Abstract
Objectives
Postoperative readmission is an increasingly scrutinized quality metric that impacts patient satisfaction and cost. Even more important is its implication for short-term prognosis. The purpose of this study is to characterize post-esophagectomy readmissions and determine their relationship with subsequent 90-day mortality.
Methods
Data were extracted for esophagectomy patients from the linked SEER-Medicare registry (2000–2009) which provides longitudinal information about Medicare beneficiaries with cancer. We assessed demographics, comorbidities, 30-day readmission, and 90-day mortality. Readmitting facility and diagnoses were identified. A hierarchical multivariable regression model clustered at the hospital level assessed the relationship between readmission within 30 days of discharge and 90-day mortality.
Results
We identified 1543 patients discharged alive after esophagectomy. Among patients discharged alive, readmission rate was 20.7% (319/1543), and 33.5% (107/319) of readmissions were to facilities that did not perform the index operation. Mortality rate at 90 days among patients discharged alive was 6.4% (98/1543). Readmission was associated with a four-fold increase in mortality (16.3% vs 3.8%, p < 0.001). Using multivariable regression, readmission was the strongest predictor of mortality (OR 6.64, p < 0.001), with a stronger association than age, Charlson score, or and index length of stay. Readmission diagnoses with the highest mortality rates were those associated with pulmonary, gastrointestinal, or cardiovascular diagnoses.
Conclusions
Patients readmitted within 30 days of discharge following esophagectomy are at exceptionally high risk for early mortality. Early recognition of life-threatening readmission diagnoses is essential in order to provide optimal care.
Keywords: Esophageal cancer, esophagectomy, postoperative readmission, postoperative mortality, outcomes
Introduction
Esophageal cancer is the fastest-growing cancer in the United States (1). For appropriate surgical candidates, esophagectomy is the principle treatment for early cancer and a vital component of multi-modal therapy for locally advanced disease. However, despite advancements in operative approach and postoperative care, esophagectomy for cancer remains a morbid operation, with early postoperative mortality ranging from 1%–13% (2–4). Reducing this risk has been an area of intensive investigation over the past fifteen years, with most studies focusing on mortality risk stratification, assessment of minimally- and less-invasive approaches, the volume-outcome relationship, and formalized postoperative management pathways (5– 7).
While the association between major surgical complications and mortality is widely recognized, using surgical complications as a quality metric is problematic due to subjective definitions (8). Data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) indicate that the primary difference between high- and low-mortality providers is not the incidence of surgical complications, but rather the mortality rates associated with these complications (9). Evidently, focus should be placed not only on avoiding complications, but also on the proper management of complications that do occur. Due to its accessibility through national databases, early readmission is increasingly recognized for its clinical and financial significance (10, 11). Readmission rate following esophagectomy is reportedly between 5% and 20% (12–14). Although readmission has been linked to early postoperative mortality in colorectal, hepatobiliary, and pulmonary surgery, its impact on early mortality following esophagectomy is underexplored (11, 15, 16).
Although initial readmitting diagnoses may not reflect the true root cause of a patient’s demise, these early indicators may represent opportunities to appropriately address potentially life-threatening conditions. The purpose of this study was to better describe the characteristics and downstream effects of readmissions occurring within 30-days of discharge following esophagectomy. In particular, we sought to determine the temporal trends in postoperative readmissions, the most common readmitting diagnoses, and the relationships between readmission and subsequent 90-day mortality. We hypothesized that readmission is associated with an increased risk of subsequent death, with certain readmission diagnoses having higher mortality rates than others.
Methods
The Surveillance, Epidemiology, and End Results (SEER) registry is a population-based collection of incident cases with cancer diagnostic, descriptive, and therapeutic information. The Medicare healthcare claims database includes survival data, as well as information abstracted from discharge records of inpatient admissions, outpatient care claims, and other healthcare event claims for which Medicare provides reimbursement. The National Cancer Institute links the SEER registry with the Medicare claims database which includes records for 93% of patients 65 years old or older, representing 24% of the total population (17). This combined database accurately records postoperative readmissions, including those taking place at facilities which did not provide the index operation. Unlike the Society of Thoracic Surgeons database, SEER-Medicare includes long-term follow-up of patient survival, thereby providing an opportunity for longitudinal studies of mortality among patients with cancer that are broadly generalizable to the Medicare population.
The 2000 to 2009 SEER-Medicare database was used to identify records for all patients age 66 years or greater with esophageal cancer of any histological type and stage who received surgical resection. Patients enrolled in a Medicare health maintenance organization were excluded. Additional exclusionary criteria included esophageal cancer diagnosed during autopsy, missing date of diagnosis, and in-hospital mortality during the operative admission. To ensure adequate records of pre-existing comorbidities, we also excluded patients who were ineligible for Medicare during the three months prior to surgery.
Demographic information included age, sex, and the operative and readmitting facilities. Clinical data included year of operation, final pathologic stage, extent of resection, and preoperative comorbidities. Because the SEER-Medicare database does not separate preoperative from postoperative diagnoses within a given admission, early postoperative complications could not be distinguished from preexisting morbidities. In order to determine causes for readmission, the first two International Classification of Diseases (ICD-9) diagnosis codes associated with each readmission were collected. If diagnosis codes belonging to more than one organ system are associated with one readmission, investigator judgement was used to determine the primary readmitting etiology. Comorbidities present prior to the index operation were identified based on the Deyo modification of the Charlson index (18) using criteria provided by the National Cancer Institute for use with inpatient files (MEDPAR), physician claims data (NCH), and outpatient files (OUTSAF) (19, 20). Preoperative induction treatment with either chemotherapy alone or chemoradiation was noted. Mortality measures were derived from Medicare death certificate records. The primary outcomes were readmission within 30 days of discharge and mortality within 90 days of surgery.
Ninety-day mortality was compared between readmitted and non-readmitted patients using the Chi-squared test. Subgroup comparisons were used to assess mortality rates based on the readmitting facility (operative vs. non-operative) and readmitting diagnoses grouped by organ system. Reporting of SEER-Medicare data requires a minimum cell size of greater than 11 patients out of consideration for confidentiality. Thus, diagnosis-specific mortality rates are not presented due to limitations to reportable cell size. A hierarchical generalized linear model was created to estimate the risk of readmission using clinically-relevant variables, including age, Charlson score, sex, operative year, induction therapy, discharge disposition, and postoperative length of stay (LOS) (21). Because the relationship between LOS and readmission is not linear, this variable was modeled using a linear spline with a knot at 20 days. To determine the impact of readmission on mortality, a second model was created for 90-day mortality with the addition of 30-day readmission as a predictive variable. Both models were adjusted for data clustered by hospital provider. Model predictors were selected a priori based on frequency of occurrence within our dataset and review of recent thoracic surgery literature. The statistical significance of each predictor was assessed using the F-test statistic. Test for provider covariance determined whether risk-adjusted readmission rates and mortality rates varied significantly across hospitals.
To determine the relationship between index postoperative LOS and likelihood of readmission, a third regression model was generated using natural cubic splines. This method allowed the interpretation of a non-linear relationship, and controlled for patient age, Charlson comorbidity index, and induction therapy. Spline knots were placed at 9, 11, 14, 18, and 25 days with boundary knots at 7 and 29 days, but results were similar with different knot placements. All analyses were performed using SAS (version 9.4; SAS Institute, Inc, Cary, NC) and R (version 3.1.2) statistical software (22). The University of Virginia Institutional Review Board for Health Sciences Research approved this study.
Results
The SEER-Medicare database included 1688 patients who underwent esophagectomy among patients diagnosed with esophageal cancer from 2000 to 2009. Overall 30-day postoperative mortality was 6.9% (116/1688), and 90-day mortality was 13.9% (234/1688). After excluding in-hospital deaths—these patients are not candidates for readmission—the readmission analysis dataset included 1543 patients. Within this cohort, 90-day mortality following successful discharge was 6.4% (98/1543). Patient demographics are presented in Table 1.
Table 1.
Patient demographics
| Demographics | Readmit n (%) |
No Readmit n (%) |
p-value |
|---|---|---|---|
| Age | |||
| 65–69 | 110 (34%) | 422 (34%) | 0.916 |
| 70–74 | 102 (32%) | 410 (34%) | |
| 75–79 | 71 (22%) | 249 (20%) | |
| 80–84 | 24 (8%) | 119 (10%) | |
| 85+ | 12 (4%) | 24 (2%) | |
| Sex | |||
| Female | 71 (22%) | 228 (19%) | 0.726 |
| Year of Diagnosis | |||
| 2000–2001 | 57 (18%) | 219 (18%) | 0.983 |
| 2002–2003 | 59 (18%) | 241 (20%) | |
| 2004–2005 | 74 (23%) | 295 (24%) | |
| 2006–2007 | 65 (20%) | 258 (21%) | |
| 2008–2009 | 64 (20%) | 211 (17%) | |
| Tumor Stage | |||
| 0 | 10 (3%) | 36 (3%) | 0.990 |
| I | 99 (32%) | 412 (34%) | |
| II | 124 (40%) | 484 (40%) | |
| III | 55 (18%) | 196 (16%) | |
| IV | 22 (7%) | 71 (6%) | |
| Comorbidities | |||
| Induction chemotherapy | 137 (43%) | 445 (36%) | 0.032 |
| COPD | 41 (13%) | 98 (8%) | 0.011 |
| Diabetes | 32 (10%) | 167 (14%) | 0.092 |
| Congestive heart failure | 7 (2%) | 34 (3%) | 0.697 |
| Myocardial infarction | 5 (2%) | 10 (1%) | 0.212 |
| Peripheral vascular disease | 8 (3%) | 18 (1%) | 0.220 |
COPD: Chronic obstructive pulmonary disease
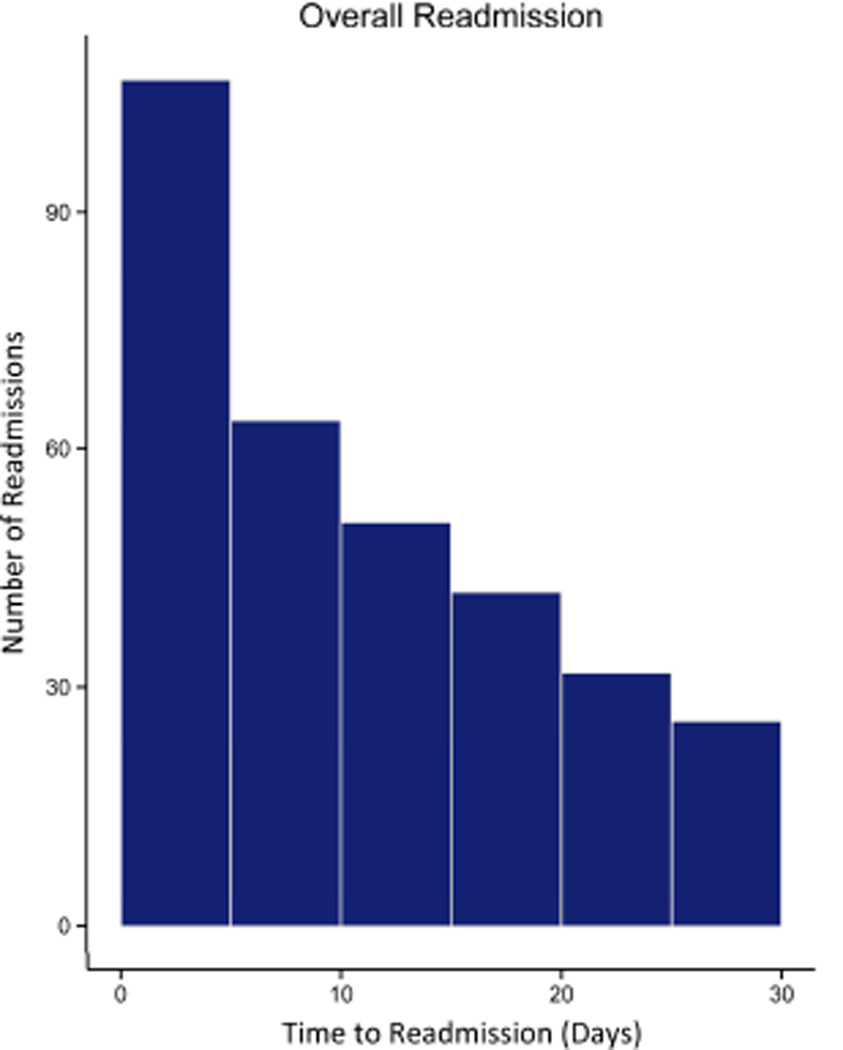
The rate of readmission within 30 days of discharge was 20.7% (319/1543). The median readmission length of stay was 4 days (interquartile range, IQR 2 – 8), and most readmissions occurred within 15 days of discharge (Figure 1). Among readmitted patients, in-hospital mortality rate was 8.8% (28/319). Ninety-day mortality rate among readmitted patients was almost five times that of non-readmitted counterparts (16.3% (52/319) vs. 3.8% (46/1224), p < 0.001). Patients who were readmitted two or more times did not have a significantly higher mortality rate (19.1% (16/84), 95% CI: 12.0% – 28.8%). Multivariable analysis demonstrated postoperative readmission to be the strongest contributor to predicting mortality risk (OR 6.64, p < 0.001, F-test statistic 33.5). The model C-statistic was 0.715 (Table 2). The test for provider covariance was not significant (p = 0.072), suggesting that risk-adjusted mortality rates did not significantly differ across providers.
Figure 1. Distribution of Readmissions Over Time.
Postoperative readmissions were most common within the first two weeks of discharge.
Table 2.
Hierarchical generalized linear model for 90-day mortality
| Variable | OR | CI | p-value | F test |
|---|---|---|---|---|
| Readmission | 6.64 | 3.49 – 12.62 | < 0.001 | 33.47 |
| Age (10 year increment) | 2.20 | 1.29 – 3.75 | < 0.001 | 8.50 |
| Discharge to home | 0.44 | 0.22 – 0.88 | 0.019 | 5.48 |
| Induction chemotherapy | 1.59 | 0.84 – 2.99 | 0.151 | 2.06 |
| Charlson-Deyo score | 0.83 | 0.52 – 1.34 | 0.448 | 0.58 |
| Sex (female) | 0.72 | 0.35 – 1.49 | 0.372 | 0.80 |
| Surgery year | 0.97 | 0.82 – 1.16 | 0.747 | 0.10 |
| Postoperative LOS | ||||
| LOS < 20 days | 1.04 | 0.97 – 1.12 | 0.291 | 1.12 |
| LOS ≥ 20 days | 0.96 | 0.88 – 1.05 | 0.376 | 0.78 |
LOS: length of stay
More than one-third of readmissions were to facilities that did not perform the index operation (33.5% 107/319). Readmissions to non-operative hospitals were significantly more likely to undergo subsequent transfer (15.0% vs 1.9%, p < 0.001). Ninety-day mortality did not differ significantly between patients readmitted to the operative facility versus those readmitted to an alternate facility (13.1% vs 17.9% p = 0.269), nor did it differ between transferred and non-transferred patients (20.0% vs 16.1%, p = 0.644). The most frequent readmitting diagnoses were pneumonia (11.8%), malnutrition/dehydration (8.1%), pleural effusion (7.5%), and aspiration pneumonitis (6.8%). Mortality at 90 days grouped by cause of readmission is presented in Table 3. Notably, more than one in five patients readmitted with a pulmonary diagnosis subsequently died within 90 days of the operation. Temporal trends indicate that postoperative readmissions remained common up to 4 weeks following discharge (Figure 1).
Table 3.
Subgroup analysis: 90-day mortality
| Readmission Diagnosis | N | 90-day Mortality (%) |
|---|---|---|
| Pulmonary | 114 | 22.8% |
| Pneumonia | ||
| Pleural effusion | ||
| Aspiration pneumonitis | ||
| Other pulmonary | ||
| Gastrointestinal | 79 | 16.5% |
| Malnutrition/dehydration | ||
| Dysphagia | ||
| Other gastrointestinal (incl. leak) | ||
| Other | 129 | 10.8% |
| Cardiovascular | ||
| Infections (non-pulmonary) | ||
| Wound | ||
| Other (incl. bleeding, AKI) |
AKI: acute kidney injury
The hierarchical logistic regression model used to estimate the risk of 30-day readmission obtained a C-statistic of 0.648. To account for the clustering effect of procedures within hospitals, the hospital provider was included as a random effect. The only significant preoperative predictor of readmission was delivery of induction therapy (OR 1.46, p = 0.032) (Table 4). As was the case for mortality, the test for provider covariance was not significant (p = 0.159), suggesting that risk-adjusted readmission rates did not differ significantly across providers.
Table 4.
Hierarchical generalized linear model for 30-day readmission
| Variable | OR | CI | p-value | F test |
|---|---|---|---|---|
| Induction chemotherapy | 1.46 | 1.03 – 2.06 | 0.032 | 4.64 |
| Discharge to home | 0.79 | 0.52 – 1.19 | 0.257 | 1.29 |
| Sex (female) | 1.24 | 0.84 – 1.83 | 0.275 | 1.19 |
| Charlson-Deyo score | 1.14 | 0.89 – 1.46 | 0.309 | 1.04 |
| Surgery year | 0.96 | 0.88 – 1.06 | 0.421 | 0.65 |
| Age (10 year increment) | 0.99 | 0.73 – 1.36 | 0.974 | 0 |
| Postoperative LOS | ||||
| LOS < 20 days | 1.02 | 0.98 – 1.06 | 0.373 | 0.80 |
| LOS ≥ 20 days | 0.99 | 0.94 – 1.04 | 0.671 | 0.18 |
LOS: length of stay
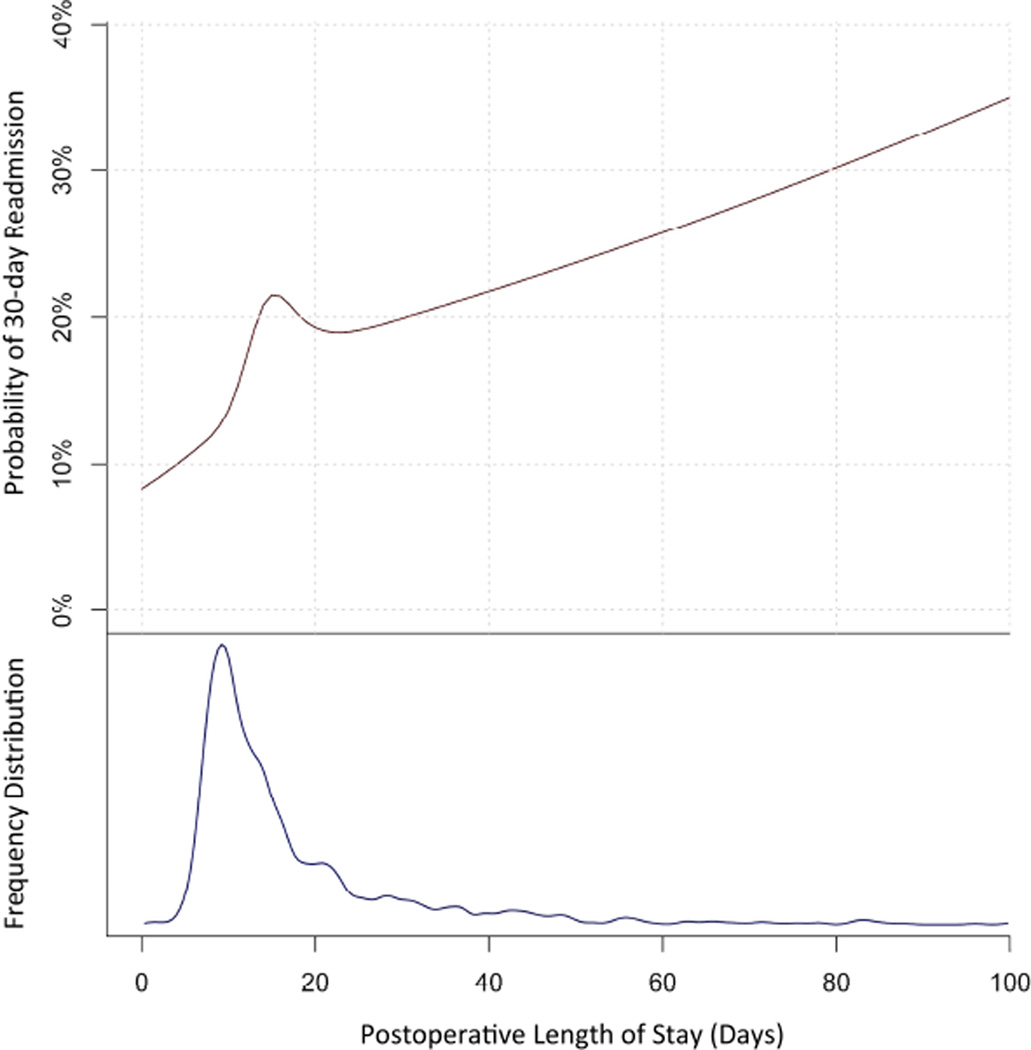
We conducted a separate analysis of the association between LOS for the index operative hospitalization and readmission. Median LOS was 13 days (IQR 9 – 20), and the most common LOS was 9 days. Logistic regression was used to assess the effect of LOS on readmission, with the effect of LOS modeled using natural cubic splines. The readmission probability for a 73 year old patient (median age) with a Charlson index of 0, and who did not receive induction therapy is shown in Figure 2; for a LOS of 9 days, we estimate an 12.6% chance of readmission. In general, the probability of readmission increases with increasing postoperative LOS (Figure 2). However, a deviation from this relationship occurred between LOS 9 and 23 days, with a peak at LOS 15 days. This suggests that there is a range of index hospitalization lengths over which patients have an uncharacteristically elevated risk of readmission.
Figure 2. Readmission Rate vs Postoperative Length of Stay.
The probability of readmission within 30 days is higher for patients with prolonged postoperative length of stay (top). For the mode length of stay (9 days), readmission rate is relatively low, at 12.6%. The frequency distribution of postoperative length of stay is provided for comparison (bottom).
Discussion
In this study, we used a large, national dataset to examine the relationship between postoperative readmissions and subsequent 90-day mortality among patients undergoing esophagectomy for cancer. Predictably, early readmissions are very common following esophagectomy. Interestingly, 33.5% of readmissions were to facilities that did not perform the index operation. This indicates that quality metrics that focus on readmissions to the index hospital where the operation was performed may substantially underestimate the true readmission rate. Moreover, not all readmissions have the same clinical significance. Among major organ systems, pulmonary complications are responsible for the largest proportion of readmissions, and portend the worst clinical outcome. Our results highlight the acuity of this extremely high-risk population, and argue for early and intensive medical attention.
We found that early readmission outperforms all commonly-reported preoperative risk factors in predicting mortality following esophagectomy. Because readmission is a post-operative risk factor, this associative finding was not entirely unexpected. However, the magnitude of the risk increase was notable, as readmitted patients had a nearly five-fold increase in mortality rate within the subsequent 2–3 months. Risk for early mortality following readmission was high regardless of the readmitting hospital or patient transfer status. In a recent study, Fernandez and colleagues reported that early readmission following esophagectomy portends a negative long-term prognosis (23). Our results indicate that much of this impact is felt within the first three months following discharge.
Analyses by readmitting diagnosis suggests that readmissions related to pulmonary complications carry the worst short-term prognosis. This finding is corroborated by prior work demonstrating negative associations between pulmonary complications and both short- and long-term survival following esophagectomy (24, 25). While these studies focused on in-hospital complications, ours is the first to demonstrate that pulmonary readmissions within 30 days of discharge are equally ominous. Patients with nonspecific dyspneic symptoms or small pleural effusions should receive aggressive care upon readmission, as more than 20% will not survive the next few months. These results reinforce the notion that a fairly benign readmitting diagnosis is often an indicator of a much more severe root process. For example, malnutrition and dehydration carried the highest mortality rate of any non-pulmonary readmission diagnosis. Among patients undergoing esophagectomy, nutritional status is often compromised to some degree preoperatively through induction treatment (26). This may explain the observation in our dataset that induction therapy was a predictor of both readmission and early mortality. While there are many interventions that can promote postoperative nutrition (27–29), a readmission due to poor dietary tolerance often indicates other complications such as infection, stenosis or anastomotic leak. Clearly, the theme that an initial readmitting diagnosis may not indicate the root cause of decompensation is pervasive. A thorough root-cause analysis should be a part of every readmission.
The most common postoperative LOS for patients in our study was 9 days. Overall, those who stayed longer tended to have a higher risk of readmission. This trend indicates that prolonged LOS may be related to unforeseen complications or sicker patients. Encouragingly, a 9-day LOS in an otherwise healthy patient was associated with a comparatively low readmission rate of 12.6% (Figure 2). Thus, patients discharged after a routine hospital course are relatively unlikely to be readmitted. We also found that the relationship between index LOS and readmission deviated from an otherwise relatively linear pattern between the range of 9 to 23 days, with a peak at index LOS 15 days. While the precise range of LOS that was associated with elevated readmission risk varied slightly depending on spline knot placement, we found that other knot placements produced very similar results. Freeman and colleagues previously reported similar findings with pulmonary lobectomy (30). These results suggests that, while the routine esophagectomy patient may be discharged expeditiously, those who have already experienced a relatively prolonged postoperative course (15 days) may benefit from additional observation. Early results from enhanced recovery programs after surgery report shorter LOS without deleterious effects on readmission rate (6, 31). Results from our study advocate for careful management of patients who deviate from the standard postoperative recovery pathway.
Despite its clinical relevancy, the validity of readmission as a quality indicator is difficult to establish. In a previous study, we found that risk-adjusted readmission rate following lung cancer resection does not vary significantly across hospital providers (11). This study further shows that risk-adjusted readmission rate following esophagectomy also does not significantly vary across providers. The incentive structure associated with adopting readmission as a quality metric is problematic. Varghese previously demonstrated that hospitals qualifying for LeapFrog case-volume criteria for esophagectomy had lower odds of 90-day mortality compared to non-qualifying hospitals, yet had higher odds of readmission (12). This may be explained by a potential tendency among LeapFrog-qualifying hospitals to provide more clinically-appropriate readmissions following complicated procedures. An earlier study using ACS-NSQIP showed similar complication rates between high and low-mortality providers, but marked differences in mortality rates associated with each complication (9). These findings suggest that appropriate management of complications—more so than prevention—is the strongest determinant of mortality outcomes. Home interventions, nutritional counseling, early provider-initiated telephone follow-up, and other discharge planning strategies have been shown to reduce readmissions and emergency department visits among medically-managed patients (32, 33). Results in the surgical population are thus far unclear; a recent multicenter trial instituting centralized telephone-based counseling following colorectal resection failed to demonstrate an impact on readmission and quality of life (34). Conversely, Nabagiez and colleagues recently reported that a physician-assistant home care program was associated with a reduction in the incidence of infection-related readmissions after cardiac surgery (35).
There are several limitations to this study. The SEER-Medicare dataset, by excluding patients less than 65 years of age, is older than the general esophagectomy population. However, the peak incidence of esophageal cancer is 75–85 years of age (36), therefore our results are relevant to the majority of patients undergoing esophagectomy for cancer. Second, SEER-Medicare does not clearly separate preoperative from postoperative diagnosis codes. Therefore, we could not incorporate postoperative complications into our predictive models of readmission or 90-day mortality. However, SEER-Medicare reliably captures readmissions to any facility and long-term mortality, making it a relevant source of data for the present study’s purpose. When performing risk-analysis with administrative databases, it is critical that only reliably-captured variables are selected for modeling. Third, the study may not adequately represent patients with an extreme index postoperative LOS, because 90-day mortality was calculated from the date of surgery rather than date of discharge. Finally, causes of readmission were based on ICD-9 diagnosis codes. These codes are often recorded early during an admission. Despite application of investigator judgement, these codes may not always represent the root complication responsible for each readmission. For example, only six readmission diagnoses were indicative of anastomotic leak, which is a complication frequently not discovered until one or more days into a readmission.
Conclusions
Early readmissions are very common following esophagectomy for cancer, especially among patients who underwent induction chemotherapy. Patients who deviate from a standard postoperative course and are considered for discharge after an index hospitalization of 15 days are at particular risk for early subsequent readmission. Readmitted patients need to be recognized for their high risk of early mortality. In particular, one in five patients readmitted with a pulmonary diagnosis will die within 90-days of surgery. Regardless of the presenting symptom, all patients readmitted following esophagectomy should undergo an exhaustive root-cause exploration, intensive monitoring, and aggressive care.
Perspective Statement.
Early readmissions are common following esophagectomy. This study assesses the temporal trends in readmissions, the most common readmitting diagnoses, and the relationships between readmission and 90-day mortality. Results highlight the clinical acuity and poor prognosis of readmitted patients, and argue for early root-cause exploration and aggressive care.
Acknowledgments
Financial Support:
Funding support provided by Agency for Healthcare Research and Quality K080HS18049 (to BDK) and NIH T32 CA163177 (to YH)
Abbreviations
- ACS-NSQIP
American College of Surgeons National Surgical Quality Improvement Program
- SEER
Surveillance, Epidemiology, and End Results
- ICD-9
International Classification of Diseases
- LOS
Length of stay
- IQR
Interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors report no potential conflicts of interest.
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swisher SG, Deford L, Merriman KW, Walsh GL, Smythe R, Vaporicyan A, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000 Jun;119(6):1126–1132. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 3.Lapar DJ, Stukenborg GJ, Lau CL, Jones DR, Kozower BD. Differences in reported esophageal cancer resection outcomes between national clinical and administrative databases. J Thorac Cardiovasc Surg. 2012 Nov;144(5):1152–1157. doi: 10.1016/j.jtcvs.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, et al. Outcomes after minimally invasive esophagectomy: Review of over 1000 patients. Ann Surg. 2012 Jul;256(1):95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: A meta-analysis. Ann Surg. 2011 Dec;254(6):894–906. doi: 10.1097/SLA.0b013e3182263781. [DOI] [PubMed] [Google Scholar]
- 6.Markar SR, Karthikesalingam A, Low DE. Enhanced recovery pathways lead to an improvement in postoperative outcomes following esophagectomy: Systematic review and pooled analysis. Dis Esophagus. 2014 Apr 3; doi: 10.1111/dote.12214. [DOI] [PubMed] [Google Scholar]
- 7.Walters DM, McMurry TL, Isbell JM, Stukenborg GJ, Kozower BD. Understanding mortality as a quality indicator after esophagectomy. Ann Thorac Surg. 2014 Aug;98(2):506–511. doi: 10.1016/j.athoracsur.2014.03.041. discussion 511–2. [DOI] [PubMed] [Google Scholar]
- 8.Luc G, Durand M, Chiche L, Collet D. Major post-operative complications predict long-term survival after esophagectomy in patients with adenocarcinoma of the esophagus. World J Surg. 2014 Sep 5; doi: 10.1007/s00268-014-2754-1. [DOI] [PubMed] [Google Scholar]
- 9.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009 Oct 1;361(14):1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 10.Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one-year mortality in community-dwelling older medicare beneficiaries. J Gen Intern Med. 2012 Nov;27(11):1467–1474. doi: 10.1007/s11606-012-2116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, McMurry TL, Isbell JM, Stukenborg GJ, Kozower BD. Readmission after lung cancer resection is associated with a 6-fold increase in 90-day postoperative mortality. J Thorac Cardiovasc Surg. 2014 Apr 18; doi: 10.1016/j.jtcvs.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese TK, Jr, Wood DE, Farjah F, Oelschlager BK, Symons RG, MacLeod KE, et al. Variation in esophagectomy outcomes in hospitals meeting leapfrog volume outcome standards. Ann Thorac Surg. 2011 Apr;91(4):1003–1009. doi: 10.1016/j.athoracsur.2010.11.006. discussion 1009–10. [DOI] [PubMed] [Google Scholar]
- 13.Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg. 2003 Aug;238(2):161–167. doi: 10.1097/01.SLA.0000081094.66659.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Shen Y, Feng M, Zhang Y, Jiang W, Xu S, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg. 2015 Apr;149(4):1006–1015. doi: 10.1016/j.jtcvs.2014.12.063. e4. [DOI] [PubMed] [Google Scholar]
- 15.Schneider EB, Hyder O, Brooke BS, Efron J, Cameron JL, Edil BH, et al. Patient readmission and mortality after colorectal surgery for colon cancer: Impact of length of stay relative to other clinical factors. J Am Coll Surg. 2012 Apr;214(4):390–398. doi: 10.1016/j.jamcollsurg.2011.12.025. discussion 398–9. [DOI] [PubMed] [Google Scholar]
- 16.Schneider EB, Hyder O, Wolfgang CL, Hirose K, Choti MA, Makary MA, et al. Patient readmission and mortality after surgery for hepato-pancreato-biliary malignancies. J Am Coll Surg. 2012 Nov;215(5):607–615. doi: 10.1016/j.jamcollsurg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-medicare data: Content, research applications, and generalizability to the united states elderly population. Med Care. 2002 Aug;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV,3–18. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.SEER-medicare: Calculation of comorbidity weights [homepage on the Internet] 2010 Available from: http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 21.Hu Y, McMurry TL, Wells KM, Isbell JM, Stukenborg GJ, Kozower BD. Postoperative mortality is an inadequate quality indicator for lung cancer resection. Ann Thorac Surg. 2014 Mar;97(3):973–979. doi: 10.1016/j.athoracsur.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R: A language and environment for statistical computing.[homepage on the Internet] Vienna, Austria: R Foundation for Statistical Computing; 2013. Available from: http://www.R-project.org/. [Google Scholar]
- 23.Fernandez FG, Khullar O, Force SD, Jiang R, Pickens A, Howard D, et al. Hospital readmission is associated with poor survival after esophagectomy for esophageal cancer. Ann Thorac Surg. 2015 Jan;99(1):292–297. doi: 10.1016/j.athoracsur.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson MK, Durkin AE. Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2002 Apr;123(4):661–669. doi: 10.1067/mtc.2002.120350. [DOI] [PubMed] [Google Scholar]
- 25.Schieman C, Wigle DA, Deschamps C, Nichols Iii FC, Cassivi SD, Shen KR, et al. Patterns of operative mortality following esophagectomy. Dis Esophagus. 2012 Sep-Oct;25(7):645–651. doi: 10.1111/j.1442-2050.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 26.Sikora SS, Ribeiro U, Kane JM, 3rd, Landreneau RJ, Lembersky B, Posner MC. Role of nutrition support during induction chemoradiation therapy in esophageal cancer. JPEN J Parenter Enteral Nutr. 1998 Jan-Feb;22(1):18–21. doi: 10.1177/014860719802200118. [DOI] [PubMed] [Google Scholar]
- 27.Baker A, Wooten LA, Malloy M. Nutritional considerations after gastrectomy and esophagectomy for malignancy. Curr Treat Options Oncol. 2011 Mar;12(1):85–95. doi: 10.1007/s11864-010-0134-0. [DOI] [PubMed] [Google Scholar]
- 28.Xiao-Bo Y, Qiang L, Xiong Q, Zheng R, Jian Z, Jian-Hua Z, et al. Efficacy of early postoperative enteral nutrition in supporting patients after esophagectomy. Minerva Chir. 2014 Feb;69(1):37–46. [PubMed] [Google Scholar]
- 29.Yamasaki M, Miyata H, Yasuda T, Shiraishi O, Takahashi T, Motoori M, et al. Impact of the route of reconstruction on post-operative morbidity and malnutrition after esophagectomy: A multicenter cohort study. World J Surg. 2014 Oct 15; doi: 10.1007/s00268-014-2819-1. [DOI] [PubMed] [Google Scholar]
- 30.Freeman RK, Dilts JR, Ascioti AJ, Dake M, Mahidhara RS. A comparison of length of stay, readmission rate, and facility reimbursement after lobectomy of the lung. Ann Thorac Surg. 2013 Aug 26; doi: 10.1016/j.athoracsur.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 31.Shewale JB, Correa AM, Baker CM, Villafane-Ferriol N, Hofstetter WL, Jordan VS, et al. Impact of a fast-track esophagectomy protocol on esophageal cancer patient outcomes and hospital charges. Ann Surg. 2014 Sep 19; doi: 10.1097/SLA.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costantino ME, Frey B, Hall B, Painter P. The influence of a postdischarge intervention on reducing hospital readmissions in a medicare population. Popul Health Manag. 2013 Oct;16(5):310–316. doi: 10.1089/pop.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001 Dec 21;111(9B):26S–30S. doi: 10.1016/s0002-9343(01)00966-4. [DOI] [PubMed] [Google Scholar]
- 34.Young JM, Butow PN, Walsh J, Durcinoska I, Dobbins TA, Rodwell L, et al. Multicenter randomized trial of centralized nurse-led telephone-based care coordination to improve outcomes after surgical resection for colorectal cancer: The CONNECT intervention. J Clin Oncol. 2013 Oct 1;31(28):3585–3591. doi: 10.1200/JCO.2012.48.1036. [DOI] [PubMed] [Google Scholar]
- 35.Nabagiez JP, Shariff MA, Khan MA, Molloy WJ, McGinn JT., Jr Physician assistant home visit program to reduce hospital readmissions. J Thorac Cardiovasc Surg. 2013 Jan;145(1):225–231. doi: 10.1016/j.jtcvs.2012.09.047. 233; discussion 232–3. [DOI] [PubMed] [Google Scholar]
- 36.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]