaptation or opposition to the stress environment.
Extracellular vesicle content of lncRNA may reflect tumor growth, metastasis, and response to treatment. LncRNA TUC339, found in extracellular vesicles derived from hepatocellular carcinoma cells (HCC), has been implicated in tumor growth, adhesion and cell cycle progression [87, 88]. Linc-ROR, another lncRNA enriched in extracellular vesicles from HCC, protects cancer cells from chemotherapy-induced apoptosis and cytotoxicity [89], and MALAT1, an lncRNA enriched in extracellular vesicles from cervical carcinoma and breast cancer cells [86], was associated with tumor metastasis and invasion [90, 91]. The potential for MALAT1 to act as a blood-based biomarker for the diagnosis of nonsmall cell lung cancer is currently being evaluated [92].
5. Conclusions
Extracellular vesicles originating from cancer cells contain several types of biomolecules including oncogenes and molecules capable of epigenetic reprogramming. They are shed to the cancer microenvironment and may promote cancer progression [93, 94]. Epigenetic regulation appears to play a major role in this process (Figure 1). Many of the mRNAs and proteins present in extracellular vesicles are ascribed GO biological processes related to epigenetic regulation [24]. Recipient cell methyltransferase and cytidine deaminase can be downregulated by onco-mRNA in microvesicles derived from leukemia cells [34]. LncRNA TUC339, found in extracellular vesicles from hepatocellular carcinoma cells, participates in tumor growth, adhesion, and cell cycle progression [87, 88]. Furthermore, detection of the biomarkers present in extracellular vesicles represents a promising, noninvasive method of cancer diagnosis. For instance, methylated LINE1 and methylated SOX17 DNA accumulated in gastric juice-derived exosomes [37], and lincRNA-p21 was detected in extracellular vesicles from cervical carcinoma cells and breast cancer cells [86]. In addition, due to their capacity to mediate intercellular communication, extracellular vesicles may represent targets for therapeutic intervention, and both native extracellular vesicles and artificially engineered vesicles represent promising new tools for drug delivery [22, 23].
Figure 1.
Various extracellular vesicles derived from donor cells play various epigenetic roles in microenvironment of cancer, including DNA modification, histone modification, and noncoding RNA regulations.
Acknowledgments
This work was supported by grants from Health Bureau of Nanjing (no. QRX11009), Nanjing science and technology project (no. 201201059), the Shanghai Government (no. 0952nm03900), Shanghai Science and Technology Committee (no. 13XD1402600), and Shanghai Jiao Tong University School of Medicine (no. BXJ201024).
Conflict of Interests
The authors declare no conflict of interests.
Authors' Contribution
Zhongrun Qian and Qi Shen contributed equally to this paper.
References
- 1.van der Vlist E. J., Nolte-'t Hoen E. N. M., Stoorvogel W., Arkesteijn G. J. A., Wauben M. H. M. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nature Protocols. 2012;7(7):1311–1326. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 2.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of Cell Biology. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Andrade A. V. G., Bertolino G., Riewaldt J., et al. Extracellular vesicles secreted by bone marrow- and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells and Development. 2015;24(11):1374–1376. doi: 10.1089/scd.2014.0563. [DOI] [PubMed] [Google Scholar]
- 4.Mytar B., Baj-Krzyworzeka M., Majka M., Stankiewicz D., Zembala M. Human monocytes both enhance and inhibit the growth of human pancreatic cancer in SCID mice. Anticancer Research. 2008;28(1):187–192. [PubMed] [Google Scholar]
- 5.Ratajczak J., Miekus K., Kucia M., et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 6.Kim H. K., Song K. S., Park Y. S., et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. European Journal of Cancer. 2003;39(2):184–191. doi: 10.1016/s0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 7.Rechavi O., Goldstein I., Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Letters. 2009;583(11):1792–1799. doi: 10.1016/j.febslet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 8.D'Agostino S., Salamone M., Di Liegro I., Vittorelli M. L. Membrane vesicles shed by oligodendroglioma cells induce neuronal apoptosis. International Journal of Oncology. 2006;29(5):1075–1085. [PubMed] [Google Scholar]
- 9.Deregibus M. C., Cantaluppi V., Calogero R., et al. Endothelial progenitor cell—derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 10.Skog J., Würdinger T., van Rijn S., et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biology. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobrie A., Colombo M., Raposo G., Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Mathivanan S., Ji H., Simpson R. J. Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Graves L. E., Ariztia E. V., Navari J. R., Matzel H. J., Stack M. S., Fishman D. A. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Research. 2004;64(19):7045–7049. doi: 10.1158/0008-5472.can-04-1800. [DOI] [PubMed] [Google Scholar]
- 14.Piccin A., Murphy W. G., Smith O. P. Circulating microparticles: pathophysiology and clinical implications. Blood Reviews. 2007;21(3):157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Smalley D. M., Sheman N. E., Nelson K., Theodorescu D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. Journal of Proteome Research. 2008;7(5):2088–2096. doi: 10.1021/pr700775x. [DOI] [PubMed] [Google Scholar]
- 16.Taylor D. D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncology. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Peinado H., Alečković M., Lavotshkin S., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Medicine. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao H., Chung J., Balaj L., et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nature Medicine. 2012;18(12):1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshioka Y., Kosaka N., Konishi Y., et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nature Communications. 2014;5, article 3591 doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im H., Shao H., Park Y. I., et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature Biotechnology. 2014;32(5):490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y., El Andaloussi S., Wood M. J. A. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics. 2012;21(1):R125–R134. doi: 10.1093/hmg/dds317.dds317 [DOI] [PubMed] [Google Scholar]
- 23.El Andaloussi S., Mäger I., Breakefield X. O., Wood M. J. A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A. Bioinformatic analysis revealing association of exosomal mRNAs and proteins in epigenetic inheritance. Journal of Theoretical Biology. 2014;357:143–149. doi: 10.1016/j.jtbi.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 26.Rabinowits G., Gerçel-Taylor C., Day J. M., Taylor D. D., Kloecker G. H. Exosomal microRNA: a diagnostic marker for lung cancer. Clinical Lung Cancer. 2009;10(1):42–46. doi: 10.3816/clc.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 27.Chik F., Szyf M., Rabbani S. A. Role of epigenetics in cancer initiation and progression. Advances in Experimental Medicine and Biology. 2011;720:91–104. doi: 10.1007/978-1-4614-0254-1_8. [DOI] [PubMed] [Google Scholar]
- 28.Fouse S. D., Costello J. F. Epigenetics of neurological cancers. Future Oncology. 2009;5(10):1615–1629. doi: 10.2217/fon.09.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalchuk O., Tryndyak V. P., Montgomery B., et al. Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications and aberrant microRNA expression. Cell Cycle. 2007;6(16):2010–2018. doi: 10.4161/cc.6.16.4549. [DOI] [PubMed] [Google Scholar]
- 30.Robertson K. D., Uzvolgyi E., Liang G., et al. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Research. 1999;27(11):2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popp C., Dean W., Feng S., et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463(7284):1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L., Wu H., Diep D., et al. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153(3):692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratajczak J., Wysoczynski M., Hayek F., Janowska-Wieczorek A., Ratajczak M. Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X., You Y., Li Q., et al. BCR-ABL1-positive microvesicles transform normal hematopoietic transplants through genomic instability: Implications for donor cell leukemia. Leukemia. 2014;28(8):1666–1675. doi: 10.1038/leu.2014.51. [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka Y., Konishi Y., Kosaka N., Katsuda T., Kato T., Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. Journal of Extracellular Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahlert C., Melo S. A., Protopopov A., et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. The Journal of Biological Chemistry. 2014;289(7):3869–3875. doi: 10.1074/jbc.c113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida Y., Yamamoto H., Morita R., et al. Detection of DNA methylation of gastric juice-derived exosomes in gastric cancer. Integrative Molecular Medicine. 2014 doi: 10.15761/IMM.1000105. [DOI] [Google Scholar]
- 38.Watanabe Y., Kim H. S., Castoro R. J., et al. Sensitive and specific detection of early gastric cancer with DNA methylation analysis of gastric washes. Gastroenterology. 2009;136(7):2149–2158. doi: 10.1053/j.gastro.2009.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oishi Y., Watanabe Y., Yoshida Y., et al. Hypermethylation of Sox17 gene is useful as a molecular diagnostic application in early gastric cancer. Tumor Biology. 2012;33(2):383–393. doi: 10.1007/s13277-011-0278-y. [DOI] [PubMed] [Google Scholar]
- 40.Vigetti D., Viola M., Karousou E., et al. Epigenetics in extracellular matrix remodeling and hyaluronan metabolism. FEBS Journal. 2014;281(22):4980–4992. doi: 10.1111/febs.12938. [DOI] [PubMed] [Google Scholar]
- 41.Kass S. U., Wolffe A. P. DNA methylation, nucleosomes and the inheritance of chromatin structure and function. Novartis Foundation Symposium. 1998;214:22–50. doi: 10.1002/9780470515501.ch3. [DOI] [PubMed] [Google Scholar]
- 42.Verma M., Srivastava S. Epigenetics in cancer: implications for early detection and prevention. The Lancet Oncology. 2002;3(12):755–763. doi: 10.1016/s1470-2045(02)00932-4. [DOI] [PubMed] [Google Scholar]
- 43.Munshi A., Shafi G., Aliya N., Jyothy A. Histone modifications dictate specific biological readouts. Journal of Genetics and Genomics. 2009;36(2):75–88. doi: 10.1016/S1673-8527(08)60094-6. [DOI] [PubMed] [Google Scholar]
- 44.Lee K. K., Workman J. L. Histone acetyltransferase complexes: one size doesn't fit all. Nature Reviews Molecular Cell Biology. 2007;8(4):284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 45.Li B., Carey M., Workman J. L. The role of chromatin during transcription. Cell. 2007;128(4):707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Hou H., Yu H. Structural insights into histone lysine demethylation. Current Opinion in Structural Biology. 2010;20(6):739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallinari P., Di Marco S., Jones P., Pallaoro M., Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Research. 2007;17(3):195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 48.Kuo M.-H., Allis C. D. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays. 1998;20(8):615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 49.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A. Novel transcriptome data analysis implicates circulating microRNAs in epigenetic inheritance in mammals. Gene. 2014;538(2):366–372. doi: 10.1016/j.gene.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 51.Mathivanan S., Fahner C. J., Reid G. E., Simpson R. J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Research. 2012;40(1):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson R. J., Kalra H., Mathivanan S. ExoCarta as a resource for exosomal research. Journal of Extracellular Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18374.18374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiera G., Di Liegro C. M., Saladino P., et al. Oligodendroglioma cells synthesize the differentiation-specific linker histone H1° and release it into the extracellular environment through shed vesicles. International Journal of Oncology. 2013;43(6):1771–1776. doi: 10.3892/ijo.2013.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Izzo A., Kamieniarz K., Schneider R. The histone H1 family: specific members, specific functions? Biological Chemistry. 2008;389(4):333–343. doi: 10.1515/bc.2008.037. [DOI] [PubMed] [Google Scholar]
- 55.Marzluff W. F., Gongidi P., Woods K. R., Jin J., Maltais L. J. The human and mouse replication-dependent histone genes. Genomics. 2002;80(5):487–498. doi: 10.1016/s0888-7543(02)96850-3. [DOI] [PubMed] [Google Scholar]
- 56.Happel N., Doenecke D. Histone H1 and its isoforms: contribution to chromatin structure and function. Gene. 2009;431(1-2):1–12. doi: 10.1016/j.gene.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Kowalski A., Pałyga J. Linker histone subtypes and their allelic variants. Cell Biology International. 2012;36(11):981–996. doi: 10.1042/cbi20120133. [DOI] [PubMed] [Google Scholar]
- 58.Zlatanova J., Doenecke D. Histone H1 zero: a major player in cell differentiation? The FASEB Journal. 1994;8(15):1260–1268. doi: 10.1096/fasebj.8.15.8001738. [DOI] [PubMed] [Google Scholar]
- 59.Gabrilovich D. I., Cheng P., Fan Y., et al. H1 histone and differentiation of dendritic cells. A molecular target for tumor-derived factors. Journal of Leukocyte Biology. 2002;72(2):285–296. [PubMed] [Google Scholar]
- 60.Lawrie C. H., Gal S., Dunlop H. M., et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. British Journal of Haematology. 2008;141(5):672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell P. S., Parkin R. K., Kroh E. M., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma R., Jiang T., Kang X. Circulating microRNAs in cancer: origin, function and application. Journal of Experimental and Clinical Cancer Research. 2012;31(1, article 38) doi: 10.1186/1756-9966-31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 64.Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 65.Huang X., Yuan T., Tschannen M., et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14(1, article 319) doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pegtel D. M., Cosmopoulos K., Thorley-Lawson D. A., et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gibbings D. J., Ciaudo C., Erhardt M., Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nature Cell Biology. 2009;11(9):1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 68.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. Journal of Biological Chemistry. 2010;285(23):17442–17452. doi: 10.1074/jbc.m110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zomer A., Vendrig T., Hopmans E. S., van Eijndhoven M., Middeldorp J. M., Pegtel D. M. Exosomes: fit to deliver small RNA. Communicative & Integrative Biology. 2014;3(5):447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rappa G., Mercapide J., Anzanello F., et al. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Experimental Cell Research. 2013;319(6):810–819. doi: 10.1016/j.yexcr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorico A., Mercapide J., Rappa G. Prominin-1 (CD133) and metastatic melanoma: current knowledge and therapeutic perspectives. Advances in Experimental Medicine and Biology. 2013;777:197–211. doi: 10.1007/978-1-4614-5894-4-13. [DOI] [PubMed] [Google Scholar]
- 72.Rappa G., Fodstad O., Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26(12):3008–3017. doi: 10.1634/stemcells.2008-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rappa G., Mercapide J., Anzanello F., Pope R. M., Lorico A. Biochemical and biological characterization of exosomes containing prominin-1/CD133. Molecular Cancer. 2013;12(1, article 62) doi: 10.1186/1476-4598-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohshima K., Inoue K., Fujiwara A., et al. Let-7 microRNA family Is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013247.e13247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rana S., Malinowska K., Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15(3):281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grange C., Tapparo M., Collino F., et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Research. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.can-11-0241. [DOI] [PubMed] [Google Scholar]
- 77.Taverna S., Amodeo V., Saieva L., et al. Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells. Molecular Cancer. 2014;13(1, article 169) doi: 10.1186/1476-4598-13-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tominaga N., Kosaka N., Ono M., et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nature Communications. 2015;6:p. 6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou W., Fong M. Y., Min Y., et al. Cancer-Secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ono M., Kosaka N., Tominaga N., et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling. 2014;7(332, article ra63) doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 81.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic micrornas regulate cancer cell metastasis. The Journal of Biological Chemistry. 2013;288(15):10849–10859. doi: 10.1074/jbc.m112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kogure T., Lin W.-L., Yan I. K., Braconi C., Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohno S.-I., Takanashi M., Sudo K., et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Molecular Therapy. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perkel J. M. Visiting ‘noncodarnia’. BioTechniques. 2013;54(6):301–304. doi: 10.2144/000114037. [DOI] [PubMed] [Google Scholar]
- 85.Grammatikakis I., Panda A. C., Abdelmohsen K., Gorospe M. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging. 2014;6(12):992–1009. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gezer U., Özgür E., Cetinkaya M., Isin M., Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biology International. 2014;38(9):1076–1079. doi: 10.1002/cbin.10301. [DOI] [PubMed] [Google Scholar]
- 87.Kogure T., Yan I. K., Lin W.-L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes & Cancer. 2013;4(7-8):261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braconi C., Valeri N., Kogure T., et al. Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):786–791. doi: 10.1073/pnas.1011098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi K., Yan I. K., Kogure T., Haga H., Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt L. H., Spieker T., Koschmieder S., et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. Journal of Thoracic Oncology. 2011;6(12):1984–1992. doi: 10.1097/jto.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 91.Lin R., Maeda S., Liu C., Karin M., Edgington T. S. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26(6):851–858. doi: 10.1038/sj.onc.1209846. [DOI] [PubMed] [Google Scholar]
- 92.Weber D. G., Johnen G., Casjens S., et al. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Research Notes. 2013;6(1, article 518) doi: 10.1186/1756-0500-6-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raimondo S., Saieva L., Corrado C., et al. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Communication and Signaling. 2015;13(1, article 8) doi: 10.1186/s12964-015-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corrado C., Raimondo S., Chiesi A., Ciccia F., De Leo G., Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. International Journal of Molecular Sciences. 2013;14(3):5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]