Abstract
Background
With increasing utilization of endovascular techniques in the treatment of both ruptured and unruptured intracranial aneurysms, the issue of obliteration efficacy has become increasingly important.
Objective
Our goal was to systematically develop a comprehensive model for predicting retreatment with various types of endovascular treatment.
Methods
We retrospectively reviewed medical records that were prospectively collected for 305 patients who received endovascular treatment for intracranial aneurysms from 2007 to 2013. Multivariable logistic regression was performed on candidate predictors identified by univariable screening analysis to detect independent predictors of retreatment. A composite risk score was constructed based on the proportional contribution of independent predictors in the multivariable model.
Results
Size (>10 mm), aneurysm rupture, stent assistance, and post-treatment degree of aneurysm occlusion were independently associated with retreatment while intraluminal thrombosis and flow diversion demonstrated a trend towards retreatment. The Aneurysm Recanalization Stratification Scale was constructed by assigning the following weights to statistically and clinically significant predictors. Aneurysm-specific factors: Size (>10 mm), 2 points; rupture, 2 points; presence of thrombus, 2 points. Treatment-related factors: Stent assistance, -1 point; flow diversion, -2 points; Raymond Roy 2 occlusion, 1 point; Raymond Roy 3 occlusion, 2 points. This scale demonstrated good discrimination with a C-statistic of 0.799.
Conclusion
Surgical decision-making and patient-centered informed consent require comprehensive and accessible information on treatment efficacy. We have constructed the Aneurysm Recanalization Stratification Scale to enhance this decision-making process. This is the first comprehensive model that has been developed to quantitatively predict the risk of retreatment following endovascular therapy.
Keywords: Intracranial aneurysm, endovascular treatment, recanalization, retreatment
Introduction
With increasing utilization of endovascular techniques in the treatment of both ruptured and unruptured aneurysms, the issue of obliteration efficacy has become increasingly important. Rates of aneurysm recanalization vary over a wide range from 17% to 90%.1 Concern for aneurysm recurrence necessitates ongoing follow-up, with resulting uncertainty, anxiety, radiation exposure, and possible dissatisfaction for patients.2 Accordingly, the purpose of this study was to systematically develop a comprehensive model for predicting retreatment following various types of endovascular treatment. This is helpful in discussions with patients regarding choice of treatment as well as frequency and type of radiographic follow-up.
To be inclusive, we evaluated a variety of aneurysm-specific factors such as size,3,4,5,6 neck width,7 dome-to-neck ratio,7 terminal or sidewall lesion, presence of intraluminal thrombus,8 and whether the aneurysm is ruptured or unruptured1,9 which influence recanalization rates. In addition, we included treatment-related factors including initial incomplete occlusion,4,5,6,10 stent-assisted coiling, and utilization of flow diversion devices that have been demonstrated to impact on aneurysm recanalization rates. Utilizing these factors, we have developed the Aneurysm Recanalization Stratification Scale to predict retreatment following endovascular treatment (Table 1).
Table 1. Aneurysm recanalization stratification scale.
| Factors | Points assigned |
|---|---|
| Aneurysm-specific factors | |
|
| |
| Size | |
| >10 mm | +2 |
| ≤10 mm | 0 |
| Ruptured | +2 |
| Unruptured | 0 |
| Thrombus | +2 |
| No thrombus | 0 |
| Treatment-related factors | |
|
| |
| Coils only | 0 |
| Stent-assistance | -1 |
| Flow diversion | -2 |
| Initial treatment result | |
| Raymond Roy 1 | 0 |
| Raymond Roy 2 | +1 |
| Raymond Roy 3 | +2 |
Methods
Patient population
This study was conducted in accordance with institutional guidelines and with the approval of the Institutional Review Board. We retrospectively reviewed medical records and imaging studies that were prospectively collected for 305 patients who received endovascular treatment for intracranial aneurysms at our center from January 2007 to December 2013. Patients with less than 3 months of angiographic follow-up were excluded from the study. Patients were classified as smokers or nonsmokers on the basis of their smoking status at the time of primary endovascular therapy. Nonsmokers were defined as patients who had never smoked.
Aneurysm anatomy
Aneurysm anatomy was assessed by digital subtraction angiography (DSA), magnetic resonance angiography (MRA), and/or computed tomography angiography (CTA) using multiple projections to determine aneurysm size, neck width, dome-to-neck ratio, location, and presence of intraluminal thrombosis before treatment. Size was defined as the largest dimension of the aneurysm (dome, length, or height). Aneurysms were categorized as small (≤10 mm) or large (>10 mm). Neck width was categorized as narrow (≤4 mm) or wide (>4 mm). Dome-to-neck ratio was categorized as ≤1.5 or >1.5. Aneurysm location was recorded as end-on (bifurcation) or sidewall. Aneurysm rupture was defined as acute subarachnoid hemorrhage (SAH) including intraprocedural rupture. Dissecting and fusiform aneurysms were excluded from the study.
Immediate angiographic result
Immediate angiographic result post coiling and stent-assisted coiling was assessed by DSA according to the Raymond Roy Occlusion Classification (RROC).11 Embolization was considered to be complete (Raymond Roy class 1) if there was no contrast filling of the dome, body, or neck of the aneurysm. Neck remnant (Raymond Roy class 2) was defined as residual filling of the neck without opacification of the aneurysmal sac. Residual aneurysm (Raymond Roy class 3) was indicated by contrast agent in the dome of the aneurysm. Immediate angiographic result was not assessed for aneurysms treated by flow diversion as the classification of Roy et. al. does not adequately apply to flow diverter therapy.12 For the purpose of multivariable regression, aneurysms treated by flow diversion were uniformly categorized as Raymond Roy class 1. No penalty was ascribed for residual filling of the aneurysm as this is very common even after technically successful flow diversion.12
Data analysis
Statistical analysis was performed using R version 3.1.1 (http://www.r-project.org). Our primary outcome of interest was retreatment as this reflects clinically significant aneurysm recanalization. In univariable analysis, variables were compared between groups by the Pearson chi-squared test. Statistical significance was defined as P < 0.05. Multivariable logistic regression was performed on candidate predictor variables to identify independent predictors of outcome. A composite risk score was then formulated by assigning weights to predictors in proportion to their coefficients.
Results
Endovascular treatment was performed on 305 patients with 333 intracranial aneurysms. Mean age at time of treatment was 57 years (range, 19–86 years). 9 fusiform and dissecting aneurysms (2.7%) were excluded from the study. Follow-up angiography (≥3 months) was available for 268 of 324 saccular aneurysms (82.7%). Average follow-up time was 19 months. Retreatment was performed for 79 aneurysms (29.5%). 120 patients (44.8%) were smokers and 81 (30.2%) were nonsmokers. Information on smoking history was unavailable for 67 patients (25.0%).
Aneurysm-specific factors
198 (73.9%) of 268 aneurysms with follow-up angiography were small and 70 (26.1%) were large. Mean aneurysm size was 7.2 mm (standard deviation [SD], 4.51 mm; range, 1.8–40.0 mm). Dome-to-neck ratio was greater than 1.5 for 133 aneurysms (49.6%). Mean dome-to-neck ratio was 1.63 (SD, 0.61; range, 0.67–5.25). 84 (31.3%) aneurysms were wide-necked. Mean neck size was 3.85 mm (SD, 2.02 mm; range, 0.7–14.0 mm). There were 102 (38.1%) ruptured aneurysms. Anterior cerebral artery (ACA), anterior communicating artery (ACoA), basilar artery, internal carotid artery (ICA), and middle cerebral artery (MCA) bifurcation aneurysms were observed. 118 (44.0%) aneurysms were end-on (bifurcastion) and 150 (56.0%) were sidewall. Intraluminal thrombosis occurred in 3 aneurysms (1.1%).
Treatment-related factors
147 aneurysms (54.9%) were treated with coils only, 107 aneurysms (39.9%) received stent-assisted coiling, and 14 aneurysms (5.2%) received flow diverter placement. Complete obliteration was achieved for 126 aneurysms (47.0%). Neck and dome residuals were observed for 132 (49.3%) and 10 (3.7%) aneurysms respectively.
Univariable analysis
In univariable analysis (Table 2), size (>10 mm) (P < 0.005), aneurysm rupture (P < 0.0001), treatment with stent-assistance or flow diversion (P < 0.0001), and immediate angiographic result (P < 0.0005) were significantly associated with retreatment, whereas neck width (>4 mm) (P = 0.130) and intraluminal thrombosis (P = 0.155) demonstrated a trend toward retreatment. Dome-to-neck ratio (≤1.5) (P = 0.454) and sidewall versus end-on location of aneurysms (P = 0.550) were not significantly associated with retreatment. Size was correlated with neck width (Spearman's rho = 0.75). After excluding patients whose smoking status was unknown, smoking was not significantly associated with retreatment (P = 0.370). Based on statistical and clinical significance, size (>10 mm), aneurysm rupture, stent placement, flow diversion, immediate angiographic result, and intraluminal thrombosis were selected for multivariable analysis. Neck width was not included in primary analysis to avoid colinearity in multivariable regression.
Table 2. Aneurysm-specific and treatment-related factors.
| Characteristic | Total n = 268 | Retreatment n = 79 | No retreatment n = 189 | P value1 |
|---|---|---|---|---|
| Aneurysm-specific factors | ||||
|
| ||||
| Size | ||||
| >10 mm | 70 (26.1%) | 30 (38.0%) | 40 (21.2%) | <0.0005 |
| ≤10 mm | 198 (73.9%) | 49 (62.0%) | 149 (78.8%) | |
| Neck width | ||||
| >4 mm | 84 (31.3%) | 30 (38.0%) | 54 (28.6%) | 0.130 |
| ≤4 mm | 184 (68.7%) | 49 (62.0%) | 135 (71.4%) | |
| Dome-to-neck ratio | ||||
| >1.5 | 133 (49.6%) | 42 (53.2%) | 91 (48.1%) | 0.454 |
| ≤1.5 | 135 (50.4%) | 37 (46.8%) | 98 (51.9%) | |
| Ruptured | 102 (38.1%) | 51 (64.6%) | 51 (27.0%) | <0.0001 |
| Unruptured | 166 (61.9%) | 28 (35.4%) | 138 (73.0%) | |
| Location | ||||
| End-on | 118 (44.0%) | 37 (46.8%) | 81 (42.9%) | 0.550 |
| Sidewall | 150 (56.0%) | 42 (53.2%) | 108 (57.1%) | |
| Thrombus | 3 (1.1%) | 2 (2.5%) | 1 (0.5%) | 0.155 |
| No thrombus | 265 (98.9%) | 77 (97.5%) | 188 (99.5%) | |
| Treatment-related factors | ||||
|
| ||||
| Coils only | 147 (54.9%) | 61 (77.2%) | 86 (45.5%) | |
| Stent-assistance | 107 (39.9%) | 17 (21.5%) | 90 (47.6%) | <0.0001 |
| Flow diversion | 14 (5.2%) | 1 (1.3%) | 13 (6.9%) | |
| Initial treatment result | ||||
| Raymond Roy 1 | 126 (47.0%) | 22 (27.8%) | 104 (55.0%) | |
| Raymond Roy 2 | 132 (49.3%) | 52 (65.8%) | 80 (42.3%) | <0.0005 |
| Raymond Roy 3 | 10 (3.7%) | 5 (6.3%) | 5 (2.6%) | |
Variables were compared between retreatment and no retreatment groups by the Pearson chi-squared test.
Multivariable regression
Multivariable logistic regression identified size (>10 mm) (odds ratio [OR] = 4.90, P < 0.001), aneurysm rupture (OR, 4.41, P < 0.0005), stent assistance (OR = 0.426, P = 0.0481), and immediate angiographic result (neck remnant: OR = 2.66, P < 0.0005; residual aneurysm: OR = 4.99, P = 0.0335) as independent predictors of retreatment, whereas flow diversion (OR = 0.122, P = 0.0948) and intraluminal thrombosis (OR = 9.56, P = 0.135) displayed a trend toward retreatment (Table 3).
Table 3. Independent predictors of aneurysm retreatment with odds ratios and P values.
| Predictor | Multivariate coefficient | Standard error | Odds ratio | P value | Points assigned |
|---|---|---|---|---|---|
| Size (>10 mm) | 1.589 | 0.388 | 4.90 (2.29–10.5) | <0.0001 | 2 |
| Rupture | 1.485 | 0.383 | 4.41 (2.08–9.36) | <0.0005 | 2 |
| Thrombus | 2.258 | 1.510 | 9.56 (0.496–185) | 0.135 | 2 |
| Stent-assistance | -0.853 | 0.432 | 0.426 (0.188–0.994) | 0.0481 | -1 |
| Flow diversion | -2.106 | 1.261 | 0.122 (0.0103–1.44) | 0.0948 | -2 |
| Neck remnant (Raymond Roy 2) | 0.977 | 0.336 | 2.66 (1.37–5.16) | <0.005 | 1 |
| Residual aneurysm (Raymond Roy 3) | 1.608 | 0.756 | 4.99 (1.13–22.0) | 0.0335 | 2 |
Composite risk score
Based on the proportional contribution of each independent predictor in the multivariable regression model, we constructed the Aneurysm Recanalization Stratification Scale with a range from -2 to +8 to predict retreatment following endovascular treatment of intracranial aneurysms (Table 1). Median risk score was 1.5 and the mean was 1.37 ± 1.70. Risk scores in this series were distributed from -2 to 6 (Figure 1). Our risk score demonstrated high predictive value with a C-statistic of 0.799 (95% confidence interval [95% CI] = 0.744–0.854). This was significantly superior to prediction using only the RROC (C-statistic = 0.641; 95% CI = 0.578–0.704) (Figure 2).
Figure 1.
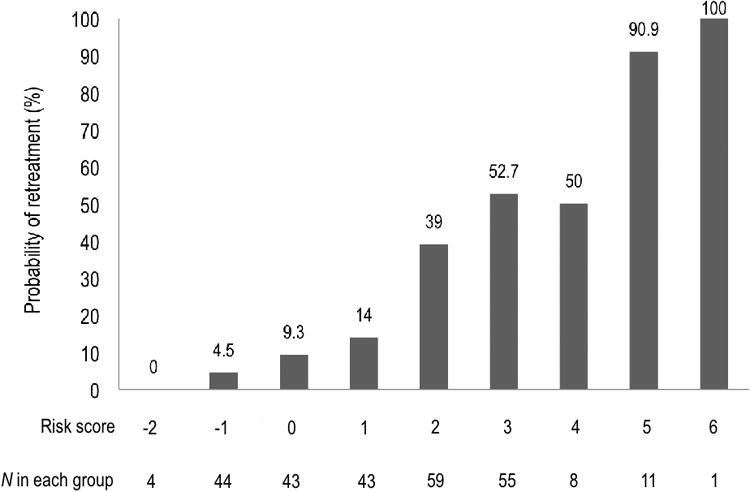
Probability of retreatment by composite risk score.
Figure 2.
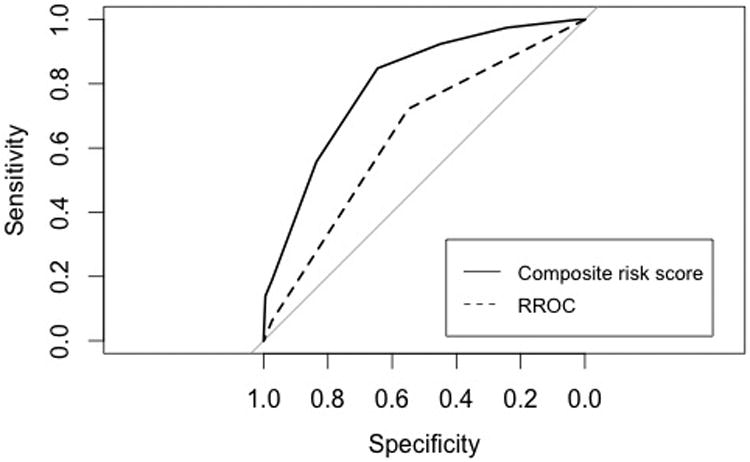
Receiver operating characteristic curves comparing performance of our composite risk score and the Raymond Roy Occlusion Classification in predicting clinically significant aneurysm recanalization.
Discussion
Surgical decision making and informed patient consent both benefit from having accurate and accessible information about treatment efficacy including the risk of retreatment following endovascular therapy for intracranial aneurysms. Endovascular therapists have traditionally relied on clinical experience augmented by epidemiological and clinical studies providing piecemeal and, at times, abstruse addendums to our understanding of aneurysmal recurrence. With increasing utilization of endovascular therapy for intracranial aneurysms, patients are in need of more comprehensive, streamlined, and accessible risk information. Accordingly, we have constructed the Aneurysm Recanalization Stratification Scale (Table 1) which allows neurosurgical providers to predict the likelihood of retreatment following endovascular therapy using accessible and widely recognized predictors as follows: -2, 0%; -1, 4.5%; 0, 9.3%; 1, 14%; 2, 39%; 3, 52.7%; 4, 50%; 5, 90.0%; 6, 100% (Figure 1). No aneurysms in the series were graded 7 or 8. This is the first comprehensive model that has been developed to quantitatively predict the risk of retreatment following endovascular therapy.
Aneurysm recurrence is only an indirect measure of the risk of subarachnoid hemorrhage. In view of increasing clinical evidence that aneurysms rarely hemorrhage following endovascular therapy,13,14,15 aneurysm recanalization defined as “any increase in size of the remnant”4 or “more than 10% increase in contrast filling of the aneurysm”3 is unlikely to be clinically meaningful for patient or provider. Findings from both single-center and multicenter studies support the notion that a proportion of aneurysmal recurrences are stable and are unlikely to enlarge and/or rebleed.4,15,16 In fact, it is possible that fewer than 50% of recurrences are of sufficient concern to warrant retreatment.4,16 All-cause retreatment as a primary endpoint is more relevant for patients as it captures the costs, anxiety, and unpleasantness associated with an additional procedure and is less likely to grossly inflate the risk of aneurysm rupture. We did not distinguish or exclude staged procedures as retreatment from any cause subjects patients to anxiety and expense. This partially accounts for the relatively high retreatment rate observed in our study. Moreover, staged treatments may be frequently performed by some centers based on the belief this will achieve better long-term occlusion outcomes.
Currently, the RROC is most commonly used to evaluate coiled aneurysms and is regarded as a significant predictor of aneurysm recurrence as it reflects packing density.4,5,6,10 In this study, aneurysm size (>10 mm), neck size (>4 mm), rupture, and stent-assistance were independently predictive of all-cause retreatment after controlling for immediate angiographic result (RROC). Flow diversion and intraluminal thrombosis trended towards statistical significance, possibly due to inadequate statistical power. The C-statistic is considered to be the most relevant measure of model success and refers to the ability of a model to discriminate cases (e.g. retreatment) from noncases. A cutoff value of 0.70 has been regarded as acceptable discrimination.17 In comparison to the RROC (C-statistic = 0.641), our composite risk score demonstrated significantly superior discrimination (C-statistic = 0.799) (Figure 2). This justifies the added complexity of a multifactorial approach.
Neck width (>4 mm) (P < 0.005, OR = 1.15) was independently associated with retreatment after controlling for aneurysm rupture, intraluminal thrombosis, stent-assistance, flow diversion, and immediate angiographic result, but was not included in our final multivariable model to avoid colinearity with aneurysm size. The latter was regarded as a superior predictor due to its larger effect size, ease of use, and consistent association with aneurysm recanalization. In contrast, neck width was not significantly associated with aneurysm recanalization in several reports.1,15 ICA and basilar bifurcation aneurysms tended to be large (P = 0.0577, 38.5% vs. 24.0%) and wide necked (P < 0.0001, 57.9% vs. 27.0%). However, sidewall versus end-on location of aneurysms was not significantly associated with retreatment in this series, possibly due to judicious selection of end-on aneurysms for endovascular treatment and increased utilization of stent-assistance (P = 0.0548, 53.8% vs. 37.6%) in an effort to reduce recanalization. Smoking was not significantly associated with retreatment in univariable analysis (P = 0.370). However, we observed a 5.7 percentage point difference in retreatment rate between smokers (29.2%) and nonsmokers (23.5%), which is suggestive of an effect from smoking. In Ortiz et. al. (2008),18 smoking was significantly associated with aneurysm recanalization. Information on smoking status was unavailable for 67 patients (25.0%) in our current series, which could partially account for the discrepancy in findings.
In Singla et. al. (2013), contrast stasis, defined as persistent contrast in the aneurysm neck or dome during the capillary or venous phase of the angiogram, was found to be a better predictor of long term aneurysm occlusion than the RROC.19 This was a single center retrospective study involving 110 patients. It has also been suggested that the RROC should be modified to distinguish between contrast within the coil interstices and contrast along the aneurysm wall.20 These findings have not, however, been validated in independent cohorts. There is presently insufficient evidence demonstrating superior predictive value of these systems, which are also more complex, to justify replacement of the RROC.
Limitations and applications of our analysis merit discussion. This is a retrospective study with a minimum required follow up time of at least 3 months. However, 250 cases (93.3%) had at least 6 months of radiographic follow up. Median time to retreatment in this series was 4 months. 52 retreatments (65.8%) were performed ≤6 months after primary treatment (Figure 3). This is consistent with previous reports.3,21 Follow up time is therefore unlikely to have significantly impacted on the internal validity of our findings.
Figure 3.
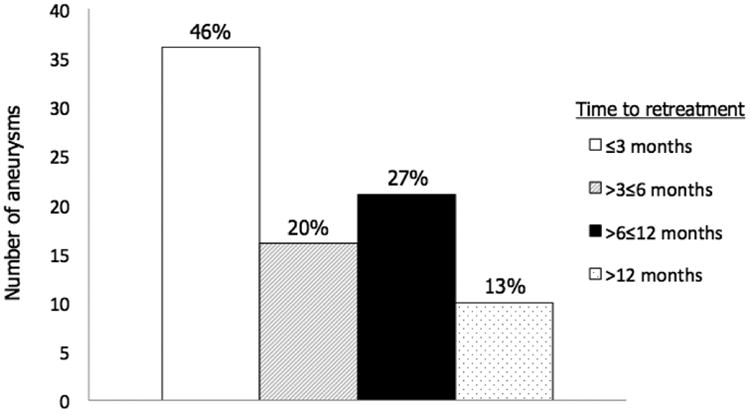
Time to retreatment after primary endovascular treatment. Percentages reflect the proportions of retreatments performed at ≤3 months, >3≤6 months, >6≤12 months, and >12 months.
Retreatment is a semi-subjective endpoint which reflects the objective presence of recanalization, a subjective assessment by the neurosurgical team of the risk of hemorrhage, as well as other idiosyncratic factors such as physician bias and/or previous bad experience.22 Recent studies have reported moderate interobserver agreement22,23,24,25 and there is a lack of available guidelines. However, retreatment as an endpoint captures the reality that patients may receive variable advice as physicians are currently unable to determine the true risk of rupture of an aneurysmal recurrence and is therefore a more meaningful outcome for patients. Our results reflect the experience and retreatment criteria of a single endovascular center. However, due to the widely recognized clinical relevance of our predictor variables, we are hopeful that our model will produce a useful stratification of retreatment risk in other centers. Using this scale, each institution may generate its own retreatment rate. We are currently in the process of validating our stratification system on independent cohorts from other endovascular centers.
Conclusion
In conclusion, surgical decision-making and patient-centered informed consent both require comprehensive and accessible information on treatment efficacy. We have constructed the Aneurysm Recanalization Stratification Scale in an effort to enhance this decision making process.
Acknowledgments
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Disclosures: There were no sources of outside funding.
Dr. Christopher Ogilvy is an author for UpToDate.
References
- 1.Nguyen TN, Hoh BL, Amin-Hanjani S, Pryor JC, Ogilvy CS. Comparison of Ruptured versus Unruptured Aneurysms in Recanalization after Coil Embolization. Surgical Neurology. 2007;68(1):19–23. doi: 10.1016/j.surneu.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Kauffmann TJ, Huston J, 3rd, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: Evaluation of 19,826 consecutive patients. Radiology. 2007;243(3):812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, Martin N, Vinuela F. Guglieimi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 5.Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J. Long-Term Follow-Up of Cerebral Aneurysms after Endovascular Therapy – Prediction and Outcome of Retreatment. American Journal of Neuroradiology. 2007;28:1755–1761. doi: 10.3174/ajnr.A0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalouhi N, Jabbour P, Singhai S, Drueding R, Starke RM, Dalyai RT, Tjoumakaris S, Gonzalez LF, Dumont AS, Ronsenwasser R, Randazzo CG. Stent-Assisted Coiling of Intracranial Aneurysms. Predictors of Complications, Recanalization, and Outcome in 508 Cases. Stroke. 2013;44:1348–1353. doi: 10.1161/STROKEAHA.111.000641. [DOI] [PubMed] [Google Scholar]
- 7.Weir B, Amidei C, Kongable G, Findlay JM, Kassell NF, Kelly J, Dai L, Karrison TG. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003;99:447–451. doi: 10.3171/jns.2003.99.3.0447. [DOI] [PubMed] [Google Scholar]
- 8.van Rooij WJ, Sprengers ME, Sluzewski M, Beute GN. Intracranial aneurysms that repeatedly reopen over time after coiling: imaging characteristics and treatment outcome. Neuroradiology. 2007;49:343–349. doi: 10.1007/s00234-006-0200-2. [DOI] [PubMed] [Google Scholar]
- 9.Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, Moret J. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 10.Grunwald IQ, Papanagiotou P, Struffert T, Politi M, Krick C, Gul G, Reith W. Recanalization after endovascular treatment of intracerebral aneurysms. Neuroradiology. 2007;49:41–47. doi: 10.1007/s00234-006-0153-5. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 12.O'Kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16(2):133–137. doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molyneux A, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 14.Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. AJNR. 2005;26:2542–2549. [PMC free article] [PubMed] [Google Scholar]
- 15.Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RSC, Sneade M, Yarnold JA, Rischmiller J, Byrne JV. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (ISAT) Stroke. 2007;38:1538–1544. doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 16.Gallas S, Pasco A, Cottier JP, Gabrillargues J, Drouineau J, Cognard C, Herbreteau D. A multicenter study of 705 ruptured intracranial aneurysms with Guglielmi detachable coils. AJNR. 2005;26:1723–1731. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz R, Stefanski M, Rosenwasser R, Veznedaroglu E. Cigarette smoking as a risk factor for recurrence of aneurysms treated by endosaccular occlusion. J Neurosurg. 2008;108:672–675. doi: 10.3171/JNS/2008/108/4/0672. [DOI] [PubMed] [Google Scholar]
- 19.Singla A, Villwock MR, Jacobsen W, Deshaies EM. Aneurysm embolization grade: A predictive tool for aneurysm recurrence after coil embolization. Acta Neurochir (Wien) 2013;155(2):231–236. doi: 10.1007/s00701-012-1554-3. [DOI] [PubMed] [Google Scholar]
- 20.Mascitelli JR, Moyle H, Oermann EK, Polykarpou MF, Patel AA, Doshi AH, Gologorsky Y, Bederson JB, Patel AB. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 2014 doi: 10.1136/neurintsurg-2014-011258.. [DOI] [PubMed] [Google Scholar]
- 21.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: Perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 22.Kallmes DF, Cloft HJ. Ready or not, here they come: Randomized trials evaluating new endovascular aneurysm therapies. AJNR. 2007;28:799–803. [PMC free article] [PubMed] [Google Scholar]
- 23.Cloft HJ, Kaufmann T, Kallmes DF. Observer agreement in the assessment of endovascular aneurysm therapy and aneurysm recurrence. AJNR. 2007;28:497–500. [PMC free article] [PubMed] [Google Scholar]
- 24.Daugherty WP, Rad AE, White JB, Meyers PM, Lanzino GL, Cloft HJ, Gordon J, Kallmes DF. Observer agreement regarding the necessity of retreatment of previously coiled recurrent cerebral aneurysms. AJNR. 2011;32:566–569. doi: 10.3174/ajnr.A2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald JS, Carter RE, Layton KF, Mocco J, Madigan JB, Tawk RG, Hanel RA, Roy SS, Cloft HJ, Klunder AM, Suh SH, Kallmes DF. Interobserver variability in retreatment decisions of recurrent and residual aneurysms. AJNR. 2013;34:1035–1039. doi: 10.3174/ajnr.A3326. [DOI] [PMC free article] [PubMed] [Google Scholar]


