Abstract
Inhalation of diacetyl, a butter flavoring, causes airway responses potentially mediated by sensory nerves. This study examines diacetyl-induced changes in sensory nerves of tracheal epithelium. Rats (n = 6/group) inhaled 0-, 25-, 249-, or 346-ppm diacetyl for 6 hr. Tracheas and vagal ganglia were removed 1-day postexposure and labeled for substance P (SP) or protein gene product 9.5 (PGP9.5). Vagal ganglia neurons projecting to airway epithelium were identified by axonal transport of fluorescent microspheres intratracheally instilled 14 days before diacetyl inhalation. End points were SP and PGP9.5 nerve fiber density (NFD) in tracheal epithelium and SP-positive neurons projecting to the trachea. PGP9.5-immunoreactive NFD decreased in foci with denuded epithelium, suggesting loss of airway sensory innervation. However, in the intact epithelium adjacent to denuded foci, SP-immunoreactive NFD increased from 0.01 ± 0.002 in controls to 0.05 ± 0.01 after exposure to 346-ppm diacetyl. In vagal ganglia, SP-positive airway neurons increased from 3.3 ± 3.0% in controls to 25.5 ± 6.6% after inhaling 346-ppm diacetyl. Thus, diacetyl inhalation increases SP levels in sensory nerves of airway epithelium. Because SP release in airways promotes inflammation and activation of sensory nerves mediates reflexes, neural changes may contribute to flavorings-related lung disease pathogenesis.
Keywords: flavorings, inhalation toxicity, airway epithelium, airway innervation, airway inflammation, cough
Introduction
Exposure to vapors from butter flavoring induces fixed airways obstruction, a condition that is commonly called “popcorn workers” lung (PWL; Kreiss et al. 2002). Affected workers have multiple symptoms of cough, shortness of breath and wheezing, and morphologic changes in the lung consisting of air trapping, marked thickening of bronchial walls, and cylindrical bronchiectasis. Some of these workers have clinical bronchiolitis obliterans, and histopathology in a small number of biopsied patients is consistent with constrictive bronchiolitis obliterans (Kreiss et al. 2002; Akpinar-Elci et al. 2004). In rats, exposure to vapors from artificial butter flavorings induced necrotizing bronchitis and necrosuppurative rhinitis, a finding that is consistent with airway epithelial injury being the underlying cause for bronchiolitis obliterans (Hubbs et al. 2008; King 1989).
Diacetyl, an α-diketone, is a major component of butter flavoring vapors and its estimated cumulative exposure correlates with lung disease in the sentinel popcorn production plant (Kreiss et al. 2002). Subsequently, clinical bronchiolitis obliterans and other forms of lung disease were identified in additional popcorn plants and in workers who made flavorings (Kanwal, 2008; Kanwal et al. 2006, 2007; van Rooy et al. 2007, 2009; Kreiss 2007). Like diacetyl-containing butter flavoring vapors, inhalation of diacetyl as a single agent exposure by rats or mice caused epithelial necrosis; the injury in rodents was generally in the upper airways (Hubbs et al. 2008; Morgan et al. 2008). Pharmacokinetic modeling indicated the reason for the different location of airway injury in rodents and humans: when inhaled, diacetyl doses to human intrapulmonary airways may be much higher than the doses to rodent intrapulmonary airways, whereas the rodent nose is efficient in scrubbing the vapor (Gloede et al. 2011; Morris and Hubbs 2009). At the appropriate dose, respiratory epithelial cells were damaged by diacetyl, and respiratory epithelial damage is considered the underlying cause for bronchiolitis obliterans (Morris and Hubbs 2009; Palmer et al. 2011; King 1989).
Respiratory symptoms, including wheezing or asthma-like symptoms, are also reported by workers exposed to flavorings (Kanwal et al. 2006; van Rooy et al. 2009). Irreversible fixed airways obstruction typical of clinical bronchiolitis obliterans is well described in studies of diacetyl-exposed workers (Kreiss et al. 2002; Akpinar-Elci et al. 2004; National Institute for Occupational Safety and Health [NIOSH] 2011). However, the full spectrum of respiratory disease in diacetyl-exposed workers may include reversible airways obstruction such as asthma (van Rooy et al. 2009; NIOSH 2011). Importantly, in vitro, diacetyl can increase airway reactivity to methacholine and is also a potential sensitizing agent (Anderson et al. 2007; Roberts, York, and Basketter 1999; Fedan et al. 2006). An understanding of the full spectrum of diacetyl-induced airways disease is needed.
Airway sensory innervation plays an important role in airway hyperresponsiveness and neurogenic inflammation. Substance P (SP) is a neuropeptide expressed extensively in peripheral sensory neurons and released from airway sensory C-fibers including those of the airway epithelium (Joos et al. 1994). SP induces airway smooth muscle contraction, vasodilatation, edema and mucus hypersecretion, symptoms associated with inflammatory airway diseases, and is also important in pathology of asthma and cough (Barnes 1986). Human airways have sensory nerve fibers that contain SP, and the lengths of these fibers are increased in asthmatics compared to normal airways (Ollerenshaw et al. 1991). In rats and guinea pigs, SP-containing nerve fibers innervate the airway epithelium and other effector structures within the airway wall and respond to noxious chemicals (Lundberg et al. 1984; Woolf and Salter 2000). The cell bodies of SP-containing vagal sensory nerves that project into airway epithelium are almost exclusively located in jugular and nodose ganglia (Pedersen et al. 1998).
Inhalation of occupational chemical irritants has been shown to stimulate the afferent sensory nerve fibers to trigger the release of neuropeptides (Lundberg and Saria 1983; Wu et al. 2002). For example, inhalation of toluene diisocyanate, which induces occupational asthma, increases SP in the sensory neurons of nasal mucosa and allergen provocation increases SP content in nasal cavity and induces neurogenic inflammation (Nieber et al. 1991; Hunter et al. 2000). Therefore, the objectives of the current study were to determine whether diacetyl inhalation alters airway innervation, in general, or specifically SP production in sensory nerves of tracheal epithelium and associated ganglia in rats. Immunocytochemical identification of SP-containing sensory C fibers was accomplished using antibodies directed against SP. General airway innervation was characterized using the neuronal marker, protein gene product 9.5 (PGP9.5), a carboxyl-terminal hydrolase selectively expressed in neural tissues, including nerves in the airways (Thompson et al. 1983; Lauweryns and van Ranst 1988).
Material and Method
Animals
Male Sprague-Dawley rats (Hilltop Lab Animals, Scottdale, PA) weighing between 200 and 250 g were housed in individually ventilated micro-isolator units ventilated with high-efficiency particulate air-filtered laminar flow air (Thoren Caging Systems, Hazleton, PA) and bedding material consisted of autoclaved Alpha-Dri™ virgin cellulose chips (Shepherd Specialty Papers, Watertown, TN) and hardwood beta-chips (NEPCO, Warrensburg, NY). Rats were provided tap water ad libitum and autoclaved Harlan Teklad Global 18% protein diet (Harlan Teklad, Madison, WI). Rats were given 7 to 16 days of acclimation before experimental exposure. The animal exposures were conducted in the Association for Assessment and Accreditation of Laboratory Animal Care International–approved animal facility at the NIOSH, and the Institutional Animal Care and Use Committee approved the animal use protocol.
Red Fluorescent Latex Microsphere Instillation
Red fluorescent microspheres (Red Fluorescent RetroBeads™; LumaFluor Inc., Durham, NC) were used as retrograde tracers revealing neurons in the nodose and jugular ganglia that project fibers to the airway epithelium. The microspheres are not cytotoxic (Katz, Burkhalter, and Dreyer 1984; Colin, Donoff, and Foote 1989). The labeling procedures were followed as previously described with minor modifications (Hunter et al. 2011). Briefly, 14 days prior to diacetyl exposure, rats were anesthetized with intraperitoneal injections of sodium brevital (50 mg/kg; Eli Lilly, Indianapolis, IN). Red fluorescent latex microspheres were diluted 1:4 with sterile water and 20 μl was instilled into the proximal trachea using a rat feeder tube. To ensure even distribution of the beads over the tracheal mucosa, the rats were rotated circularly 3 times on their anterior–posterior axis.
Diacetyl Exposure
Fourteen days after microsphere instillation, rats were exposed to diacetyl vapors in a whole-body inhalation chamber as previously described (Hubbs et al. 2008) with modifications. Liquid diacetyl (product B85307, lot 03798LJ, Aldrich, St. Louis, MO) was injected from a computer-controlled syringe pump (Model 210; KD Scientific Inc., Holliston, MA) through a septum and into a heated cross section of stainless steel tubing with a conditioned diluent airstream (20 lpm). The tubing’s external surface was heated to 150°C, which resulted in an air temperature of 70°C where the liquid diacetyl was injected and vaporized. The air–vapor mix then passed into the exposure chamber and the diacetyl concentration was measured with a diacetyl-calibrated volatile organic meter (PGM-7600, RAE Systems, San Jose, CA). The inhalation chamber temperature for both diacetyl-exposed and air-exposed rats was maintained in the thermoneutral zone for the rat at 27.0 to 27.8°C using a flow temperature controller (National Research Council 2011). Chamber relative humidity was controlled between 30 and 40% for animal comfort. Gravimetric filter measurements, a scanning mobility particle sizer (TSI, St. Paul, MN) and an aerodynamic particle sizer (TSI, St. Paul, MN), were utilized to determine if any of the diacetyl had condensed into an aerosol. All measurements indicated negligible aerosol formation.
Rats (n = 6/exposure group) were exposed to diacetyl target concentrations of 0 (air control), 25 (low), 250 (mid), or 350 (high) ppm for 6-hr continuous exposure. Each exposure group was divided into 2 blocks (3 rats/block). The resultant time-weighted average exposure concentrations (mean ± standard deviation [SD]) were low-dose block 1: 25.0 ± 3.5; low-dose block 2: 25.6 ± 8; mid-dose block 1: 248.2 ± 20.2; mid-dose block 2: 249.5 ± 18.4; high-dose block 1: 342.3 ± 29.6; and high-dose block 2: 349.8 ± 30. After exposure completion, the rats were returned to the individually ventilated cages. Rats were necropsied the following day, 18 to 20 hr after removal from the exposure chamber. The doses were chosen based on the previously published study in our laboratory showing that diacetyl concentrations of 224 ppm or greater induced epithelial necrosis and inflammation primarily in the trachea while bronchi were damaged at concentrations greater than 294.6 ppm (Hubbs et al. 2002, 2008). Exposures approaching 100 ppm have been measured in the workplace and differences in the anatomic location of respiratory epithelial injury in rodents and man can be explained by pharmacokinetic modeling (Kreiss et al. 2002; Kanwal et al. 2006; Morris and Hubbs 2009; Gloede et al. 2011; Morris 2012).
Preparation of Tissues for Immunocytochemical Procedures
Rats were euthanized with intraperitoneal injection of Sleep-away (Fort Dodge Animal Health, Fort Dodge, IA). The right and left jugular and nodose ganglion complex and the trachea were removed. Tissues were processed according to the procedures previously published (Wilfong and Dey 2004). All tissues were fixed immediately by immersion in 15% saturated picric acid, 2% paraformaldehyde, and 0.15 M phosphate buffer for 3 hr at 4°C, rinsed in 0.1 M phosphate-buffered saline containing 0.3% Triton-X-100 (PBS-Tx) overnight at 4°C. The next day, tissues were frozen in isopentane cooled with dry ice and stored in airtight polythene bags at −70°C for further analysis. Tracheas were sectioned longitudinally in order to maximize the amount of epithelium in each section. Two serial 12-μm cryostat sections of trachea were sampled at 100-μm intervals, and each serial section was collected separately on 2 different gelatin-coated coverslips (6 sections per coverslip). One coverslip was used for SP immunostaining and the other for PGP9.5 immunostaining. The entire thicknesses of nodose and jugular ganglia were serially sectioned together longitudinally and every fourth section was collected and stained with SP antibody.
Immunocytochemistry
The immunocytochemical methodology used was a slight modification of the procedure that has been described previously (Dey, Satterfield, and Altemus 1999). Briefly, 100 μl of SP polyclonal rabbit primary antiserum (diluted 1:200; Peninsula Laboratories, LLC, San Carlos, CA) was spread over a coverslip and incubated for 30 min at 37°C in a humidified chamber and then rinsed 3 times with PBS-Tx; 100 μl of a 1:50 dilution of donkey anti-rabbit Alexa 488 secondary antibody (Molecular Probes/Invitrogen, Carlsbad, CA) was spread on the section, incubated for 30 min at 37°C in a humidified chamber and rinsed as above. Coverslips were mounted on glass slides in Fluoromount (Southern Biotechnology, Birmingham, AL). Similar staining conditions were followed for PGP9.5 except that incubation of primary antibody (diluted 1:50; Millipore, Billerica, MA) was extended to overnight at 4°C.
Analysis of Immunoreactivity
Because the microspheres instilled in the trachea become distributed throughout the conducting airways, their presence in cell bodies of sensory ganglia reflects retrograde transport from throughout the tracheobronchial tree where epithelial innervation is present. The microspheres identify only nerve cell bodies of neurons with fibers projecting to the epithelium and not fibers distributed to the submucosa (Hunter and Undem 1999; Hunter et al. 2000). Fluorescent images of the jugular and nodose neurons were randomly captured using a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) using multitract rhodamine and fluorescein filters. Fluorescent images of each field were saved as single image files displaying three different frames consisting of 1 with the fluorescein filter (displayed as green in Figure 1A), the rhodamine filter (displayed as red in Figure 1B), and the third image as a digitally combined image of the 2 original images (Figure 1C).
Figure 1.
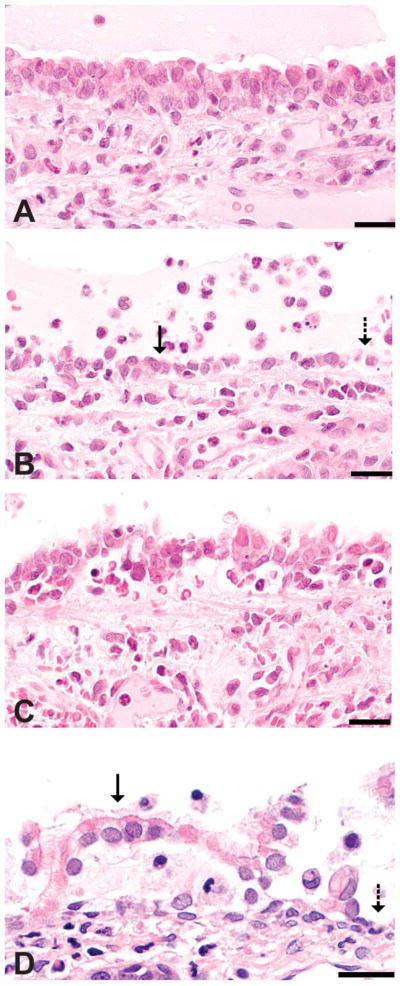
Classification of histopathologic alterations in respiratory epithelium. Images are from tracheas of rats inhaling 356 ppm in a previous study (Hubbs et al. 2008) and the rat nose from a high-dose animal in this study. (A) Intact respiratory epithelium in a diacetyl-exposed rat trachea. (B) Attenuated epithelium (solid arrow) in the diacetyl-exposed trachea may represent an attempt at sliding repair of denuded epithelium (dashed arrow). (C) Tracheal epithelial necrosis with detachment. (D) Respiratory epithelial detachment (solid arrow) and denuded basement membrane (dashed arrow) in the nose of a rat in the high-dose group from the current study. Hematoxylin and eosin stain; bar = 20 μm.
For determination of SP-positive neurons, multiframed image files were opened in image analysis software (Optimas 6.5, Media Cybernetics, Bethesda, MD) and converted to gray scale. Neurons with red fluorescent microspheres were identified, and the perimeter of that same neuron image was outlined in the fluorescein image. The intensity of SP immunoreactivity was determined by calculating mean gray value (MGV) of each neuron using Optimas software. Neurons with MGV ≥ 50 were considered positive for SP immunoreactivity. The threshold MGV was set by initial observation of several positive or negative neurons in the jugular and nodose ganglia and then by digital analysis. Percentage of SP-positive neurons innervating the airway epithelium was determined by dividing the number of SP-positive red fluorescent microsphere-containing neurons (i.e., SP-positive airway-projecting neurons) by the total number of red fluorescent microsphere-labeled neurons (total airway projecting neurons). A total of 199 total neurons projecting to airways were counted with an average of 9.95 + 6.22 (mean + SD) per ganglion.
SP and PGP9.5 Nerve Fiber Density (NFD)
Sections of tracheal mucosa immunostained for SP and PGP9.5 were observed under Zeiss LSM 510 confocal microscope. Fifteen random images of the tracheal epithelium were captured from each coverslip. Threshold operations were performed to capture only those pixels representing SP-IR and PGP9.5-IR nerve fibers in the epithelium. Total nerve fiber area in the entire epithelial region of the image was recorded using Optimas image analysis software. In addition, the corresponding entire length basement membrane was measured. The NFD was calculated by dividing SP or PGP9.5 nerve fiber area by corresponding basement membrane length. Therefore, NFD in our study is the ratio of nerve fiber area and basement membrane length. NFD was further subdivided into groups based on the extent of epithelial damage resulting from diacetyl exposure (Hubbs et al. 2008). Regions identified were classified as intact epithelium (intact), which appear normal in the fluorescence images; foci of denudation (denuded); intact epithelium adjacent to denuded foci (adjacent to denuded); foci where epithlium was detaching from the basement membrane (detaching); and foci of attenuated epithelium (attenuated). The classification was based upon the spectrum of histopathologic alterations previously observed in the trachea and nose of rats inhaling 345 to 365-ppm diacetyl for 6 hr and necropsied the follow day (Figure 1; Hubbs et al. 2008).
Statistical Analysis
The data were analyzed using SAS/STAT software, Version 9.2 of the SAS System for Windows. Treatment comparisons were performed with one-way analysis of variance using Proc Mixed. Post hoc pairwise comparisons between treatment groups were performed using Fisher’s Least Significant Difference tests. All differences were considered significant at p ≤ .05.
Results
SP Immunoreactivity in Neurons of Jugular and Nodose Ganglia Following Diacetyl Exposure
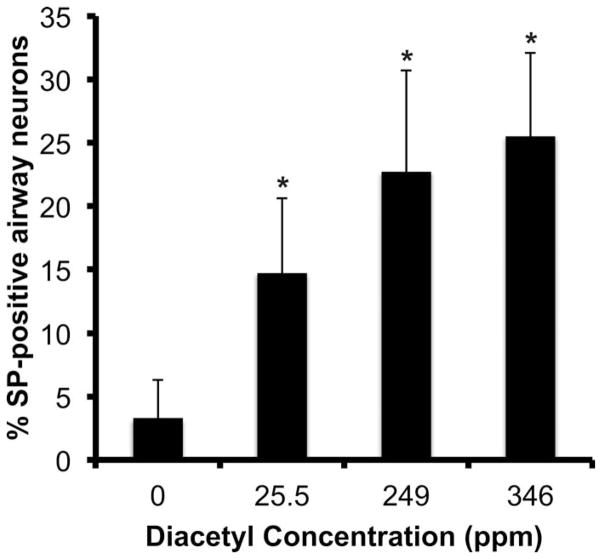
SP-containing neurons (Figure 2A) of nodose and jugular ganglia innervating respiratory epithelium of the trachea were identified by the presence of red microspheres in the cell bodies (Figure 2B). Digitally superimposed images of SP and microspheres confirmed that some airway neurons were SP-positive (solid arrows in Figure 2C) and others were negative (dashed arrows in Figure 2C). Diacetyl inhalation caused a dose-dependent increase in the percentage of SP-immunoreactive airway neurons in jugular ganglia as compared to air-exposed controls (Figure 3). In control animals, the percentage of SP-IR airway neurons (i.e., contained red microspheres) was 3.3 ± 3.0%. SP-IR airway neurons increased significantly to 14.7 ± 5.9, 22.7 ± 8.4, and 25.5 ± 6.6% in 25-, 249-, and 346-ppm diacetyl-exposed animals, respectively.
Figure 2.
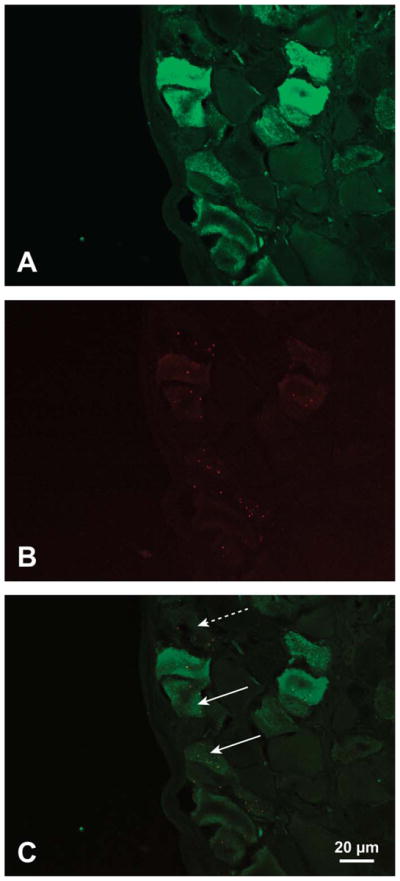
Immunofluorescence staining for SP (green) and red fluorescent microspheres in nerve cell bodies of jugular ganglion using rabbit anti-SP primary and fluorescein-labeled goat anti-rabbit secondary. (A) SP immunoreactivity in neurons of a jugular ganglion. (B) Red fluorescent microspheres in cell bodies of jugular ganglion from A. The microspheres indicate cell bodies of neurons projecting to the airway epithelium. (C) Merged images from A and B with SP-positive airway neurons identified by fluorescent microspheres. Solid arrows indicate two SP-positive airway neurons. Dashed arrow indicates airway neuron lacking SP immunoreactivity. SP = substance P.
Figure 3.
Percentage of SP-positive airway neurons in nodose–jugular ganglia of rats 18 to 20 hr after air or 25.5-, 249-, or 346-ppm diacetyl exposure. SP = substance P.
*p ≤ .05.
SP and PGP9.5 NFD in Tracheal Epithelium Following Diacetyl Exposure
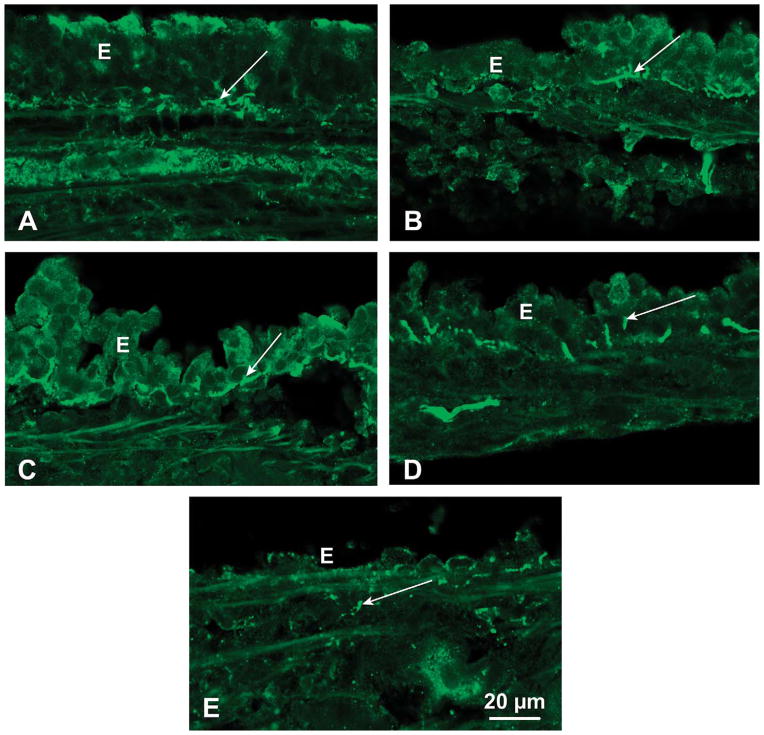
Only the medium and high doses of diacetyl caused identifiable damage of airway epithelium. Examples of intact, denuded, attenuated, detaching, and regions adjacent to denuded are shown in Figure 4. Foci of tracheal epithelial denudation and foci of epithelial attenuation were most frequently observed in rats inhaling 346-ppm diacetyl, were sometimes seen in rats inhaling 249-ppm diacetyl, and were absent in rats inhaling air or 25-ppm diacetyl.
Figure 4.
SP-immunoreactive nerves (arrows) in intact or damaged regions of tracheal epithelium (E): (A) SP-IR nerves in the intact epithelium. (B) SP-positive nerves in epithelium adjacent to denuded regions after 346-ppm diacetyl. (C) SP innervation in region of detaching epithelium. (D) SP nerves in attenuated epithelium. (E) Lack of SP-immunoreactive nerves in regions where epithelium was denuded. A few positive nerves are located below the basement membrane (arrow). SP = substance P; SP-IR = SP immunoreactive.
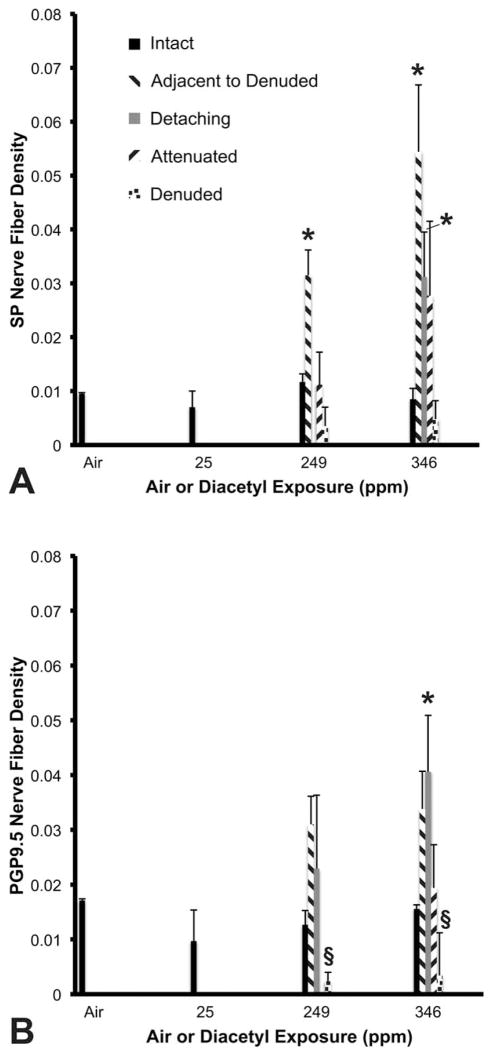
Figure 5A shows the results of diacetyl exposure on SP NFD. After inhaling 346-ppm diacetyl, SP NFD was unchanged in the fields with intact tracheal epithelium. However, SP NFD was significantly increased from air-exposed controls (0.01 ± 0.002%) when the intact epithelium was adjacent to denuded foci (0.05 ± 0.01%) and in foci with detaching epithelium (0.03 ± 0.01%). Similarly, in animals exposed to 249 ppm of diacetyl, SP NFD was unchanged in the fields with intact, attenuated, or denuded epithelium (areas of detaching epithelium were insufficient for measurement). However, the innervation was significantly increased in the intact epithelium adjacent to denuded foci (0.03 ± 0.01%) compared to air exposure.
Figure 5.
SP and PGP9.5 nerve fiber density in five different regions of epithelial damage. (A) SP is increased in the epithelium adjacent to denuded epithelium. In the highest exposure group, SP is also increased in the detaching epithelium. Air and 25-ppm exposure contained only intact epithelium. Insufficient data for significance of detaching epithelium in 249-ppm group. (B) PGP9.5 nerve fiber density. Insufficient data for attenuated epithelium in 249-ppm group. SP = substance P; PGP9.5 = protein gene product 9.5.
*Significantly greater than the control. §Significantly less than the control. p ≤ .05.
Figure 5B shows the effects of diacetyl inhalation on PGP9.5 NFD. Inhalation of 346-ppm diacetyl significantly increased PGP9.5 NFD in the detaching regions of tracheal epithelium (0.04 ± 0.01%) compared to air-exposed controls (0.02 ± 0.0003%). Nerve density bordered on significance in the epithelium adjacent to denuded foci (0.03 ± 0.007%; p = .0560) compared to controls. NFD was not statistically different in fields with intact or attenuated epithelium. Exposure at 249-ppm diacetyl was associated with an increase in PGP9.5 NFD in tracheal epithelium adjacent to denuded foci that bordered on statistical significance (0.03 ± 0.01%; p = .0560), but minimal changes not approaching significance were observed in intact or detaching epithelium compared to control (0.01 ± 0.003%). Interestingly, PGP9.5 NFD was significantly decreased in fields of denuded epithelium in animals exposed to either 249-ppm (0.002 ± 0.002%) or 346-ppm (0.003 ± 0.008%) diacetyl compared to control. PGP9.5 NFD in rats exposed to 25 ppm of diacetyl did not differ from controls rats.
Discussion
The results in the present study indicate that diacetyl inhalation dose dependently increases the number of SP-containing neurons in the nodose and jugular ganglia that project into airway epithelium and increases SP sensory nerve fibers innervating the tracheal epithelium. Increased SP NFD and increased SP production (as shown by increased percentage of SP-positive airway neurons in nodose and jugular ganglia) would be expected to favor the development of airway inflammation. For example, SP released by airway sensory nerves is involved in neurogenic inflammation and is associated with clinical symptoms such as coughing and wheezing (Pernow 1985; Barnes 1990; Nieber et al. 1992). In addition, SP increases ozone- and nerve growth factor-induced airway responsiveness in ferret trachea, strongly supporting the role of SP in enhancing airway reactivity (Wu, Satterfield, and Dey 2003; Wu and Dey 2006). SP was recently shown to increase in smokers with chronic obstructive pulmonary disease relative to those without evidence of obstruction (Vatrella et al. 2010). Therefore, increased SP production may contribute to flavorings-related lung disease associated with diacetyl inhalation. Often in patients with bronchiolitis obliterans, symptoms start with persistent coughing, wheezing, and dyspnea before progressing into shortness of breath (Akpinar-Elci et al. 2004). Previous studies have confirmed the role of C-fiber pathways in producing cough (Canning, Mori, and Mazzone 2006). Further, airway inflammation may increase sensitivity of SP-containing C-fibers to allergens and irritants (Lee et al. 2002). Importantly, SP is present in human airway sensory C-fiber nerves within and beneath the epithelium, similar to the location in the epithelium of rats (Sekizawa et al. 1996).
Previous studies suggest some factors that may influence the diacetyl-induced changes in SP production. Acute diacetyl exposure is associated with significant neutrophilic inflammation and necrosis of upper airways (Hubbs et al. 2008; Morgan et al. 2008; Palmer et al. 2011). Interleukin-1 (IL-1), an inflammatory cytokine, increases SP gene expression and release from neurons in sensory and autonomic ganglia (Kessler et al. 1993; Inoue et al. 1999; Wu et al. 2002; Skoff, Zhao, and Adler 2009). Thus, IL-1 released from diacetyl-induced inflammatory tissues could potentially increase the production and accumulation of SP in sensory nerve terminals in the immediate adjacent tissue, although prior reports that IL-1 is produced during diacetyl exposure were not found. Another possibility is diacetyl could directly stimulate sensory nerve endings or indirectly evoke an irritant response through a receptor-mediated pathway to increase SP production from nodose or jugular ganglia (Trevisani et al. 2007). However, our data suggest another potential mechanism. Diacetyl-induced increases in SP- and PGP9.5-positive nerve fibers were only seen in foci of detaching epithelium or in foci adjacent to denuded epithelium. Conversely, SP and PGP9.5 nerves were significantly decreased in the denuded regions. This suggests that both sensory nerves and epithelial cells were injured in those sites of extensive epithelial damage represented as denudation. Axonal injury can increase SP production, particularly at sites of inflammation and suggests that mild or moderate injury at sites of epithelial damage may contribute to increased SP production (Weissner et al. 2006; Wong and Oblinger 1991; Skoff, Zhao, and Adler 2009). However, at sites of extensive damage, such as denuded regions, nerve and epithelial damage may be so extensive that the fibers are no longer identifiable.
In our current study, diacetyl dose dependently increased the number of SP-positive neurons in retrogradely labeled airway neurons of vagal ganglia. This result suggests that diacetyl directly affects sensory neurons that project into airways, which, in the trachea, originate from jugular and nodose ganglia. However, it should be noted that even though nodose ganglia are a major source of sensory airway innervation into airways, neurons that contain SP in the jugular ganglion are more responsive to irritants than those in nodose ganglion of rat and guinea pig (Hunter et al. 2011; Hunter and Undem 1999). These findings support our conclusion that the flavorings exposure affected SP-containing sensory C-fibers originating in the jugular ganglia (although we did not distinguish these two ganglia in our study). The increase in PGP9.5-positive nerves in the epithelium adjacent to denuded or detaching regions, similar to the distribution of SP fibers, further substantiates this effect of diacetyl exposure on airway epithelial nerves. A similar increase in PGP9.5 in guinea pig airways was reported with toluene diisocyanate (Mapp et al. 1998), known to cause occupational asthma in some exposed individuals.
Therefore, the current findings suggest that diacetyl exposure increases sensory innervation and SP production in injured regions of tracheal epithelium. This may contribute to diacetyl-induced airway toxicity and associated symptoms in diacetyl-exposed workers. However, the role of axonal injury, the potential role and mechanisms by which sensory nerves become sensitized, and the potential contribution of cytokines released by inflammatory cells and necrotic epithelial cells remain to be investigated. Our findings suggest that airway epithelial injury from diacetyl exposure changes sensory innervation and SP production. Although not directly demonstrated in the current study, altered airway responsiveness and respiratory pathologies associated with diacetyl exposures (Hubbs et al. 2008; Palmer et al. 2011; Morgan et al. 2008) may be at least partially mediated through the actions of SP released from sensory nerves.
Acknowledgments
This project was supported by NIOSH contract/order 212-2009-M-30140 to West Virginia University and by NIOSH intramural NORA projects Pathophysiology of Popcorn Workers’ Lung and Identifying the Causes of Flavorings-Related Lung Disease.
Abbreviations
- IL-1
interleukin-1
- lpm
liters per minute
- MGV
mean gray value
- NFD
nerve fiber density
- NIOSH
National Institute for Occupational Safety and Health
- PBS-Tx
0.1 M phosphate-buffered saline containing 0.3% Triton-X-100
- PGP9.5
protein gene product 9.5
- ppm
parts per million
- PWL
popcorn workers’ lung
- SP
substance P
- SP-IR or PGP9.5-IR
SP-immunoreactive or PGP9.5-immunoreactive
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of brand name does not constitute product endorsement.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24:298–302. doi: 10.1183/09031936.04.00013903. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Wells J, Fedorowicz A, Butterworth L, Meade B, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol Sci. 2007;97:355–63. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Asthma as an axon reflex. Lancet. 1986;1:242–45. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neurogenic inflammation in airways and its modulation. Arch Int Pharmacodyn Ther. 1990;303:67–82. [PubMed] [Google Scholar]
- Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223–42. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Colin W, Donoff RB, Foote WE. Fluorescent latex microspheres as a retrograde tracer in the peripheral nervous system. Brain Res. 1989;486:334–39. doi: 10.1016/0006-8993(89)90520-9. [DOI] [PubMed] [Google Scholar]
- Dey RD, Satterfield B, Altemus JB. Innervation of tracheal epithelium and smooth muscle by neurons in airway ganglia. Anat Rec. 1999;254:166–72. doi: 10.1002/(SICI)1097-0185(19990201)254:2<166::AID-AR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Fedan JS, Dowdy JA, Fedan KB, Hubbs AF. Popcorn worker’s lung: In vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol Appl Pharmacol. 2006;215:17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Gloede E, Cichocki JA, Baldino JB, Morris JB. A validated hybrid computational fluid dynamics-physiologically based pharmacokinetic model for respiratory tract vapor absorption in the human and rat and its application to inhalation dosimetry of diacetyl. Toxicol Sci. 2011;123:231–46. doi: 10.1093/toxsci/kfr165. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, Jones WG. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharmacol. 2002;185:128–35. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol Pathol. 2008;36:330–44. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol. 2011;45:359–65. doi: 10.1165/rcmb.2010-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Satterfield BE, Huang J, Fedan JS, Dey RD. Toluene diisocyanate enhances substance P in sensory neurons innervating the nasal mucosa. Am J Respir Crit Care Med. 2000;161:543–49. doi: 10.1164/ajrccm.161.2.9812083. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–48. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–13. [PubMed] [Google Scholar]
- Joos GF, Germonpre PR, Kips JC, Peleman RA, Pauwels RA. Sensory neuropeptides and the human lower airways: Present state and future directions. Eur Respir J. 1994;7:1161–71. [PubMed] [Google Scholar]
- Kanwal R. Bronchiolitis obliterans in workers exposed to flavoring chemicals. Curr Opin Pulm Med. 2008;14:141–46. doi: 10.1097/MCP.0b013e3282f52478. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Kullman G, Piacitelli C, Boylstein R, Sahakian N, Martin S, Fedan K, Kreiss K. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup Environ Med. 2006;48:149–57. doi: 10.1097/01.jom.0000194152.48728.fb. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Kullman G, Sahakian N, Harber H, Materna B, Kreiss K. Severe fixed obstructive lung disease in flavoring workers. Am J Respir Crit Care Med. 2007;175:A427. [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Kessler JA, Freidin MM, Kalberg C, Chandross KJ. Cytokines regulate substance P expression in sympathetic neurons. Regul Pept. 1993;46:70–75. doi: 10.1016/0167-0115(93)90014-y. [DOI] [PubMed] [Google Scholar]
- King TE., Jr Bronchiolitis obliterans. Lung. 1989;167:69–93. doi: 10.1007/BF02714935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss K. Flavoring-related bronchiolitis obliterans. Curr Opin Allergy Clin Immunol. 2007;7:162–67. doi: 10.1097/ACI.0b013e3280298235. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–38. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Lauweryns JM, van Ranst L. Protein gene product 9.5 expression in the lungs of humans and other mammals. Immunocytochemical detection in neuroepithelial bodies, neuroendocrine cells and nerves. Neurosci Lett. 1988;85:311–16. doi: 10.1016/0304-3940(88)90584-8. [DOI] [PubMed] [Google Scholar]
- Lee LY, Kwong K, Lin YS, Gu Q. Hypersensitivity of bronchopulmonary C-fibers induced by airway mucosal inflammation: Cellular mechanisms. Pulm Pharmacol Ther. 2002;15:199–204. doi: 10.1006/pupt.2002.0338. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T, Martling CR, Saria A, Cuello C. Substance P-immunoreactive sensory nerves in the lower respiratory tract of various mammals including man. Cell Tissue Res. 1984;235:251–61. doi: 10.1007/BF00217848. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983;302:251–53. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- Mapp CE, Lucchini RE, Miotto D, Chitano P, Jovine L, Saetta M, Maestrelli P, Springall DR, Polak J, Fabbri LM. Immunization and challenge with toluene diisocyanate decrease tachykinin and calcitonin gene-related peptide immunoreactivity in guinea pig central airways. Am J Respir Crit Care Med. 1998;158:263–69. doi: 10.1164/ajrccm.158.1.9704061. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 2008;103:169–80. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JB. Biologically-based modeling insights in inhaled vapor absorption and dosimetry. Pharmacol Ther. 2012;136:401–13. doi: 10.1016/j.pharmthera.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hubbs AF. Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol Sci. 2009;108:173–83. doi: 10.1093/toxsci/kfn222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academic Press; Washington, DC: 2011. [Google Scholar]
- Nieber K, Baumgarten CR, Rathsack R, Furkert J, Oehme P, Kunkel G. Substance P and beta-endorphin-like immunoreactivity in lavage fluids of subjects with and without allergic asthma. J Allergy Clin Immunol. 1992;90:646–52. doi: 10.1016/0091-6749(92)90138-r. [DOI] [PubMed] [Google Scholar]
- Nieber K, Baumgarten CR, Witzel A, Rathsack R, Oehme P, Brunnee T, Kleine-Tebbe J, Kunkel G. The possible role of substance P in the allergic reaction, based on two different provocation models. Int Arch Allergy Appl Immunol. 1991;94:334–38. doi: 10.1159/000235397. [DOI] [PubMed] [Google Scholar]
- NIOSH. Criteria for a Recommended Standard: Occupational Exposure to Diacetyl and 2, 3-Pentanedione (external review draft, August 12, 2011) Department of Health and Human Services; Washington, DC: 2011. pp. 1–321. [Google Scholar]
- Ollerenshaw SL, Jarvis D, Sullivan CE, Woolcock AJ. Substance P immunoreactive nerves in airways from asthmatics and nonasthmatics. Eur Respir J. 1991;4:673–82. [PubMed] [Google Scholar]
- Palmer SM, Flake GP, Kelly FL, Zhang HL, Nugent JL, Kirby PJ, Foley JF, Gwinn WM, Morgan DL. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One. 2011;6:e17644. doi: 10.1371/journal.pone.0017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KE, Meeker SN, Riccio MM, Undem BJ. Selective stimulation of jugular ganglion afferent neurons in guinea pig airways by hypertonic saline. J Appl Physiol. 1998;84:499–506. doi: 10.1152/jappl.1998.84.2.499. [DOI] [PubMed] [Google Scholar]
- Pernow B. Role of tachykinins in neurogenic inflammation. J Immunol. 1985;135:812s–815s. [PubMed] [Google Scholar]
- Roberts DW, York M, Basketter DA. Structure-activity relationships in the murine local lymph node assay for skin sensitization: Alpha, beta-diketones. Contact Dermatitis. 1999;41:14–17. doi: 10.1111/j.1600-0536.1999.tb06201.x. [DOI] [PubMed] [Google Scholar]
- Sekizawa K, Jia YX, Ebihara T, Hirose Y, Hirayama Y, Sasaki H. Role of substance P in cough. Pulm Pharmacol. 1996;9:323–28. doi: 10.1006/pulp.1996.0042. [DOI] [PubMed] [Google Scholar]
- Skoff AM, Zhao C, Adler JE. Interleukin-1alpha regulates substance P expression and release in adult sensory neurons. Exp Neurol. 2009;217:395–400. doi: 10.1016/j.expneurol.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5—a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983;278:224–28. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–24. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 2007;176:498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- van Rooy FG, Smit LA, Houba R, Zaat VA, Rooyackers JM, Heederik DJ. A cross-sectional study of lung function and respiratory symptoms among chemical workers producing diacetyl for food flavourings. Occup Environ Med. 2009;66:105–110. doi: 10.1136/oem.2008.039560. [DOI] [PubMed] [Google Scholar]
- Vatrella A, Montagnani S, Calabrese C, Parrella R, Pelaia G, Biscione GL, Corcione N, Marsico SA, Guerra G. Neuropeptide expression in the airways of COPD patients and smokers with normal lung function. J Biol Regul Homeost Agents. 2010;24:425–32. [PubMed] [Google Scholar]
- Weissner W, Winterson BJ, Stuart-Tilley A, Devor M, Bove GM. Time course of substance P expression in dorsal root ganglia following complete spinal nerve transection. J Comp Neurol. 2006;497:78–87. doi: 10.1002/cne.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol. 2004;30:793–800. doi: 10.1165/rcmb.2003-0303OC. [DOI] [PubMed] [Google Scholar]
- Wong J, Oblinger MM. NGF rescues substance P expression but not neurofilament or tubulin gene expression in axotomized sensory neurons. J Neurosci. 1991;11:543–52. doi: 10.1523/JNEUROSCI.11-02-00543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–69. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Dey RD. Nerve growth factor-enhanced airway responsiveness involves substance P in ferret intrinsic airway neurons. Am J Physiol Lung Cell Mol Physiol. 2006;291:L111–18. doi: 10.1152/ajplung.00377.2005. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Satterfield BE, Dey RD. Substance P released from intrinsic airway neurons contributes to ozone-enhanced airway hyperresponsiveness in ferret trachea. J Appl Physiol. 2003;95:742–50. doi: 10.1152/japplphysiol.00109.2003. [DOI] [PubMed] [Google Scholar]
- Wu ZX, Satterfield BE, Fedan JS, Dey RD. Interleukin-1beta-induced airway hyperresponsiveness enhances substance P in intrinsic neurons of ferret airway. Am J Physiol Lung Cell Mol Physiol. 2002;283:L909–17. doi: 10.1152/ajplung.00363.2001. [DOI] [PubMed] [Google Scholar]