Abstract
Objectives
Anxiety sensitivity (AS) is related to the development and maintenance of posttraumatic stress disorder (PTSD) among cigarette smokers, and is also implicated in the amplification of acute nicotine withdrawal symptoms. The present study sought to examine the role of nicotine withdrawal in moderating the association between AS and PTSD symptom severity among a sample of treatment-seeking smokers with PTSD.
Method
Participants (n = 117) were enrolled in a randomized controlled trial for the treatment of PTSD and nicotine dependence. Cross-sectional data were randomly sampled from three different study time points. A series of multiple regression models were tested.
Results
Results revealed main effects of both AS and withdrawal severity on PTSD severity after controlling for gender, assessment time-point, negative affectivity, and biochemically verified smoking (expired carbon monoxide). The interaction of AS and withdrawal was also significant, and appeared to be specific to PTSD avoidance and hyperarousal symptoms. However, contrary to expectations, the association between AS and PTSD symptoms was only significant at relatively lower levels of nicotine withdrawal.
Conclusions
These findings highlight the complex interplay between AS, nicotine withdrawal, and their synergistic effect in terms of the exacerbation of PTSD symptomology.
Keywords: posttraumatic stress disorder, anxiety sensitivity, nicotine withdrawal, trauma-exposed smokers
While cigarette smoking has significantly declined over the past five decades (Ng et al., 2014), the prevalence of smoking remains high among individuals with psychiatric disorders compared to non-psychiatric populations (e.g., anxiety disorders: Lasser et al., 2000; posttraumatic stress disorder [PTSD]: Hapke et al., 2005; schizophrenia: de Leon & Diaz, 2005; depression: Strong et al., 2014). Furthermore, smoking is associated with exacerbation of mental health symptoms (Farrell et al., 2003), greater overall functional impairment (Schnoll, Goren, Annunziata, & Suaya, 2013), and higher health care costs due to higher incidence of physical illness (US Burden of Disease Collaborators, 2013). Conversely, smoking cessation is associated with reduction of psychiatric symptoms (Taylor et al., 2014). Given these associations and the recognized need to develop smoking cessation treatments tailored for psychiatric populations (Ziedonis et al., 2008), there has been an increased focus on better understanding the factors that may explain the impact of smoking on mental health symptoms (see for example: Richards et al., 2013).
In particular, smokers with PTSD are more likely to smoke heavily and report higher levels of nicotine dependence than those without the disorder (Feldner, Babson, & Zvolensky, 2007; Hapke et al., 2005). In fact, heavy smoking and higher levels of nicotine dependence are implicated in the development and exacerbation of PTSD symptomology (Feldner et al., 2007), especially avoidance and hyperarousal symptoms (Beckham et al., 1997). Compared to non-psychiatric smokers, daily smokers with PTSD report more difficulty quitting, as indexed by greater number of failed lifetime quit attempts, more severe withdrawal quit problems (Marshall et al., 2008), and poorer outcomes in smoking cessation programs (Zvolensky et al., 2008; Beckham, Calhoun, Dennis, Wilson, & Dedert, 2013).
Recent research has aimed to identify cognitive-affective vulnerability factors that underlie the associations between PTSD and smoking (Cook, McFall, Calhoun, & Beckham, 2007). One factor, anxiety sensitivity (AS), has gained attention as a possible explanatory factor for the development and maintenance of PTSD (Marshall, Miles, & Stewart, 2010). AS is posited as a trait-like cognitive vulnerability factor defined as one's tendency to catastrophically (mis)interpret the meaning of internal bodily anxiety-relevant sensations or interoceptive perturbation (i.e., “fear of fear”; Reiss et al., 1986). Indeed, AS is a risk factor for the development of anxiety and related disorders including PTSD (e.g., Olatunji & Wolitzky-Taylor, 2009). Specifically, data suggest that AS amplifies negative affective and physiological states, and exacerbates PTSD symptom severity in both non-clinical (Berenz, Vujanovic, Coffey, & Zvolensky, 2012) and clinical (Marshall et al., 2010; Lang, Kennedy, & Stein, 2002) trauma-exposed samples.
Moreover, initial work suggests that among trauma-exposed smokers, AS is related to the exacerbation of PTSD symptom severity (at least cross-sectionally), specifically hyperarousal symptoms (Farris, Vujanovic, Hogan, Schmidt, & Zvolensky, 2014). Additionally, among trauma-exposed smokers, smoking at heavier rates in the context of elevated AS, gives rise to more severe PTSD symptomology (Feldner et al., 2008). It is important to also note that AS is directly related to various aspects of cigarette smoking (Leventhal & Zvolensky, in press). That is, data suggest that AS is linked to several indices of smoking behavior, including smoking frequency (e.g. number of cigarettes a day; Dedert et al., 2012; Fu et al., 2007), nicotine dependence (Zvolensky, Farris, Schmidt, & Smits, 2014), severity of smoking urges during abstinence (Zvolensky, Farris, Guillot, & Leventhal, 2014), and duration of abstinence after attempting to quit (Assayag, Bernstein, Zvolensky, Steeves, & Stewart, 2012; Brown et al., 2001; Mullane et al., 2008; Zvolensky et al., 2009). In addition, AS has been posited as a possible central mechanism for explaining the association between emotional disorders and smoking (Zvolensky, Farris, Leventhal, & Schmidt, 2014). Specifically, while emotional disorder symptoms were found to predict higher and more severe nicotine dependence symptoms, this relationship was mediated (indirectly explained) by AS.
AS is also directly related to the experience of nicotine withdrawal symptoms (e.g., frustration, restlessness, anxiousness, irritability). Given that nicotine withdrawal is conceptualized as both a physiologically and psychologically distressing experience (Hughes, Hatsukami, Pickens, & Svikis, 1984), it is perhaps not surprising that various cognitive-affect processes appear to impact the subjective experience of withdrawal (e.g., thought suppression, experiential avoidance; Erskine et al., 2012; Farris, Zvolensky, & Schmidt, in press), including AS. In general, data suggest that higher levels of AS are associated with higher levels of both historically-reported nicotine withdrawal (Zvolensky, Baker, Leen-Feldner, Bonn-Miller, & Feldner, 2004) and acute (quit-day) nicotine withdrawal severity after deprivation (e.g., Marshall, Johnson, Bergman, Gibson, & Zvolensky, 2009; Zvolensky, Farris, Guillot, & Leventhal, 2014) and during quitting (Langdon et al., 2013). In fact, AS is associated with shorter latency to re-initiating smoking after nicotine deprivation in the context of the amplified expression of nicotine withdrawal (Zvolensky, Farris, Guillot, & Leventhal, 2014). Generally, research suggests that one's tendency to misinterpret the meaning of bodily sensations (such as symptoms experienced in the context of nicotine withdrawal) may importantly influence smoking re-initiation/maintenance.
While it logically follows that elevated AS may independently impact the experience of subjective nicotine withdrawal and concurrent PTSD symptom severity, little research has examined the interplay between AS and nicotine withdrawal in terms of PTSD (Morissette, Tull, Gulliver, Kamholz, & Zimering, 2007). One study found that among smokers with PTSD, relative to those without psychopathology, the subjective experience of nicotine withdrawal severity during acute deprivation predicted anxiety following an interoceptive exposure task (voluntary hyperventilation challenge; Feldner, Vujanovic, Gibson, & Zvolensky, 2008). Thus, initial data suggest that in the context of nicotine withdrawal, smokers with PTSD may be more vulnerable to experiencing anxiety states due to, in part, their interpretation of nicotine withdrawal symptoms, although this finding was not replicated in another experimental study (Vujanovic, Marshall-Berenz, Beckham, Bernstein, & Zvolensky, 2010). Theoretically, nicotine withdrawal could amplify the perceived threat of feared internal sensations (in other words, AS), which in turn may potentiate severity of PTSD symptoms among trauma-exposed smokers. Such a model therefore suggests that nicotine withdrawal (a state-dependent variable) acts as the moderator in the relationship between AS (a trait-like construct) and its prediction of PTSD symptoms. Given the highlighted importance of AS as a cognitive vulnerability factor for both PTSD and smoking, further research is warranted to test such a proposed model.
The current study aimed to fill this gap by examining relations between AS, nicotine withdrawal, and PTSD symptoms in a sample of treatment-seeking adult daily smokers diagnosed with PTSD. It was hypothesized that independent effects of higher AS and nicotine withdrawal would directly contribute to the severity of PTSD symptoms. A second aim of this study was to examine whether higher nicotine withdrawal symptom severity would moderate the strength of association between AS and PTSD symptom severity, based on the hypothesized “amplification” effect that more intense withdrawal states may have on AS (i.e., AS in the context of higher withdrawal would show a stronger effect on PTSD severity). Based on the existing literature (Berenz et al., 2011; Farris et al., 2014; Joseph et al., 2012), it was expected that any observed moderating effects would be evidenced most strongly in avoidance and hyperarousal PTSD symptoms.
Material and methods
Participants
Adult daily smokers with PTSD (n = 117) were recruited to participate in a randomized-controlled trial comparing standard smoking cessation treatment (varenicline and supportive counseling) versus combined standard smoking cessation with treatment of PTSD (varenicline, supportive counseling and Prolonged Exposure Therapy). Participants were eligible for the trial on the basis of being between ages of 18-65 years, self-reporting smoking at least 10 cigarettes per day, having a primary diagnosis of PTSD as defined by the DSM-IV-TR (APA, 2000) with symptom duration of at least three months, and having a total score ≥ 20 on the PTSD Symptom Scale Interview (PSS-I; Foa, Riggs, Dancu, & Rothbaum, 1993). Primary exclusion criteria were: alcohol or non-nicotine substance use disorder in the past 3 months, psychosis, prominent suicidal ideation, continuing intimate relationship with an abusive partner, and certain medical conditions contraindicated with the use of varenicline (e.g., cardiovascular disease or uncontrolled hypertension).
Procedure
Participants were recruited through public advertising (e.g. flyers, advertisements in a free city newspaper) and direct referrals from healthcare providers. Potentially eligible participants completed an in-person baseline assessment of PTSD symptoms, co-morbid Axis I psychological disorders per the Structured Clinical Interview of DSM-IV Disorders (SCID-I; First et al., 2002), and smoking history, which included biochemical verification of smoking status. After baseline (Week 0), eligible participants were prescribed varenicline and asked to set a quit date within a week of this visit. They were then randomized to the 12-week treatment protocol which started in Week 1 on their quit date. Participants were then re-assessed at Week 12 and again at Week 27 with the identical assessment battery. At each time point, masters- or doctoral-level independent evaluators, blinded to participants’ treatment condition, administered the PTSD Symptom Scale Interview (PSS-I), and participants completed a battery of self-report assessments, which included measures of AS, PTSD severity, and negative affectivity. Participants also met with the study nurse at each time point to assess medication compliance and side effects, along with completion of a measure of withdrawal symptoms and provided a biochemical verification of smoking status (per carbon monoxide expired breath sample). This study was conducted at the University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center (VAMC). All participants provided written informed consent prior to initiating any study procedures and the study protocol was approved by Institutional Review Board at the University of Pennsylvania and the Philadelphia VAMC. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Measures
Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986)
The ASI is a 16-item self-report measure that assesses fear of anxiety-related sensations and beliefs about the negative consequences of anxiety. Items are rated on a 5-point Likert scale ranging from 0 (very little) to 4 (very much). A total sum scored is derived with higher scores indicating higher levels of AS. The ASI has strong documented psychometric properties including good discriminant and predictive validity (Taylor, Koch, & Crockett, 1991), adequate test-retest reliability and good internal consistency (Reiss et al., 1986). Internal consistency for the ASI in the present study was excellent (α = .94).
Withdrawal Symptoms Checklist-Weekly (WSC-W; Hughes et al., 1984)
The WSCW is a 20-item questionnaire that assesses the presence and severity of nicotine withdrawal symptoms over the past 7 days. In the present study, the WSC-W was administered by the study nurse. The checklist includes 20 items (e.g. insomnia, nausea, and impatience) that are each rated on a 4-point scale from 0 (not present) to 3 (severe), and one additional item (not scored) that assesses the extent to which withdrawal symptoms have caused significant distress or interference in the respondent's life. Responses are summed to derive a total index of withdrawal severity. The WSC-W has adequate psychometric properties including construct validity and inter-rater reliability between self/collateral reporters (Hughes et al., 1984). In the present study, the WSC-W was found to have good internal consistency (α = .86).
PTSD Symptom Scale Interview (PSS-I; Foa, Riggs, Dancu, & Rothbaum, 1993)
The PSS-I is a 17-item semi-structured interview that assesses the severity of PTSD symptoms according to DSM-IV criteria. Respondents are asked about the nature and frequency of the symptoms experienced over the previous 2 weeks, with responses coded on a 4-point scale from 0 (not at all) to 3 (5 or more times per week/very much). The PSS-I yields a total score (possible range 0-51) and three subscale scores: re-experiencing (five items, range 0-15), avoidance (seven items, range 0-21) and hyperarousal (five items, range 0-15). The PSS-I has strong psychometric properties including good internal consistency, test-retest reliability, and convergent validity (Foa et al., 1993). Internal consistency in the current study for the PSS-I total and subscales scores was good to excellent: PSS-I total (α = .91), re-experiencing (α = .83), avoidance (α = .83), and hyperarousal (α = .74).
Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988)
The PANAS is a 20-item self-report measure that requires participants to rate the extent to which they currently experience (i.e., “to what extent you feel this way right now”) 20 different feelings and emotions (e.g., nervous, interested) based on a Likert-scale that ranges from 1 (“Very slightly or not at all”) to 5 (“Extremely”). The measure yields two factors, negative and positive affect, and has strong documented psychometric properties (Watson et al., 1988). For the current study, only the negative affect subscale total score (comprising of 10 items) was used, and internal consistency for this subscale was good (Cronbach's α = .92).
Carbon Monoxide Levels
Biochemical verification of smoking status was completed by Carbon Monoxide (CO) analysis of breath samples. Expired air CO levels were assessed at each study visit using a Carbon Monoxide Monitor (Serial #: BC26098; Vitalograph) which provided a digital reading of breath CO levels ranging from 0-99 in the standard unit of measure (parts per million; ppm).
Statistical Analyses
Because participants were individuals seeking treatment for PTSD, all participants had elevated PSS-I scores at baseline. In order to avoid restricting variability of our dependent variable (PTSD severity), data were cross-sampled from the week 0, week 12, and week 27 assessment points. Cases (and associated study variables collected at each time point) were randomly selected without replacement from each time point such that each participant was selected from one of the three assessment points. This means that each data point was unique (i.e. no person provided more than one data point, and all measures were taken from that same time-point for that participant). Such a method allowed us to examine associations among the variables of interest at various cross-sections of the sample, and therefore the stage of treatment does not affect the analyses. The number of cases selected at each point was based on the proportion of total cases with available data at that assessment point: 43 cases selected from week 0 (of 117 available), 38 from week 12 (of 102 available), and 37 from week 27 (of 102 available). This cross-sampling technique has been used before in other studies with trauma-exposed substance users (Gillihan, Farris, & Foa, 2011).
Initial descriptive statistics and bivariate correlations were conducted between variables of interest. Data were normally distributed, thus hierarchical linear regressions were utilized to evaluate the contribution of the ASI, the WSC-W, and their statistical interaction in terms of the PSS-I (total score, and the re-experiencing, avoidance, and hyperarousal symptom subscales). Separate regression models were tested for each criterion variable, thus a total of four models were conducted.
Analyses were conducted using PROCESS, a conditional process modeling program that utilizes an ordinary least squares-based analytical framework (Hayes, 2013). In all models, assessment time point (Week 0, Week 12, or Week 27) was included as a covariate to account for any differences in PTSD symptom severity as a result of treatment. Gender was also included as a covariate in all models based on findings showing differential conditional associations of AS on PTSD symptoms severity by gender (Feldner, Zvolensky, Schmidt, & Smith, 2008). To adjust for smoking status at each time point, expired CO level on the day of the assessment was included as a covariate. The PANAS-Negative affect subscale was also included as a covariate in order to account for the state tendency to experience negative affective states, a construct that has been linked to greater smoking relapse rates in individuals with PTSD (Beckham et al., 2013). AS (per ASI) was entered as the predictor (X) and severity of nicotine withdrawal (per WSC-W) was entered as the moderator (M). In order to test whether a model of X's effect contingent on M is a better fitting model than if the effect of X is constrained to be unconditional on M, a hierarchical regression approach was utilized. First, the direct effect of these variables on the criterion outcomes was tested (model 1; yields R21). In the second stage, the conditional effect of withdrawal in terms of AS on PTSD symptom severity (effect of ASI*WSC-W) was added to the model (to create model 2; yields R22). The difference in R22-1 (a descriptive measure of model fit), was computed (ΔR2).
An examination of the assumptions required for inclusion of all predictors (i.e., absence of multicollinearity [VIF < 10], linearity, low incidence of outliers with standard residuals between -3.3 and 3.3, and homogeneity of variance) revealed that none of these criteria were violated in any of the regression models tested.
Results
Baseline Characteristics of Sample
Of the 117 participants, 70 (59.9%) were male and 47 (40.2%) were female. The mean age was 41.9 years (SD = 10.4 years). The majority of participants (76.1%) were Black or African American, with the rest of the participants identifying as White (22.2%) and American Indian or Alaskan Native (1.7%). The mean number of cigarettes smoked per day in the week prior to the baseline assessment point examined in the study was 18.2 (SD = 10.4), and the average expired CO level at the baseline assessment visit was 14.4 ppm (SD = 5.8). The mean negative affect subscale score on the PANAS was 22.8 (SD = 8.4). Further, the mean PSS-I score at baseline was 28.5 (SD = 6.5), indicating moderately severe PTSD at the start of the study. The mean ASI score at baseline was in the clinical range (M = 27.7, SD = 13.6), and participants reported low levels of nicotine withdrawal in the week prior to the baseline assessment (M = 12.7, SD = 5.3).
Correlational analyses
Table 1 shows the direction and strength of associations using bivariate correlations among all independent and dependent variables. Briefly, higher levels of AS were moderately associated with higher levels of general negative affect (r = .46, p < .001), expired CO levels (r = .29, p = .001), PTSD symptom severity across subscales (r's range = .36 - .52, all p < .001) and nicotine withdrawal symptom severity (r = .36, p < .001). In addition, higher nicotine withdrawal symptom severity was moderately associated with expired CO (r = .45, p < .001), negative affect (r = .47, p < .001), and more severe PTSD symptoms (r's range = .46 - .56, p < .001).
Table 1.
Zero-order Correlations among Study Variables
| Mean (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | 40.2% | -- | |||||||||
| 2. Time Point | -- | −.281** | -- | ||||||||
| 3. Negative Affect | 19.4 (9.05) | .064 | −.300** | -- | |||||||
| 4. Expired CO | 8.4 (7.09) | .107 | −.525** | .354** | 1 | ||||||
| 5. ASI | 23.3 (15.22) | .125 | −.331** | .463** | .293** | 1 | |||||
| 6. WSC-W | 8.2 (5.98) | .097 | −.644** | .470** | .449** | .357** | 1 | ||||
| 7. PSS-I-Total | 20.0 (12.36) | .229* | −.477** | .583** | .408** | .502** | .558** | 1 | |||
| 8. PSS-I-Re-Exper. | 4.7 (3.82) | .138 | −.412** | .488** | .446** | .360** | .482** | .830** | 1 | ||
| 9. PSS-I-Avoid | 7.8 (5.71) | .208* | −.377** | .540** | .286** | .448** | .464** | .926** | .645** | 1 | |
| 10. PSS-I-Hyperar. | 7.4 (4.35) | .255** | −.498** | .519** | .393** | .523** | .552** | .896** | .635** | .753** | 1 |
Note. Gender = Percent listed is female, coded male=1 and female=0; Time Point = Assessment time point (coded 0-2 for week 0, week 12 and week 27 respectively); Negative Affect = Positive and Negative Affect Scale – Negative Affect subscale; Expired CO = Expired carbon monoxide breath sample; ASI = Anxiety Sensitivity Index total score; WSC-W = Withdrawal Symptom Checklist –Weekly; PSS-I-Total = PTSD Symptom Scale Interview – total score; PSSI-I-Re-Exper. = PTSD Symptom Scale Interview - Re-experiencing subscale; PSS-I-Avoid. = PTSD Symptom Scale Interview - Avoidance subscale; PSS-I-Hyperar. = PTSD Symptom Scale Interview - Hyperarousal subscale.
p <0.05
p <0.01
Moderator analysis
Results from the regression models are presented in Table 2.
Table 2.
Hierarchical Multiple Regression Analyses Assessing for Main and Interactive Effects of AS and Withdrawal on PTSD Symptom Clusters
| DV | Predictors | R2 | b | se | t | p |
|---|---|---|---|---|---|---|
| PSS-I Total | Gender | .548a | 2.543 | 1.718 | 1.480 | .142 |
| Time Point | −.178 | .147 | −1.213 | .228 | ||
| Negative Affect | 439 | .108 | 4.054 | < .001 | ||
| Expired CO | .103 | .137 | .754 | .453 | ||
| ASI | .361 | .096 | 3.760 | < .001 | ||
| WSC-W | 1.048 | .289 | 3.627 | < .001 | ||
| ASI*WSC-W | .028b | −.023 | .009 | −2.613 | .010 | |
| PSS-I Re-Experiencing | Gender | .376a | .374 | .624 | .601 | .549 |
| Time Point | −.030 | .053 | −.564 | .574 | ||
| Negative Affect | .113 | .039 | 2.864 | .005 | ||
| Expired CO | .109 | .050 | 2.194 | .030 | ||
| ASI | .034 | .035 | .978 | .330 | ||
| WSC-W | .163 | .105 | 1.556 | .123 | ||
| ASI*WSC-W | .001b | −.002 | .003 | −.502 | .617 | |
| PSS-I Avoidance | Gender | .436a | 1.170 | .887 | 1.319 | .190 |
| Time Point | −.064 | .076 | −.841 | .402 | ||
| Negative Affect | .218 | .056 | 3.903 | < .001 | ||
| Expired CO | −.029 | .071 | −.406 | .686 | ||
| ASI | .168 | .050 | 3.387 | .001 | ||
| WSC-W | .465 | .149 | 3.120 | .002 | ||
| ASI*WSC-W | .033b | −.011 | .004 | −2.510 | .014 | |
| PSS-I Hyperarousal | Gender | .545a | .999 | .607 | 1.646 | .103 |
| Time Point | −.084 | .052 | −1.625 | .107 | ||
| Negative Affect | .108 | .038 | 2.833 | .006 | ||
| Expired CO | .023 | .048 | .473 | .637 | ||
| ASI | .159 | .034 | 4.692 | < .001 | ||
| WSC-W | .419 | .102 | 4.113 | < .001 | ||
| ASI*WSC-W | .043b | −.010. | .003 | −3.216 | .002 | |
Note. Gender = coded male=1 and female=0; Time Point = Assessment time point (coded 0-2 for baseline, post-treatment, and three-month follow-up respectively); Negative Affect = Positive and Negative Affect Scale – Negative Affect subscale; Expired CO = Expired carbon monoxide breath sample; ASI = Anxiety Sensitivity Index total score; WSC-W = Withdrawal Symptom Checklist - Weekly; PSS-I Total, PSS-I Re-experiencing, PSS-I Avoidance, and Hyperarousal = PTSD Severity Scale Interview Total, Re-experiencing subscale, Avoidance subscale, and Hyperarousal subscale scores.
Total model R2
R2 increase due to interaction.
PTSD total score
Regression analyses revealed a significant main effect of both AS (b = .361, SE = .096, t = 3.60, p < .001) and nicotine withdrawal (b = .1.048, SE = .289, t = 3.627, p < .001) on total PTSD symptom severity, after controlling for gender, assessment time-point, negative affect and expired CO. In the model with the interaction term, the total model effect was significant (total R2 = .548, F(7,109) = 18.879, p < .001) and the addition of the interaction variable was also significant (b = -.023, SE = .009, t = -2.613, p = .010) accounting for an additional unique 2.8% of variance in PTSD symptom severity.
PTSD cluster scores
Results from the model with re-experiencing symptoms revealed a non-significant direct effect of AS (b = .034, SE = .035, t = .978, p = .330) or nicotine withdrawal (b = .163, SE = .105, t = 1.556, p = .123) on re-experiencing symptom severity. In the model that included the interaction term, the full model was significant (R2 = .376, F(7,109) = 9.400, p < .0001), although there was a non-significant incremental effect of the interaction term (ΔR2 = .001).
Results from the model with avoidance symptoms revealed a significant direct effect of AS (b = .168, SE = .050, t = 3.387, p = .001) and nicotine withdrawal (b = .465, SE = .149, t = 3.120, p = .002) on avoidance symptom severity, after controlling for gender and assessment time-point, negative affect and expired CO level. The full model with the interaction term was significant (R2 = .436, F(7,109) = 12.055, p < .001). The interaction accounted for a significant unique 3.3% of variance in PTSD avoidance symptom severity (b = -.011, SE = .004, t = -2.510, p = .014).
Results from the model with hyperarousal symptoms revealed a significant direct effect of AS (b =.159, SE = .034, t = 4.692, p < .001) and nicotine withdrawal (b = .419, SE = .102, t = 4.113, p < .001) on hyperarousal symptom severity, after controlling for gender, assessment time-point, negative affect and expired CO level. The full model with the interaction term was significant (R2 = .545, F(7,109) = 18.675, p < .001). The interaction effect accounted for a unique 4.3% of variance in PTSD hyperarousal symptoms (b = -.010, SE = .003, t = -3.216, p = .002).
Post-hoc tests of significance
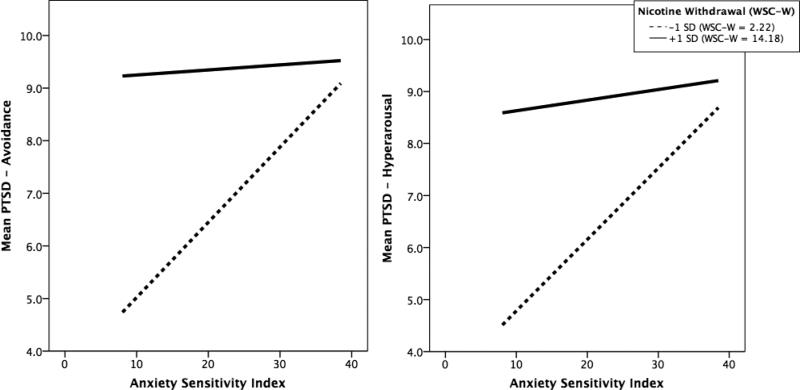
The significant subscale interactive effects (for PTSD avoidance and hyperarousal) were subjected to follow-up testing. Specifically, the form of the interactions for these models was examined graphically and statistically. First, Figure 1 illustrates the interaction by depicting the regression lines of the relation between AS and PTSD avoidance (Figure 1a) and hyperarousal (Figure 1b) symptom severity at scores on the moderator (nicotine withdrawal) that are +/− 1 SD. While descriptively the highest levels of PTSD avoidance and hyperarousal symptoms were yielded from the combination of higher AS and +1 SD nicotine withdrawal, the form of the interactions revealed that the steepest slope of the regression lines were at -1 SD levels of nicotine withdrawal (WSC-W M = 2.2). That is, the associations between AS and PTSD avoidance and hyperarousal symptom severity was strongest at lower levels of nicotine withdrawal (relative to mean scores), although also still statically significant (in the same direction) when nicotine withdrawal was +1 SD; (WSC-W M = 14.2).
Figure 1.
Impact of AS on PTSD avoidance and hyperarousal symptoms at low and high levels of nicotine withdrawal.
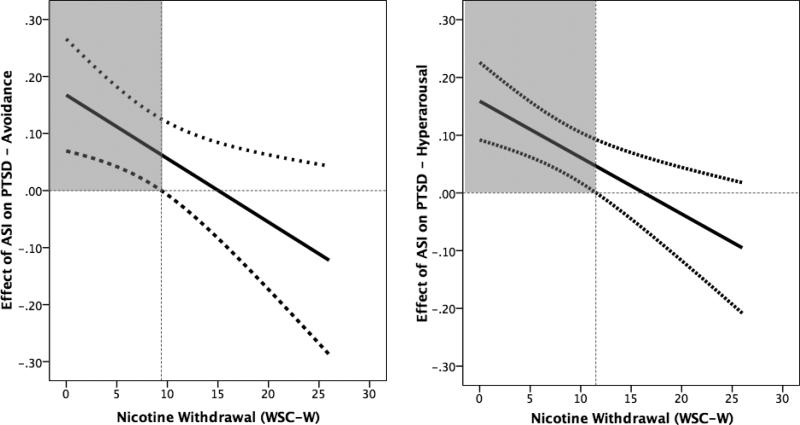
In order to further characterize the nature of the interaction, the Johnson–Neymann (J–N) technique was utilized (per recommendations by Hayes, 2013). The J–N technique statistically identifies points in the range of the continuous moderator variable where the effect of the predictor on the criterion variable transitions from being statistically significant to non-significant (rather than the “pick-a-point” technique used in selected +/− 1 SD). The J-N technique identifies the value of the moderator variable for which the ratio of the conditional effect to its standard error is equal to the critical t score. Here, it is first important to note that when the full range of values on the moderator variable is examined, there is an overall negative slope for the conditional effect of AS on PTSD avoidance (see Figure 2a) and hyperarousal (see Figure 2b) symptom severity. This indicates that the strength of the association between AS and both PTSD subscales decreases as scores on the moderator (nicotine withdrawal) increase. Specifically, the conditional effect of AS on PTSD avoidance symptom severity was only significant at WSC-W scores of ≤ 9.4 (Figure 2a) – and was actually strongest at the lowest levels of nicotine withdrawal (even within this range; as illustrated in Figures 1a and 1b). Thus, the conditional effect AS on PTSD avoidance symptoms was only significant when WSC-W scores were at or below this threshold (which included 62.4% of the sample distribution) but not above this threshold (which included 37.6% of the sample distribution). The conditional effect of AS on PTSD hyperarousal symptom severity was only significant at WSC-W scores of ≤ 11.5 (Figure 2b); the conditional effect AS on PTSD hyperarousal symptoms was significant when WSC-W scores were at or below this threshold (which included 74.4% of the sample distribution) but not above this threshold (which included 25.6% of the sample distribution).
Figure 2.
Location of the change in significance of the conditional effect of nicotine withdrawal on the association between AS and PTSD avoidance and hyperarousal symptoms.
Discussion
The current study examined the interplay between AS and PTSD symptom severity among a clinical sample of smokers with PTSD, and the role played by nicotine withdrawal in determining the strength of this association. As hypothesized, after adjusting for the variance accounted for by gender, assessment time-point, expired CO levels (which indicate current smoking status), and state negative affectivity, higher levels of AS were positively associated with increased PTSD symptom severity (at least cross-sectionally). These findings are unique in that these effects were documented in a clinical sample of smokers with PTSD, and are broadly consistent with previous studies conducted in non-clinical trauma-exposed samples (Feldner et al., 2005; Marshall et al., 2010; Farris et al., 2014). Interestingly, the main effect of AS was observed only in the avoidance and hyperarousal PTSD symptom cluster scores, but not in re-experiencing symptoms. The lack of finding of direct effects of AS in re-experiencing symptoms is consistent with these previous studies that have only found a relationship of AS to avoidance and hyperarousal symptoms of PTSD.
Additionally, it is important to note the significant direct effect of the moderator, nicotine withdrawal, on PTSD symptom severity, although this appeared to be specific to the PTSD avoidance and hyperarousal symptom subscales. A main effect of nicotine withdrawal on total PTSD severity has been found in non-clinical samples of trauma-exposed smokers (e.g., Feldner et al., 2008; Dedert et al., 2012; Beckham et al., 1996), therefore this result is consistent with a priori hypotheses. The specific effects of nicotine withdrawal on the avoidance and hyperarousal symptoms of PTSD are novel, and the reasons for this finding warrant further examination and replication in other clinical samples. Overall, these main effects suggest that AS and nicotine withdrawal (independently) are important to assess in smokers with PTSD, and may similarly contribute to the exacerbation of PTSD avoidance and hyperarousal symptoms, but may be different in the etiological risk for the expression of PTSD re-experiencing symptoms. However,given that all participants included in this study were either beginning, just ending, or recently on medication for smoking cessation (varenicline) and some type of psychological therapy, it is also important to note the current findings were observed within this context.
Further, we hypothesized that higher levels of nicotine withdrawal would moderate the effect of AS on PTSD symptom severity (specifically avoidance and hyperarousal symptoms). This was based on our proposed conceptual model describing the relationship among the variables of interest, suggesting that within the context of uncomfortable interoceptive sensations (as seen with nicotine withdrawal), the effects of AS on PTSD severity would be even stronger. The hypothesized reason for this is that withdrawal amplifies the “interoceptive threat” to increase the cognitive vulnerability of AS, in turn further exacerbating PTSD hyperarousal symptoms and PTSD avoidance symptoms of such negative internal sensations. Indeed, a significant moderation effect was found for withdrawal on changing the association between AS and PTSD in both avoidance and hyperarousal symptoms. However, contrary to expectations, the conditional effect of AS on both PTSD avoidance and hyperarousal symptoms was strongest and significant only at lower levels of nicotine withdrawal. That is, as levels of nicotine withdrawal increased, the impact of AS on PTSD symptom severity diminished, and after the identified withdrawal ‘cut-points’ (WSC-W scores = 9.4-11.5) the effect of AS on PTSD was no longer significant.
Therefore, higher levels of nicotine withdrawal (causing smoking-related interoceptive distress) did not further “amplify” the effect of having an elevated pre-existing tendency to misinterpret the meaning of interoceptive sensations (i.e., high AS) in terms of PTSD symptom expression as initially expected. It is conceivable that among smokers with PTSD, experiencing very high levels of nicotine withdrawal may actually serve to override any influence that AS has on PTSD symptoms; that is, at higher levels of withdrawal, physical sensations may be perceived as being directly attributable to abstinence from smoking, thus perhaps being less ambiguous to individuals with high AS (i.e., withdrawal symptoms are less likely to be misinterpreted because the cause of the interoceptive distress is known). It is worth noting that only a small minority of participants actually reported nicotine withdrawal symptom severity above the identified cut-offs (25-38% of the study sample), meaning that AS appeared to compound PTSD symptoms for the majority of participants. Given the scarcity of investigation into this important area, future examination testing alternative theoretical/conceptual explanations for role of nicotine withdrawal in the AS-PTSD interplay is warranted.
It is worth noting that while the direction of the moderational effect was counter to what was expected, the specificity of the effect to PTSD avoidance and hyperarousal symptoms was partially consistent with expectations and consistent with other studies. For instance, Farris et al. (2014) observed that in a sample of trauma-exposed treatment-seeking smokers, individuals high in AS with lower tolerance of physical distress (i.e., one's actual capacity to tolerate aversive physical sensations) was associated with highest levels of hyperarousal symptoms of PTSD, but not other symptom clusters. Indeed, there is certainly conceptual overlap between one's ability to tolerate aversive physical states and the subjective experience of nicotine withdrawal (i.e., attention/perceived tolerance to this noxious internal state; Leyro, Zvolensky, & Bernstein, 2010). The finding for a significant interaction between AS and withdrawal symptoms on the avoidance cluster of symptoms has not been found elsewhere; however, this finding should be interpreted with caution, because this subscale as defined by the DSM-IV measures used in the present study consists of heterogeneous items that have since been pulled out and added to the new cluster (cognition/emotion) in DSM-5. This avoidance cluster therefore does not particularly represent pure avoidance symptoms.
Related to this last point, it is important to note the limitations of the present study. Specifically, the variables of interest could only be examined cross-sectionally instead of longitudinally or by examining changes in these relationships due to PTSD treatment, given the trial is still in its ending stages of long-term follow-up data collection (as of October 2014). However, the cross-sampling technique across several time-points allowed for examination of how these variables processes relate to each other in a given point of time, and evidenced significant findings even after statistically controlling for passage of time in the study. Yet, it is important to note that the time frames for the variables of interest varied to some degree (ranging from right now to the past 2 weeks, depending on the measure), which could further impact how we interpret these cross-sectional findings. Indeed, if more nuanced and consistently timed data were available to examine, it would be helpful to investigate the effect of AS on the course of nicotine withdrawal after quitting in terms of the severity of PTSD symptoms observed in a specific and defined time frame. Such a fine-tuned analysis would aid in exploring the possibility that smokers low in AS may experience either shorter or less severe courses of withdrawal after quitting, which may correspond to reductions in PTSD symptom severity (particularly in the avoidance and hyperarousal symptom domains). Consequently, smokers high in AS may be at higher risk for relapse and protracted PTSD symptoms due to a more long-drawn or severe withdrawal period following smoking abstinence. These important implications of the relationship between AS and nicotine withdrawal on PTSD recovery require more attention and empirical examination.
Another consideration when interpreting the current findings is that when a person is attempting to abstain from nicotine, the withdrawal symptoms that occur (e.g. irritability and difficulties concentrating) reflect a more state-like measurement (unlike the trait-like quality of AS), one that is sensitive to more rapid changes over time and experienced more ubiquitously across all smokers when abstaining from cigarettes (Hughes et al., 1984). Indeed, nicotine withdrawal is dependent on inter-individual variations in smoking frequency and specific conditions (acute withdrawal, overnight withdrawal, withdrawal associated with a quit attempt), which were not examined here. Thus, testing this same model with changes in withdrawal within one consistent period of time in the smoking cessation process is important. Further, it would be useful to control the effects of nicotine dependence severity while examining these changes in withdrawal. Unfortunately, the present study only administered a measure of nicotine dependence (Fagerstrom Test of Nicotine Dependence; FTND) at one time-point (week 0), so this was not able to be included as a covariate in the cross-sectional analyses conducted. However, this study did examine a biological measure of smoking frequency occurring around the measurements of smoking withdrawal (CO levels), which captures smoking behavior on the assessment day specifically (whereas FTND examines symptoms beyond the same day of the assessment). CO measurement also allows for biochemical verification of smoking status which is superior to subjective self-report (Shiffman et al., 1997). Indeed, the results regarding the impact of nicotine withdrawal severity and AS were still evident, even after controlling for smoking status/rate as measured by CO levels (Langdon et al., 2013). In addition, it is important to note that the effects of AS and nicotine withdrawal were significant beyond the effects of general negative affectivity, which itself has been implicated in higher nicotine dependence and relapse among smokers with PTSD (Beckham et al., 2013), and is inter-related with AS and nicotine withdrawal (these constructs shared 46-47% of variance with negative affect). Thus, while negative affect appears to be related to AS and nicotine withdrawal and relevant to PTSD symptom severity, there appears to be incremental predictive validity of AS and nicotine withdrawal (and the moderation effect) in terms of PTSD symptom severity.
Finally, an important aspect to note in the current study is the conceptual model being tested, with AS (a trait-like variable) being defined as a predictor and smoking withdrawal (a state-like variable) being defined as a moderator. Kraemer and colleagues have recommended that moderators should temporally precede predictors (which is not the case here), and should not be associated with each other (but AS and smoking withdrawal are weakly correlated) (Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001). However, Hayes (2013) has argued that in the moderation model tested here, the temporal patterning of the predictor and moderator does not matter because all of the variables (AS, smoking withdrawal, and PTSD) are measured cross-sectionally, at the same time-points. The conditional process model analysis refers to statistical prediction of PTSD severity by AS, not longitudinal prediction by AS, so temporal precedence of the moderator over the predictor is less relevant. In addition, the association between predictor and moderator is only concerning when the correlations are strong (Kenny and Judd, 1984; Judd, Kenny, & McClleland, 2001), which is not the case in the current study. Thus, taken together, the conceptual model tested and temporal patterning of variables observed in the current study do not pose significant problems.
In summary, the current investigation attempted to further elucidate the impact of one cognitive-affective risk factor (AS) as a linking factor in the comorbidity of smoking and PTSD. To our knowledge, this study is the first to examine the relationships between these factors in a treatment-seeking sample of smokers with PTSD, lending a unique view into how withdrawal symptoms and AS interact with one another to impact PTSD symptom severity. The current study also provided a comprehensive and nuanced examination of how specifically nicotine withdrawal conditionally impacts the AS-PTSD association. To further extend and understand the nature of the observed relationships between these processes, it would be important for future studies to examine how varying levels of AS, nicotine withdrawal, and their interaction predict treatment outcome on both measures of PTSD and smoking cessation outcomes (e.g., quit day abstinence; latency to lapse/relapse, and other smoking milestones; Shiffman et al., 2006). In addition, it is important for future studies to consider the role of other possible cognitive-affective risk processes (e.g., distress tolerance) related to specific symptom clusters of PTSD, at multiple time-points throughout treatment. Related to this, examination into how AS and nicotine withdrawal specifically impact the newly devised DSM-5 PTSD cluster of cognition and mood (APA, 2013) is needed, to expand our understanding into the unique relationships of AS and nicotine withdrawal with certain clusters of PTSD symptoms.
Acknowledgments
The authors express their sincerest appreciation to the study nurse Patricia Imms, research assistants on this study Michelle Capozzoli, Janice Paton, Catherine Coogan and Elizabeth Alpert, and to the patients who were brave enough to share their struggles with our research team by participating in this study.
Footnotes
Conflicts of Interest
Dr. Edna B. Foa received funding for this study by a National Institute of Drug Abuse grant (R01-DA023507-01A1). Ms. Samantha Farris received support from a pre-doctoral National Research Service Award from the National Institute of Drug Abuse (F31-DA035564). Dr. Anu Asnaani, Mr. Joseph Carpenter, and Dr. Laurie Zandberg have no conflict of interest.
Statement of Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. All participants provided written informed consent prior to initiating any study procedures and the study protocol was approved by Institutional Review Board at the University of Pennsylvania and the Philadelphia VAMC.
Statement of Animal Rights
No animal studies were carried out by any of the authors for this paper.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. text revision Washington, DC.: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC.: 2013. [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine & Tobacco Research. 2013;15(6):1122–1129. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, Fairbank JA. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addictive Behaviors. 1997;22(5):637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Berenz EC, Vujanovic AA, Coffey SF, Zvolensky MJ. Anxiety sensitivity and breath-holding duration in relation to PTSD symptom severity among trauma exposed adults. Journal of Anxiety Disorders. 2012;26(1):134–139. doi: 10.1016/j.janxdis.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Mcfall MM, Calhoun PS, Beckham JC. Posttraumatic stress disorder and smoking relapse: A theoretical model. Journal Of Traumatic Stress. 2007;20(6):989–998. doi: 10.1002/jts.20275. doi:10.1002/jts.20275. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Harper LA, Dutton CE, McClernon FJ, Beckham JC. Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine & Tobacco Research. 2012;14(3):372–376. doi: 10.1093/ntr/ntr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76(2-3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Erskine JAK, Ussher M, Cropley M, Elgindi A, Zaman M, Corlett B. Effect of thought suppression on desire to smoke and tobacco withdrawal symptoms. Psychopharmacology. 2012;219(1):205–211. doi: 10.1007/s00213-011-2391-4. [DOI] [PubMed] [Google Scholar]
- Farrell M, Howes S, Bebbington P, Brugha T, Jenkins R, Lewis G, Marsden J, Taylor C, Meltzer H. Nicotine, alcohol and drug dependence, and psychiatric comorbidity-results of a national household survey. International Review of Psychiatry. 2003;15:50–56. doi: 10.1080/0954026021000045949. [DOI] [PubMed] [Google Scholar]
- Farris SG, Vujanovic AA, Hogan J, Schmidt NB, Zvolensky MJ. Main and interactive effects of anxiety sensitivity and physical distress intolerance with regard to PTSD symptoms among trauma-exposed smokers. Journal of Trauma & Dissociation. 2014;15:254–270. doi: 10.1080/15299732.2013.834862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Zvolensky MJ, Schmidt NB. Smoking-specific experiential avoidance cognition: Explanatory relevance to pre- and post-cessation nicotine withdrawal, craving and negative affect. Addictive Behaviors. doi: 10.1016/j.addbeh.2014.07.026. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and post-traumatic stress: A critical review of the empirical literature. Clinical Psychology Review. 2007;27(1):14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Schmidt NB, Smith RC. A prospective test of anxiety sensitivity as a moderator of the relation between gender and posttraumatic symptom maintenance among high anxiety sensitive young adults. Depression and Anxiety. 2008;25(3):190–199. doi: 10.1002/da.20281. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Vujanovic AA, Gibson LE, Zvolensky MJ. Posttraumatic stress disorder and anxious and fearful reactivity to bodily arousal: A test of the mediating role of nicotine withdrawal severity among daily smokers in 12-hr nicotine deprivation. Experimental and Clinical Psychopharmacology. 2008;16:144–155. doi: 10.1037/1064-1297.16.2.144. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The posttraumatic diagnostic scale. Psychological Assessment. 1997;9(4):445–451. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6(4):459–473. [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM. Post-traumatic stress disorder and smoking: A systematic review. Nicotine & Tobacco Research. 2007;9(11):1071–1084. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farris SG, Foa EB. The effect of anxiety sensitivity on alcohol consumption among individuals with comorbid alcohol dependence and posttraumatic stress disorder. Psychology of Addictive Behaviors. 2011;25(4):721–726. doi: 10.1037/a0023799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapke U, Schumann A, Rumpf HJ, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. Journal of Nervous and Mental Disease. 2005;193(12):843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; New York, NY.: 2013. [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Svikis DS. Consistency of the tobacco withdrawal syndrome. Addictive Behaviors. 1984;9(4):409–412. doi: 10.1016/0306-4603(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Joseph AM, McFall M, Saxon AJ, Chow BK, Leskela J, Dieperink ME, Beckham JC. Smoking intensity and severity of specific symptom clusters in posttraumatic stress disorder. Journal of Traumatic Stress. 2012;25(1):10–16. doi: 10.1002/jts.21670. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-participant designs. Psychological Methods. 2001;6:115–134. doi: 10.1037/1082-989x.6.2.115. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Judd CM. Estimating the nonlinear and interactive effects of latent variables. Psychological Bulletin. 1984;96:201–210. [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How Do Risk Factors Work Together? Mediators, Moderators, and Independent, Overlapping, and Proxy Risk Factors. American Journal of Psychiatry. 2001;158(6):848–856. doi: 10.1176/appi.ajp.158.6.848. doi:10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Kennedy CM, Stein MB. Anxiety sensitivity and PTSD among female victims of intimate partner violence. Depression and Anxiety. 2002;16(2):77–83. doi: 10.1002/da.10062. [DOI] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: Prospective relationships to nicotine withdrawal symptoms during smoking cessation. Journal of the Study of Alcohol and Drugs. 2013;74:469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd J, Woolhandler S, Himmelstein D, McCormick D, Bor D. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leyro TM, Zvolensky MJ, Bernstein A. Distress tolerance and psychopathological symptoms and disorders: A review of the empirical literature among adults. Psychological Bulletin. 2010;136(4):576–600. doi: 10.1037/a0019712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. doi: 10.1037/bul0000003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EC, Johnson K, Bergman J, Gibson LE, Zvolensky MJ. Anxiety sensitivity and panic reactivity to bodily sensations: Relation to quit-day (acute) nicotine withdrawal symptom severity among daily smokers making a self-guided quit attempt. Experimental and Clinical Psychopharmacology. 2009;17(5):356–364. doi: 10.1037/a0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GN, Miles JV, Stewart SH. Anxiety sensitivity and PTSD symptom severity are reciprocally related: Evidence from a longitudinal study of physical trauma survivors. Journal of Abnormal Psychology. 2010;119(1):143–150. doi: 10.1037/a0018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, Bernstein A. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. Journal of Anxiety Disorders. 2008;22(7):1214–1226. doi: 10.1016/j.janxdis.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychological Bulletin. 2007;133(2):245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopex AD, Murray CJL, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. Journal of the American Medical Association. 2014;311(2):183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Wolitzky-Taylor KB. Anxiety sensitivity and the anxiety disorders: A meta-analytic review and synthesis. Psychological Bulletin. 2009;135(6):974–999. doi: 10.1037/a0017428. [DOI] [PubMed] [Google Scholar]
- Richards CS, Cohen LM, Morrell HE, Watson NL, Low BE. Treating depressed and anxious smokers in smoking cessation programs. Journal of Consulting and Clinical Psychology. 2013;81(2):263–273. doi: 10.1037/a0027793. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Goren A, Annunziata K, Suaya JA. The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction. 2013;108(11):1989–2000. doi: 10.1111/add.12285. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting And Clinical Psychology. 1997;65(2):292–300. doi: 10.1037/0022-006x.65.2.292.a. doi:10.1037/0022-006X.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74(2):276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Strong DR, Uebelacker L, Fokas K, Saritelli J, Matsko S, Abrantes AM, Schonbrun Y. Utilization of evidence-based smoking cessation treatments by psychiatric inpatient smokers with depression. Journal of Addiction Medicine. 2014;8(2):77–83. doi: 10.1097/ADM.0000000000000027. [DOI] [PubMed] [Google Scholar]
- Taylor S, Koch WJ, Crockett DJ. Anxiety sensitivity, trait anxiety, and the anxiety disorders. Journal of Anxiety Disorders. 1991;5(4):293–311. [Google Scholar]
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: Systematic review and meta-analysis. British Medical Journal. 2014;348:g1151. doi: 10.1136/bmj.g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Burden of Disease Collaborators The State of US Health, 1990-2010: Burden of Diseases, Injuries, and Risk Factors. Journal of the American Medical Association. 2013;310(6):591–606. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Marshall-Berenz E, Beckham JC, Bernstein A, Zvolensky MJ. Posttraumatic stress symptoms and cigarette deprivation in the prediction of anxious responding among trauma- exposed smokers: A laboratory test. Nicotine & Tobacco Research. 2010;12(11):1080–1088. doi: 10.1093/ntr/ntq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Zvolensky MJ. Anxiety sensitivity, acute nicotine withdrawal symptoms, and anxious and fearful responding to bodily sensations: A laboratory test. Experimental and Clinical Psychopharmacology. 2009;17(3):181–190. doi: 10.1037/a0016266. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, Riley WT. Tobacco use and cessation in psychiatric disorders: National institute of mental health report. Nicotine & Tobacco Research. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Baker KM, Leen-Feldner E, Bonn-Miller M, Feldner MT, Brown RA. Anxiety sensitivity: Association with intensity of retrospectively-rated smoking-related withdrawal symptoms and motivation to quit. Cognitive Behaviour Therapy. 2004;33(3):114–125. doi: 10.1080/16506070310016969. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Leventhal AM, Schmidt NB. Anxiety sensitivity mediates relations between emotional disorders and smoking. Psychology of Addictive Behaviors. 2014;28(3):912–920. doi: 10.1037/a0037450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Schmidt NB, Smits JAJ. The role of smoking inflexibility/avoidance in the relation between anxiety sensitivity and tobacco use and beliefs among treatment-seeking smokers. Experimental and Clinical Psychopharmacology. 2014;22(3):229–237. doi: 10.1037/a0035306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Guillot CR, Leventhal AM. Anxiety sensitivity as an amplifier of the subjective and behavioral tobacco abstinence effects. Drug and Alcohol Dependence. doi: 10.1016/j.drugalcdep.2014.06.023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Feldner MT. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research. 2008;10(8):1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]