Abstract
Background
There is an urgent need for gut lengthening secondary to massive resections of the gastrointestinal (GI) tract. In this study, we propose to evaluate the remodeling, vascularization and functionality of a chitosan-based tubular neuro-muscular tissue upon subcutaneous implantation in the back of athymic rats.
Methods
Aligned innervated smooth muscle sheets were bioengineered using human smooth muscle and neural progenitor cells. The innervated sheets were wrapped around tubular chitosan scaffolds. The engineered tubular neuro-muscular tissue was implanted subcutaneously in the back of athymic rats. The implant was harvested after 14 days and assessed for morphology, vascularization and functionality.
Results
Gross examination of the implants showed healthy color with no signs of inflammation. The implanted tissue became vascularized as demonstrated by gross and histological analysis. Chitosan supported the luminal patency of the tissue. The innervated muscle remodeled around the tubular chitosan scaffold. Smooth muscle maintained its circumferential alignment and contractile phenotype. Functionality of the implant was further characterized using real time force generation. Cholinergic response was demonstrated by robust contraction in response to Ach. VIP and EFS caused relaxation. In the presence of neurotoxin TTX, the magnitude of Ach-induced contraction and VIP-induced relaxation was attenuated while EFS-induced relaxation was completely abolished, indicating neuronal contribution to the response.
Conclusion
Our results indicated the successful subcutaneous implantation of engineered tubular neuro-muscular tissues. The tissues became vascularized and maintained their myogenic and neurogenic phenotype and function. This provides potential therapeutic prospects for providing implantable replacement GI segments for treating GI motility disorders.
Keywords: Intestinal tissue engineering, gut lengthening, chitosan, smooth muscle, neural progenitor cells, implantation
1. Introduction
Short bowel syndrome (SBS) is the result of massive resections (over 70%) of the small intestine [1]. Severity and treatment of SBS are dependent on the specific regions of the bowel that are resected [2]. Due to reduced absorptive surface area following resection of the intestine, patients usually suffer from dehydration, weight loss, disturbances in electrolytes and deficiencies in minerals and vitamins [1, 3]. Current standard treatments for SBS require extensive nutritional management [4]. Parenteral nutrition is usually associated with liver failure and catheter-related complications. Intestinal transplantation provides an alternative treatment, however complications including graft rejection and failure still exist [5]. Therefore, there is a clinical need for gut lengthening to restore intestinal function. Previous studies demonstrated the feasibility of re-implanting a mechanically-lengthened intestinal segment in pig models [6]. This resulted in mucosal and muscle function. Tissue engineering is another viable option to restore gut continuity. The goal is to duplicate the architecture and function of all cell types of the GI tract using a combination of cells and scaffolds.
The GI tract is a complex system with multiple cell types, organized in different layers. The smooth muscle is considered as the basic unit of the musculature of the tract. The alignment and the phenotype of gut smooth muscle are critical for proper function [7]. The GI tract has its own intrinsic regulatory apparatus which includes the enteric nervous system and the interstitial cells of Cajal (ICC). Smooth muscle receives and interprets signals from the regulatory apparatus required for proper function [8]. Another major component of the GI tract includes the epithelium which is required for enzyme secretion, nutrient absorption and defense barrier. All these functions are dictated by specialized cell types. Taken together, an ideal functional gut replacement requires the regeneration of all of these cell types.
In our previous studies, sphincteric ring structures were engineered using the co-culturing system of smooth muscle and neural progenitor cells [9]. Those ring structures became vascularized and maintained their phenotype and function following their implantation around the internal anal sphincter of rats [10]. Results presented in this work demonstrate that we can successfully develop tubular tissues as a first stage for future gut replacements. We engineered innervated human smooth muscle sheets. We also introduced a tubular chitosan scaffold as support for those sheets. The tubular tissues were implanted subcutaneously in athymic rats and evaluated for vascularization, remodeling and functionality. Following 14 days of subcutaneous implantation, the engineered tubular neuro-muscular tissues became vascularized and maintained their myogenic and neurogenic characteristics as demonstrated by histological and physiological analysis.
2. Materials and methods
2.1. Reagents
Cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Smooth muscle growth medium consisted of Dulbecco's modified Eagle medium (DMEM), 10% fetal bovine serum (FBS), 1.5% antibiotic-antimycotic, and 0.6% l-glutamine. Neural progenitor cell growth medium consisted of neurobasal, N2 supplement and antibiotic–antimycotic. Neural differentiation media consisted of neurobasal medium-A supplemented with 2% fetal calf serum, 1X B27 supplement and 1X antibiotic–antimycotic. Medium molecular weight chitosan (75–85% deacetylation), acetylcholine (ACh), vasoactive intestinal peptide (VIP), tetrodotoxin (TTX), and nNOS-blocker Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) were purchased from Sigma (St. Louis, MO). Sylgard [poly(dimethylsiloxane); PDMS] was purchased from World Precision Instruments (Sarasota, FL). Type I collagen was purchased from BD Biosciences.
2.2. Cell Isolation
Human intestinal tissues were ethically obtained from organ donors through Carolina Donor Services and Wake Forest Baptist Medical Center (IRB No. 00007586).
2.2.1. Human circular smooth muscle cells
A segment of 10 cm of the duodenum right below the pyloric sphincter was consistently obtained from all donors for cell isolation. Human smooth muscle cells were isolated from human duodenum following the protocols previously described by our lab [11]. Briefly, human duodena were cleaned of any luminal content and washed extensively in ice-cold Hank's balanced salt solution. The circular smooth muscle layer was stripped off and separated from the mucosa and the longitudinal muscle layer. The circular smooth muscle was minced and digested twice in type II collagenase (Worthington, Lakewood, NJ) and DNAse (Roche, Indianapolis, IN) for one hour each. Digested cells were washed, resuspended in smooth muscle growth media and cultured on tissue culture flasks until confluent.
2.2.2. Human enteric neural progenitor cells
Human enteric neural progenitor cells were isolated following previously published protocols [9, 10, 12]. Briefly, human intestinal tissues were extensively washed in ice-cold Hank's balanced salt solution supplemented with gentamicin and antibiotics/antimycotics. The tissues were finely minced followed by additional washing. Tissues were then subjected to digestion using a mixture of collagenase type II, dispase, and DNAse I. Cells were then passed through 70 μm followed by 40 μm cell strainers. Cells were recovered by centrifugation and cultured in non-tissue culture treated plates in neural growth medium that enhances cell proliferation. Cells were maintained at 37°C and 7% CO2 with regular media change. These cultured cells formed floating clusters known as neurospheres. These neurospheres stained positive for neural crest-derived cell marker p75 [9].
2.3. Engineering tubular neuro-muscular tissue
Tubular chitosan scaffolds were engineered as described previously [11]. Briefly, chitosan solution was prepared in 0.2 M acetic acid and mixed with type I collagen. Chitosan/collagen mixture was poured into a custom made tubular mold with a central opening to create the lumen of the scaffold. The whole assembly was frozen at −80 °C for 3 h and then lyophilized for 24 h. The scaffolds were neutralized in NaOH and washed extensively with PBS and distilled water.
Innervated smooth muscle sheets (2×4 cm) were bioengineered following our previously published method [13]. Briefly, smooth muscle cells were trypsinized and collected. A suspension of 500,000 smooth muscle cells was seeded onto a Sylgard wavy mold coated with laminin and cultured in muscle growth medium. Smooth muscle cells were left in culture for 5 days to allow smooth muscle cell alignment along the grooves of the mold. On day 5, human enteric neuronal progenitor cells were suspended in a collagen gel mixture, which was overlaid on top of the aligned smooth muscle cells. Innervated smooth muscle sheets were formed through the process of delamination of the smooth muscle cell layer. Neural differentiation media was supplied every other day to enhance neural differentiation. The innervated smooth muscle sheets were then wrapped around the tubular chitosan scaffolds and prepared for implantation.
2.4. Implantation of the engineered tubular neuro-muscular tissues
The engineered tubular neuro-muscular tissues were implanted subcutaneously in the back of female athymic rats (n=5). All surgical procedures were performed following the guidelines set forth by IACUC. This surgical procedure has been previously described [14]. Athymic rats were anesthetized under continuous isoflurane masking. Surgical area in the back of anesthetized rats were shaved and aseptically prepared. A 1 cm transverse incision was made in the upper back of the rat. A subcutaneous pocket was created into which the engineered tissue was inserted and fixed using 5-0 prolene sutures in order to locate it at the time of harvest. Tubular chitosan scaffolds that were not seeded with the innervated smooth muscle were implanted in a similar fashion and served as control. The rats were allowed to recover in their cages in standard fashion.
2.5. Implant harvest
The rats were euthanized 14 days post-implantation. The site of incision was reopened and the tissues were located using the 5-0 prolene sutures. The neuro-muscular tissues and the control scaffolds were dissected from the surrounding tissue. The harvested tissues and scaffolds were evaluated as described below.
2.6. Histological and immunofluorescence evaluation of the implant
Following harvest, the tissues were fixed in formaldehyde, processed and paraffin embedded. Cross sections of 6 μm thickness were obtained, deparaffinized and hydrated in water. Hematoxylin and eosin (H&E) and Masson's Trichrome stains were perfomed for morphological analysis. Immunofluorescence studies were designed to evaluate (i) smooth muscle phenotype using primary antibodies directed against α-smooth muscle actin and smooth muscle specific heavy-Caldesmon and (ii) differentiated neurons using β-III tubulin.
2.7. Physiological analysis of the implant
Cross sections of the harvested neuro-muscular tissues were tested for physiological functionality using a force transducer apparatus (Harvard Apparatus, Holliston, MA) following our previously published protocols [15]. The physiological functionality of the implanted neuro-muscular tissues was compared to forces generated by native rat intestines of same size as the implants. The tissues were incubated in a warm tissue bath and connected to the measuring arm of the transducer.
The tests were designed to evaluate the (i) electromechanical coupling integrity of the smooth muscle using potassium chloride (KCl), (ii) cholinergic responsiveness of the smooth muscle using Acetylcholine (Ach) and (iii) relaxation of smooth muscle in response to vasoactive intestinal peptide (VIP) and electrical field stimulation (EFS). The tissues were washed after each experiment. KCl response was evaluated in the absence and presence of calcium channel blocker, Nifedipine. Neural contribution to cholinergic contraction, VIP and EFS relaxation was tested in the absence and presence of neurotoxin, tetrodotoxin (TTX). EFS-induced relaxation was further characterized for nitrergic neurotransmitters involved in the response by pre-treating the tissues with nitric oxide synthase (nNOS) blocker Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME).
2.8. Statistical analysis
Analysis of acquired force data was acquired using Powerlab and exported to GraphPad Prism 5.0 for Windows (GraphPad Software, San Diego CA; www.graphpad.com). Second order Savitzky–Golay smoothing was applied to data. Student paired t-test was used to compare the means of forces in the absence and presence of inhibitors. Student t-test was used to compare the forces generated by the implant and the native intestine. A p-value less than 0.05 was considered significant. All values were expressed as means ± SEM (n=5).
3. Results
3.1. Implantation and harvest of the tubular neuro-muscular tissues
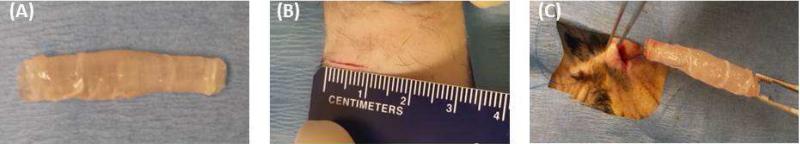
Innervated smooth muscle sheets were cultured in the presence of neural differentiation media to allow neural differentiation. The Sheets were then wrapped around sterile tubular chitosan scaffolds and prepared for implantation (Figure 1A). A 1 cm incision was made in the back of the rat (Figure 1B) and a pocket was created to implant the tubular neuro-muscular tissues (Figure 1C). The implants were secured in place by sutures and the skin was closed. Tubular chitosan scaffolds without innervated muscle were implanted as control.
Figure 1.
(A) Engineered tubular neuro-muscular tissue pre-implantation. Engineered aligned smooth muscle sheet was wrapped around tubular chitosan scaffold to form the tubular neuro-muscular tissue. (B) A 1 cm incision was made in the back of nude athymic rats. (C) A pocket was created and the tubular neuro-muscular tissue was inserted into the subcutaneous tissue.
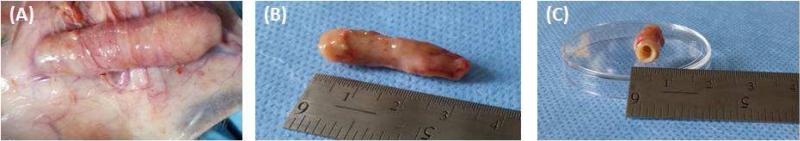
All rats maintained normal activity and survival was 100%. Incision sites were completely healed at the time of harvest with no signs of infection. Gross inspection of the neuro-muscular tissues showed no signs of inflammation, abscess formation or necrosis (Figure 2A). The tissues appeared healthy in color and surrounded by blood vessels. The tubular neuro-muscular tissues were 3 cm in length (Figure 2B). The tissues maintained their luminal patency following implantation, with an internal diameter of 0.3 cm and external diameter of 0.5 cm (n=5) (Figure 2C).
Figure 2.
(A) The tubular neuro-muscular tissue was harvested after 14 days of implantation. The implant was healthy and vascularized. (B) The tubular neuro-muscular tissue was 3 cm in length and (C) 0.3 cm internal diameter. The tubular neuro-muscular tissue maintained luminal patency during the implantation period.
3.2. Histological and immunofluorescence evaluation of the implant
3.2.1. H&E and Masson's Trichrome
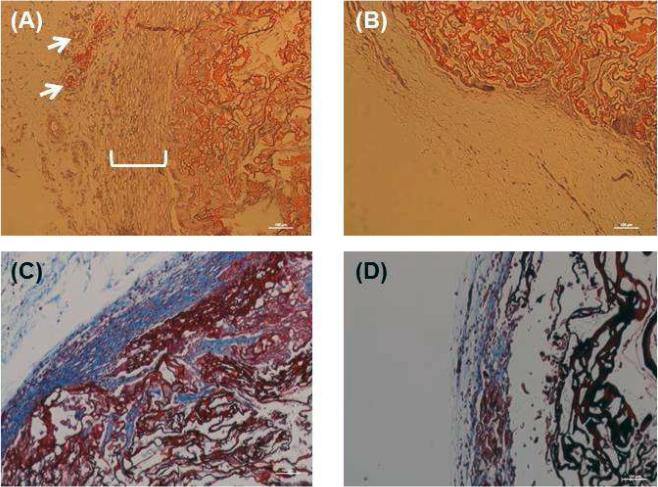
Harvested implants were fixed in formaldehyde, processed and paraffin embedded. Cross sections of 6 μm thickness were prepared. Representative H&E staining of the neuro-muscular tissues after implantation is shown in Figure 3A. Stains showed preservation of circumferentially aligned smooth muscle layer around the lumen. Blood vessel structures were observed in the periphery of the implant (white arrows). H&E of scaffolds only showed lack of cellular alignment around the scaffold (Figure 3B). Masson's trichrome staining demonstrated deposition of collagen in the tubular neuro-muscular tissues (Figure 3C) when compared to scaffolds only without smooth muscle (Figure 3D).
Figure 3.
H&E analysis of (A) the tubular neuro-muscular tissue showed maintenance of alignment of smooth muscle around the lumen with blood vessels (white arrows) and (B) absence of smooth muscle in the scaffold only (control). Masson's trichrome analysis revealed high and more compact collagen around the tubular neuro-muscular tissue (C) when compared to control scaffold (D).
3.2.2. Immunofluorescence
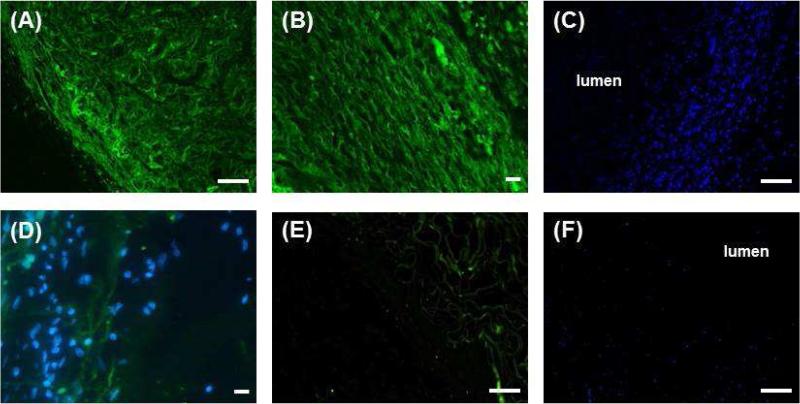
Implant sections were deparaffinized and rehydrated. Sections stained positive with α-smooth muscle actin (Figure 4A) and smooth muscle specific heavy Caldesmon (Figure 4B). This indicated that smooth muscle contractile phenotype was maintained during the 2 week period of implantation. DAPI staining showed maintenance of concentric alignment of the smooth muscle around the lumen of the tubular scaffold (Figure 4C). Sections of the neuro-muscular tissues stained positive for βIII tubulin, indicating the presence of differentiated mature neurons (Figure 4D). Scaffolds that served as control stained negative for α-smooth muscle actin (Figure 4E) and showed no specific cellular alignment around the scaffold (Figure 4F).
Figure 4.
Immunofluorescence analysis of the tubular neuro-muscular tissue showed (A) positive stain for α-smooth muscle actin and (B) smooth muscle specific heavy Caldesmon indicating the maintenance of smooth muscle contractile phenotype. (C) DAPI staining (blue) showed maintenance of alignment of smooth muscle around the lumen. (D) Differentiated neurons were demonstrated by positive stain for β-III tubulin. DAPI stain shows the location of the smooth muscle with respect to neurons. (E) Tubular chitosan scaffold alone (control) showed negative stain for α-smooth muscle actin and (F) lack of any cellular alignment around the lumen of the scaffold. Scale bars are 100 μm.
3.3. Physiological studies
Further confirmation of functionality of the neuro-muscular tissues is critical for successful outcomes. Cross sections of the tissues were obtained after harvest for real time force generation. Cross sections of similar sizes were obtained from rat intestines for comparison. One end of the tissue was connected to the fixed pin of the organ bath while the other end was connected to the measuring arm of the force transducer. The tissues were allowed to establish baseline. Tissues were washed with fresh warm buffer after every experiment.
3.3.1. Electromechanical coupling integrity
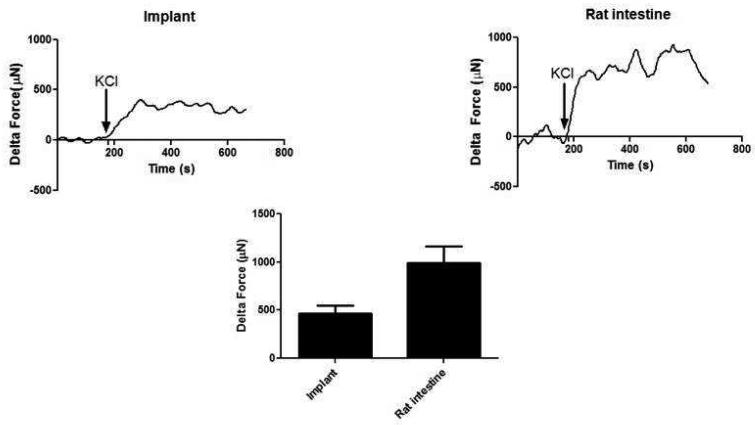
After establishing baseline, the tissues were treated with 60 mM KCl. A robust and rapid contraction of 470 ± 78 μN (n=5) was observed in the neuro-muscular tissues compared to 992 ± 170 μN in the native tissue (Figure 5). In the presence of calcium channel blocker, Nifedipine, the response to the same concentration of KCl was significantly diminished. The response of the implanted tissue to KCl demonstrated that smooth muscle component was maintained and the calcium channels were preserved.
Figure 5.
Electromechanical coupling integrity was evaluated using potassium chloride (KCl). KCl caused a rapid and robust sustained contraction in both the implant and the native intestine. KCl response in the implants was around 50% of the force generated in the native rat intestine.
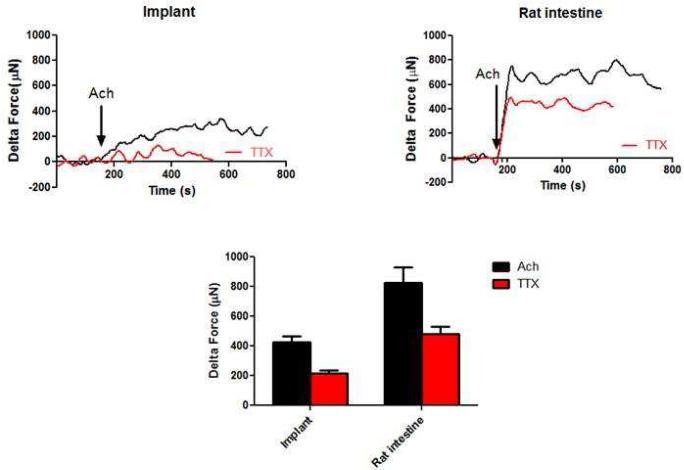
3.3.2. Cholinergic response
Smooth muscle contraction was tested using the major contractile neurotransmitter in the gut, Acetylcholine (Ach). As the tissues established baseline, 1 μM Ach induced a rapid contraction of 427 ± 39 μN in the implants (n=5) compared to 827 ± 103 μN generated in the native intestine (Figure 6). In the presence of 1 μM TTX, the same dose of Ach caused a significant decrease in the magnitude of contraction (215 ± 18 μN in the implants and 482 ± 48 μN in native intestine) (Figure 6 - red trace). This indicated that the contractile response in the implants is mediated by both a myogenic and neurogenic component.
Figure 6.
Cholinergic contraction was evaluated by addition of exogenous contractile neurotransmitter Ach. Ach caused a rapid contraction in both the implant and the native intestine. In the presence of TTX, contraction was significantly attenuated (red trace), indicating both myogenic and neurogenic contribution to the response. TTX significantly attenuated the Ach-induced contraction (p<0.05). Ach-induced contraction seen in the implants was around 50% that of the native rat intestine.
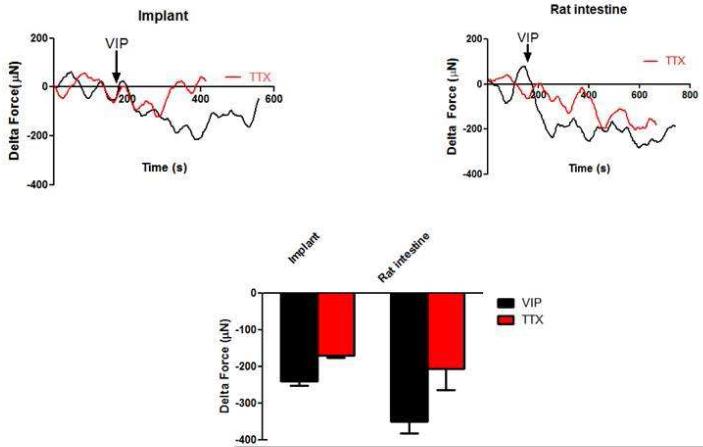
3.3.3. VIPergic relaxation
Relaxation of the tissues was studied using vasoactive intestinal peptide (VIP). Upon addition of 1 μM VIP, tissues rapidly relaxed (Figure 7). The average maximal relaxation magnitude in the implants was −240 ± 12 μN (n=5) compared to −350 ± 31 μN in the native intestines. In the presence of TTX, the magnitude of relaxation was significantly attenuated to −182 ± 14 μN (n=5), indicating that VIP-induced relaxation in the implants has myogenic and neurogenic components (Figure 7 – red trace). VIP-relaxation of native intestine in the presence of TTX was −205 ± 59 μN.
Figure 7.
Relaxation was evaluated using the neurotransmitter VIP. Both the implanted tissue and the native intestine relaxed upon addition of 1μM of VIP. In the presence of TTX, the response was significantly reduced (red trace, p<0.05). This indicates that relaxation was mediated by both the smooth muscle and the neurons. The force generated by the implants was 30% of the force generated by the native rat intestine.
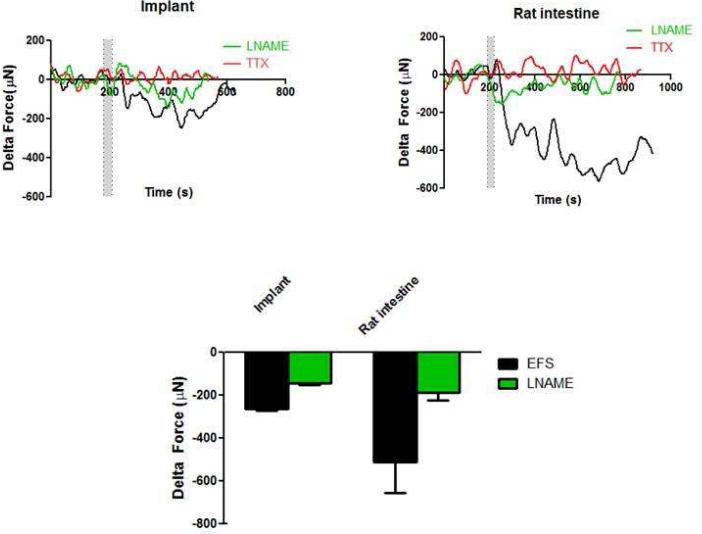
3.3.4. Electrical Field Stimulation (EFS)
Neuronal excitation was performed using EFS (8 Hz and 0.5 ms). Upon stimulating the nerves with electrical field, a rapid and robust relaxation was observed (Figure 8). The maximal relaxation averaged −250 ± 16 μN (n=5) in the implants versus −510 ± 145 μN in the native intestine. In the presence of TTX, the relaxation response was completely abolished (Figure 8 – red trace). This indicated that the relaxation was purely neuronally mediated and caused by neurotransmitters released from the neurons upon stimulation by electrical field. In the presence of nNOS inhibitor LNAME, the EFS-induced relaxation was significantly reduced to -163 ± 14 μN (n=5) in the implants and to −187 ± 36 μN in the native intestines (green trace). This inhibition indicates the presence of functional nitrergic neurons.
Figure 8.
Relaxation was further induced using electrical field stimulation (EFS). The 2 bars indicate the time of applying EFS. The implants and the native intestine exhibited a rapid relaxation. When the tissues were pre-treated with TTX (red trace), relaxation was completely inhibited. This indicates that the response was purely neuronally mediated and that the neurons are functional. In the presence of nNOS blocker (L-NAME), EFS-induced relaxation was significantly attenuated indicating the functionality of nitrergic neurons (green trace, p<0.05). EFS-induced relaxation in the implants was 50% of that seen in the native rat intestine.
4. Discussion
The challenges and limitations of current surgical treatments have switched the focus to a tissue engineering approach for generating functional segments to lengthen the gut. The use of scaffolds in combination with cells has shown promising outcomes in tissue engineering. Chitosan is a natural biomaterial used in different tissue engineering application [16]. Recently, we introduced tubular chitosan scaffolds in the field of GI tissue engineering and evaluated its biocompatibility in vitro [11, 15]. The next step in order to move this study toward translational aspects was to evaluate the tubular tissues in vivo. In our previous studies, we implanted engineered innervated smooth muscle rings for sphincter replacements. In this study, we sought an approach to develop tubular gut tissues. We developed tubular neuro-muscular tissues by wrapping sheets of innervated smooth muscle around 3 cm long tubular chitosan scaffolds. As early as 2 weeks following subcutaneous implantation, the tubular tissues were vascularized, maintained luminal patency and preserved viability and functionality. This is essential for maintenance of survival in future long term implantation studies. Since the engineered tubular neuro-muscular tissue is human derived, nude athymic rats were used in this study in order to avoid any immune rejection of the implant.
The smooth muscle is the basic effector responsible for contraction and relaxation [17]. Plasticity of smooth muscle and the switch between synthetic and contractile phenotype is an extensive area of study [7, 18, 19]. Different smooth muscle markers exist and their expression varies [18, 20]. At 2 weeks post-implantation, the engineered neuro-muscular tissue showed positive expression of α-smooth muscle actin. This is consistent with a previous study where α-smooth muscle actin expression was extensive at 2 weeks [19]. The implant also exhibited smooth muscle specific heavy Caldesmon expression. Smooth muscle specific heavy Caldesmon is a smooth muscle marker of later events of contractility and maturation [21]. The positive stain indicates that the implanted neuro-muscular tissue maintained contractile phenotype for 2 weeks in vivo.
Previous attempts to develop gut segments have used scaffolds seeded with organoid units isolated from different parts of the GI tract [22-25]. Massive bowel resection was performed in rats followed by anastomosis to tissue-engineered small intestine [26]. The engineered intestine grew into a mature tissue with differentiated epithelial, muscular and neural components. Organoid units were shown to be successful in generating a muscularis layer, ganglionated plexi and a differentiated functional epithelium in small and large animal studies. However, the functionality of the innervated muscularis layer was not evaluated, which is essential for motility purposes. Additionally, early reports investigated the ability to regenerate the musculature of the GI tract but showed lack of proper smooth muscle alignment or phenotype [27-29]. In our study, we generated tubular neuro-muscular tissues of 3 cm in length with luminal patency. Our immunostaining studies demonstrated differentiated phenotype of the muscle and the neurons. The innervated muscle maintained proper alignment and displayed key aspects of GI physiology when treated with exogenous contractile/relaxant neurotransmitters that are normally found in the GI tract. Both the myogenic and neurogenic components of the tissue proved to be preserved. KCl-induced contraction was reversed in the presence of calcium channel blocker Nifedipine, which is consistent with previous studies [30]. The inhibition of the Ach, VIP and EFS responses in the presence of TTX indicated that the responses were partially mediated by the neural component. All of these responses were consistent with the in vitro functionality studies [15]. The ability of our engineered tissues to generate force indicates that the innervated muscle remodeled around the scaffold and was able to respond to pharmacological stimuli.
We demonstrated the successful development of a viable and functional tubular neuro-muscular tissue in athymic rat models. This tissue is a first step towards generating a gut segment that contains the epithelial component. Our technique is advantageous by providing a pre-aligned innervated smooth muscle around tubular scaffolds to regenerate the neuro-musculature of the gut. However, this study has some limitations that need to be considered in the future. The first limitation is that out engineered tissue lacks the epithelial component, which is crucial for complete gut function. The epithelial part is important for patients receiving intestinal replacement to ensure proper absorption and secretion balance. Our engineered hollow tubular neuro-muscular tissue is a first step towards developing a complete functional gut segment that can be used as tubular replacement for different parts of the GI tract. Further investigations on the regeneration of the epithelial component are currently being conducted in our lab. There are different approaches in the literature for the regeneration of the epithelium, including the use of organoid units or isolated epithelial cells [31-35]. Additionally, findings from previous reports demonstrated the proliferation of the epithelial cells following mechanical lengthening of an intestinal segment after its isolation from the intestinal continuity [6]. The capacity of the epithelium to proliferate and grow following resection of the intestine provides promising insights for our study. It will be interesting in the future to determine the ability of the epithelium to grow and proliferate into our engineered tissues following anastomosis with the native intestine. The second limitation of our study is the site of implantation. Even though the tissues became vascularized in the back of the rats, it is important to test vascularization in a more clinically relevant area. Our previous in vivo studies using engineered IAS have shown the capacity of our constructs to become vascularized when implanted subcutaneously in the back or in situ. This current study was intended to provide a proof that the engineered neuro-muscular tissues survived around tubular chitosan scaffolds of 3 cm length. We have initiated studies in our lab where the tissues are implanted in the abdominal area and vascularization was achieved. The outcomes of this paper will allow us to extend our studies to further evaluate the tissue following anastomosis with the native intestine. The third limitation is the lower force generated by our engineered tissues compared to native rat intestines. A recent study reported force values generated by an implanted engineered intestine to be one-fourth of native intestine [36]. The forces generated by our engineered tissues were 50%-70% those of the native intestine. Even though the forces generated by our engineered tissues were lower than native rat intestines, our engineered tissues displayed similar neuro-motility characteristics of native intestine. KCl and Ach induced sustained contraction in both types of tissues. Also, the responses were inhibited to the same level in the presence of TTX and LNAME. The lower force generated in our case might be due to the short period of implantation (2 weeks) which did not allow complete maturation of the tissue. Longer implantation periods are needed to confirm our hypothesis. In conclusion, we provided a custom-made multi-purpose system to engineer 3 cm tubular neuro-muscular human tissues using smooth muscle and neural progenitor cells obtained from the same donor. Our tubular tissues can be used for applications including small intestine and/or large intestine. The engineered tissues presented in this work are considered as a first successful stage of engineering the neuro-musculature of a gut. Next, we aim to incorporate the epithelial component in order to develop a functional gut.
Acknowledgment
This work was supported by NIH/NIDDK R01DK071614 and Wake Forest School of Medicine Institutional Funds.
*Dr. Mostafa Elbahrawy is a visiting medical doctor from Al-Azhar University, School of Medicine, Cairo, Egypt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sundaram A, Koutkia P, Apovian CM. Nutritional management of short bowel syndrome in adults. Journal of clinical gastroenterology. 2002;34:207–20. doi: 10.1097/00004836-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yang I, Boushey RP. Complexities in Colorectal Surgery. Springer; 2014. Short Bowel Syndrome. pp. 447–62. [Google Scholar]
- 3.Wilmore DW, Robinson MK. Short bowel syndrome. World journal of surgery. 2000;24:1486–92. doi: 10.1007/s002680010266. [DOI] [PubMed] [Google Scholar]
- 4.Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clinical Nutrition. 2013;32:368–74. doi: 10.1016/j.clnu.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Lao OB, Healey PJ, Perkins JD, Horslen S, Reyes JD, Goldin AB. Outcomes in children after intestinal transplant. Pediatrics. 2010;125:e550–e8. doi: 10.1542/peds.2009-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga H, Sun X, Yang H, Nose K, Somara S, Bitar KN, et al. Distraction-induced intestinal enterogenesis: Preservation of intestinal function and lengthening after re-implantation into normal jejunum. Annals of surgery. 2012;255:302. doi: 10.1097/SLA.0b013e318233097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M, Wu BM, Stelzner M, Reichardt HM, Dunn JC. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue engineering Part A. 2008;14:1395–402. doi: 10.1089/ten.tea.2007.0232. [DOI] [PubMed] [Google Scholar]
- 8.Bitar KN, Raghavan S, Zakhem E. Tissue engineering in the gut: developments in neuromusculature. Gastroenterology. 2014;146:1614–24. doi: 10.1053/j.gastro.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmont RR, Raghavan S, Somara S, Bitar KN. Bioengineering of physiologically functional intrinsically innervated human internal anal sphincter constructs. Tissue engineering Part A. 2014;20:1603–11. doi: 10.1089/ten.tea.2013.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghavan S, Miyasaka EA, Gilmont RR, Somara S, Teitelbaum DH, Bitar KN. Perianal implantation of bioengineered human internal anal sphincter constructs intrinsically innervated with human neural progenitor cells. Surgery. 2014;155:668–74. doi: 10.1016/j.surg.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakhem E, Raghavan S, Gilmont RR, Bitar KN. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials. 2012;33:4810–7. doi: 10.1016/j.biomaterials.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–96. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghavan S, Gilmont RR, Bitar KN. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials. 2013;34:6649–58. doi: 10.1016/j.biomaterials.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashish M, Raghavan S, Somara S, Gilmont RR, Miyasaka E, Bitar KN, et al. Surgical implantation of a bioengineered internal anal sphincter. Journal of pediatric surgery. 2010;45:52–8. doi: 10.1016/j.jpedsurg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakhem E, Raghavan S, Bitar KN. Neo-innervation of a bioengineered intestinal smooth muscle construct around chitosan scaffold. Biomaterials. 2014;35:1882–9. doi: 10.1016/j.biomaterials.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Cho HS, Park E, Kim S, Lee SY, Kim CS, et al. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology. 2008;250:109–15. doi: 10.1016/j.tox.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annual review of physiology. 2006;68:345–74. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 18.Huber A, Badylak SF. Phenotypic changes in cultured smooth muscle cells: limitation or opportunity for tissue engineering of hollow organs? Journal of tissue engineering and regenerative medicine. 2012;6:505–11. doi: 10.1002/term.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin HH, Dunn JC. Small intestinal submucosa seeded with intestinal smooth muscle cells in a rodent jejunal interposition model. The Journal of surgical research. 2011;171:e21–6. doi: 10.1016/j.jss.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kofler K, Ainoedhofer H, Tausendschön J, Höllwarth M, Saxena A. Esophageal smooth muscle cells dedifferentiate with loss of α-smooth muscle actin expression after 8 weeks of explant expansion in vitro culture: Implications on esophagus tissue engineering. European Surgery. 2011;43:168–73. [Google Scholar]
- 21.Frid MG, Shekhonin BV, Koteliansky VE, Glukhova MA. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Developmental biology. 1992;153:185–93. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- 22.Grant CN, Salvador GM, Sala FG, Hill JR, Levin DE, Speer AL, et al. Human and Mouse Tissue-Engineered Small Intestine Both Demonstrate Digestive And Absorptive Function. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2015:ajpgi, 00111. doi: 10.1152/ajpgi.00111.2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. Journal of Surgical Research. 2009;156:205–12. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 24.Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Engineering Part A. 2011;17:1841–50. doi: 10.1089/ten.tea.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Annals of surgery. 2003;238:35. doi: 10.1097/01.SLA.0000074964.77367.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Annals of surgery. 2004;240:748. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hori Y, Nakamura T, Matsumoto K, Kurokawa Y, Satomi S, Shimizu Y. Tissue engineering of the small intestine by acellular collagen sponge scaffold grafting. The International journal of artificial organs. 2001;24:50–4. [PubMed] [Google Scholar]
- 28.Hori Y, Nakamura T, Kimura D, Kaino K, Kurokawa Y, Satomi S, et al. Experimental study on tissue engineering of the small intestine by mesenchymal stem cell seeding. The Journal of surgical research. 2002;102:156–60. doi: 10.1006/jsre.2001.6294. [DOI] [PubMed] [Google Scholar]
- 29.Nakase Y, Hagiwara A, Nakamura T, Kin S, Nakashima S, Yoshikawa T, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue engineering. 2006;12:403–12. doi: 10.1089/ten.2006.12.403. [DOI] [PubMed] [Google Scholar]
- 30.Janssen LJ, Tazzeo T, Zuo J, Pertens E, Keshavjee S. KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. American journal of physiology Lung cellular and molecular physiology. 2004;287:L852–8. doi: 10.1152/ajplung.00130.2004. [DOI] [PubMed] [Google Scholar]
- 31.Grikscheit T, Ochoa ER, Srinivasan A, Gaissert H, Vacanti JP. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. The Journal of thoracic and cardiovascular surgery. 2003;126:537–44. doi: 10.1016/s0022-5223(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 32.Maemura T, Ogawa K, Shin M, Mochizuki H, Vacanti JP. Assessment of tissue-engineered stomach derived from isolated epithelium organoid units. Transplantation proceedings. 2004;36:1595–9. doi: 10.1016/j.transproceed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Annals of surgery. 2004;240:748–54. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Annals of surgery. 2003;238:35–41. doi: 10.1097/01.SLA.0000074964.77367.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckstead BL, Pan S, Bhrany AD, Bratt-Leal AM, Ratner BD, Giachelli CM. Esophageal epithelial cell interaction with synthetic and natural scaffolds for tissue engineering. Biomaterials. 2005;26:6217–28. doi: 10.1016/j.biomaterials.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Nakao M, Ueno T, Oga A, Kuramitsu Y, Nakatsu H, Oka M. Proposal of intestinal tissue engineering combined with Bianchi's procedure. Journal of pediatric surgery. 2014 doi: 10.1016/j.jpedsurg.2014.11.035. [DOI] [PubMed] [Google Scholar]