Abstract
Background
Chronic social adversity activates a conserved transcriptional response to adversity (CTRA) marked by increased expression of pro-inflammatory genes and decreased expression of antiviral- and antibody-related genes. Recent findings suggest that some psychological resilience factors may help buffer CTRA activation, but the relative impact of resilience and adversity factors remains poorly understood. Here we examined the relative strength of CTRA association for the two best-established psychological correlates of CTRA gene expression – the risk factor of perceived social isolation (loneliness) and the resilience factor of eudaimonic well-being (purpose and meaning in life).
Methods
Peripheral blood samples and validated measures of loneliness and eudaimonic well-being were analyzed in 108 community-dwelling older adults participating in the longitudinal US Health and Retirement Study (56% female, mean age 73). Mixed effect linear model analyses quantified the strength of association between CTRA gene expression and measures of loneliness and eudaimonic well-being in separate and joint analyses.
Results
As in previous studies, separate analyses found CTRA gene expression to be up-regulated in association with loneliness and down-regulated in association with eudaimonic well-being. In joint analyses, effects of loneliness were completely abrogated whereas eudaimonic well-being continued to associate with CTRA down-regulation. Similar eudaimonia-dominant effects were observed for positive and negative affect, optimism and pessimism, and anxiety symptoms. All results were independent of demographic and behavioral health risk factors.
Conclusions
Eudaimonic well-being may have the potential to compensate for the adverse impact of loneliness on CTRA gene expression. Findings suggest a novel approach to targeting the health risks associated with social isolation by promoting purpose and meaning in life.
Keywords: social genomics, psychoneuroimmunology, gene expression, transcriptome, microarray, stress, social support, psychological well-being, eudaimonia, positive psychology
1 Introduction
Within the realm of conscious experience, negative events generally outweigh the impact of positive events on our overall experience of well-being (Lyubomirsky, 2010). Regardless of whether they are expressed as losses vs. gains (Kahneman and Tversky, 1979) or negative vs. positive emotions (Baumeister et al., 2001; Houben et al., 2015), adverse events generally have a greater impact on subjective psychological well-being than do positive events. Much less is known, however, about the relative impact of negative and positive events on physiological well-being and health (Friedman, 2012). Epidemiologic data suggest that both positive and negative psychological processes may have distinct influences on the physical well-being (Chida and Steptoe, 2008; Friedman, 2012), but no previous studies have examined the relative magnitude of their effects on the molecular processes underlying human health and disease risk.
In the present analyses, we compared the relative strength of association between the normal range of individual differences on two established social adversity and resilience factors with a functional genomics-based measure of “nonconsciously evaluated molecular well-being” in the form of a transcriptome profile known as the conserved transcriptional response to adversity (CTRA) (Cole, 2013; Cole, 2014). Across a wide range of adverse life circumstances, transcriptome profiling studies have found exposed individuals to show a general pattern of up-regulated expression of pro-inflammatory genes and down-regulated expression of innate antiviral and antibody-related genes in peripheral blood leukocytes (Cole, 2013; Cole, 2014). Experimental studies in animal models have shown a causal impact of adverse social conditions on CTRA gene expression (Cole et al., 2012; Powell et al., 2013; Tung et al., 2012), and mechanistic analyses have identified sympathetic nervous system (SNS)-mediated gene regulation as a key mediator of such effects (Powell et al., 2013). Randomized controlled experiments in human samples have also shown that CTRA gene expression profiles can be reduced by positive psychological interventions such as cognitive-behavioral stress management (Antoni et al., 2012), meditation (Black et al., 2012), yoga (Bower et al., 2014), and Tai Chi (Irwin et al., 2014). Recent observational studies have also found down-regulated CTRA gene expression profiles in people who experience comparatively high levels of eudaimonic well-being, or a sense of purpose and meaning in their lives (Fredrickson et al., 2015; Fredrickson et al., 2013). However, no study has yet quantified the relative impacts of an adverse social risk factor with that of a positive psychological resilience factor to determine the extent to which positive processes might match the impact of negative processes. Do adverse psychological conditions such as social isolation (Cacioppo et al., 2015; Cacioppo and Hawkley, 2009; Holt-Lunstad et al., 2010) loom so large in the psychobiological regulation of leukocyte gene transcription that they overwhelm any salutary effects of positive psychological conditions such as eudaimonic well-being (Fredrickson et al., 2015; Fredrickson et al., 2013; Friedman, 2012; Ryff, 2014; Ryff et al., 2004)? Or might it be the case that psychological resilience factors such as eudiamonia can potentially match or outweigh the impact a relatively common “everyday” social adversity such as loneliness? The present analyses addressed this question in a population-representative sample of older adults from the United States Health and Retirement Study (HRS).
2 Methods
2.1 Participants, Recruitment, and Procedure
Data were collected from a pilot sample of 121 participants in the HRS, which is a longitudinal study of a representative sample of U.S. individuals > 50 years of age and their spouses (Sonnega et al., 2014). An “RNA sub-study pilot sample” was derived as previously described (Levine et al., 2015) by approaching 200 study participants who had completed the HRS 2010 interview and were randomly selected from within a set of 1000 HRS respondents who had completed a face-to-face HRS interview in either 2006 or 2008. Selected participants were asked to provide a blood sample in the near future. At the time of the approach interview, 15.9% declined to participate. An additional 14.8% did not complete the subsequent blood draw, resulting in 122 participants with blood samples. Of these, 1 blood sample yielded insufficient RNA for analysis, and 13 participants had missing data on one or more of the measured covariates, leaving a final analyzed sample of 108 individuals. All participants provided written informed consent prior to participation, and all procedures were approved by the Institutional Review Board of the University of Michigan, which administers the HRS under a collaborative agreement the United States National Institute on Aging. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
2.2 Materials
2.2.1 Loneliness
Perceived social isolation (loneliness) was assessed using abbreviated variants of the UCLA Loneliness Scale (Hughes et al., 2004; Russell et al., 1980; Smith et al., 2013), which were administered as part of the HRS Psychosocial and Lifestyle questionnaire to half of the sample in 2006 and the other half in 2008 (Smith et al., 2013). Respondents were asked “how much of the time do you feel…” loneliness-indicating experiences such as “you lack companionship,” “left out”, and “isolated from others.” Responses were given on a 3-point scale; 1=Often, 2=Some of the time, 3=Hardly ever or never. The scale average showed adequate reliability, with Cronbach α = .87 and 4-year test-retest stability (estimated for the 2006-assessed sub-sample, which was re-assessed in 2010) of r = .62. Gene expression analyses utilized z-score standardized scores on the loneliness scale.
2.2.2 Eudaimonic well-being
Eudaimonic well-being was assessed using the Purpose in Life scale of the Ryff Measures of Psychological Well-being (Keyes et al., 2002; Ryff and Keyes, 1995; Smith et al., 2013). This 7-item scale was administered in parallel with the Loneliness scale as part of the HRS interview in 2006 (half sample) and 2008 (other half) (Smith et al., 2013). Respondents were presented with statements such as “I have a sense of direction and purpose in my life,” “I am an active person in carrying out the plans I set for myself,” and “I enjoy making plans for the future and working to make them a reality,” and asked to “decide the extent to which each statement describes you.” Responses were given on a 6-point scale: 1=Strongly disagree, 2=Somewhat disagree, 3=Slightly disagree, 4=Slightly agree, 5=Somewhat agree, 6=Strongly agree. The scale average showed adequate reliability (α = .77) and a 4-year test-retest r = .52. Gene expression analyses utilized z-score standardized scores on the eudaimonic well-being scale.
2.2.3 Demographic, biometric, and behavioral covariates
Age was measured coincident with blood sample collection in 2010. Sex was based on self-report and coded as 1 for females and 0 for males. Dummy variables for Non-Hispanic Black race and Hispanic ethnicity were coded based on self-reports. Socioeconomic status was assessed by (log-transformed) annual household income. Presence of a major chronic illness was coded as 1 if participants reported any history of diabetes, cancer, cardiovascular disease, or stroke, and 0 otherwise. Body mass index (BMI) was represented as a continuous measure (kg/m2). A dummy variable for smoking history was coded 1 if participants reported smoking ≥ 100 cigarettes during their lifetime, and a dummy variable for heavy alcohol consumption was coded 1 if participants reported consuming an average of ≥ 14 alcoholic beverages per week.
2.2.4 Other dimensions of affect and well-being
Ancillary analyses examined measures of positive and negative affect (items from the PANAS) (Smith et al., 2013; Watson et al., 1988), symptoms of depression (Center for Epidemiologic Studies Depression scale; CES-D) (Radloff, 1977) and anxiety (5 items from the Beck Anxiety Inventory) (Beck et al., 1988; Smith et al., 2013), optimism and pessimism (6 items from the Revised Life Orientation Test) (Scheier et al., 1994; Smith et al., 2013), and general life satisfaction (Satisfaction With Life Scale) (Diener et al., 1985), each collected in parallel with measures of loneliness and eudaimonia. Measurement details and psychometric characteristics in the HRS sample are reported in (Smith et al., 2013).
2.2.5 Transcriptome profiling
Blood samples were collected into PAXgene RNA tubes and shipped overnight to a central laboratory for storage at −80C. Samples were subsequently shipped in batch to the UCLA Social Genomics Core Laboratory, where RNA was extracted (Qiagen QIAcube), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent Bioanalyzer), converted to fluorescent cRNA (Ambion TotalPrep) and hybridized to Illumina Human HT-12 v4 BeadArrays following the manufacturer’s standard protocol in the UCLA Neuroscience Genomics Core Laboratory. All samples were assayed in a single batch and yielded valid results according to standard quality assurance metrics (e.g., median probe fluorescence intensity > 100 units). The microarray-based transcriptome profiling approach used here does not require any normalization to a specific internal housekeeping control because the quantile-based data normalization employed at the outset of data analysis (see below) standardizes total assayed RNA mass across samples at the level of the whole transcriptome.
2.3 Statistical analysis
Gene expression values were quantile-normalized (Bolstad et al., 2003) and log2-transformed for mixed effect linear model analyses (McCulloch et al., 2008) quantifying association between expression of 53 CTRA indicator transcripts (with inverse components weighted negatively as described below) and z-score standardized measures of loneliness and/or eudaimonic well-being (Fredrickson et al., 2015). All analyses controlled for systematic differences across indicator genes as well as participant age, sex, race/ethnicity, socio-economic status, chronic illness, BMI, alcohol consumption, smoking history, and expression of 8 mRNA transcripts indicting the relative prevalence of major leukocyte subsets within the circulating blood cell pool (CD14 for monocytes, CD3D, CD3E, CD4, and CD8A for T lymphocyte subsets, CD19 for B lymphocytes, and CD56/NCAM1 and CD16/FCGR3A for natural killer cells). The 53 CTRA indicator genes were comprised of 2 a priori-defined gene sets (Fredrickson et al., 2015; Fredrickson et al., 2013; Vedhara et al., 2015): 19 pro-inflammatory genes (IL1A, IL1B, IL6, IL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, RELB) weighted +1 as positive indicators of the CTRA profile, and 34 genes involved in Type I interferon responses (GBP1, IFI16, IFI27, IFI27L1–2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1–3, IFIT5, IFIT1L, IFITM1–3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7–8, MX1–2, OAS1–3, OASL) and antibody synthesis (IGJ, IGLL1, IGLL3) weighted −1 as inverse indicators (Fredrickson et al., 2015; Fredrickson et al., 2013). Models were estimated by maximum likelihood using SAS PROC MIXED, with the 53 indicator transcripts treated as repeated measurements with a fully parameterized (unstructured) covariance matrix (Fredrickson et al., 2015). Effect sizes for the resulting linear model coefficients (metric: difference in predicted gene expression per loneliness/eudaimonia SD) were graphed as the range-spanning magnitude of difference in predicted CTRA indicator gene abundance over a standardized range of observed continuous loneliness/eudaimonia scores (i.e., the expected magnitude of difference in gene expression over the range from 2 SD above the mean loneliness/eudaimonia score to 2 SD below the mean, corresponding to the difference in expected gene expression over the 4 SD range that captures 95% of observations in a normal distribution). Gene expression data are publicly available as Gene Expression Omnibus series GSE68526. The statistical significance level was set at p < .05, and analyses followed established statistical guidelines in controlling for multiple comparisons in exploratory analyses of many related hypotheses (e.g., correlations among covariates and loneliness/eudaimonia) but not in analyses of distinct substantive hypotheses (e.g., testing associations between loneliness and/or eudaimonia and specific a priori predicted differences in CTRA gene expression). Initial model specification tests confirmed previous observations of significant heteroscedasticity across the 53 CTRA indicator transcripts (Levene’s test: F(52,6360) = 20.97, p < .0001), which was accounted for by specification of fully parameterized (unstructured) covariance matrices as noted above (McCulloch et al., 2008). CTRA gene expression varied significantly as a function of both a priori-specified sets of demographic and behavioral covariates (F(9,90) = 10.88, p < .0001) and mRNA indicators of leukocyte subset prevalence (F(8,90) = 35.63, p < .0001), so both a priori-specified covariate sets were retained in all subsequent analyses. Specification analyses showed comparable CTRA associations with loneliness or eudaimonia across the 2006 and 2008 measurement waves (i.e., no significant wave x loneliness or wave x eudaimonia interaction). For all primary analyses, Studentized residual distributions were verified to be approximately normal as determined by near-linearity of quantile-quantile plots and normal score correlation coefficients > .99.
3. Results
3.1 Sample characteristics
As shown in Table 1, the analyzed sample comprised 108 community-dwelling older adults who were broadly representative of the HRS sample of older Americans: average age 73, predominately white, with a slight majority of females, moderate BMI, and low rates of heavy alcohol consumption. Consistent with current norms for this age cohort, slight majorities reported a history of smoking and one or more chronic illness (cardiovascular disease and diabetes being the most prevalent). Average levels of loneliness fell well below the response scale mid-point, with only 18% of respondents reporting average rates of loneliness-indicating experiences “some of the time” or “often.” Average levels of eudaimonic well-being fell well above its response scale mid-point, with 52% of the sample reporting an average score that indicated “somewhat” or “strongly” experiencing a sense of purpose in life. Loneliness and eudaimonic well-being were inversely correlated (Figure 1; r = −.40, p < .0001), but each measure also tapped significant unique variance distinct from the other (loneliness: unique = 82% of systematic score variance; eudaimonia: unique = 79% of systematic score variance).
Table 1.
Sample characteristics
| Mean (SD) or % | |
|---|---|
| Age (years) | 73.4 (9.3) |
| Female | 55.5 % |
| Race/ethnicity | |
| White | 92.6 % |
| Black | 4.6 % |
| Hispanic | 2.8 % |
| Income (log2 $/yr) | 15.3 (1.4) |
| Body mass index (kg/m2) | 27.7 (6.5) |
| Chronic illness | 63.0 % |
| Smoking history | 62.0 % |
| Heavy alcohol consumption | 3.7 % |
| Loneliness (1–3 scale) | 1.37 (0.53) |
| Eudaimonia (1–6 scale) | 4.77 (0.94) |
Figure 1.
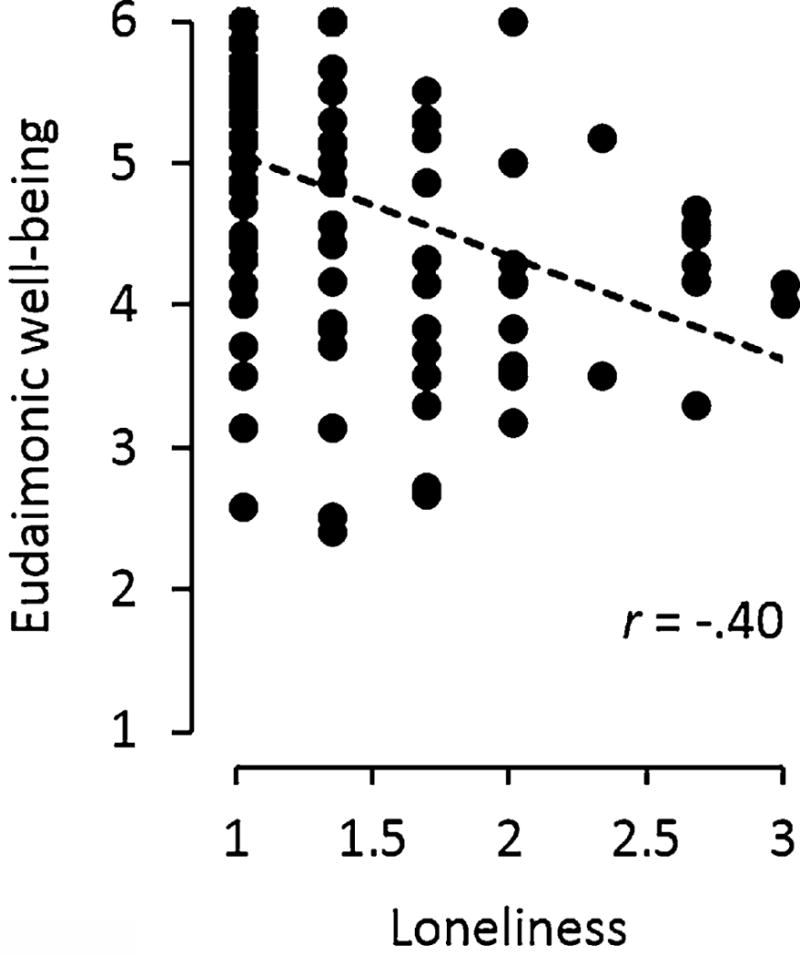
Loneliness and eudaimonic well-being
3.2 CTRA Gene Expression
Primary analyses examined the relationship between average expression of the 53 CTRA indicator genes and continuously measured individual differences in loneliness and eudaimonic well-being while controlling for demographic characteristics (age, sex, race/ethnicity, socioeconomic status) and health-related risk factors (BMI, smoking history, heavy alcohol consumption, chronic illness). CTRA gene expression was significantly up-regulated in association with increasing degrees of loneliness (p = .038; Table 2). In contrast, CTRA gene expression was significantly down-regulated in association with increasing degrees of eudaimonic well-being (p < .001; Table 2).
Table 2.
CTRA relationship to loneliness and eudaimonia
| Separate | Mutually adjusted | |||
|---|---|---|---|---|
| b1 (SE) | p value | b1 (SE) | p value | |
| Loneliness | .0033 (.0016) | .0379 | −.0002 (.0016) | .9080 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0090 (.0016) | < .0001 |
Linear model parameter estimate: log2 CTRA RNA abundance / predictor SD
To determine whether either loneliness or eudaimonia showed a significant unique relationship to CTRA gene expression (i.e., above and beyond their 16% shared variance), an additional “mutually adjusted” model analyzed the effects of both variables simultaneously (Table 2, final column). After controlling for individual differences in loneliness, eudaimonic well-being continued to associate with down-regulated CTRA gene expression (p < .001), with no appreciable change in the estimated strength of association (< 1% change in parameter estimate). In contrast, controlling for individual differences in eudaimonia completely abrogated CTRA association with loneliness (p = .908, 106% reduction in parameter estimate).
Figure 2 shows results from additional sensitivity analyses that deleted all observations from 3 participants who showed unusually heterogeneous gene expression values (generating aberrant residuals). In analyses of loneliness and eudaimonia in isolation (Figure 2A), results again linked loneliness to up-regulated CTRA gene expression (p < .001) and eudaimonia to down-regulated CTRA continued to associate with down-regulated CTRA gene expression (p < .001; < 10% change in parameter estimate), whereas CTRA associations with loneliness were rendered non-significant (p = .232, 64% reduction in strength of association).
Figure 2. Loneliness, eudaimonia, and CTRA gene expression.
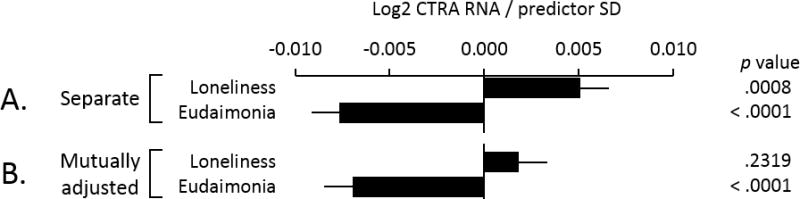
Data represent strength of association (b ± SE) between indicated predictor variables and the 53-gene CTRA indicator contrast in (A.) separate analyses of loneliness and eudaimonia and (B.) analyses in which each was adjusted for covariance with the other.
To assess the specificity of eudaimonia’s association with CTRA gene expression, we conducted additional exploratory analyses of other dimensions of affect and well-being that correlated with eudaimonia including positive and negative affect, symptoms of depression and anxiety, optimism and pessimism, and general life satisfaction (all .35 < |r| < .56, all p < .001). Results (Table 3, Column 1) identified CTRA down-regulation in association with positive affect (p = .010) and a similar trend for optimism (p = .053), as well as CTRA up-regulation in association with negative affect and anxiety symptoms (both p < .001) and pessimism (p = .006). CTRA gene expression was not substantially associated with depressive symptoms or general life satisfaction (both p > .490). Among the constructs that did individually associate with CTRA gene expression, all relationships except anxiety were rendered non-significant by inclusion of eudaimonia in the model (Table 3, Column 2). In contrast, eudaimonia continued to show a robust independent relationship to CTRA gene expression in all models that controlled for other dimensions of affect and well-being (all p < .001 and all parameter estimates reduced by < 14%; Table 3, Column 2).
Table 3.
CTRA, eudaimonia, and other aspects of affect and well-being
| Separate | Mutually adjusted | |||
|---|---|---|---|---|
| b1 (SE) | p value | b1 (SE) | p value | |
| Positive Affect | −.0042 (.0016) | .0101 | .0006 (.0018) | .7449 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0090 (.0018) | < .0001 |
| Negative Affect | .0065 (.0016) | < .0001 | .0019 (.0017) | .2674 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0079 (.0017) | < .0001 |
| Depression | −.0002 (.0018) | .9155 | −.0045 (.0018) | .0147 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0103 (.0016) | < .0001 |
| Anxiety | .0070 (.0015) | < .0001 | .0047 (.0015) | .0028 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0077 (.0015) | < .0001 |
| Optimism | −.0032 (.0016) | .0528 | −.0012 (.0017) | .4834 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0088 (.0016) | < .0001 |
| Pessimism | .0049 (.0017) | .0063 | −.0007 (.0019) | .6963 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0093 (.0017) | < .0001 |
| Life Satisfaction | .0011 (.0016) | .4970 | .0024 (.0016) | .1323 |
| Eudaimonia | −.0089 (.0015) | < .0001 | −.0092 (.0016) | < .0001 |
Linear model parameter estimate: log2 CTRA RNA abundance / predictor SD
4. Discussion
These results show an unanticipated relationship between two previously distinct research literatures identifying loneliness as a psychological risk factor for CTRA up-regulation (Cole et al., 2011; Cole et al., 2007; Creswell et al., 2012) and eudiamonic well-being as a psychological resilience factor for CTRA down-regulation (Fredrickson et al., 2015; Fredrickson et al., 2013): whereas both factors associated with gene expression profiles when considered in isolation, eudiamonic well-being clearly dominated the effects of loneliness in simultaneous analyses. CTRA associations with loneliness were largely abrogated by control for individual differences in eudaimonia, whereas eudaimonia continued to show a highly significant association with CTRA gene expression in analyses controlling for loneliness. The asymmetry of these effects is remarkable in light of the fact that loneliness was only moderately associated with eudaimonia in this sample (r = −.40) and thus shares only a small fraction of variance in common with it (approximately 16%). That small fraction of shared variance nevertheless carried much of loneliness’ association with CTRA gene expression (and very little of eudaimonia’s association with CTRA gene expression). These results were independent of general demographic and health-related risk factors, independent of differences in major leukocyte subset distributions, and unaffected by the removal of aberrant gene expression profiles that may reflect disease processes. Loneliness and eudaimonic well-being constitute two of the best-established psychological correlates of CTRA gene expression, and these results suggest that, at least in the present context, the positive psychological resilience factor has the potential to at least match and perhaps even outweigh the effects of a robust psychological risk condition such as perceived social isolation.
The present results highlight the close conceptual relationship between psychological wellbeing (of which purpose is a part) and social well-being (of which social integration is a part), and suggest that their shared components may be particularly relevant to molecular physiology and health. These findings are consistent with previous observations linking CTRA gene expression to the variance shared in common by psychological well-being and social well-being (Fredrickson et al., 2015). Eudaimonia and social integration are clearly distinct constructs, but both contribute to overall human thriving (Keyes, 1998; Keyes et al., 2002; Keyes and Simoes, 2012). The two domains are functionally related in that social engagements provide a major source of purpose in life (Baumeister et al., 2013; Lambert et al., 2013; Stillman et al., 2009) and eudaimonic purpose can promote social engagement (Ryff, 2014). There is also a conceptual relationship between eudaimonia (as a self-transcendent orientation outward and toward others) (Baumeister et al., 2013) and the social perceptual dynamics underlying loneliness (which involves a generalized sense of social mistrust and perceived social threat, resulting in a more self-focused and defensive orientation) (Cacioppo et al., 2015; Cacioppo et al., 2015; Cacioppo and Hawkley, 2009; Lambert et al., 2013; Stillman et al., 2009). The current findings suggest that such shared conceptual and functional relationships may also play a key role in engaging the biological signaling pathways that regulate CTRA gene expression in immune cells (Cole, 2014). If this pattern of results continues to be substantiated in future research, it could provide a rationale for the development of new intervention strategies to mitigate the health risks associated with loneliness not by directly targeting social interaction per se, but rather by promoting social well-being indirectly via the development of pro-social eudaimonic well-being. Such purpose-based interventions have already shown promise in several studies (Ryff, 2014), and research involving experimental induction of eudaimonic well-being represents a promising area for future research.
Another important topic for future research is the identification of the specific neural and endocrine pathways by which eudaimonic well-being might regulate peripheral physiological function. Previous research has shown that SNS activity plays a key role in activating the CTRA gene expression profile (Cole, 2014; Powell et al., 2013). No measures of SNS activity are available in the present study, but it is plausible that eudaimonic well-being might reduce SNS activity either by reducing the sense of threat or uncertainty that stimulates sympathetic activity (Weiner, 1992) or by stimulating the pro-social care-giving system (Churchland, 2011; Eisenberger and Cole, 2012) that is associated with parasympathetic activity (Porges, 1998; Porges, 2011) (which physiologically antagonizes SNS effects). Future research using pharmacologic antagonists and experimental models will be required to determine whether such effects are mediated by such sympathetic/parasympathetic dynamics or perhaps by other neural or endocrine signaling pathways that may be engaged by eudaimonic well-being (Ryff, 2014; Ryff et al., 2006; Ryff et al., 2004; Taylor, 2006). Additional insights could also come from mapping the functional interactions between the CNS substrates of eudaimonia (Heller et al., 2013; Kong et al., 2015; Lewis et al., 2014; Telzer et al., 2014) and those involved in autonomic nervous system regulation (Beissner et al., 2013). Ancillary analyses identified CTRA associations with several other dimensions of affect and social perception, including positive and negative affect, anxiety symptoms, optimism (marginally), and pessimism. CTRA gene expression was not significantly associated with either depressive symptoms or general life satisfaction. Moreover, among the affective dimensions that were associated with CTRA expression, their relationships were generally rendered non-significant by statistical control for correlated variations in eudaimonia whereas eudaimonia continued to show significant CTRA association. The sole exception to this general pattern involved anxiety symptoms, which showed some residual significant CTRA association in parallel with eudaimonia’s significant CTRA association. These findings replicate previous observations linking anxiety to elevated expression of the “Axis 2” component of the Blood-Informative Transcript set, which shares considerable variance with the CTRA profile (Wingo and Gibson, 2015). More broadly, these results also suggest that a wide variety of correlated affective, social, and perceptual constructs may serve as at least partial proxies for eudaimonia’s relationship with molecular well-being.
It is important to note several limitation of this study including it its correlational design, which precludes drawing any causal conclusions (e.g., it is conceivable that CTRA pro-inflammatory signaling might modulate loneliness and/or eudaimonia (Dantzer et al., 2008; Eisenberger et al., 2010)), and the older adult sample, which increases the potential for confounding by disease. The present sample excluded participants with serious illness requiring institutional residence, but the sample did include people with chronic illness diagnoses prevalent among community-dwelling adults in this age cohort (predominately cardiovascular disease and diabetes). Although all analyses controlled for the presence of such diagnoses and additional sensitivity analyses confirmed the stability of findings after removal of atypical gene expression profiles that could reflect disease, undiagnosed disease processes may well exist and simultaneously affect both psychological processes and gene expression. However, such effects would not explain the asymmetric pattern of CTRA associations noted for loneliness vs. eudaimonia. In addition, parallel CTRA associations have been previously observed in younger samples with little or no disease exposure (Cole et al., 2011; Cole et al., 2007; Creswell et al., 2012; Fredrickson et al., 2015; Fredrickson et al., 2013). Nevertheless, the present results should be regarded as most relevant to older Americans from this demographic/historical cohort and future studies will be required to evaluate the generality these findings. No assessments of clinical health outcomes or immune function are available in this sample, so the implications of the observed transcriptome differences for health and immune function remain to be determined in future analyses. However, the present data are consistent with previous epidemiologic observations relating eudaimonic well-being to favorable health outcomes (Boyle et al., 2009; Boyle et al., 2010; Boyle et al., 2010; Friedman et al., 2007; Hill and Turiano, 2014; Kim et al., 2014; Kim et al., 2013; Ryff, 2014; Yu et al., 2015) and loneliness or social isolation to elevated disease risk and mortality (Cacioppo et al., 2015; Holt-Lunstad et al., 2010; Luo et al., 2012). This study tested an a priori hypothesis involving the CTRA gene set as a whole (Cole, 2014; Fredrickson et al., 2015; Fredrickson et al., 2013; Vedhara et al., 2015). No discovery-based analyses were attempted to identify individual gene transcripts that might empirically associate with either loneliness or eudaimonia, and no statistical testing was conducted at the level of individual genes. As such, other genes besides those examined here may well be regulated by loneliness and eudaimonia (either individually or as elements of other empirically defined gene sets such as the Blood-Informative Transcripts (Preininger et al., 2013)). Discovery of additional gene modules linked to eudaimonic well-being and loneliness represents an important topic for future research.
Despite these limitations, the present results identify eudaimonia as a stronger correlate of CTRA gene expression than loneliness (and a variety of related affective characteristics) in a representative sample of US older adults. These findings are consistent with the possibility that psychological resilience factors can, at least in some circumstances, outweigh the effects of a well-established and quantitatively robust psychological risk factor such as perceived social isolation (Cacioppo et al., 2015; Cacioppo and Hawkley, 2009; Holt-Lunstad et al., 2010; Luo et al., 2012). Given the central role of “inflammaging” and immunosenescence in most major diseases of aging (Finch, 2007), the present findings may also provide a molecular framework for understanding how generativity and pro-social engagement in life can potentially support health and well-being in older adults (Boyle et al., 2009; Boyle et al., 2010; Boyle et al., 2010; Gruenewald et al., 2012; Gruenewald et al., 2015; Hill and Turiano, 2014; Kim et al., 2014; Kim et al., 2013; Kim and Ferraro, 2014; Yu et al., 2015).
Highlights.
Purpose in life (eudaimonic well-being) outcompetes the adverse effects of loneliness in predicting leukocyte gene expression profiles.
Acknowledgments
Financial support: This research was supported grants from the United States National Institutes of Health (P30-AG107265, R01-AG033590, R24-AG037898, U01-AG009740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The funders had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the article for publication.
References
- Antoni MH, Lutgendorf SK, Blomberg B, Stagl J, Carver CS, Lechner S, Diaz A, Arevalo JMG, Cole SW. Transcriptional modulation of human leukocytes by cognitive-behavioral stress management in women undergoing treatment for breast cancer. Biological Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Baumeister RF, Vohs KD, Aaker JL, Garbinsky EN. Some key differences between a happy life and a meaningful life. Journal of Positive Psychology. 2013 in press. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, Khalsa DS, Lavretsky H. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Arevalo J, Cole SW. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Barnes LL, Buchman AS, Bennett DA. Purpose in life is associated with mortality among community-dwelling older persons. Psychosom Med. 2009;71:574–579. doi: 10.1097/PSY.0b013e3181a5a7c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Barnes LL, Bennett DA. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry. 2010;67:304–310. doi: 10.1001/archgenpsychiatry.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Bennett DA. Purpose in life is associated with a reduced risk of incident disability among community-dwelling older persons. Am J Geriatr Psychiatry. 2010;18:1093–1102. doi: 10.1097/JGP.0b013e3181d6c259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015 doi: 10.1146/annurev-psych-010814-015240. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Cacioppo S, Cole SW, Capitanio JP, Goossens L, Boomsma DI. Loneliness across phylogeny and a call for comparative studies and animal models Perspectives on Psychological. Science. 2015 doi: 10.1177/1745691614564876. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. Epub 2009 Aug 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–756. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression: mechanisms and implications for public health. Am J Public Health. 2013;103(Suppl 1):S84–92. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Human social genomics. PLoS Genet. 2014;10:e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Ruggerio AM, Heckman JJ, Suomi S. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci U S A. 2012;109:20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:1–13. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. Journal of Personality Assessment. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Algoe SB, Firestine AM, Arevalo JMG, Ma J, Cole SW. Psychological well-being and the human conserved transcriptional response to adversity. PLoS One. 2015;10:e0121839. doi: 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Coffey KA, Algoe SB, Firestine AM, Arevalo JM, Ma J, Cole SW. A functional genomic perspective on human well-being. Proc Natl Acad Sci U S A. 2013;110:13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM. Well-being, aging, and immunity. In: Segerstrom S, editor. The Oxford Handbook of Psychoneuroimmunology. New York: Oxford University Press; 2012. [Google Scholar]
- Friedman EM, Hayney M, Love GD, Singer BH, Ryff CD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Liao DH, Seeman TE. Contributing to others, contributing to oneself: perceptions of generativity and health in later life. J Gerontol B Psychol Sci Soc Sci. 2012;67:660–665. doi: 10.1093/geronb/gbs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Tanner EK, Fried LP, Carlson MC, Xue QL, Parisi JM, Rebok GW, Yarnell LM, Seeman TE. The Baltimore Experience Corps Trial: Enhancing Generativity via Intergenerational Activity Engagement in Later Life. J Gerontol B Psychol Sci Soc Sci. 2015 doi: 10.1093/geronb/gbv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, van Reekum CM, Schaefer SM, Lapate RC, Radler BT, Ryff CD, Davidson RJ. Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychol Sci. 2013;24:2191–2200. doi: 10.1177/0956797613490744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PL, Turiano NA. Purpose in life as a predictor of mortality across adulthood. Psychol Sci. 2014;25:1482–1486. doi: 10.1177/0956797614531799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben M, Van Den Noortgate W, Kuppens P. The Relation Between Short-Term Emotion Dynamics and Psychological Well-Being: A Meta-Analysis. Psychol Bull. 2015 doi: 10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Research on Aging. 2004;26:655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Olmstead R, Breen E, Witarama T, Carrillo C, Sadeghi N, Arevalo JMG, Ma J, Nicassio P, Ganz PA, Bower JE, Cole SW. Tai Chi Chih reduces cellular and genomic markers of inflammation in breast cancer survivors with insomnia. Journal of the National Cancer Institute. 2014 doi: 10.1093/jncimonographs/lgu028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. Econometrica. 1979;47:263–292. [Google Scholar]
- Keyes CL. Social well-being. Social Psychology Quarterly. 1998;61:121–140. [Google Scholar]
- Keyes CL, Shmotkin D, Ryff CD. Optimizing well-being: the empirical encounter of two traditions. J Pers Soc Psychol. 2002;82:1007–1022. [PubMed] [Google Scholar]
- Keyes CL, Simoes EJ. To flourish or not: positive mental health and all-cause mortality. Am J Public Health. 2012;102:2164–2172. doi: 10.2105/AJPH.2012.300918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Strecher VJ, Ryff CD. Purpose in life and use of preventive health care services. Proc Natl Acad Sci U S A. 2014;111:16331–16336. doi: 10.1073/pnas.1414826111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Sun JK, Park N, Peterson C. Purpose in life and reduced incidence of stroke in older adults: ‘The Health and Retirement Study’. J Psychosom Res. 2013;74:427–432. doi: 10.1016/j.jpsychores.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Kim S, Ferraro KF. Do productive activities reduce inflammation in later life? Multiple roles, frequency of activities, and C-reactive protein. Gerontologist. 2014;54:830–839. doi: 10.1093/geront/gnt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Liu L, Wang X, Hu S, Song Y, Liu J. Different neural pathways linking personality traits and eudaimonic well-being: a resting-state functional magnetic resonance imaging study. Cogn Affect Behav Neurosci. 2015;15:299–309. doi: 10.3758/s13415-014-0328-1. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Stillman TF, Hicks JA, Kamble S, Baumeister RF, Fincham FD. To belong is to matter: sense of belonging enhances meaning in life. Pers Soc Psychol Bull. 2013;39:1418–1427. doi: 10.1177/0146167213499186. [DOI] [PubMed] [Google Scholar]
- Levine ME, Cole SW, Weir DR, Crimmins EM. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med. 2015;130:16–22. doi: 10.1016/j.socscimed.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Kanai R, Rees G, Bates TC. Neural correlates of the ‘good life’:eudaimonic well-being is associated with insular cortex volume. Soc Cogn Affect Neurosci. 2014;9:615–618. doi: 10.1093/scan/nst032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and mortality in old age: a national longitudinal study. Soc Sci Med. 2012;74:907–914. doi: 10.1016/j.socscimed.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S. Hedonic adaptation to negative and positive experiences. In: Folkman S, editor. The Oxford Handbook of Stress, Health, and Coping. New York: Oxford University Press; 2010. [Google Scholar]
- Porges SW. Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23:837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preininger M, Arafat D, Kim J, Nath AP, Idaghdour Y, Brigham KL, Gibson G. Blood-informative transcripts define nine common axes of peripheral blood gene expression. PLoS Genet. 2013;9:e1003362. doi: 10.1371/journal.pgen.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:386–401. [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Ryff CD. Psychological well-being revisited: advances in the science and practice of eudaimonia. Psychother Psychosom. 2014;83:10–28. doi: 10.1159/000353263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Love GD, Muller D, Urry H, Friedman EM, Davidson R, Singer B. Psychological well-being and ill-being: do they have distinct or mirrored biological correlates? Psychother Psychosom. 2006;75:85–95. doi: 10.1159/000090892. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer BH, Dienberg Love G. Positive health: connecting well-being with biology. Philos Trans R Soc Lond B Biol Sci. 2004;359:1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Smith J, Fisher G, Ryan L, Clarke P, House J, Weir D. HRS psychosocial and lifestyle questionnaire 2006–2010: documentation report core section LB. 2013 http://hrsonline.isr.umich.edu/sitedocs/userg/HRS2006-2010SAQdoc.pdf, Accessed April 4 2015.
- Sonnega A, Faul J, Ofstedal MB, Langa K, Phillips J, Weir D. Cohort profile: the Health and Retirement Study (HRS) International Journal of Epidemiology. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman TF, Baumeister RF, Lambert NM, Crescioni AW, Dewall CN, Fincham FD. Alone and without purpose: life loses meaning following social exclusion. J Exp Soc Psychol. 2009;45:686–694. doi: 10.1016/j.jesp.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend: biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15:273–277. [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proc Natl Acad Sci U S A. 2014;111:6600–6605. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci U S A. 2012;109:6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Gill S, Eldesouky L, Campbell BK, Arevalo JM, Ma J, Cole SW. Personality and gene expression: Do individual differences exist in the leukocyte transcriptome? Psychoneuroendocrinology. 2015;52:72–82. doi: 10.1016/j.psyneuen.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Gibson G. Blood gene expression profiles suggest altered immune function associated with symptoms of generalized anxiety disorder. Brain Behav Immun. 2015;43:184–191. doi: 10.1016/j.bbi.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle PA, Wilson RS, Levine SR, Schneider JA, Bennett DA. Purpose in life and cerebral infarcts in community-dwelling older people. Stroke. 2015;46:1071–1076. doi: 10.1161/STROKEAHA.114.008010. [DOI] [PMC free article] [PubMed] [Google Scholar]


