Abstract
Pseudomonas aeruginosa infection is a hallmark of lung disease in cystic fibrosis. Acute infection with P. aeruginosa profoundly inhibits alveolar macrophage clearance of apoptotic cells (efferocytosis) via direct effect of virulence factors. During chronic infection, P. aeruginosa evades host defense by decreased virulence, which includes the production or, in the case of mucoidy, overproduction of alginate. The impact of alginate on innate immunity, in particular on macrophage clearance of apoptotic cells is not known.
We hypothesized that P. aeruginosa strains that exhibit reduced virulence impair macrophage clearance of apoptotic cells and we investigated if the polysaccharide alginate produced by mucoid P. aeruginosa is sufficient to inhibit alveolar macrophage efferocytosis.
Rat alveolar or human peripheral blood monocyte (THP-1)-derived macrophages cell lines were exposed in vitro to exogenous alginate or to wild type or alginate-overproducing mucoid P. aeruginosa prior to challenge with apoptotic human Jurkat T-lymphocytes. The importance of LPS contamination and that of structural integrity of alginate polymers was tested using alginate of different purities and alginate lyase, respectively.
Alginate inhibited alveolar macrophage efferocytosis in a dose- and time-dependent manner. This effect was augmented but not exclusively attributed to lipopolysaccharide (LPS) present in alginates. Alginate-producing P. aeruginosa inhibited macrophage efferocytosis by more than 50%. Although alginate lyase did not significantly restore efferocytosis in the presence of exogenous alginate, it had a marked beneficial effect on efferocytosis of alveolar macrophages exposed to mucoid P. aeruginosa.
Despite decreased virulence, mucoid P. aeruginosa may contribute to ongoing airway inflammation through significant inhibition of alveolar clearance of apoptotic cells and debris. The mechanism by which mucoid bacteria inhibit efferocytosis may involve alginate production and synergy with LPS, suggesting that alginate lyase may be an attractive therapeutic approach to airway inflammation in cystic fibrosis and other chronic obstructive pulmonary diseases characterized by P. aeruginosa colonization.
Keywords: Pseudomonas aeruginosa, cystic fibrosis, macrophage, phagocytosis, efferocytosis, mucoid, alginate lyase
Introduction
Cystic fibrosis (CF) is the most common autosomal recessive disorder with an estimated incidence of 1 in 3,000 Caucasians [1]. The majority of individuals with CF have positive sputum cultures for Pseudomonas aeruginosa. Infection with P. aeruginosa is associated with significant morbidity and mortality [2, 3]. Neutrophils (PMN) constitute an initial line of defense against P. aeruginosa infection via phagocytosis of bacteria, which is followed by neutrophil apoptosis. In turn, apoptotic cell clearance (efferocytosis) from the airways by alveolar macrophages (AM) is an essential step in the resolution of inflammation [4, 5].
It has been established that the acute infectious (planktonic) P. aeruginosa increases PMN apoptosis but decreases their clearance by AM by mechanisms involving pyocyanin and Type III Secretion System (T3SS) production, classical virulence factors of this bacteria [6, 7]. This mechanism may be partly responsible for chronic PMN infiltration of CF airways, increased mucous viscosity, and perpetuation of airway inflammation. However, with the transition to chronicity, microbes accumulate mutations rendering CF airways frequently colonized by P. aeruginosa strains with lower virulence [8-10]. These strains associated with chronic airway infections produce more biofilms and down-regulate T3SS and pyocyanin production, attempting to evade host immunity [11]. Despite this decrease in expression of classical virulence factors, mucoid P. aeruginosa, an alginate overproducing mutant [12] often found in CF sputum cultures, is associated with higher morbidity and mortality in persons with CF [13]. The alginate-containing biofilm produced by the mucoid strain has been shown to impair antibiotic penetration and to inhibit phagocytosis of bacteria, whereas treatment with alginate lyase improves antibiotic responses and sputum viscosity [14-16]. However, bacterial and apoptotic cell phagocytosis do not share similar mechanisms. The effects of alginate on AM efferocytosis is unknown and could play an essential role in airway inflammation and therefore the morbidity of chronic P. aeruginosa infection. Accordingly, restoration of inflammatory clearance by means of mitigating alginate production or breaking down its polymers with alginate lyase might provide the rationale for potential therapeutic interventions, especially in the setting of increased antibiotic resistance.
We hypothesize that alginate produced by less virulent, mucoid P. aeruginosa will decrease AM efferocytosis and that alginate lyase treatment will improve AM engulfment of apoptotic cells in the presence of mucoid bacteria.
Materials and Methods
Reagents
All reagents were from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. LPS, from E. coli strain O111:B4 was from Thermo Scientific (Asheville, NC).
Cells
Rat alveolar macrophages (AM) cell line NR8383 (ATCC, Manassas, VA) was maintained in Ham’s F12K medium (ATCC) containing L-glutamine (2 mM), sodium bicarbonate (1.5 g/L), and heat-inactivated fetal bovine serum (FBS, 15%). Select experiments (as noted) were performed with monocyte derived macrophages from THP-1 (ATCC) differentiated with phorbol 12-myristate 13-acetate (PMA; 5 nM, 48h). Human acute T cell leukemia cell line Jurkat (ATCC) was maintained in RPMI-1640 supplemented with heat-inactivated FBS (10%), penicillin (100 U/ml), and streptomycin (0.1 mg/ml). All cells were maintained in an incubator at 37°C, 5% CO2.
Treatments
Cells were treated with sodium alginate (Spectrum Chemical, New Brunswick, NJ) at 0.2 mg/l, 0.5 mg/ml, 1.0 mg/ml, or 2.5 mg/ml or its vehicle (sterile water) for 0, 4, or 24 h in F12K media with 2% FBS at 37°C, 5% CO2. These alginate concentrations are within ranges used in studies investigating alginate interactions with P. aeruginosa [17], as well as similar to alginate levels produced by certain mucoid P. aeruginosa strains [18, 19]. After treatment, AM were either immediately used, or were first rinsed in PBS and then used, or were rinsed and then recovered for 24 h in full culture media and then used in efferocytosis experiments, as indicated. Alginate preparations are often contaminated with LPS due to processing conditions. Thus, to investigate the role of LPS contamination, alginate was assayed using a Limulus Amebocyte Lysate (LAL) based kit (Thermo Scientific) and compared to other alginate preparations (Pronova-Nova Matrix, Ewing, NJ), which were ultra-purified (LPS-free), as noted.
Efferocytosis
Apoptotic targets were obtained from Jurkat cells labeled with Cell Tracker Orange (Invitrogen, Grand Island, NY; 0.5 mM) and exposed to UV radiation (30,000 μJ/cm2) using a HL-2000 HybriLinker, followed by incubation for 3.5 h at 37°C, 5% CO2 in serum-free media. Apoptotic Jurkat cells were co-cultured (1 h at 37°C) with AM in a 5:1 ratio. Afterwards, cells were collected and extracellular fluorescence (of membrane-bound but non-engulfed apoptotic cells or bodies) was quenched with Trypan Blue (Sigma; 500μl; 0.04% in PBS) [20]. Cells were then fixed with 1% paraformaldehyde. Efferocytosis was evaluated by flow cytometry using Cytomics FC500 cytofluorimeter with CXP software (Beckman Coulter, Fullerton, CA), as described (Fig. 1A) [20].
Fig. 1. Effect of sodium alginate on efferocytosis.
(A) Schematic of efferocytosis flow-cytometry method. Macrophage population was gated on FSC/SSC (gate B) and set for negative fluorescence (autofluorescence) on FSC/FL2 panel (gate AJ). When assessing the engulfment, the macrophages which engulfed apoptotic targets can be seen as a population of larger and more complex events in panel FSC/SSC (black) which were then evaluated (and measured as % cells which engulfed fluorescent targets) in panel FSC/FL2. The quenching with trypan blue discriminated engulfed from potentially attached apoptotic targets. (B-D) Efferocytosis of apoptotic Jurkat cells by rat alveolar macrophages (NR8383 cell line) in the presence of alginate at the indicated concentrations (B, 24 h) and time points (C, 2.5 mg/ml). Efferocytosis was expressed as relative phagocytic index (% of vehicle control; Mean + SEM; n=3; (Mean+SEM of control 47.35+2.49); B: ANOVA, p ≤ .0001; Tukey’s post-hoc * p ≤ 0.0001 and # p ≤ 0.01); (C) (Mean+SEM of control 35.55+2.55); ANOVA, p ≤ .0001; Tukey’s post-hoc * p ≤ 0.0001. (D) Efferocytosis index of rat alveolar macrophages (NR8383) following exposure to alginate (2.5 mg/ml; 4 h). Engulfment was assessed in co-cultures in the presence of alginate (present), immediately following removal of alginate (rinsed), or 24h following removal of alginate (rinsed & recovered). (Mean + SEM of control 29.9+3.24); n=3; ANOVA, p ≤ .0001; Tukey’s post-hoc # p ≤<0.0001, $ p<0.01,* p< 0.05. (E-F) Metabolic activity measured by MTT assay in macrophages in the following conditions: untreated, vehicle treated (water) and alginate (2.5 mg/ml) treated for 4 h and 24h, respectively (Mean + SEM, n=3).
Bacteria
The strains of P. aeruginosa used were PA14 (a highly virulent human clinical isolate) [21], PA01 (the standard laboratory reference strain) [22], and FRD1 (a mucoid strain isolated from a CF patient) [23]. The following mutants were used: isogenic deletion mutant for the genes phzM and exsA in PA14 (PAKO), which lacks pyocyanin and T3SS, an algD mutant in PA01 to eliminate alginate production [24, 25]. Isogenic deletions in phzM and exsA were created using an allelic replacement technique, as previously described [24]. For phzM mutation, we used the primers PhzMLfor (5′ TCG ACT GAG CCT TTC GTT TTA TTT GAT GCC TGG CAG TTC CGA TCC TCG GTC TCG AAG ATC 3′), PhzMLrev (5′ CAG CCG TTG AGA GTT CCG GTC TCT CGT TAC ACA TTT CCG T 3′), PhzMRfor (5′ ACG GAA ATG TGT AAC GAG AGA CCG GAA CTC TCA ACG GCT G 3′), and PhzMRrev (5′ GGA ATT GTG AGC GGA TAA CAA TTT CAC ACA GGA AAC AGC TAG TGG GAA ATC GAC CTG TTC 3′). Deletions were confirmed by PCR using primers PhzMfor (5′ GTT GTT TCC GCA ACG AGA TC 3′) and PhzMrev (5′ GCA ACG CGC TCA ACC AAC TG 3′). Deletion of exsA was described previously [25]. The algD mutant was kindly donated by Dr. George O’Toole, Geisel School of Medicine at Dartmouth. Bacteria were grown overnight in liquid LB media and then working suspensions were prepared to an OD600 of 0.6, as measured by spectrometry. These bacterial suspensions and apoptotic cells were co-cultured with macrophages for 1 h. Where indicated, alginate lyase was added to bacterial suspensions alone for 4 h before efferocytosis experiments.
Alginate lyase activity
Alginate lyase was obtained from Sphingobacterium multivorum (Sigma-Aldrich), and has specificity for cleaving manuronic acid bonds. The reaction was initiated by adding alginate lyase (in PBS, pH 7.4) to alginate (1:30 vol:vol). The enzyme activity (4h at 37 °C) was measured with a SpectraMax M2 plate reader (Molecular Devices Inc., Sunnyvale, CA) at 235 nm.
Cellular toxicity was determined using an in vitro MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay, according to the manufacturer’s (Sigma) protocol. The absorbance of formazan was measured at 570 nm with a SpectraMax M2 plate reader.
Statistical methods
Differences among groups were assessed using either t-test for two groups or ANOVA for 3 or more groups with post-hoc Tukey’s testing for significant differences between selected groups. Statistical difference was considered significant when p<0.05. Analyses were performed using Prism Software (GraphPad Software, Inc. La Jolla, CA).
Results
Effect of alginate on AM efferocytosis
We first determined if alginate, a complex copolymer present in the biofilm formed by P. aeruginosa during chronic infections, is sufficient to impair AM efferocytosis. We exposed rat AM (NR8383 cell line) to exogenous sodium alginate suspensions of varying concentrations (Fig. 1B) and for varying amount of time (Fig. 1C), followed by AM co-culture with apoptotic targets. Alginate inhibited efferocytosis of apoptotic targets (fluorescently-labeled UV-irradiated Jurkat T-cells) in a dose- and time-dependent fashion, as measured by flow cytometry. The highest dose of alginate tested (2.5 mg/ml) exerted an inhibitory effect on AM efferocytosis after as early as 5 minutes of treatment, and was most profound after 24 h of incubation.
To determine the persistence of efferocytosis impairment following treatment with sodium alginate, rat AM efferocytosis was investigated following the removal of alginate. After alginate treatment, cells were either rinsed and immediately co-cultured with apoptotic targets, or were rinsed and allowed to recover in fresh media for 24 h prior to co-culture with apoptotic targets (Fig. 1D). The immediate removal of alginate from the co-culture did not significantly change the inhibitory impact on AM efferocytosis. Interestingly, cells that were allowed to recover overnight exhibited only partial (20%) restoration of their efferocytosis potential compared with cells from which alginate was not rinsed, and only a non-significant (9%, p=0.4) recovery in efferocytosis compared to cells that were tested immediately after alginate was rinsed. Given the persistence of the effect of alginate on efferocytosis, to rule out a nonspecific toxic effect, we tested if alginate (2.5 mg/ml) altered macrophage viability, as measured by MTT assay (Fig. 1E). Macrophages exposed to alginate did not show decreased metabolic toxicity, suggesting that alginate is a potent inhibitor of alveolar macrophage efferocytosis and that its effect is not merely due to the physical presence of alginate in co-cultures or due to a non-specific irreversible toxic effect on AM.
Effects of LPS on AM efferocytosis
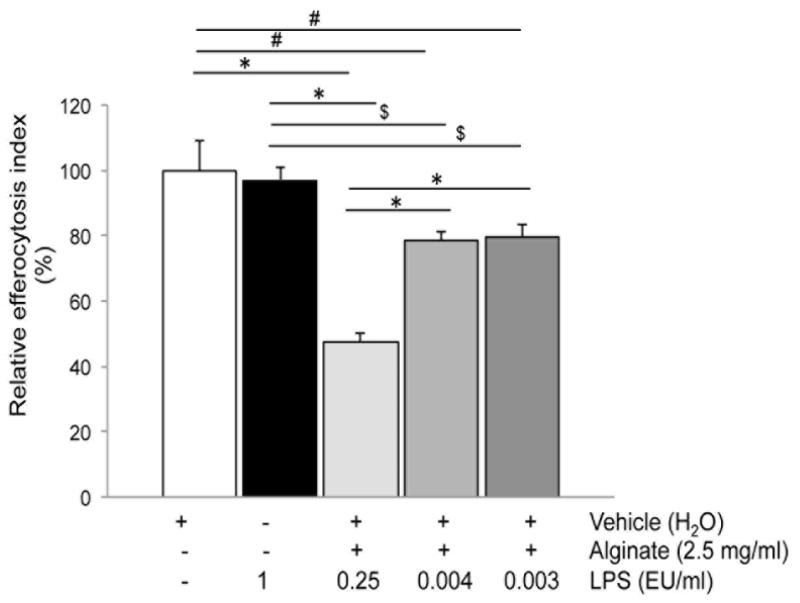
Since purified alginate may be contaminated with varying levels of lipopolysaccharide (LPS) [26], we investigated the levels and potential contribution of LPS contamination of alginate on efferocytosis. The alginate used in the experiments above was tested using the limulus amebocyte assay and found to contain 100 EU LPS/g of alginate, which is considered a low level of contamination [26]. We then compared the effect of this alginate (1 EU LPS/ml alginate) with that of highly purified alginates (0.3 and 0.4 EU LPS/ml alginate, respectively) on efferocytosis. We noted that alginate containing lower levels of LPS had less inhibitory effect on efferocytosis (Fig. 2), suggesting a contributory effect of LPS on alginate-induced AM dysfunction. However, comparable exposures (4h) to LPS alone without alginate, in concentrations even higher than those found in alginate did not inhibit AM efferocytosis (Fig. 2). These results suggest a cooperative effect of LPS and alginate on macrophage engulfment of apoptotic cells.
Fig. 2. Impact of lipopolysaccharide (LPS) in alginate on efferocytosis.
Efferocytosis index of alveolar macrophages (NR8383) following exposure to alginate (2.5 mg/ml; 4h; 0.25 EU LPS /ml of media) compared to alginate preparations with low LPS contamination (final concentrations of LPS in media as indicated), or to LPS alone (1 EU/ml). Mean + SEM; (Mean + SEM of control 53.17+7.31); n=3; ANOVA, p < 0.0001; Tukey’s post-hoc * p <0.0001 # p<0.005 and $ p<0.01.
Effects of P. aeruginosa on AM efferocytosis
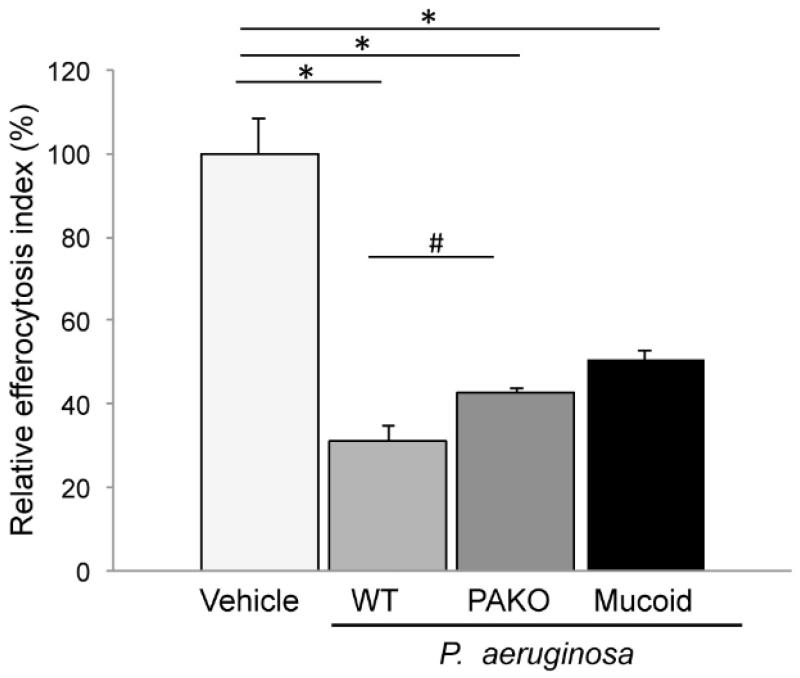
We investigated if alginate-producing (mucoid) strains of P. aeruginosa, typically present in the biofilm of CF chronic airway infection, inhibit efferocytosis. We studied the effect of a mutant P. aeruginosa lacking the pyocyanin and T3SS toxins (PAKO) relative to the wild-type (PA14) P. aeruginosa strain, which is known to inhibit efferocytosis via production of toxins such as pyocyanin [7]. The toxin-deficient strain significantly inhibited efferocytosis by more than 50% (Fig. 3). This inhibitory effect was less potent compared to wild type bacteria which depressed efferocytosis by 70%, but it was remarkably similar to the effect of alginate alone. As a comparison, we also tested a CF-adapted, mucoid strain (FRD1). Importantly, this mucoid strain also inhibited efferocytosis by more than 50% (Fig. 3).
Fig. 3. Efferocytosis of alveolar macrophages exposed to P. aeruginosa strains.
Efferocytosis index of rat alveolar macrophages (NR8383) during exposure to the following strains of P. aeruginosa: wild type (WT; non-mucoid; PA14), mutated (virulence-attenuated PAKO), or mucoid (alginate overproducer; FRD1). The ratio of bacteria: macrophages; apoptotic cells during co-culture was 1:5:5. Mean + SEM; (Mean + SEM of control 50.13+4.8;) n=3; ANOVA p < 0.0001; Tukey’s post-hoc *p <0.0001; # p<0.01.
Impact of alginate lyase (AL) treatment on efferocytosis
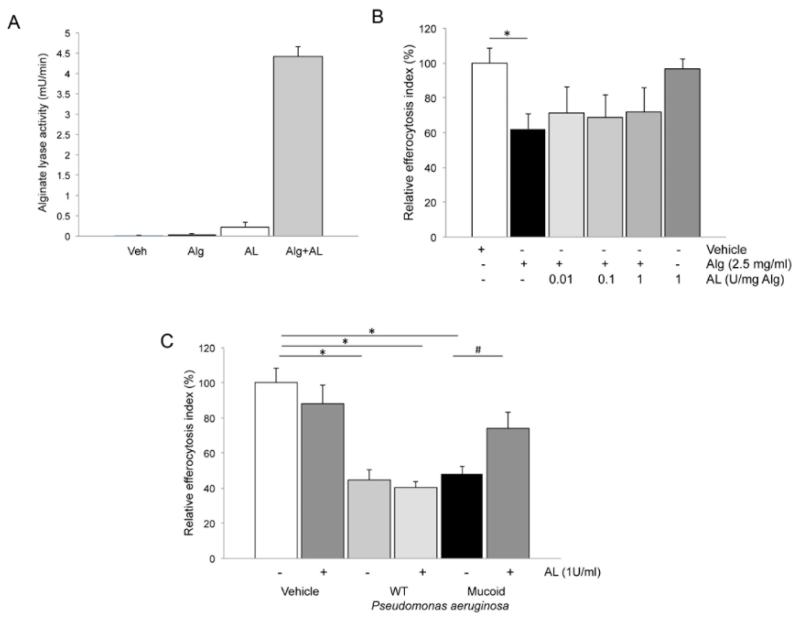
We tested if the breakdown of high-molecular weight alginate polymers with AL will prevent its inhibitory effect on efferocytosis. After testing the activity of purified AL at neutral pH (Fig. 4A), we incubated AL with exogenous alginate prior to exposure of AM. Whereas AL treatment did not affect the inhibitory effect of alginate on AM efferocytosis (Fig. 4B), it significantly attenuated the effect of mucoid, alginate producing (FRD1) P. aeruginosa on efferocytosis (Fig. 4C). However, AL had no impact on efferocytosis inhibition in response to wild type P. aeruginosa or an alginate non-producer mutant strain (data not shown). These data suggest that alginate is in large part responsible for the efferocytosis inhibitory effect of mucoid P. aeruginosa.
Fig. 4. Effect of alginate lyase (AL) on efferocytosis inhibition by alginate and alginate-producing P. aeruginosa.
(A) AL activity (mU /min; pH 7.4) measured during incubation of purified AL (1U/ml) with its substrate alginate (Alg, 2.5 mg/ml), compared to controls such as vehicle (Veh), alginate alone, or AL in the absence of substrate. Mean + SEM; n=3. (B) Effect of pre-incubation of AL with alginate (at the indicated concentrations; 1:30 v:v ratio AL:alginate; 45 min.) on efferocytosis of alveolar macrophages (NR8383 cells) exposed to alginate (2.5 mg/ml; 4h). Mean + SEM; (Mean + SEM of control 40.62+4.3); n=6; t-test *p< 0.05. (C) Effect of pre-incubation of AL (1U/ml; 4 h) with mucoid P. aeruginosa (alginate overproducer; FRD1) on efferocytosis of human peripheral blood monocyte (THP-1)-derived macrophages. The ratio of bacteria: macrophages:apoptotic cells was 2.5:1:5. Mean + SEM; (Mean + SEM of control 48.19+5.03); n=3; ANOVA p < 0.0001; Tukey’s post-hoc *p <0.01, #p< 0.05.
Discussion
Whereas the pathogenic effects of P. aeruginosa during acute infections are attributed in large part to toxin production, the production of virulent toxins is markedly decreased during chronic bacterial persistence in biofilm [27, 28]. Whereas the low virulence of P. aeruginosa in biofilm infections accounts for decreased anti-bacterial host responses, it is becoming increasingly evident that biofilm-state P. aeruginosa continues to exert pathogenic effects on the airways. The main finding of this report is that even P. aeruginosa with low virulence inhibits the clearance of apoptotic cells by macrophages, a critical innate host defense mechanism. We further report that this pathogenic effect may be due to a synergistic effect of alginate and LPS.
Similar to findings of Bianchi et al., our data indicate that wild type, pyocyanin-producing virulent P. aeruginosa has profound inhibitory effect on efferocytosis [7]. In addition, we show that even bacteria that lack these virulence factors can inhibit efferocytosis, albeit to a lesser degree. The dose-dependent effect of alginate on efferocytosis implicates this complex copolymer as one of the mediators by which mucoid P. aeruginosa inhibits macrophage engulfment of apoptotic cells. Of the three exopolymers secreted by mucoid P. aeruginosa Pel, Psl, and alginate, only the latter is found in bacteria adapted to the CF lung [29]. Alginate has been involved in other effects of mucoid P. aeruginosa on the innate immunity, such as decreased phagocytosis of bacteria by PMN, in addition to decreasing local antibiotic penetration, or, at higher concentrations, modulating the T3SS of the bacteria [17]. Secreted in abundance by mucoid P. aeruginosa, alginate is an unbranched polysaccharide composed of monomers of L-guluronic acid and D-mannuronic acid [30]. While pure alginate does not contain LPS, the steps necessary to purify alginate from various sources are associated with varied degrees of contamination of alginate with LPS. For example, alginate purified from cultured mucoid bacteria is typically contaminated with approximately 900 EU/ml LPS [31]. The commercial alginate preparation purified from algae used in our study contained 4-fold less LPS than alginate typically purified from bacteria. Although bacterial LPS alone at similar concentrations did not inhibit macrophage efferocytosis, we demonstrated a cooperative effect of LPS with alginate to inhibit efferocytosis. Whether this phenomenon could be explained by common signaling pathways engaged by alginate and LPS, such as TLR4 receptor activation, will need experimental confirmation in future investigations. One cannot exclude the possibility that other contaminants in the alginate extract, or other immunogenic components of the biofilm in addition to alginate, may impact efferocytosis [32]. However, our results showing the effect of alginate-producing P. aeruginosa, which was diminished by AL, suggest alginate-specific inhibition of efferocytosis. Although not directly compared in our model, our results also suggest that both bacteria producing alginate and algae alginate have inhibitory effects on efferocytosis, despite structural dissimilarities of the two exopolymers. The mechanism by which alginate impairs efferocytosis may include alternation of expression or accessibility of efferocytosis-required receptors at the plasma membrane. Such a candidate could be ABCA1, one of the twelve members of the ABC transporter system that plays an important role in efferocytosis [33], since alginate molecules interact via the ABC system [34] and ABCA1 has been shown to be downregulated by LPS [35].
It is interesting that the enzymatic breakdown of the copolymer by incubation with AL did not rescue efferocytosis impaired by marine alginate, whereas the enzyme was effective in rescuing efferocytosis inhibited by alginate-overproducing bacteria. It is possible that the effectiveness of the enzyme may be related to reaction conditions in vitro or to substrate accessibility, given the variability in monomer type predominance and acetylation of different alginates [36]. In particular, the AL used in our experiments has specificity for manuronic acid. The fact that guluronate is more prevalent in algal alginate and mannuronate is more abundant in Pseudomonas alginate may explain why AL was more effective in cleaving bacterial alginate. Although AL may be inhibited by acetylation [37] and bacterial alginate is heavily acetylated, treatment of alginate overproducing P. aeruginosa strain FRD1 with AL led to significant increases (and partial recovery) in efferocytosis and support the notion that bacterial alginate, like algal alginate, contributes to the detrimental effect of mucoid P. aeruginosa on this innate immune function.
The approach used in our studies relied on ex vivo observations of macrophage function, due to the difficulty to establish chronic mucoid P. aeruginosa airway infection or colonization in animals [38]. Nevertheless, these results may be relevant to human lung diseases characterized by airway colonization and chronic biofilm infection with P. aeruginosa, such as CF, COPD, and non-CF bronchiectasis. Chronic airway infection with P. aeruginosa is known to decrease lung function and increased mortality in CF and is characterized by production of a biofilm containing the polysaccharide alginate and by decreased microbial virulence, in attempt to evade host immunity. Indeed, the biofilm matrix, composed of extracellular polysaccharides such as alginate, Pel, and Psl [29] along with DNA, protein, and other host components, protect the constituent bacteria from immune attack and from antibiotic treatment [9]. Despite the decreased virulence, mucoid P. aeruginosa is still associated with decline in lung function. We have shown that alginate decreases the removal of dead cells by macrophages, which may cause prolonged airway inflammation and lung dysfunction. Since mucoid biofilm infections are characterized by marked secretion of alginate [39, 40], our data implicate the mucoid P. aeruginosa in airway inflammation, associated with alginate inhibition of alveolar macrophage efferocytosis. The impairment of lung macrophage efferocytosis contributes to airway inflammation through mechanisms that include release of pro-inflammatory mediators from non-engulfed apoptotic cells (via secondary necrosis), increased mucus viscosity from spilled DNA, and loss of anti-inflammatory phenotype of alveolar macrophages associated with apoptotic cell engulfment. Several studies suggest that airway inflammation further propagates bacterial biofilm persistence. The released intracellular proteins and nucleic acids, which increase airway mucus viscosity, enhance the bacterial biofilm matrix that surrounds and protects bacteria [41]. Biofilm polysaccharides themselves exert a positive feedback on further biofilm synthesis [42]. In addition, persistently activated immune cells, which may include macrophages with impaired efferocytosis, exert oxidative stress which promotes P. aeruginosa mutations (mucA) that stimulate alginate overexpression [9, 43]. These dysregulated host-microbe interactions support that alginate lyase or other methods to mitigate alginate accumulation in the airway may decrease airway inflammation and biofilm formation in chronic P. aeruginosa infection in CF and other bronchiectatic patients.
Acknowledgements
We acknowledge the following funding sources: The Cystic Fibrosis Foundation Fellowship Grant (CM) and HL 077328 (IP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Salvatore D, et al. An overview of international literature from cystic fibrosis registries. Part 3. Disease incidence, genotype/phenotype correlation, microbiology, pregnancy, clinical complications, lung transplantation, and miscellanea. J Cyst Fibros. 2011;10(2):71–85. doi: 10.1016/j.jcf.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Emerson J, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–8. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 4.Vandivier RW, et al. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L677–86. doi: 10.1152/ajplung.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi SM, et al. Granulocyte apoptosis in the pathogenesis and resolution of lung disease. Clin Sci (Lond) 2006;110(3):293–304. doi: 10.1042/CS20050178. [DOI] [PubMed] [Google Scholar]
- 6.Dacheux D, et al. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect Immun. 1999;67(11):6164–7. doi: 10.1128/iai.67.11.6164-6167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi SM, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2008;177(1):35–43. doi: 10.1164/rccm.200612-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith EE, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci US A. 2006;103(22):8487–92. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez MI, et al. Opportunistic infections in lung disease: Pseudomonas infections in cystic fibrosis. Curr Opin Pharmacol. 2007;7(3):244–51. doi: 10.1016/j.coph.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Gibson RL, et al. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 11.Gooderham WJ, et al. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol Rev. 2009;33(2):279–94. doi: 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- 12.Wozniak DJ, et al. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2003;100(13):7907–12. doi: 10.1073/pnas.1231792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry RL, et al. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12(3):158–61. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 14.Song Z, et al. Pseudomonas aeruginosa alginate is refractory to Th1 immune response and impedes host immune clearance in a mouse model of acute lung infection. J Med Microbiol. 2003;52(Pt 9):731–40. doi: 10.1099/jmm.0.05122-0. [DOI] [PubMed] [Google Scholar]
- 15.Alkawash MA, et al. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. APMIS. 2006;114(2):131–8. doi: 10.1111/j.1600-0463.2006.apm_356.x. [DOI] [PubMed] [Google Scholar]
- 16.Mrsny RJ, et al. Addition of a bacterial alginate lyase to purulent CF sputum in vitro can result in the disruption of alginate and modification of sputum viscoelasticity. Pulm Pharmacol. 1994;7(6):357–66. doi: 10.1006/pulp.1994.1042. [DOI] [PubMed] [Google Scholar]
- 17.Horsman SR, et al. Calcium chelation by alginate activates the type III secretion system in mucoid Pseudomonas aeruginosa biofilms. PLoS One. 2012;7(10):e46826. doi: 10.1371/journal.pone.0046826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laux DC, et al. Lysophosphatidic acid inhibition of the accumulation of Pseudomonas aeruginosa PAO1 alginate, pyoverdin, elastase and LasA. Microbiol. 2002;148(Pt 6):1709–23. doi: 10.1099/00221287-148-6-1709. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann N, et al. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun. 2005;73(4):2504–14. doi: 10.1128/IAI.73.4.2504-2514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrusca DN, et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem. 2010;285(51):40322–32. doi: 10.1074/jbc.M110.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati NT, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A. 2006;103(8):2833–8. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 23.Ohman DE, Chakrabarty AM. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun. 1981;33(1):142–8. doi: 10.1128/iai.33.1.142-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanks RM, et al. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol. 2006;72(7):5027–36. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson GG, et al. The Pseudomonas aeruginosa magnesium transporter MgtE inhibits transcription of the type III secretion system. Infect Immun. 2010;78(3):1239–49. doi: 10.1128/IAI.00865-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dusseault J, et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J Biomed Mater Res A. 2006;76(2):243–51. doi: 10.1002/jbm.a.30541. [DOI] [PubMed] [Google Scholar]
- 27.Ventre I, et al. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci U S A. 2006;103(1):171–6. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furukawa S, et al. Keeping their options open: acute versus persistent infections. J Bacteriol. 2006;188(4):1211–7. doi: 10.1128/JB.188.4.1211-1217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin MJ, et al. Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front Microbiol. 2011;2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey DM, Wozniak DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Molec Microbiol. 2005;56(2):309–22. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 31.Kashef N, et al. Synthesis and characterization of Pseudomonas aeruginosa alginate-tetanus toxoid conjugate. J Med Microbiol. 2006;55(Pt 10):1441–6. doi: 10.1099/jmm.0.46696-0. [DOI] [PubMed] [Google Scholar]
- 32.Paredes-Juarez GA, et al. The role of pathogen-associated molecular patterns in inflammatory responses against alginate based microcapsules. J Control Release. 2013;172(3):983–92. doi: 10.1016/j.jconrel.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Hamon Y, et al. ABCA1 and the engulfment of apoptotic cells. Biochim Biophys Acta. 2002;1585(2-3):64–71. doi: 10.1016/s1388-1981(02)00325-6. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto W, et al. Molecular identification of oligoalginate lyase of Sphingomonas sp. strain A1 as one of the enzymes required for complete depolymerization of alginate. J Bacteriol. 2000;182(16):4572–7. doi: 10.1128/jb.182.16.4572-4577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin K, et al. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med. 2010;16(9-10):438–49. doi: 10.2119/molmed.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ertesvag H, et al. Biochemical properties and substrate specificities of a recombinantly produced Azotobacter vinelandii alginate lyase. J Bacteriol. 1998;180(15):3779–84. doi: 10.1128/jb.180.15.3779-3784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley LM, et al. Structural and functional characterization of Pseudomonas aeruginosa AlgX: role of AlgX in alginate acetylation. J BiolChem. 2013;288(31):22299–314. doi: 10.1074/jbc.M113.484931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher JT, et al. Comparative biology of cystic fibrosis animal models. Met Molec Biol. 2011;742:311–34. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjarnsholt T, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(6):547–58. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Solano L, et al. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1526–33. doi: 10.1086/593186. [DOI] [PubMed] [Google Scholar]
- 41.Walker TS, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73(6):3693–701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irie Y, et al. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2012;109(50):20632–6. doi: 10.1073/pnas.1217993109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman AL, et al. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Develop Cell. 2004;7(5):745–54. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]