Abstract
The specific role of C5a in cancer, especially in melanoma, has yet to be determined. Differential effects of C5a could be cancer-specific. In the host defense system, C5a functions to protect the body from harmful entities via a plethora of mechanisms. Yet, C5a may also serve to potentiate cancerous process. C5a facilitates cellular proliferation and regeneration by attracting myeloid-derived suppressor cells and supporting tumor promotion. In this article, we critically reviewed the properties, mechanisms of action, and functions of C5a, with particular emphasis on cancer inhibition and promotion, and clinical application of such knowledge in better management of cancer patients. Outstanding questions and future directions in regard to the function of C5a in melanoma and other cancers are discussed.
Keywords: C5a, Cancer, Complement, Chronic inflammation, Immunosuppression, Melanoma
Introduction
C5a is an anaphylatoxin formed via the cleavage of the complement protein, C5 [1]. The C5, a 1,676 amino acid protein, is primarily produced in the liver and resides in the circulation system as a zymogen [2,3]. When cleaved by a C5 convertase, C5 produces two active derivatives, C5a and C5b. The C5b complexes with C6-9 of the complement system to form the membrane attack complex (MAC), while C5a functions as an anaphylatoxin [4].
C5a is composed of 74 amino acids but is rapidly metabolized into a 73 amino acid form, C5a des-Arg. C5a is converted to C5a des-Arg via carboxypeptidases, which are located on cell surfaces and in plasma and function by removing the C terminal arginine group from C5a [5]. Both C5a and C5a des-Arg can act on two different types of 7-transmembrane domain receptors, CD88 and C5a receptor-like 2 (C5L2). CD88 is a G protein-coupled receptor, while C5L2 is a non-G protein-coupled receptor [6]. CD88 and C5LR receptors are generally expressed on similar cell types; however, fewer C5LR receptors tend to be expressed than CD88. The cell types on which these two receptors are expressed include both myeloid and non-myeloid-derived cells, including lymphocytes, phagocytes, and hepatocytes [7]. C5a binds with a similar affinity to CD88 and C5L2 receptors. But, while C5a and C5a des-Arg have approximately the same binding affinity for C5L2 receptors, C5a des-Arg has a considerably lower affinity for CD88 receptors than C5a [6]. Therefore, C5a des-Arg is a less potent form of C5a [8]. As a part of the complement system, C5a is produced in serum during activation of the complement cascade. However, C5a can also be produced locally via cleavage by phagocytes and thrombin [9, 10].
C5a in the Complement System
C5a is one of over 30 proteins of the complement system. The proteins of the complement system are produced in liver and macrophages and are activated sequentially as a part of the innate immune response. Functioning as opsonins, pro-inflammatory molecules, and in the formation of the membrane attack complex, most proteins of the complement system circulate within the blood stream as zymogens. Traditionally, C5a can be produced via complement system activation, which occurs via three pathways: the classical, alternative, and lectin pathway (Table 1). All three pathways result in the cleavage of C3 into C3a and C3b and therefore, also result in the activation of C5, C6, C7, C8, and C9 [4, 11]. More recent research indicates that C5 can also be activated by two additional mechanisms: thrombin and activated neutrophils functioning as a C5 convertase (Table 1) [9, 10].
Table 1.
Pathways activating C5a and activating stimuli
| Mechanism of Activation | Activating Stimulus |
|---|---|
| Classical Pathway | Antibody-antigen interaction |
| Lectin Pathway | Mannose-MBL or oligosaccharide-ficolin interactions |
| Alternative Pathway | Spontaneous hydrolysis of C3 |
| Extrinsic Coagulation Pathway | Tissue Damage |
| Inducible Serine Proteases | Macrophage activation of the protease |
Activation of C5
The Classical, Lectin, and Alternative Pathways
The classical pathway via antibody-antigen interactions activates the complement system. Antibody-antigen complexes are recognized by C1 fragment of the complement system which when bound to the Fc region of an antibody is capable of cleaving C4 protein and activating the rest of the complement cascade. The lectin pathway via two possible interactions activates the complement system: mannose-mannose binding lectin (MBL) interactions and oligosaccharides-ficolin interactions. Mannose and specific oligosaccharides displayed on the cell surface of foreign bodies (i.e. fungi or bacteria) are recognized by the host proteins, MBL and ficolin, respectively. Both MBL and ficolin are complexed with mannan-binding lectin serine protease 1 (MASP1) and mannan-binding lectin serine protease 2 (MASP2). Upon the recognition and binding of MBL to mannose and ficolin to an oligosaccharide, MASP1 and MASP2 are activated. MASP2 functions to cleave both C4 and C2, thus producing C3 convertase (C4bC2a). The complement system is activated by the alternative pathway via the spontaneous hydrolysis of C3 into C3a and C3b [4, 12, 13].
Thrombin and Phagocytic Cells Functioning as C5 Convertase
Huber-Lang and colleagues [10] examined the concentration of C5a in mice with a genetic deficiency of C3. Without C3, C5 convertase of the classical and lectin pathways (C4bC2aC3b) and C5 convertase of the alternative pathway (C3bBbC3b) could not be formed. Interestingly, functionally active C5a may still be produced in the genetically deficient mice in both the in vitro and in vivo studies [10]. Furthermore, the mice genetically deficient in C3 had a 3 fold higher activity of plasma thrombin than those without C3 deficiency. These findings suggested that the C5 convertase activity necessary for the production of C5a can be elicited in thrombin [10]. Thrombin, the final product of the extrinsic coagulation pathway, is itself activated by tissue damage. Thus, the coagulation pathway and complement pathway are perhaps interwoven at this C5 junction.
Huber-Lang and colleagues [9] have also implicated activated rat alveolar macrophages and human neutrophils as cells with C5 convertase capabilities. By incubating rat alveolar macrophages and human neutrophils with C5, C5a was generated. It is proposed that C5a is produced by local cleavage of C5 via phagocytes, which utilize an inducible serine protease [9]. Serine proteases are enzymes that cleave peptide bonds via the use of the amino acid, serine, which serves as the necessary electron donor [14].
Function of C5a
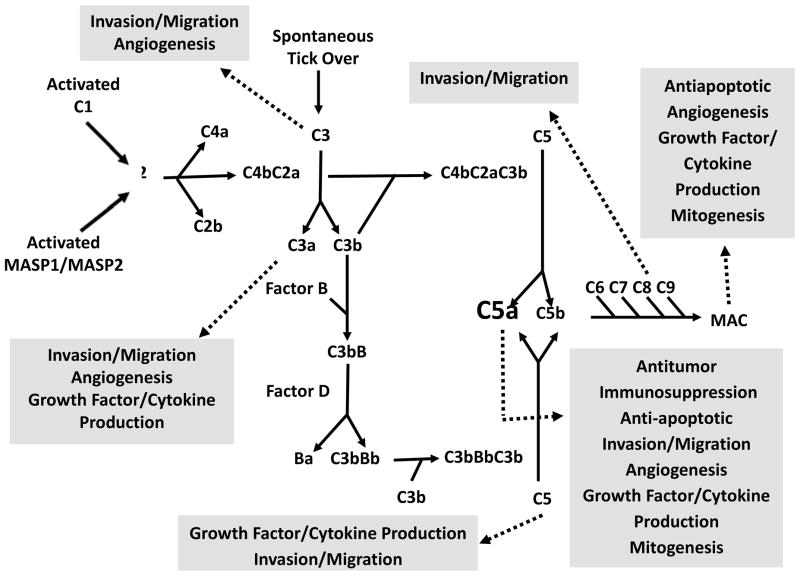
C5a is a powerful anaphylatoxin that functions in multiple ways to induce inflammation; C5a acts as a chemotactic agent for inflammatory cells, stimulates respiratory burst, cytokine and chemokine release, and functions to increase vascular permeability [15]. Additionally, C5a has been found to stimulate angiogenesis (Figure 1) [16].
Figure 1.
Activation of the complement cascade and its role in tumorigenesis. The complement cascade can be activated via the classical, lectin, or alternative pathway; each of which results in the production of tumorigenic complement proteins. C3, C3a, C5, C5a, C9, and the membrane attack complex (MAC). Many of these complement fragments have been implicated as pro-tumorigenic molecules since they can enhance tumor angiogenesis or inhibit apoptosis, and thus facilitate tumor progression.
A cornerstone of the immunological effect of C5a is its ability to stimulate the release of histamine from mast cells. Histamine, a vasoactive amine, stimulates vasodilation and contraction of venular endothelial cells, thus increasing vascular permeability. This effect takes place in coordination with the function of C5a as a chemotactic factor by facilitating the extravasation of the leukocytes, such as basophils, neutrophils, monocytes, and eosinophils, attracted by C5a [3]. Furthermore, histamine also stimulates the production of VEGF-A, which induces angiogenesis. Ryuji and co-investigators [16] have found C5a to directly stimulate angiogenesis via promoting the migration of human microvascular endothelial cells (HMEC-1) both in vitro and in vivo [16]. Thus, C5a could indirectly induce angiogenesis either via production of VEGF-A or directly via its effect on the migration of endothelial cells.
C5a also stimulates the lipoxygenase pathway of arachidonic acid metabolism. This pathway involves the conversion of phospholipids into arachidonic acid, which is metabolized into two types of eicosanoids, leukotrienes and lipoxins. It is unclear whether C5a stimulates the lipoxygenase pathway directly by simply binding to its CD88 receptors or involves other mechanisms. This obviously requires further attention. None-the-less, in this process neutrophils and macrophages serve as local sources of the eicosanoids. Leukotriene B4 and 5-hydroxyeicosatetraenoic acid (5-HETE) are eicosanoids that both function in chemotaxis and leukocyte adhesion. Leukotrienes C4, D4, and E4 stimulate vasoconstriction, an initial and ephemeral stage of inflammation, and increased vascular permeability. Lipoxins serve as inflammatory antagonists and gradually begin to be produced as cells switch from the production of inflammatory mediators to that of anti-inflammatory mediators [3].
C5a also has the ability to trigger degranulation of leukocytes, such as neutrophils, and stimulate respiratory burst [17]. Degranulation of neutrophils causes the release of inflammatory substances such as toxic mediators and matrix metalloprotease-9 [18]. Respiratory burst causes the release of reactive oxygen species. Both the mediators released from granules and reactive oxygen species kill harmful entities in the body.
Role of C5a in Tumor Suppression
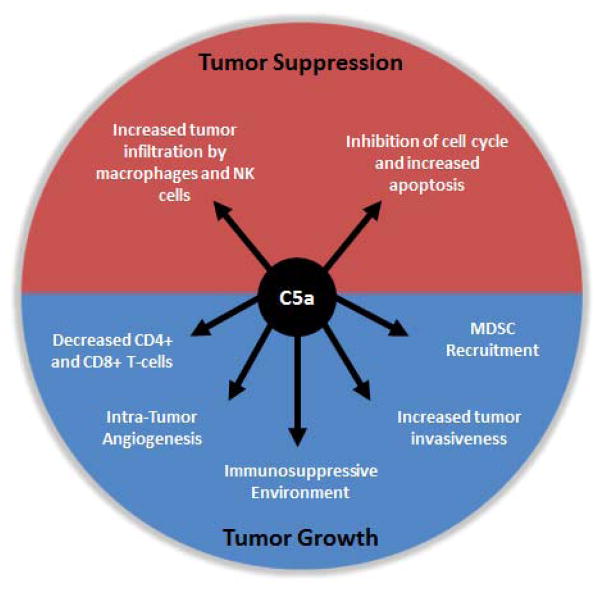
C5a has been experimentally found to inhibit tumor growth via either stimulating innate immune cells or halting cell cycle progression (Figure 2) [19, 20]. However, the inhibitory functions of C5a were observed only when amounts of C5a were low. In fact, not only did C5a in low dose act as a tumor suppressor,, C5a present in high concentrations was actually observed to have significantly accelerated tumor progression [19]. Thus, the concentration of local C5a within a tumor may dictate the effect of C5a on tumor suppression or tumor progression.
Figure 2.
Opposing roles of C5a in tumorigenesis. The role of C5a has been found to vary according to its concentration at the tumor site. At low concentrations, C5a inhibits tumor growth via facilitating the infiltration of natural killer (NK) cells and macrophages in the tumor, as well as directly altering the cancer cell cycle. At higher concentrations, C5a enhances tumor growth by decreasing the T-cell population, stimulating tumor angiogenesis and invasion, recruiting myeloid-derived suppressor cells, and generating an immunosuppressive environment.
Gunn and colleagues [19] demonstrated with the ovarian SKOV-3 xenograft model, which uses immune suppressed mice, that low levels of C5a in the tumor microenvironment attract both M1 macrophages and natural killer (NK) cells to tumor sites [19]. The M1 macrophages have cytotoxic effects and function as anti-tumorigenic cells [21]. NK cells are also cytotoxic to tumor cells [22]. When low levels of C5a from lymphoma cells were released into the tumor microenvironment, both increased tumor infiltration and cytotoxic function was observed of macrophages and NK cells, which presumably led to reduced tumor growth. It should be noted that SVOV-3 tumor cells do not express CD88 receptors. Thus, C5a could only have been functioning indirectly to inhibit tumor growth.
Kim and co-investigators [20] also observed a correlation between low levels of C5a and reduced tumor growth using the murine mammary cancer model. Complete tumor regression was seen in 1/3rd of the mice that were administered with cells expressing low levels of C5a. Their results supported the hypothesis that the tumor regression was due to inhibition of the cell cycle and increased rates of apoptosis.
Role of C5a in Tumor Promotion
Gunn and colleagues [19] also demonstrated that high levels of C5a (~500ng/ml) stimulate tumor progression [20]. Using immune-competent mice, it was found that higher levels of C5a correlated with more Gr-1+CD11b+ myeloid cells in the spleen and decreased number of CD4+ and CD8+ T-cells in the tumor, tumor-draining lymph nodes and spleen, and ultimately increased tumor growth. However, other research has been done to understand the specific mechanisms by which C5a stimulates tumor growth. The mechanisms, including increased recruitment of myeloid-derived suppressor cell (MDSC), creation of an immunosuppressive microenvironment, increased intra-tumor angiogenesis, and enhanced tumor invasiveness, could be attributed to the effect of C5a in tumor progression [23-25].
Antitumor Immunity Suppression via the Inhibition of T-lymphocytes
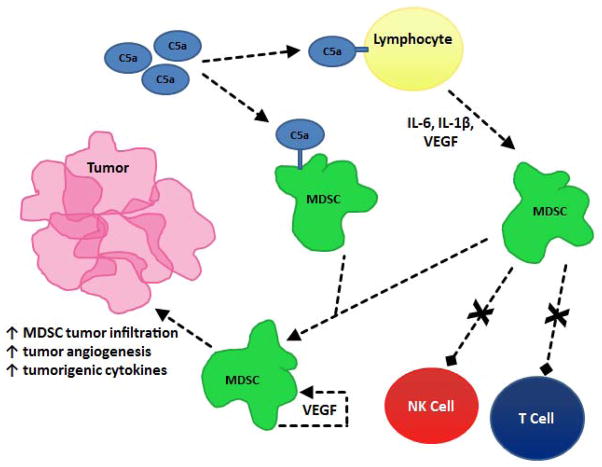
C5a activates myeloid-derived suppressor cells (MDSCs) both directly and indirectly. The presence of C5a receptors on the cell surface of MDSCs allows C5a to directly bind and stimulate MDSCs. The degree of C5a receptor expression on MDSCs was directly proportional to the extent of tumor infiltration by MDSCs. Therefore, a greater number of C5a receptors lead to greater stimulation of MDSCs by C5a and resultantly, increased immunosuppression [23]. MDSCs are also induced by IL-6, IL-1β and VEGF and C5a stimulates the production of these mediators [24, 26, 27]. Thus, C5a may have the capacity to doubly stimulate MDSCs via both direct and indirect stimulation. Furthermore, MDSCs themselves produce VEGF and therefore, could potentially be self- activating as well [28, 29].
As a consequence of C5a stimulation, MDSCs are attracted to the tumor site and enhance tumor growth by inhibiting T-cell activation and NK cell cytotoxicity, inducing the production of tumorigenic cytokines, and increasing tumor angiogenesis [30]. For example, C5a-induced release of VEGF inhibits the maturation of dendritic cells (DCs) and therefore, increases the population of MDSCs. Interleukin-1β directly stimulates the production of MDSCs [31]. C5a has been found to modulate the production of reactive oxygen and nitrogen species in MDSCs [23].
Recent studies in both tumor models and cancer patients have shown that the activity of NADPH oxidase in MDSCs is upregulated in tumor environments, which results in increased production of reactive species. The resulting reactive oxygen and nitrogen species suppress antitumor CD8+ T-cell responses [32]. In particular, the production of peroxynitrite leads to the binding of nitrate to T-cell receptors (TCRs), preventing the receptor from binding to tumor antigens. Additionally, MDSCs hinder T-cell receptor function by causing downregulation of zeta chain expression. This downregulation is achieved via high levels of arginase in MDSCs. Because arginase functions to breakdown arginine, MDSCs regulate arginine levels; as an amino acid critical for the development of the zeta chain of the TCR, arginine is vital for T-cells to function properly [31, 33].
MDSCs also inhibit T-cells by sequestering cysteine from the tumor microenvironment. T-cells lack both cystathionase, which converts methionine to cysteine, and the xC- transporter, which functions to bring cystine into the cell to be reduced into cysteine. Consequently, acquisition of cysteine by T-lymphocytes is dependent on the exportation of cysteine by antigen presenting cells (APCs). MDSCs reduce the level of cysteine in the environment by importing cystine but due to lack of the necessary ASC transporter, fail to export cysteine back into the environment, reducing the amount of available cysteine for T-lymphocytes [34] (Figure 3).
Figure 3.
The interaction between C5a and myeloid-derived suppressor cells (MDSCs). C5a attracts MDSCs to tumor sites by directly binding to receptors on the MDSC surface. C5a also indirectly activates MDSCs by binding to other cell types, inducing the release of cytokines that attract MDSCs tumor sites. The presence of MDSCs at tumor sites leads to increased tumor angiogenesis and increased concentration of tumorigenic cytokines. MDSCs also inhibit the activation of T Cells and NK Cell cytotoxicity.
Studies have demonstrated that when C5aR signaling is blocked the number of CD8+ T-cells significantly increases with decrease in tumor size [23]. This is hypothesized to be due to decreased MDSC stimulation and therefore, increased antitumor immune system function.
Stimulation of Angiogenesis
It has been observed that C5a generates a microenvironment favorable for tumor growth [24]. This microenvironment was characterized by increased angiogenesis and expression of IL-6, IL-10, LAG3, PDL1, ARG1, and CTLA-4, most of which are related to an immunosuppressive state [24]. The vascular density of a given tumor strongly correlates with the likelihood of tumor to metastasize. New vessel formation allows the tumor to access the circulation and metastasize throughout the body. The process of angiogenesis is most commonly found to be associated with two families of growth factors, basic fibroblast growth factor (bFGF or FGF1), vascular endothelial growth factor A (VEGF-A), and VEGF-C [35 – 37].
VEGF-A has been credited as the most important angiogenic cytokine and plays a critical role in tumor metastasis [36]. Not only is it commonly overexpressed in solid cancers; its concentration is directly proportional to malignancy [38–40]. VEGF-A increases vascular permeability and has 50,000 times greater potency than that of histamine [36, 41]. VEGF-A also functions to inhibit apoptosis in endothelial cells and stimulate chemotaxis of monocytes and macrophages and endothelial cell migration and division [36, 42, 43].
C5a has been found to stimulate VEGF in the spontaneously arising retinal pigment epithelia cell line “ARPE-19.” Likewise, C5a can also stimulate VEGF production in vivo by intravitreous injection in mice [44]. Accordingly, use of a C5a antagonist blocked the production of VEGF [44, 45]. One potential hypothesis for the mechanism in which C5a induces VEGF release is via the cytokines IL-1β, IL-6 and IL-8. C5a stimulates monocytes to release IL-1, IL-6, IL-8 and TNF-α [46]. IL-1, IL-6, and IL-8 are known potentiators of VEGF production [47, 48]. Although IL-8 is traditionally thought of as a chemoattractant for neutrophils that stimulates neutrophil degranulation, the expression of IL-8 has been found to be associated with tumor tumorgenicity, angiogenesis, and metastasis in vivo [49].
Corrales et al. [24] observed that C5a stimulates angiogenesis in vitro by augmenting the formation of tube-like structures and increasing cell migration. When a C5a receptor antagonist was introduced, the expression of these molecules and formation of vessels decreased significantly. Furthermore, there was also a significant decrease in tumor growth [24].
Stimulation of Cancer Invasion and Migration
MMPs play an important role in tumor angiogenesis, invasion and metastasis by degrading the extracellular matrix, inducing of tumor resistance to apoptotic signals, and increasing tumor evasion of the host defense [50, 51]. MMP concentration can be correlated to the presence and aggressiveness of various cancers, such as renal cell carcinoma, lung, colorectal, and breast cancer [52–55]. It is thought that the stromal cells surrounding a tumor, and not the cancerous cells themselves, are responsible for releasing MMPs [56]. Transcription of MMPs is not common under normal cellular conditions but can be stimulated by cytokines, specifically IL-1 and TNF-α, oncogene products, changes in cell shape, and mechanical stress [51, 56–58].
C5a has been shown to increase cancer cell invasion and migration via the induction of cell motility and matrix metalloproteases (MMPs); cancer cells that expressed C5a receptors had 13x greater invasiveness than their controls [25]. Although C5a receptors are not normally expressed on epithelial cells, their expression can be stimulated by inflammation and expression has been seen in certain cancers [59, 60]. Nitta et al. [25] found C5aR expression on cancerous epithelium of the esophagus, stomach, colon, liver, bile duct, pancreas, bladder, prostate and mammary glands, whereas noncancerous versions of these tissues do not express C5aR [25]. It is suggested that the expression of C5a in epithelium is the result of malignant transformation. Blocking of the C5aR with the use of an antibody against C5aR inhibits cancer invasion enhanced by C5a. Furthermore, the extent of cell invasion stimulated by C5a directly correlated with the concentration of MMP-8. C5a has also been found to stimulate MMP-1 and MMP-9 in macrophages in vitro [61].
Inhibition of Apoptosis
C5a may also play a role in tumor progression by functioning as an antiapoptotic mediator [62]. C5a has been found to prevent activation of apoptotic caspase 3 and therefore DNA fragmentation, in murine cortico-hippocampal neuronal cultures. It is hypothesized that the binding of C5a to the C5aR triggers the mitogen-activated protein kinase (MAPK) pathway, which prevents glutamate-induced apoptosis [63].
In addition to being neuroprotective, C5a has also been implicated in liver regeneration. Daveau et al. [64] found C5a stimulation of both normal and regenerating liver to be associated with increased expression of hepatocyte growth factor and c-Met mRNAs [64]. Interestingly, high levels of hepatocyte growth factor and c-Met receptor expression have both been associated with tumor metastasis and invasiveness [65].
C5a serves as a transcriptional signal for tumor necrosis factor (TNF) and IL-1β [66]. TNFα is a stimulator of NF-κB, a transcription factor that functions in cell proliferation and is correlated to tumor growth [67]. It has also been proposed that the membrane-associated IL-1α stimulates anti-tumor immune response, whereas secreted IL-1β from the malignant cells or within the tumor microenvironment might induce pro-inflammatory effects leading to increased tumor invasiveness and tumor-mediated immune suppression [68]. In addition to TNF and IL-1β, C5a also regulates IL-6 [69]. Due to the fact that most of the genes that are regulated by IL-6 are involved in stimulation of the cell cycle and inhibition of apoptosis, IL-6 is potentially another way in which C5a plays a hand in tumor progression [70].
Therapeutic Use of C5a
Currently, C5a has proved useful in cancer vaccine development via its role in the enhancement of NK cells, dendritic cells, and T-cell activation, all of which aid in tumor suppression. The C5a containing vaccines have been found to prevent tumor growth, specifically in melanoma, lymphoma, and E.G7 OVA tumors [71–73].
A study was conducted in which the effectiveness of vaccines was compared [73]. Among the three vaccines used in this study, one vaccine was composed of the antigen “SIINFEKL” from ovalbumin and an endogenous ligand for toll-like receptor 4 (TLR4). SIINFEKL is an epitope for cytotoxic T lymphocytes (CTLs) and TLRs function as important activators of the innate immune system. The second vaccine contained SIINFEKL, the TLR4 ligand, and C5a. Results showed that the vaccine containing C5a elicited a significantly higher CTL response than the vaccine containing only the TLR4 ligand and SIINFEKL antigen. Furthermore, the C5a containing vaccine also stimulated greater NK cell activity. These results paralleled with their findings regarding the ability of the two vaccines to prevent E.G7-OVA tumor growth; 61% of the mice vaccinated with the vaccine containing C5a, while only 35% of mice immunized with the vaccine containing only TLR4 ligand were protected against tumor challenge in regard to tumor size and death.
C5a agonists have also been used in vaccines for tumor prevention [71, 72]. Kollessery et al. [72] demonstrated that vaccines composed of either the C5a agonist EP54 or EP67 protected mice from RAW117-H10 lymphoma. Despite being injected with a lethal dose of the RAW117-H10 cells, all vaccinated mice lived over year as compared to the controls, which died within 17 days. Results suggest that the vaccines prevented visible metastasis to the liver and furthermore, induced CTLs with cytotoxic specificity to RAW117-H10.
Likewise, Floreani et al. [71] also used C5a agonists to inhibit tumor growth. By covalently linking the C5a agonist, YSKFDMP(MeL)aR, to two different melanoma antigens separately, TRP2-P2 and TYR, and injecting these two agonist-antigens into mice, melanoma tumor growth was inhibited. However, when mice were injected with only one of the agonist-antigens, tumor growth inhibition did not proceed past 17 days [71].
In addition to cancer therapy, C5a has also been utilized in the treatment of arthritis. As C5a deficiency is protective against arthritis, two different anti-C5a vaccines were developed and have been tested experimentally in murine models. Both vaccines were successful in combating different facets of the disease.
One vaccine has been generated by fusing maltose to C5a. This fusion protein vaccine was tested in three different arthritic murine models. Overall, the vaccine was successful in stimulating specific C5a antibodies and decreasing the incidence and severity of the inflammation generated in arthritis. However, the antibodies generated were unsuccessful in preventing C5a activation and formation of MAC. The vaccine also failed to inhibit the anti-collagen antibodies characteristic of collagen antibody induced arthritis (CAIA) [74].
A second vaccine was produced by substituting the amino acid “p-nitrophenylalanine (4NPA)” for a tyrosine at the 35 position within C5a. This anti-C5a vaccine was exclusively tested in the collagen-induced arthritis (CIA) model. The vaccine was successful in abating the severity of the arthritis but was only partially protective against CIA development. The vaccine itself lead to a loss of B and T-cell tolerance to C5a in mice whose cells expressed the particular receptor “class II MHC H-2(q).” Both the indigenous C5a and modified C5a induced antibodies capable of effectively neutralizing C5a. The high titers of IgG prevented disease development, but did not reverse the course of ongoing disease [75].
Lastly, C5a has been successfully utilized in antibacterial therapy. Using the CD88 agonist “EP67,” researchers were capable of limiting Staphylococcus infections via the enhancement of cytokine release and neutrophil infiltration. EP65 has also been effective in Streptococcus killing, specifically group B Streptococcus (GBS). Successful killing occurred in mice lacking both CD88 and CXCr2 (a neutrophil receptor); thus, it is hypothesized that the mechanism of action of EP65 against GBS is not due to enhancement of the immune response, like with Staphylococcus, but rather by direct bacterial killing [76].
Expert Commentary and Five year Review
The role of C5a in tumorigenesis is complex and much is still unknown; however, some key characteristics have been determined. The function of C5a in cancer is dose-dependent and whether it inhibits or supports tumor progression is contingent on the concentration of C5a at the tumor site. At low levels, C5a inhibits tumor growth via enhancing NK cell and M1 macrophage tumor infiltration and cytotoxicity. C5a potentiates tumorigenesis through a plethora of mechanisms. Possibly the most crucial function of C5a in tumor promotion is its ability to activate and attract MDSCs to the tumor site. MDSCs are responsible for inhibiting T-lymphocyte activation and NK cell cytotoxicity leading to an immunosuppressed state. C5a also stimulates tumor angiogenesis, invasion, and migration. Perhaps, it is by inducing the release of growth factors, such as VEGF, that C5a facilitates the formation of the primary tube-like structures characteristic of developing vessels. C5a promotes invasion and migration by stimulating MMP release from epithelial cells aberrantly expressing C5a receptors. Lastly, C5a has been implicated as an anti-apoptotic mediator.
Although the function of C5a has been observed to vary with its concentration, additional studies are warranted to confirm and compare the effects of C5a at various concentrations, and perhaps in diverse microenvironment, in regard to its pro- and antitumor activity. It is also important to acknowledge that each function of C5a may require a separate threshold concentration. Thus, it is critical to determine the functional levels of C5a for individual cancers. Theoretically, this information could be used to test and therapeutically manipulate C5a concentration in patients in the antitumor range. If the effect of C5a is concentration-dependent, then it is also important to determine the underlying mechanism(s) of C5a production in cancer. Once identified, the specific pathway could be therapeutically interrupted to inhibit C5a production. Additionally, the tumor microenvironment may elicit synergistic or antagonistic effects on the action of C5a.
Due to the fact that the vascular density of a tumor is directly proportional to the degree of metastasis, further knowledge as to exactly how C5a stimulates VEGF release could be highly relevant. The inflammatory cytokines, including IL-1β, IL-6, and IL-8, could be critical to serve as a potential bridge between C5a and VEGF release. C5a is known to stimulate the release of IL-1β, IL-6, and IL-8 from monocytes and these cytokines are known potentiators of VEGF production. None-the-less, additional studies are warranted to accept or refute this hypothesis.
Key Issues.
If the concentration of C5a determines its anti-tumorigenic or tumorigenic effect, what is the threshold concentration?
Is the effect of C5a in various concentrations dependent on the tumor microenvironment and cancer type?
Studies are required to completely elucidate the underlying mechanisms of the anti-tumorigenic or pro-tumorigenic effects of C5a.
Comparative role of C5a receptors, CD88 and C5L2, in tumor invasive ness and tumor progression needs further investigation.
What is the mechanism by which C5a stimulates VEGF release?
What is the effect of pro-inflammatory cytokines in angiogenesis induced by the interaction between C5a and VEGF?
Footnotes
Disclaimer
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial & competing interest disclosure
This work was supported by research grants from the National Institutes of Health, USA to DK Agrawal. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special interest have been highlighted as:
• Of interest
•• Of considerable Interest
- 1.Manthey H, Woodruff T, Taylor S, Monk P. Complement Component 5a (C5a) Int J of Biochem Cell Biol. 2009;41:2114–7. doi: 10.1016/j.biocel.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Wetsel R, Lemons R, Le Beau M, et al. Molecular Analysis of Human Complement Component C5: Localization of the Structural Gene to Chromosome 9. Biochemistry. 1988;27:1474–482. doi: 10.1021/bi00405a012. [DOI] [PubMed] [Google Scholar]
- 3.Vinay K, Abbas A, Aster J, Robbins S. Robbins Basic Pathology. Philadelphia: Elsevier Saunders; 2013. [Google Scholar]
- 4.Kaufmann S, Rouse B, Sacks D. The Immune Response to Infection. Washington: ASM Press; 2011. [Google Scholar]
- 5.Bokisch V, Müller-Eberhard H. Anaphylatoxin Inactivator of Human Plasma: Its Isolation and Characterization as a Carboxypeptidase. J Clin Invest. 1970;49:2427–436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monk P, Scola A, Madala P, Fairlie D. Function, Structure and Therapeutic Potential of Complement C5a Receptors. Br J of Pharmacol. 2007;152:429–48. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Neff T, Guo R, et al. Evidence for a Functional Role of the Second C5a Receptor C5L2. FASEB J. 2005;19:1003–005. doi: 10.1096/fj.04-3424fje. [DOI] [PubMed] [Google Scholar]
- 8.Higginbottom A, Cain S, Woodruff T, et al. Comparative Agonist/antagonist Responses in Mutant human C5a Receptors Define the Ligand Binding Site. J Biol Chem. 2005;280:17831–7840. doi: 10.1074/jbc.M410797200. [DOI] [PubMed] [Google Scholar]
- 9.Huber-Lang M, Younkin E, Sarma J, et al. Generation of C5a by Phagocytic Cells. Am J Pathol. 2002;161:1849–859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber-Lang M, Sarma J, Zetoune F, et al. Generation of C5a in the Absence of C3: A New Complement Activation Pathway. Nat Med. 2006;12:682–87. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 11.Noris M, Remuzzi G. Overview of Complement Activation and Regulation. Semin Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy K, Travers P, Walport M, Janeway C. Janeway’s Immunobiology. New York: Garland Science; 2012. [Google Scholar]
- 13.Rossi V, Cseh S, Thielens N, et al. Interaction properties of human mannan-binding lectin (MBL)-associated serine proteases-1 and -2, MBL-associated protein 19, and MBL. J Immunol. 2001;166:5068–77. doi: 10.4049/jimmunol.166.8.5068. [DOI] [PubMed] [Google Scholar]
- 14.Hedstrom L. Serine Protease Mechanism and Specificity. Chem Rev. 2002;102:4501–524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Ward P. Role of C5A in Inflammatory Responses. Annu Rev of Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 16.Kurihara R, Yamaoka K, Sawamukai N, et al. C5a Promotes Migration, Proliferation, and Vessel Formation in Endothelial Cells. Inflamm Res. 2010;59:659–66. doi: 10.1007/s00011-010-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan U. Endothelial Cells. FL: CRC Press; 1988. [Google Scholar]
- 18.Lacy P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin Immunol. 2006;2:98–108. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Gunn L, Ding C, Liu M, et al. Opposing Roles for Complement Component C5a in Tumor Progression and the Tumor Microenvironment. J Immunol. 2012;189:2985–994. doi: 10.4049/jimmunol.1200846. C5a functions in a dose-dependent manner. At low doses C5a has been found to support tumor suppression. C5a accelerates tumor progression when present locally in high doses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Martin C, Lee S, Martin B. Expression of Complement Protein C5a in a Murine Mammary Cancer Model: Tumor Regression by Interference with the Cell Cycle. Cancer Immunol Immunother. 2005;54:1026–037. doi: 10.1007/s00262-005-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamagna C, Aurrand-Lions M, Imhof B. Dual Role of Macrophages in Tumor Growth and Angiogenesis. J of Leukoc Bio. 2006;80:705–13. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 22.Chan C, Andrews D, Smyth M. Can NK Cells Be a Therapeutic Target in Human Cancer? Eur J Immunol. 2008;38:2964–968. doi: 10.1002/eji.200838764. [DOI] [PubMed] [Google Scholar]
- 23••.Markiewski M, DeAngelis R, Benencia F, et al. Modulation of the Antitumor Immune Response by Complement. Nat Immunol. 2008;9:1225–235. doi: 10.1038/ni.1655. C5a can directly activate myeloid-derived suppressor cells (MDSCs) by binding to the MDSC surface. The level of C5a receptor expression on MDSC surface is directly proportional to the extent of tumor infiltration by MDSCs and immunosuppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Corrales L, Ajona D, Rafail A, et al. Anaphylatoxin C5a Creates a Favorable Microenvironment for Lung Cancer Progression. J Immunol. 2012;189:4674–683. doi: 10.4049/jimmunol.1201654. C5a has been found to stimulate angiogenesis in vitro via two mechanisms: enhancement of the formation of tube-like structures and cell migration. Use of a C5a antagonist inhibited these processes and led to a decrease in tumor growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidetoshi N, Wada Y, Kawano Y, et al. Enhancement of Human Cancer Cell Motility and Invasiveness by Anaphylatoxin C5a Via Aberrantly Expressed C5a Receptor (CD88) Clin Cancer Res. 2013;19:2004–013. doi: 10.1158/1078-0432.CCR-12-1204. [DOI] [PubMed] [Google Scholar]
- 26.Lechner M, Liebertz D, Epstein A. Characterization of Cytokine-induced Myeloid-derived Suppressor Cells from Normal Human Peripheral Blood Mononuclear Cells. J Immunol. 2010;185:2273–284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okusawa S, Dinarello C, Yancey K, et al. C5a Induction of Human Interleukin 1. Synergistic Effect with Endotoxin or Interferon-gamma. J Immunol. 1987;139:2635–640. [PubMed] [Google Scholar]
- 28.Yang Li, DeBusk L, Fukuda K, et al. Expansion of Myeloid Immune Suppressor Gr+CD11b+ Cells in Tumor-bearing Host Directly Promotes Tumor Angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Melani C, Chiodoni C, Forni G, Colombo M. Myeloid Cell Expansion Elicited by the Progression of Spontaneous Mammary Carcinomas in C-erbB-2 Transgenic BALB/c Mice Suppresses Immune Reactivity. Blood. 2003;102:2138–145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 30.Ostrand-Rosenberg S. Cancer and Complement. Nature Biotechnology. 2008;26:1348–349. doi: 10.1038/nbt1208-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx J. Cancer’s Bulwark against Immune Attack: MDS Cells. Science. 2008;319:154–56. doi: 10.1126/science.319.5860.154. [DOI] [PubMed] [Google Scholar]
- 32.Corzo C, Cotter M, Cheng P, et al. Mechanism Regulating Reactive Oxygen Species in Tumor-induced Myeloid-derived Suppressor Cells. J Immunol. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popovic P, Zeh H, Ochoa J. Arginine and Immunity. J Nutr. 2007;137:1681S–686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava M, Sinha P, Clements V, et al. Myeloid-derived Suppressor Cells Inhibit T-cell Activation by Depleting Cystine and Cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zetter B. Angiogenesis and Tumor Metastasis. Annu Rev Med. 1998;49:407–24. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak H. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy. J Clin Oncol. 2002;20:4368–380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 37.Chien M, Ku C, Johansson G. Vascular Endothelial Growth Factor-C (VEGF-C) Promotes Angiogenesis by Induction of COX-2 in Leukemic Cells via the VEGF-R3/JNK/AP-1 Pathway. Carcinogenesis. 2009;30:2005–013. doi: 10.1093/carcin/bgp244. [DOI] [PubMed] [Google Scholar]
- 38.Brown L, Berse B, Jackman R, et al. Expression of vascularpermeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 39.Guidi A, Abu-Jawdeh G, Berse B, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J Natl Cancer Inst. 1995;87:1237–1245. doi: 10.1093/jnci/87.16.1237. [DOI] [PubMed] [Google Scholar]
- 40.Wong M, Cheung N, Yuen S, et al. Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer. 1999;81:845–850. doi: 10.1002/(sici)1097-0215(19990611)81:6<845::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Dvorak HF, Nagy JA, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin LE, Golijanin D, Itin A, et al. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: A tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Nozaki M, Raisler BJ, Sakurai E. Drusen Complement Components C3a and C5a Promote Choroidal Neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–333. doi: 10.1073/pnas.0408835103. C5a stimulates VEGF in retinal pigment epithelium. Critical in tumor metastasis, increased VEGF levels are directly proportional to tumor malignancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortright D, Meade R, Waters S, Chenard B, Krause J. C5a, But Not C3a, Increases VEGF Secretion in ARPE-19 Human Retinal Pigment Epithelial Cells. Curr Eye Res. 2009;34(1):57–61. doi: 10.1080/02713680802546658. [DOI] [PubMed] [Google Scholar]
- 46.Martina B, Koraka P, Osterhaus A. Dengue Virus Pathogenesis: An Integrated View. Clin Microbio Rev. 2009;22:564–81. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular Endothelial Growth Factor (VEGF) and Its Receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 48.Martin D, Galisteo R, Gutkind J. CXCL8/IL8 Stimulates Vascular Endothelial Growth Factor (VEGF) Expression and the Autocrine Activation of VEGFR2 in Endothelial Cells by Activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) Complex. J Biol Chem. 2009;284:6038–42. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waugh D, Wilson C. The Interleukin-8 Pathway in Cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 50.Sternlicht M, Lochter A, Sympson CJ. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Overall C, López-Otín C. Strategies for Mmp Inhibition in Cancer: Innovations for the Post-trial Era. Nat Rev Cancer. 2002;2:657–72. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 52.Miyata Y, Iwata T, Maruta S, et al. Expression of matrix metalloproteinase-10 in renal cell carcinoma and its prognostic role. Eur Urol. 2007;52:791–797. doi: 10.1016/j.eururo.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 53.Justilien V, Regala R, Tseng I, et al. Matrix Metalloproteinase-10 is Required for Lung Cancer Stem Cell Maintenance, Tumor Initiation and Metastatic Potential. PLoS ONE. 2012;7(4):e35040. doi: 10.1371/journal.pone.0035040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murnan M, Cai J, Shuja S, Mcaneny D, et al. Active MMP-2 Effectively Identifies the Presence of Colorectal Cancer. Int J Cancer. 2009;125:2893–902. doi: 10.1002/ijc.24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H, Kato Y, Erzinger S, et al. The Role of MMP-1 in Breast Cancer Growth and Metastasis to the Brain in a Xenograft Model. BMC Cancer. 2012;12:583. doi: 10.1186/1471-2407-12-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coussens L, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 57.Fini M, Cook J, Mohan R, Brinckerhoff C. Matrix Metalloproteinases. New York: Academic Press; 1998. [Google Scholar]
- 58.Kheradmand F, Werner E, Tremble P, et al. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 59.Zwirner J, Fayyazi A, Gotze O. Expression of the Anaphylatoxin C5a Receptor in Non-myeloid Cells. Mol Immunol. 1991;36:877–84. doi: 10.1016/s0161-5890(99)00109-1. [DOI] [PubMed] [Google Scholar]
- 60.Buchner R, Hugli T, Ember J, Morgan E. Expression of Functional Receptors for Human C5a Anaphylatoxin (CD88) on the Human Hepatocellular Carcinoma Cell Line HepG2. Stimulation of Acute-phase Protein-specific MRNA and Protein Synthesis by Human C5a Anaphylatoxin. J Immunol. 1995;155:308–15. [PubMed] [Google Scholar]
- 61•.Speidl W, Kastl S, Hutter R, et al. The Complement Component C5a Is Present in Human Coronary Lesions in Vivo and Induces the Expression of MMP-1 and MMP-9 in Human Macrophages in Vitro. FASEB J. 2011;25:35–44. doi: 10.1096/fj.10-156083. C5a prevents the activation of apoptotic caspase 3 and DNA fragmentation and therefore, may function as an antiapoptotic. As an antiapoptotic, C5a may potentially aid in tumor progression via stimulation of the mitogen-activated protein kinase (MAPK) pathway. [DOI] [PubMed] [Google Scholar]
- 62•.Rutkowski M, Sughrue M, Kane A, et al. Cancer and the Complement Cascade. Mol Cancer Res. 2010;8:1453–465. doi: 10.1158/1541-7786.MCR-10-0225. C5a as well as other complement proteins have been implicated in tumorigenesis. Collectively, these proteins have been found to cause dysregulation of mitogenic signaling pathways, sustained cellular proliferation, insensitivity to apoptosis, angiogenesis, and invasion and migration. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee P, Pasinetti G. Complement Anaphylatoxin C5a Neuroprotects through Mitogen-activated Protein Kinase-dependent Inhibition of Caspase 3. J Neurochem. 2001;77:43–49. doi: 10.1046/j.1471-4159.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- 64.Daveau M, Benard M, Scotte M, et al. Expression of a functional C5a receptor in regenerating hepatocytes and its involvement in a proliferative signaling pathway in rat. J Immunol. 2004;173:3418–24. doi: 10.4049/jimmunol.173.5.3418. [DOI] [PubMed] [Google Scholar]
- 65.Cheng T, Chang M, Huang S. Overexpression of Circulating C-Met Messenger RNA Is Significantly Correlated With Nodal Stage and Early Recurrence in Non-Small Cell Lung Cancer. Chest. 2005;128:1453–460. doi: 10.1378/chest.128.3.1453. [DOI] [PubMed] [Google Scholar]
- 66.Schindler R, Gefland J, Dinarello C. Recombinant C5a Stimulates Transcription Rather than Translation of Interleukin-1 (IL-1) and Tumor Necrosis Factor: Translational Signal Provided by Lipopolysaccharide or IL-1 Itself. Blood. 1990;76:1631–638. [PubMed] [Google Scholar]
- 67.Luo J, Maeda S, Hsu L, et al. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNF-α to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 68.Apte R, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 69.Markiewski M, DeAngelis R, Strey C, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412–8. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Floreani A, Gunselman S, Heires A, et al. Novel C5a Agonist-Based Dendritic Cell Vaccine in a Murine Model of Melanoma. Cell Cycle. 2007;6:2835–839. doi: 10.4161/cc.6.22.4899. [DOI] [PubMed] [Google Scholar]
- 72.Kollessery G, Nordgren T, Mittal A, et al. Tumor-specific Peptide-based Vaccines Containing the Conformationally Biased, Response-selective C5a Agonists EP54 and EP67 Protect against Aggressive Large B Cell Lymphoma in a Syngeneic Murine Model. Vaccine. 2011;35:5904–910. doi: 10.1016/j.vaccine.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 73.Rudilla F, Fayolle C, Cesares N, et al. Combination of a TLR4 Ligand and Anaphylatoxin C5a for the Induction of Antigen-specific Cytotoxic T Cell Response. Vaccine. 2012;30:2848–4858. doi: 10.1016/j.vaccine.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 74.Nandakumar KS, Jansson A, Xu B, et al. A recombinant vaccine effectively induces c5a-specific neutralizing antibodies and prevents arthritis. PLoS ONE. 2010;5(10):e13511. doi: 10.1371/journal.pone.0013511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kessel C1, Nandakumar KS, Peters FB, et al. A single functional group substitution in c5a breaks B cell and T cell tolerance and protects against experimental arthritis. 2014;66(3):610–21. doi: 10.1002/art.38237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cavaco CK, Patras KA, Zlamal JE, et al. A novel C5a-derived immunobiotic peptide reduces Streptococcus agalactiae colonization through targeted bacterial killing. 2013;57:5492–9. doi: 10.1128/AAC.01590-13. [DOI] [PMC free article] [PubMed] [Google Scholar]