Abstract
Communication between cardiomyocytes depends upon Gap Junctions (GJ). Previous studies have demonstrated that electrical stimulation induces GJ remodeling and modifies histone acetylases (HAT) and deacetylases (HDAC) activities, although these two results have not been linked. The aim of this work was to establish whether electrical stimulation modulates GJ-mediated cardiac cell-cell communication by acetylation-dependent mechanisms. Field stimulation of HL-1 cardiomyocytes at 0.5 Hz for 24 hours significantly reduced Connexin43 (Cx43) expression and cell-cell communication. HDAC activity was down-regulated whereas HAT activity was not modified resulting in increased acetylation of Cx43. Consistent with a post-translational mechanism, we did not observe a reduction in Cx43 mRNA in electrically stimulated cells, while the proteasomal inhibitor MG132 maintained Cx43 expression. Further, the treatment of paced cells with the HAT inhibitor Anacardic Acid maintained both the levels of Cx43 and cell-cell communication. Finally, we observed increased acetylation of Cx43 in the left ventricles of dogs subjected to chronic tachypacing as a model of abnormal ventricular activation.
In conclusion, our findings suggest that altered electrical activity can regulate cardiomyocyte communication by influencing the acetylation status of Cx43.
Keywords: Pacing, Gap Junctions, Acetylation, Connexin43
1. INTRODUCTION
Considerable evidence indicates that exposure to electric fields has an impact on different cellular functions. It is in fact well recognized that processes such as cell migration [1], development [2], wound healing [3], nerve growth, angiogenesis and metastases are modulated by bioelectric signals [4]. Recent reports also indicate that electrical activity can have a direct impact on transcriptional response of excitable cells. The mechanisms that link changes in membrane potential to the activity of epigenetically active enzymes responsible for DNA and histone modifications have just begun to be characterized.
Histone acetylases (HATs) and deacetylases (HDACs) are enzymes able to modify the chromatin condensation status, thus altering cell transcriptional activity [5].
Electrical activity-dependent regulation of HATs and HDACs has been described for neurons, where depolarizing stimuli can lead to the activation of the HAT CBP (chromatin binding protein, p300) by increasing intracellular Ca2+ concentration via the activation of L-type Ca2+ channels [6,7]. In addition to histone proteins, HAT and HDAC enzymes can also target non histone-proteins located in the nucleus as well as in the cytoplasm [8]. Further, it has been recently shown that electrical activity modulates the subcellular localization of class II HDACs (i.e. HDAC4 and HDAC5) [9], thus possibly representing a fine regulator for controlling the access of these enzymes to their cytoplasmic versus nuclear substrates.
From the point of view of cardiac cell function, cell pacing can promote the transcriptional regulation of a variety of genes such as GATA4, Adss1 (adenylosuccinate synthetase 1) [10] and STAT3 [11]. Further, high frequency stimulation has been shown to induce hypertrophy of cultured cardiomyocytes (CMs) [12,13].
Of note, altered expression and/or distribution of Connexin43 (Cx43), which is the major component of Gap Junctions (GJs) connecting working CMs [14], has been described in cardiac rhythm disturbances, such as atrial fibrillation (AF) and tachycardia-associated remodeling [15]. To date, different groups have used tachypaced CMs as in vitro models to study AF [16] and GJ remodeling [17]. However, how electrical stimuli may affect Cx43 function and distribution in pathologies associated with rhythm disturbances is still largely unknown.
Importantly, one recent report has shown that tachypacing causes CM loss of function and electrical remodeling partly through HDAC6 activation and subsequent deacetylation-induced depolymerization of alpha-tubulin [16]. Nevertheless, the activation of epigenetic enzymes following electrical stimulation, and more specifically their action on cytoplasmic substrates, is still poorly understood. Recent work has demonstrated that HDAC4 and PCAF play a role in the acetylation-dependent regulation of cardiac myofilament contraction [18]. Further, lysine acetylation alters Cx43 expression and intracellular distribution, thus possibly impacting cell to cell communication and cardiac function [19].
Thus, the aim of this research was to assess whether electrical stimulation could impact GJ remodeling and function through acetylation/deacetylation-based mechanisms.
2. MATERIALS AND METHODS
2.1 HL-1 cardiomyocyte culture
HL-1 mouse atrial cardiomyocytes [20] were kindly donated by William Claycomb (Louisiana State University, New Orleans) and cultured in Claycomb medium (all from Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (FBS), 4 mM L-Glutamine, 100 U/mL Penicillin, 100 mg/mL Streptomycin, 0.3 mM Ascorbic Acid and 10 mM Norepinephrine as previously described [20]. Cells were plated onto gelatin/fibronectin-coated 35 mm Petri dishes at a density of 10000 cells/cm2. After 48 hours cells reached approximately 90% confluence and were used for subsequent pacing experiments.
2.2 Pacing conditions
HL-1 cells were stimulated at 0.5, 1 and 3 Hz for 90 minutes, 24 hours and 4 days with a C-Pace EP equipped with a C-Dish able to accommodate six 35 mm Petri dishes (IonOptix Corp, Ireland). A biphasic square-wave stimulus was chosen in order to minimize electrolysis at the electrodes [21]. Pulse duration and width were set at 5 msec and 20 V respectively, as with this combination it was possible to capture all beating areas evident by microscopic inspection. The strength of the applied electric field was approximately 10 V/cm.
Administration of Verapamil (Ver, 10 μM, Sigma-Aldrich, USA), Anacardic Acid (AA, 0.5 μM, Sigma-Aldrich, USA) and MG132 (10 μM, Sigma-Aldrich, USA) was performed once just before starting electric stimulation. Not-stimulated cells (NS) and NS cells with respective treatments (NS+Ver, NS+AA and NS+MG132) were considered as controls.
2.3 Western Blot analysis
Whole cells lysates were obtained by harvesting cells after electrical stimulation and treatment with Laemmli buffer containing the phosphatase inhibitors NaF (10 mM) and Na3VO4 (0.4 mM) and a protease inhibitor cocktail (all from Sigma-Aldrich, USA). The protein concentration was determined using the Bio-Rad protein assay reagent, following manufacturer’s instructions. Subsequently, 30 μg of protein extracts were separated by SDS-PAGE on precast gradient (4–12%) gels (Invitrogen) using MES running buffer (Invitrogen) and transferred onto nitrocellulose membranes (GE Healthcare, USA) in 20 mM Tris-HCl (pH 8.0), containing 150 mM glycine and 20% (v/v) methanol. Membranes were blocked with 5% non-fat dry milk in 1× PBS containing 0.1% Tween 20 (PBS-T) for 1 hour at room temperature and incubated overnight in the same solution at 4°C with antibodies against total Cx43 (1:5000, Abcam Cat# ab11370, UK), pS368Cx43 (1:500, Santa Cruz Cat# sc-101660, USA), Cx45 (1:100, Abcam Cat# ab70365, UK), Cx40 (1:1000, Millipore Cat# AB1726, USA), Troponin T-c (TnT-c), (1:200, Santa Cruz Cat# sc-20025, USA), anti-acetyl lysine (pan-Ac-K) (1:1000, Abcam Cat# ab21623, UK), Ac-α-tubulin (1:1000, Sigma-Aldrich Cat# T6793, USA), histone H3 acetyl K9 (H3K9ac) (1:500, Abcam Cat# ab10812, UK), histone H3 acetyl K14 (H3K14ac) (1:1000, Abcam Cat# ab46984, UK), α-tubulin (1:2000, Sigma-Aldrich Cat# T9026, USA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2000 Sigma-Aldrich Cat# G8795, USA), histone H3 (1:1000, Cell Signaling, Cat# 3638, USA) and β-actin (1:5000, Sigma-Aldrich, Cat# A5441, USA). To detect typical phosphorylation banding patterns an home made antibody against amino acids 1–20 of Cx43 (Cx43NT1 1:500 [22]) was used, together with specific condition for SDS-PAGE; specifically, 30 μg of protein extracts were separated by SDS-PAGE on 8% gels in 20 mM Tris-HCl (pH 8.0), containing 150 mM glycine and 0.1% SDS. Membranes were blocked and incubated as indicated before in 1% non-fat dry milk in deionized water, without detergent. Membranes were washed three times in 1× PBS-Tween buffer, followed by the incubation with the appropriate HRP-linked IgG for 1h at room temperature. Specific proteins were then visualized using the enhanced chemiluminescence (ECL) detection kit (Supersignal West Dura Extended Duration Substrate, ThermoScientific, USA). Results of Western Blot were quantified either by ImageJ v1.28 or Uvitec analyzer softwares. Optical density values were internally normalized on α-tubulin, GAPDH, histone H3 or β-actin expression. Specifically, the band density was determined considering the total optical density integrated in the selected area (OD *mm2) and subtracting the blank in a region of the blot near but outside the lanes.
2.4 HAT & HDAC Activity Assays
The HAT activity in the nuclear extract of HL-1 cells was quantified using the colorimetric HAT activity assay kit (BioVision Research Products, USA) according to the manufacturer’s instructions. Briefly, 50 μg of nuclear extract diluted in 40 μl ddH2O was incubated with HAT substrates I and II and NADH-generating enzyme in HAT assay buffer for 2 h at 37 °C. Absorbance was determined at 440 nm in an ELISA plate reader. HAT activity was estimated as relative OD values per μg of protein sample.
HDAC activity assays were performed using a kit from BioVision (BioVision Research Products, USA) according to manufacturer’s instructions. Whole HL-1 cell extracts (50 μg) were diluted in 85 μL of ddH2O; then, 10 μL of 10× HDAC assay buffer was added followed by addition of 5 μL of the colorimetric substrate; samples were incubated at 37° for 1 hour. Subsequently, the reaction was stopped by adding 10 μL of lysine developer and left for additional 30 min at 37°C. Samples were then read in an ELISA plate reader at 405 nm. Both HAT and HDAC activity assay kits contained negative and positive controls.
2.5 Dye transfer assays
Cell-to-cell gap junction mediated communication was determined by two independent fluorescent dye transfer assays.
a) Calcein-DilC12 transfer
As described previously with minor modifications [19], two independent populations of HL-1 cardiomyocytes were subjected to 24 hours electrical pacing as described above. Six hours prior the end of the experiment, one cell population was incubated for 1 hour at 37 °C with the DilC12 red fluorescent tracer (25 μg/mL). The second cell preparation was incubated with the Calcein AM green dye (30 min at 37 °C; 1 μM). Green cells were then trypsinized and plated on red cells at a ratio of 1:5. Cells were then allowed to settle and establish cell-to-cell communication under electrical stimulation. Dye transfer was evaluated 6 hours later by confocal microscopy counting of the number of double-stained fluorescent cells (yellow) in 12 randomly chosen fields. Experiments were repeated three times in duplicate.
b) Lucifer Yellow transfer
For each experiments, cells were electrically stimulated, as described above, either in the presence or absence of Anacardic Acid (AA, 20 nM). Lucifer yellow (2 mM in a standard intracellular solution composed by KAspartate 122 mM, KCl 20 mM, MgCl2 1 mM, HEPES 10 mM, EGTA 5 mM, CaCl2 1.6 mM, pH 7.3 KOH, was injected by a whole cell patch clamp technique (Axopatch Multiclamp 700B, Axon Instruments, USA) into clustered HL-1 cells perfused with an extracellular solution containing: NaCl 135 mM, KCl 4.5 mM, CaCl2 1 mM, MgCl2 1.8 mM, NaH2PO4 0.4 mM, HEPES 10 mM, Glucose 10 mM, pH 7.4 NaOH. A patch was maintained on the target cell for 15 minutes to allow the internal pipette solution to diffuse into the patched and surrounding connected cells. Cell-to-cell transfer was monitored using an inverted microscope (NIKON TS100, Japan) equipped with fluorescent illumination and images were acquired by a digital camera (Infinity 1, Lumenera Corporation, Canada). The extent of coupled cells was determined by counting the number of adjacent cells containing the tracer and the time dependence of the spreading of the dye into the recipient cell (ImageJ, v1.28 software). Only first order recipient cells, directly in contact with the donor LY-microinjected cell, were used for kinetic evaluation.
2.6 High frequency cardiac stimulation in a canine model
Dogs (n=4) were chronically instrumented with pacing leads as previously described [23]. The left ventricle was paced at 210 to 240 beats/min. This protocol leads to dilated cardiomyopathy with systolic and diastolic dysfunction after 4 weeks of tachypacing [23]. Chronically instrumented dogs that did not undergo cardiac pacing were used as normal controls (n=4). After euthanasia, tissue samples from the left ventricular free wall were harvested and frozen in liquid nitrogen for subsequent analysis. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Temple University and conform to the guiding principles for the care and use of laboratory animals published by the National Institutes of Health.
2.7 Confocal histological analysis
Cryosections from control and tachypaced dog heart tissue were used for immunofluorescence analysis. After fixation with 4% paraformaldehyde for 10 min, sections were washed with PBS and incubated for 1h with 10% BSA/PBS to block non-specific protein-binding sites and overnight at 4°C with anti Cx43 antibody (1:400, Abcam, Cat# ab11370, UK). After a brief rinse, sections were incubated with FITC-conjugated secondary antibody (1:200, Jackson immunoResearch, USA). Images were acquired with a confocal laser scanning system (TCS-SP2, Leica Microsystems, Germany) equipped with an Ar/ArKr laser for 488-nm excitation and HeNe laser for 543-nm excitation. DAPI staining was imaged during two-photon excitation (740 nm, <140 fs, 90 MHz) performed with an ultrafast, tunable, mode-locked Ti: Sapphire laser (Chameleon, Coherent Inc., USA).
2.8 Co-immunoprecipitation experiments
HL-1 protein extracts for immunoprecipitation were obtained after lysis in 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Igepal CA630, 1% sodium deoxycholate (DOC), 0.1% SDS and 1% Glycerol supplemented with protease/phosphatase inhibitor mix. Dog tissues were lysed in a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 2mM MgCl2 and 1% DOC supplemented with 1 mM PMSF and protease inhibitor mix. Identification of acetylated Cx43 was performed by immunoprecipitation using the antibody anti-Ac-K (4 μg for 500 μg of total proteins; Abcam Cat# ab21623, UK) and anti-Cx43 (Abcam Cat# ab66151, UK). Ademtech’s Bioadembeads (Ademtech, USA) paramagnetic beads system was used to immunoprecipitate the specific proteins according to the manufacturer’s instructions. Negative controls were performed with the same amount of protein extract immunoprecipitated with the corresponding purified IgG antisera (Santa Cruz, USA) in absence of primary antibody. Immunoprecipitated samples were resolved by SDS–PAGE, transferred onto nitrocellulose membrane (GE Healthcare, USA) and Western Blot performed as described above.
2.9 Statistical Analysis
Data were represented as the means of at least three independent experiments ± s.e.m. Statistical analysis was performed using one-way ANOVA, followed by Bonferroni’s multiple comparison test or unpaired two-tailed Student t-test when appropriate (see figure captions for the test used for each specific experiment). A p-value < 0.05 was considered statistically significant.
2.10 Additional methods
An expanded Methods section is available as Supplementary Material.
3. RESULTS
3.1 Validation of HL-1 cell pacing model
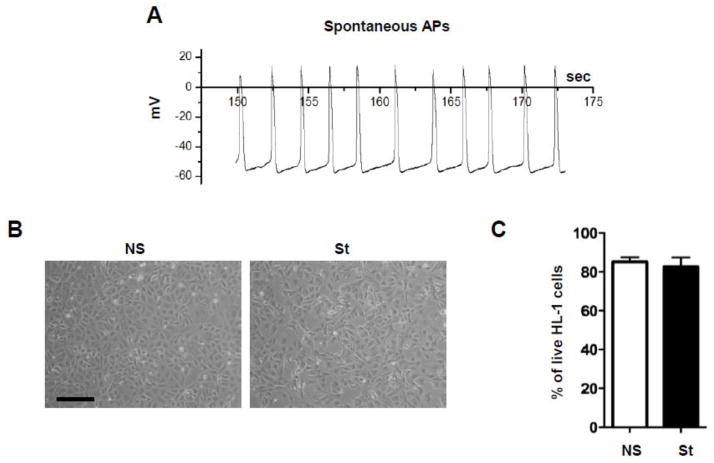
The spontaneous activation frequency of HL-1 cells at the density used in this series of experiments (Fig. 1) was approximately 0.5 Hz as measured using whole cell I-clamp (Fig. 1A) and optical map experiments (Supplementary Fig. 1). Not stimulated (NS) were allowed to spontaneously generate action potentials for the whole duration of the experiments. Nevertheless, spontaneous activity in HL-1 cells sometimes stopped for many seconds (Supplementary Fig. 1). Spontaneously active HL-1 cells were exposed for 90 minutes, 24 hours and 4 days to electrical field stimulation at different ranges of frequencies, namely 0.5, 1 and 3 Hz. In our experimental settings we found that the longer stimulation (4 days) resulted in cell death (> 95%) at each frequency tested (Supplementary Fig. 2). Also, when electrically stimulated for 24 hours at 3 Hz, HL-1 cells exhibited a significant loss of viability (resulting in approximately 40% cell death, Supplementary Fig. 2), likely because of myolysis induced by calcium overload [24]. However, after 24 hours of field stimulation at 0.5 and 1 Hz, HL-1 cells did not show either morphological changes (Fig. 1B and Supplementary Fig. 2) or blebbing, nor exhibited significant loss of viability (Fig 1.C and Supplementary Fig. 2). Therefore, we decided to stimulate HL-1 cells at their intrinsic firing rate of 0.5 Hz for 24 hours as our main experimental condition.
Fig. 1. Characteristics of cultured HL-1 cells.
(A) Spontaneous action potentials (APs) measured in single HL-1 cell. (B) Morphology of non-stimulated (NS) HL-1 cells and after 24 hours of electrical stimulation at 0.5 Hz (St). Scale bar = 100 μm. (C) Propidium Iodide (PI) staining on NS and St HL-1 cells did not reveal any change in cell viability after pacing (n=3). Data are presented as mean ± s.e.m, unpaired Student t-test: * p < 0.05.
3.2 Cx43 expression and intracellular localization in response to electrical stimulation
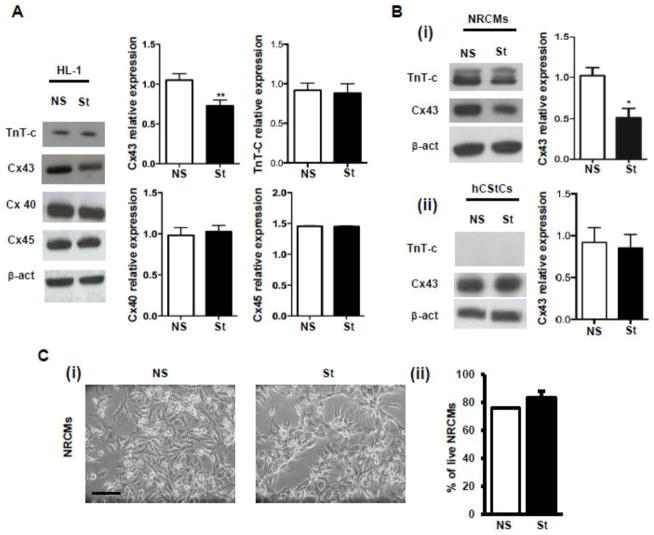
Field stimulation of HL-1 cells at 0.5 Hz for 24 hours caused a significant reduction in the expression of Cx43 protein (Fig. 2A). The expression of Cx40, Cx45 and Troponin T-c (TnT-c) was unchanged (Fig. 2A). Significant Cx43 reductions were also observed when HL-1 cells were exposed to 24 hours at 1 Hz, while shorter stimulation time (i.e. 90 min) did not affect Cx43 expression at any frequency tested (Supplementary Fig. 2). We also observed Cx43 reduction in neonatal rat cardiomyocytes (NRCMs) exposed to the same experimental conditions, but not in human cardiac fibroblasts (hCStCs) (Fig. 2B). NRCMs were exposed to 0.5 Hz stimulation and exhibited normal contractile behavior during and after 24 hour treatment. Of note, electrical pacing did not induce cell death nor evident morphological changes in either NRCMs (Fig. 2C) or hCStCs (data not shown).
Fig. 2. Effect of chronic pacing on Cx43 expression.
(A) Western Blot analysis shows Cx43 (n=20), Cx45 (n=3), Cx40 (n=3) and TnT-c (n=3) expression in NS and St (24 hours at 0.5 Hz) HL-1 cells. Densitometries are reported in the bar graphs on the right. (B) (i) Western blot analysis shows Cx43 (n=3) and TnT-c (n=3) expression in NS and St NRCMs. (ii) Western Blot analysis shows Cx43 (n=3) and TnT-c (n=3) expression in NS and St hCStCs. Densitometry analysis is shown on the right. Data are presented as mean ± s.e.m, unpaired Student t-test: * p < 0.05, ** p < 0.01. (C) (i) Morphology of NS and St NRCMs. Scale bar = 50 μm. (ii) Propidium Iodide (PI) staining of NS and St NRCMs (n=3).
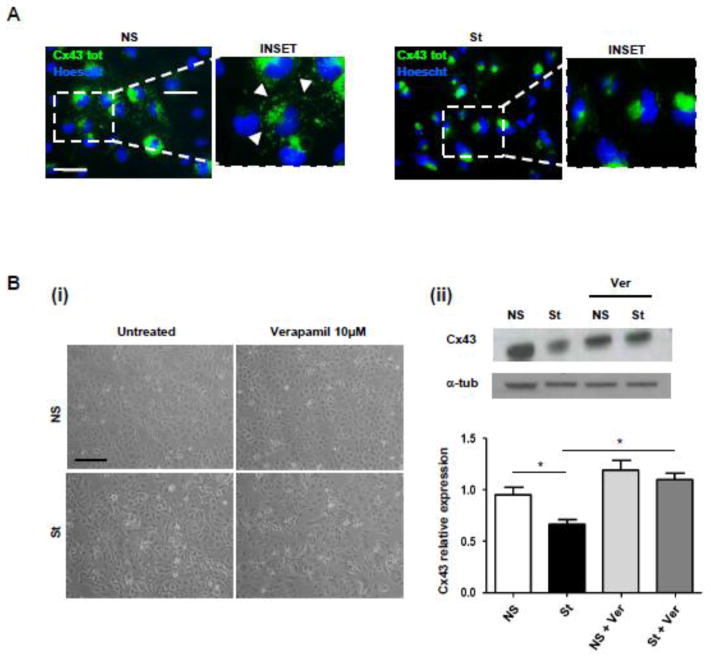
Confocal analysis confirmed (Fig. 3A) that Cx43 also relocalized from GJs and cell membrane and accumulated in the cytoplasm mainly in the peri-nuclear region. The pacing-induced reduction of Cx43 expression was prevented when the L-Type Ca2+ blocker Verapamil was added to HL-1 cardiomyocytes during electrical stimulation (Fig. 3B).
Fig. 3. Effect of chronic pacing on Cx43 localization and treatment with Verapamil, L-Type Ca2+ blocker.
(A) Confocal immunofluorescence assays performed on HL-1 cells revealed that St cells re-localize Cx43 from intercellular appositional membranes to the cytoplasm mainly in the peri-nuclear region. Experiments were repeated three times with similar results. Nuclei were counterstained with Hoescht (blue). Scale bar = 25 μm. (B) (i) Representative images of NS and St HL-1 cells treated with Verapamil (10 μM) for 24 h. (ii) Western Blot analysis of NS and St HL-1 whole cellular lysates treated with 10 μM of Verapamil. Scale bar = 100 μm. Densitometric analysis is reported below (n=5). Data are presented as mean ± s.e.m, One-way ANOVA, followed by Bonferroni’s multiple comparison: * p < 0.05.
As previously shown [19], the treatment of HL-1 cells with the HDAC inhibitors suberoylanilide hydroxamic acid (SAHA) and Trichostatin A (TSA) mimicked the effect of electrical stimulation on Cx43 protein expression and localization (Supplementary Fig. 3) [25], thus suggesting the hypothesis that electrical stimulation could promote Cx43 acetylation. However, the adjunct of Verapamil 10 μM to SAHA treated HL-1 cells failed to rescue Cx43 localization at appositional membranes (data not shown).
3.3 Electrical stimulation resulted in Cx43 direct acetylation
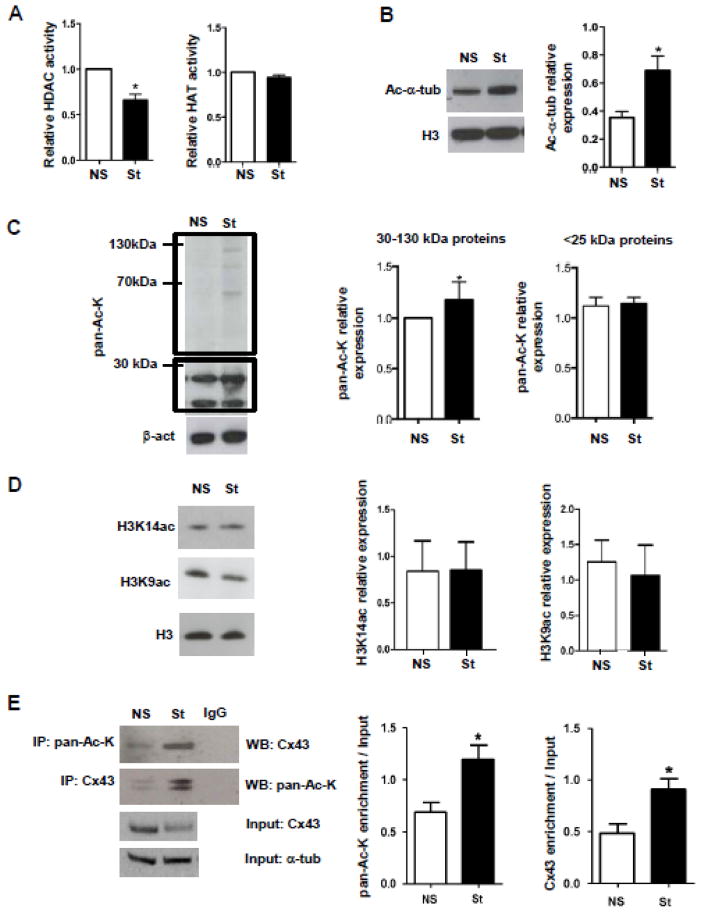
Electrical stimulation of HL-1 cells significantly down-regulated HDAC activity up to 30%, whereas HAT activity was not modified (Fig. 4A); the net effect was an overall increase in the acetylation of many proteins between 25 and 130 kDa molecular mass, as confirmed by western blot analysis of Ac-α-tubulin (Fig. 4B) and pan-Ac-K (Fig. 4C). Acetylation in proteins below 25 kDa, compatible with histones, remained unchanged (Fig. 4C), as confirmed also by western blot for H3 acetylation at residues K14 (H3K14ac) and K9 (H3K9ac) (Fig. 4D). The pacing-dependent acetylation of Cx43 was shown by reciprocal immunoprecipitation and immunoblotting with anti-Cx43 and pan-Ac-K antibodies (Fig. 4E).
Fig. 4. Effect of electrical pacing on acetylation/deacetylation balance.
(A) Bar graphs summarizing the results of HAT and HDAC activity assays performed on NS and St HL-1 nuclear (for HAT) and whole (for HDAC) cellular lysates (n=3). O.D. values for stimulated cells was normalized on O.D. values of corresponding non stimulated cells. O.D. = optical density. (B) Western blot analysis of NS and St HL-1 cells for Ac-α-tub (n=4). (C) Western Blot analysis of NS and St HL-1 whole cellular lysates showed an increase of pan-Ac-K only in proteins with molecular weights ranging between approximately 30 to 130 kDa and not in proteins below 25 kDa. Densitometry values are on the right (n=4). (D) Western Blot and densitometric analysis of NS and St HL-1 whole lysates for H3 acetylation at the specific residues K14 (n=6) and K9 (n=4). (E) Immunoprecipitation experiment showing Cx43 acetylation increases after electrical stimulation (n=6). Densitometry values are on the right. Data are presented as mean ± s.e.m, unpaired Student t-test: * p < 0.05.
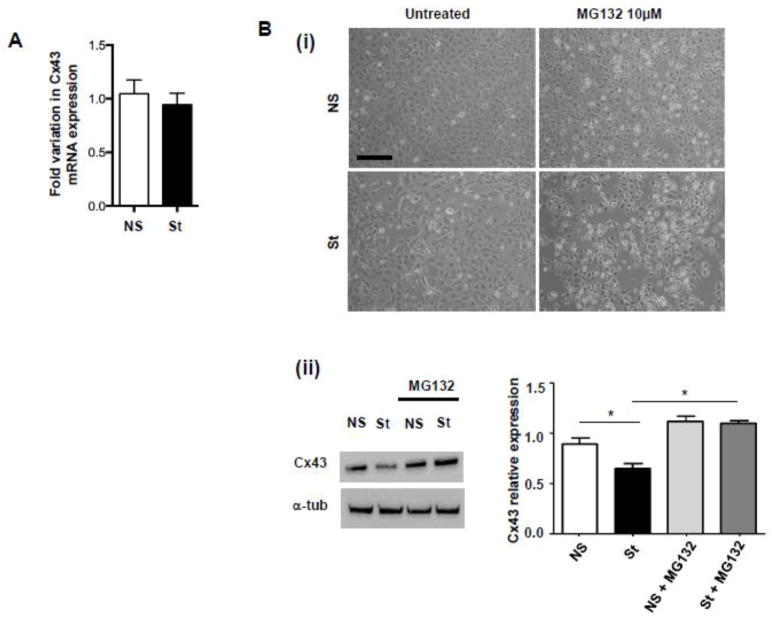
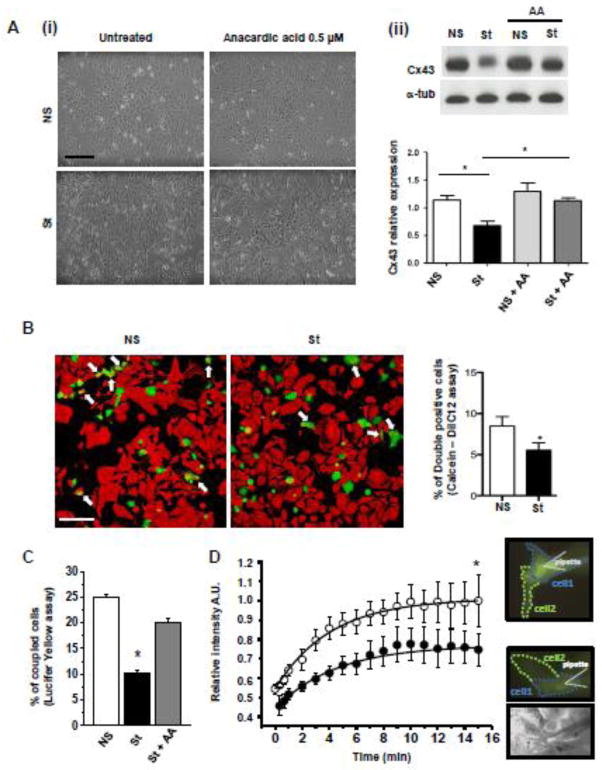
Notably, Real Time RT-PCR showed that Cx43 mRNA expression was not influenced by electrical pacing (Fig. 5A), while the Cx43 protein reduction induced by field stimulation was rescued by treatment with the proteasome inhibitor MG132 (Fig. 5B). Taken together these observations confirm that pacing-induced Cx43 down-modulation depends on the activation of post-translational mechanisms specifically associated with direct Cx43 acetylation, as confirmed by the fact that the treatment of stimulated cells with the general HAT inhibitor AA was able to rescue Cx43 expression (Fig. 6A), further confirming the involvement of acetylase activity in the observed phenomenon.
Fig. 5. Gene expression analysis of Cx43 and treatment with proteasome inhibitor MG132.
(A) Real Time RT-PCR for Cx43 mRNA expression in NS and St HL-1 cells (n=3). (B) (i) Representative pictures and (ii) Western Blot analysis of NS and St HL-1 treated with 10 μM of proteasome inhibitor MG132 (n=5). Data are presented as mean ± s.e.m, One-way ANOVA, followed by Bonferroni’s multiple comparison correction: * p < 0.05. Scale bar = 100 μm.
Fig. 6. Electrical stimulation leads to a reversible reduction in cell-cell communication.
(A) (i) Representative images and (ii) Western Blot analysis of NS and St HL-1 cells treated with 0.5 μM of HAT inhibitor Anacardic Acid AA (n=5). Data are presented as mean ± s.e.m, One-way ANOVA, followed by Bonferroni’s multiple comparison correction: * p < 0.05. Scale bar = 100 μm. (B) Representative confocal images showing reduced dye diffusion in St HL-1 cells compared with NS. White arrows indicate double positive cells (Calcein-AM+/DilC12+). The bar graph on the right shows the average number of double-positive cells per field (n=3 independent Calcein-DilC12 transfer experiments, counting cells in an average of 12 fields per experiment). Scale bar = 100 μm. (C) Bar graph representing the percentage of coupled cells receiving the Lucifer Yellow (LY) from the micro-injected donor cell (at least 23 cells from 4 independent experiments for each condition). (D) Time course of fluorescence relative intensity (A.U. = arbitrary units) averaged in a region of interest set to equal the area of the receiving cells. White and black dots represent the average time course in NS and St HL-1 cells, respectively (at least 46 cells from 10 independent experiments for each condition at different time points). On the right, fluorescence and transmission images of NS (top) and St (bottom) HL-1 clusters acquired 15 minutes after the pipette break-in.
Interestingly, electrical stimulation also enhanced the extent of Cx43 phosphorylation, as evident in the representative Western Blot reported in Supplementary Fig. 4 showing the typical banding pattern of multiple phosphoisoforms [22]. Phosphorylation also increased at the inhibitory residue S368 [26], thus supporting the hypothesis that the reduced expression of Cx43 is associated with diminished cell-cell communication (Supplementary Fig. 4).
3.4 Effect of field stimulation on cell-cell communication
Cell-cell communication was first assessed by a fluorescent dye-transfer method [19]. Two cell populations were labeled with two different dyes, GJ permeant Calcein (green) and Lipophilic and non-GJ diffusible DilC12 tracer (red). The percentage of double positive stained HL-1 cardiomyocytes (taken as an index of intercellular signal transmission) after 24 hours of electrical stimulation, was reduced by approximately 50% (Fig. 6B). Specifically, n=3 independent Calcein-DilC12 transfer experiments have been performed, counting cells in an average of 12 fields per experiment. Comparable results were obtained when cell-to-cell coupling was assessed by intracellular microinjection of Lucifer yellow (LY, Fig. 6C). Of note, we evaluated the number of cells receiving Lucifer yellow (LY) from the donor cells and the kinetic of LY diffusion in at least 46 cells from 10 independent experiments. In this case, the percentage of coupling, defined as the percentage of injections resulting in efficient LY transfer to the neighboring cell [27], was significantly reduced in electrically stimulated HL-1 cardiomyocytes. Fig. 6C shows that 25.0 ± 0.6% of unstimulated cells were coupled; upon stimulation, this value decreased to 10.3 ± 0.4% (p < 0.05). Consistent with reduced GJ-mediated communication, the quantity of dye transferred from the donor cell to the recipient cells, measured as LY relative intensity, was reduced of about 25% in the stimulated vs. unstimulated cells (Fig. 6D). However, the kinetics of the process did not vary; fitting the time course of the fluorescence intensity in recipient cells indicated a τ of 4.00 ± 0.60 sec for control experiments and 4.40 ± 0.54 sec after electrical stimulation.
Of note, residual communication was evident among cells after 24 hours stimulation (Fig. 6B–C), potentially due to the remaining Cx43 expression and/or the limited cell-cell coupling mediated by Cx40 and Cx45.
Importantly, AA treatment led to the rescue of functional cell communication, as evident by the recovery of coupling percentage up to 20.0±1.0% in LY microinjected cell clusters (Fig. 6C).
3.5 Cx43 acetylation in a dog model of chronic cardiac tachypacing
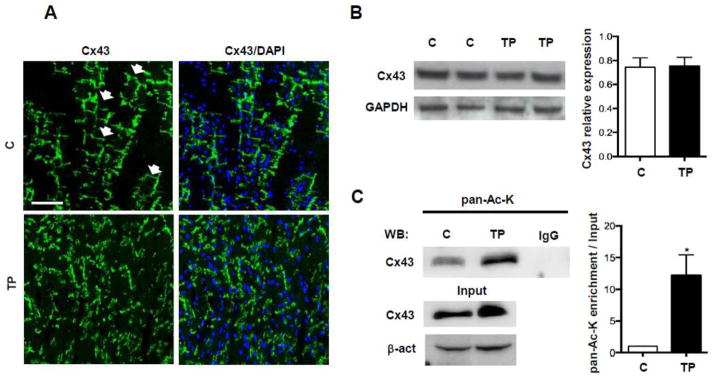
To assess whether Cx43 acetylation could be modulated in the context of altered electrical activity in an in vivo setting, we examined left ventricular tissue harvested from dogs after 4 weeks of ventricular tachypacing. In agreement with previous reports [28,29], confocal analysis of left ventricle thin sections showed that chronic ventricular tachypacing caused extensive Cx43 reorganization compared to control hearts (Fig. 7A), although Western Blot analysis showed that Cx43 expression remained unchanged (Fig. 7B). Immunoprecipitation analysis from these tissues corroborated our in vitro findings of significantly increased Cx43 acetylation in hearts subjected to tachypacing compared to control (n=4, Fig. 7C).
Fig. 7. Tachypacing-induced cardiomyopathy in dogs is associated with Cx43 acetylation.
(A) Panels showing representative Cx43 distribution in left ventricles of control dogs (C) and dogs after tachypacing (TP). Note Cx43 disorganization in TP hearts, compared to the regular distribution of the protein in control hearts (white arrows). Nuclei were counterstained with DAPI (blue). Scale bar = 50 μm (B) Western Blot analysis of Cx43 expression in left ventricles of C and TP dogs. Densitometry values are on the right (n=4). (C) Immunoprecipitation showing significant of Cx43 acetylation in heart samples from TP dogs compared to controls (n=4). Data are presented as mean ± s.e.m, unpaired Student t-test: * p < 0.05.
4. DISCUSSION
The present study describes how the culture of HL-1 cells under field-stimulation at their intrinsic frequency (approximately 0.5 Hz) for 24 hours results in the acetylation-dependent reduction of Cx43 expression and, consequently, of cell-to-cell communication.
HL-1 atrial cardiomyocytes were chosen as the primary cell model since they are easy to culture and they represent a commonly accepted model of cardiomyocytes [16,19,30]. However, pacing dependent Cx43 reduction was also evident in NRCMs but not in hCStCs, suggesting that the observed effect depends on signaling only present in excitable cells. The hypothesis for an important role of extracellular calcium entry via voltage-gated channels in the observed phenomenon is supported by the evidence that Verapamil, an antagonist of L-type calcium channels (ICa, L) [31] suppresses the pacing-induced reduction of Cx43 expression and it is consistent with the observation that electrical activity-dependent epigenetic response induced in neurons is related to calcium [6].
The effect that we observed in paced cells is only in part comparable to that induced by the pan-class II HDAC inhibitor Suberoylanilide Hydroxamic Acid (SAHA) and Trichostatin A (TSA). Intriguingly, it has been recently reported that β-hydroxybutyrate, a fundamental contributor to cardiac energy metabolism in conditions of prolonged exercise [32] can cause HDAC inhibition and consequent protection against oxidative stress [33]. It is therefore conceivable that the factor linking electrical activity to reduced HDAC activity is a metabolic factor. In agreement with this hypothesis different reports have provided evidence that electrical remodeling is associated with oxidative stress [34,35].
Our results are consistent with a prior study [19] that showed SAHA induces Cx43 protein down-modulation and lateralization in CMs. However, Veenstra and coworkers showed that SAHA also induced Cx43 mRNA reduction in neonatal mouse cardiomyocytes, consistently with the decreased association of Cx43 promoter with RNA Pol II and the increased association with HDACI [25]. In our model, increased acetylation was only evident for proteins with molecular weights ranging from 25 to 130 kDa, while the signals for anti-pan-Ac-K remained stable at molecular weights below 25 kDa, compatible with histones. This observation is supported by the evidence that acetylation in H3 at specific residues K14 and K9 remained unchanged. These results, along with the fact that Cx43 mRNA expression was stable and that the Cx43 protein reduction is rescued by the treatment with the proteasome inhibitor, suggests that electrical pacing might not significantly affect the acetylation status of histones associated with Cx43 promoter, thus acting on Cx43 mainly through post-translational mechanisms. Nevertheless, we cannot exclude that the net increase in acetylation observed in stimulated cells could also have a general impact on histones and, consequently, on other protein expression.
The hypothesis that Cx43 was affected mainly by post-translational mechanisms is also partially supported by the observation that Cx43 phosphorylation at the inhibitory residue S368 is increased following electrical stimulation. However, it also seems that electrical stimulation provokes a net increase in Cx43 phosphorylation, which has been in general associated with open pores [22]. Nevertheless, given that phosphorylation at over a dozen different Cx43 residues can have opposing effects on stability of gap junctions and conductance [36], it is beyond the scope of this manuscript to specifically determine the effects of electrical stimulation dependent Cx43 phosphorylation on junctional communication. However, our observation that pacing can affect Cx43 phosphorylation is in line with a previous report showing that electrical stimulation can induce kinase pathway activation [37].
Importantly, the fact that the net increase in Cx43 acetylation correlates with its increased degradation in a proteasomal-dependent manner is not surprising. It is known that lysine acetylation can either prevent or promote polyubiquitination and lysosomal degradation of different substrates [38,39] or can serve as a direct signal for proteasomal degradation [40]. More specifically, lysine acetylation was recently identified as an endogenous regulator of proteolytic activity in the heart, suggesting that inhibitors of cardiac proteasomal function might represent a new promising class of pharmacological agents [41]. Different reports have also shown that proteasomal activity can be regulated by calcium in neurons [42] and platelets [43]. Recently, Park and coworkers [44] reported that proteasomal activation responsible for axonal degeneration directly depends on extracellular calcium influx. This observation is in line with our result that Cx43 downregulation can be rescued both by preventing extracellular calcium influx through Verapamil application and by blocking the proteasomal pathway using the specific inhibitor MG132. Of note, in our experimental setting, the amount of Cx40 and Cx45 expression apparently did not vary. The fact that Cx40 and Cx45 can be present in junctions with Cx43 and their stability seems not to be affected might indicate that acetylation prevents the migration of newly synthesized Cx43 to the membrane, rather than favoring its loss from already formed junctional plaques. This potential mechanism is consistent with the previous report by Colussi and coworkers [19] showing that cells expressing Cx43 with specific lysines mutated to glutamine to mimic acetylation exhibited Cx43 localization predominantly in the cytoplasm. The relationship of the stimulation frequency for cells in culture to the in vivo electrical phenotype is difficult to determine. Stimulating cultured cells at 0.5 Hz may actually correspond to a strong bradychardic stimulation (compared to the 10 Hz physiological pace of the mouse heart) or it may resemble to a “quasi” normo-frequency stimulation if compared to the intrinsic firing rate of cultured HL-1 cells. High frequency stimulation of cultured cardiomyocytes results in cell death due to abnormal calcium homeostasis leading to increased diastolic calcium levels and subsequent activation of apoptotic pathways [24].
Interestingly, other groups have reported that electrical stimulation promotes an increase in the expression of Cx43 when cells are exposed to similar frequency and stimulation time [17,45]. This suggests that other variables than stimulation frequency, including the strength of the applied electric field, the electrode material and the shape of the applied wave may have an important effect on the experimental output. Of note, there is a substantial lack of standardization in studies where electrical stimulation of cultured cells and/or sliced tissue is performed [46]. Also, in some cases cardiomyocytes were placed in a bioreactor under continuous perfusion [47], thus making it difficult to define whether the reported increase in Cx43 expression depends purely on the electrical stimulus or may be the result of the combined electrical and mechanical stimulation which indeed has been shown to increase Cx43 expression. In addition, often no clear indication about the intensity (in V/cm) of the applied electric field is provided, thus preventing a direct comparison with previous results.
Of note, even if superimposed field stimulation was set at the same frequency of HL-1 cell intrinsic firing rate, it should be emphasized that spontaneous activity and field stimulation are different. In fact, spontaneous activity in cultured cells starts at one specific point on the periphery of the cell monolayer and propagates through gap junctions like a 2-dimensional wave (Supplementary Fig. 1) [48,49]. Conversely, field stimulation induces an almost instantaneous excitation across the cell monolayer and, consequently, pulse propagation does not occur. However, the intensity of the electric field necessary to induce the almost instantaneous excitation of all the cells is usually higher than the intensity required to perform a point stimulation in a cell monolayer [50]. Finally, we found that spontaneous activity in HL-1 cells sometimes stops for many seconds (Supplementary Fig. 1), while field stimulation was set to be continuous for the entire duration of the experiments.
Therefore, one limitation of the present study is that the observed decrease in Cx43 expression could be due to the use of an artificial (field) stimulation and might not have a role in in vivo normal pacemaker electrical activity. However, the application of abnormal external electrical pulses is widely used in clinical settings such as defibrillator and pace-maker implantation and is known to cause significant deterioration of the myocardial substrate. Importantly, the cardiac tissue damage caused by current flow has not only a fibrotic component [51,52], but also a junctional component, as demonstrated by different reports showing that ventricular tachypacing, either induced by direct ventricular activation [28] or by rapid atrial pacing [29], can cause gap junctional remodeling and cardiomyocyte uncoupling [53].
Therefore, in order to provide evidence that electrical activity dependent acetylation of Cx43 might also have patho-physiological relevance, we performed the analysis of Cx43 acetylation in an in vivo model of altered ventricular activation. To accomplish this goal, we used a canine model of tachycardia-induced cardiomyopathy. In agreement with previous reports [28,29], our confocal analysis confirmed that tachypacing caused extensive Cx43 reorganization and lateralization. This was associated with an increase in Cx43 acetylation, which has already been shown to be responsible for Cx43 loss from the plasma membrane in the mdx dystrophic heart mouse model [19]. Thus, our results provide for the first time a link connecting previously reported GJ remodeling in response to altered ventricular activation [28,29] to the acetylation/deacetylation balance of cardiomyocytes. Although the histone acetylase P300/CBP associated factor (PCAF) has been reported to be responsible for Cx43 acetylation [19], further experiments are needed to understand whether the same enzyme plays a major role in the context of electrical activity-dependent Cx43 acetylation. Of note, HL-1 cell gap junctions do not behave the same as cardiac gap junctions from primary atrial or ventricular cardiomyocyte cultures in response to inactivating or reactivating transjunctional voltage gradients or gap junction agonists [14], a difference that may be partially attributed to differences in the acetylation state of Cx43 in primary versus immortalized cardiomyocyte cell lineages.
5. CONCLUSIONS
In conclusion, the present work provides the proof of principle that the junctional remodeling resulting from altered electrical activity can be at least partly explained by alteration of the acetylation/deacetylation balance of cardiomyocytes. We show that electrical pulses can modulate Cx43 acetylation in cardiac cells both in vivo and in vitro, resulting in Cx43 down-modulation and intracellular relocalization. Our findings suggest that electrical activity-dependent alteration of protein acetylation might be a novel mechanism for the regulation of cardiomyocyte communication and, thus, support the hypothesis that HAT/HDAC modulators could play a role in new strategies for rhythm disturbance regulation.
Supplementary Material
Highlights.
Electrical activity modulates the acetylation/deacetylation balance of excitable cells.
Electrical activity modulates the acetylation status of Cx43.
Electrical activity modulates myocardial cell-cell communication through Cx43 acetylation.
Acknowledgments
6. FUNDING
This work was supported by the Italian Ministry of Health (Ricerca Corrente), by the Department of Educational Assistance, University and Research of the Autonomous Province of Bolzano, by the South Tyrolean Sparkasse Foundation, by Fondo di Ateneo per la Ricerca (FAR) and the US National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Dr. Mirko Prelogar for technical assistance in the initial set-up of pacing experiments.
Abbreviations
- GJ
gap junctions
- Cx43
connexin43
- HAT
histone acetylase
- HDAC
histone deacetylase
- CM
cardiomyocyte
- NRCM
neonatal rat cardiomyocyte
- hCStC
human cardiac stromal cell
Footnotes
8. CONFLICTS OF INTEREST
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. BIBLIOGRAPHY
- 1.McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. BioEssays. 1997;19:819–26. doi: 10.1002/bies.950190912. [DOI] [PubMed] [Google Scholar]
- 2.Guo A, Song B, Reid B, Gu Y, Forrester JV, Jahoda CA, et al. Effects of physiological electric fields on migration of human dermal fibroblasts. J Invest Dermatol. 2010;130:2320–7. doi: 10.1038/jid.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ET, Zhao M. Regulation of tissue repair and regeneration by electric fields. Chin J Traumatol. 2010;13:55–61. [PubMed] [Google Scholar]
- 4.Robinson KR, Messerli MA. Left/right, up/down: the role of endogenous electrical fields as directional signals in development, repair and invasion. BioEssays. 2003;25:759–66. doi: 10.1002/bies.10307. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–31. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riccio A. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci. 2010;13:1330–7. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- 7.Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–98. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- 8.Peserico A, Simone C. Physical and functional HAT/HDAC interplay regulates protein acetylation balance. J Biomed Biotechnol. 2011;2011:371832. doi: 10.1155/2011/371832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem. 2003;85:151–9. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, McMillin JB, Lewis A, Moore M, Zhu WG, Williams RS, et al. Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways and up-regulates the adenylosuccinate synthetase 1 gene. J Biol Chem. 2000;275:1855–63. doi: 10.1074/jbc.275.3.1855. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Q, Ni B, Shi J, Han Z, Qi R, Xu W, et al. Down-regulation of ATBF1 activates STAT3 signaling via PIAS3 in pacing-induced HL-1 atrial myocytes. Biochem Biophys Res Commun. 2014;449:278–83. doi: 10.1016/j.bbrc.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 12.McDonough PM, Glembotski CC. Induction of atrial natriuretic factor and myosin light chain-2 gene expression in cultured ventricular myocytes by electrical stimulation of contraction. J Biol Chem. 1992;267:11665–8. [PubMed] [Google Scholar]
- 13.Ivester CT, Kent RL, Tagawa H, Tsutsui H, Imamura T, Cooper Gt, et al. Electrically stimulated contraction accelerates protein synthesis rates in adult feline cardiocytes. Am J Physiol. 1993;265:H666–74. doi: 10.1152/ajpheart.1993.265.2.H666. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Gemel J, Glass A, Zemlin CW, Beyer EC, Veenstra RD. Connexin40 and connexin43 determine gating properties of atrial gap junction channels. J Mol Cell Cardiol. 2010;48:238–45. doi: 10.1016/j.yjmcc.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molica F, Meens MJ, Morel S, Kwak BR. Mutations in cardiovascular connexin genes. Biol Cell. 2014;106:269–93. doi: 10.1111/boc.201400038. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Wu CT, Qi X, Meijering RA, Hoogstra-Berends F, Tadevosyan A, et al. Activation of histone deacetylase-6 induces contractile dysfunction through derailment of alpha-tubulin proteostasis in experimental and human atrial fibrillation. Circulation. 2014;129:346–58. doi: 10.1161/CIRCULATIONAHA.113.005300. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima T, Ohkusa T, Okamoto Y, Yoshida M, Lee JK, Mizukami Y, et al. Rapid electrical stimulation causes alterations in cardiac intercellular junction proteins of cardiomyocytes. Am J Physiol Heart Circ Physiol. 2014;306:H1324–33. doi: 10.1152/ajpheart.00653.2013. [DOI] [PubMed] [Google Scholar]
- 18.Gupta MP, Samant SA, Smith SH, Shroff SG. HDAC4 and PCAF bind to cardiac sarcomeres and play a role in regulating myofilament contractile activity. J Biol Chem. 2008;283:10135–46. doi: 10.1074/jbc.M710277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colussi C, Rosati J, Straino S, Spallotta F, Berni R, Stilli D, et al. Nepsilon-lysine acetylation determines dissociation from GAP junctions and lateralization of connexin 43 in normal and dystrophic heart. Proc Natl Acad Sci U S A. 2011;108:2795–800. doi: 10.1073/pnas.1013124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claycomb WC, ALN, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger HJ, Prasad SK, Davidoff AJ, Pimental D, Ellingsen O, Marsh JD, et al. Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol. 1994;266:H341–9. doi: 10.1152/ajpheart.1994.266.1.H341. [DOI] [PubMed] [Google Scholar]
- 22.Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119:3435–42. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitacchione G, Powers JC, Grifoni G, Woitek F, Lam A, Ly L, et al. The gut hormone ghrelin partially reverses energy substrate metabolic alterations in the failing heart. Circ: Heart Failure. 2014;7:643–51. doi: 10.1161/CIRCHEARTFAILURE.114.001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brundel BJ, Kampinga HH, Henning RH. Calpain inhibition prevents pacing-induced cellular remodeling in a HL-1 myocyte model for atrial fibrillation. Cardiovasc Res. 2004;62:521–8. doi: 10.1016/j.cardiores.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Lin X, Andrews L, Patel D, Lampe PD, Veenstra RD. Histone deacetylase inhibition reduces cardiac connexin43 expression and gap junction communication. Front Pharmacol. 2013;4:44. doi: 10.3389/fphar.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–11. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- 27.Chung TH, Wang SM, Wu JC. 17beta-Estradiol Reduces the Effect of Metabolic Inhibition on Gap Junction Intercellular Communication in Rat Cardiomyocytes Via the Estrogen Receptor. J Mol Cell Cardiol. 2004;37:1013–22. doi: 10.1016/j.yjmcc.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Patel PM, Plotnikov A, Kanagaratnam P, Shvilkin A, Sheehan CT, Xiong W, et al. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12:570–7. doi: 10.1046/j.1540-8167.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhong JQ, Zhang W, Gao H, Li Y, Zhong M, Li D, et al. Changes in connexin 43, metalloproteinase and tissue inhibitor of metalloproteinase during tachycardia-induced cardiomyopathy in dogs. Eur J Heart Fail. 2007;9:23–9. doi: 10.1016/j.ejheart.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Shen W, Rottman JN, Wikswo JP, Murray KT. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J Mol Cell Cardiol. 2005;38:299–308. doi: 10.1016/j.yjmcc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Peng S, Lacerda AE, Kirsch GE, Brown AM, Bruening-Wright A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J Pharmacol Toxicol Methods. 2010;61:277–86. doi: 10.1016/j.vascn.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Dedkova EN, Blatter LA. Role of beta-hydroxybutyrate, its polymer poly-beta-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front Physiol. 2014;5:260. doi: 10.3389/fphys.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–4. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 35.Schild L, Bukowska A, Gardemann A, Polczyk P, Keilhoff G, Tager M, et al. Rapid pacing of embryoid bodies impairs mitochondrial ATP synthesis by a calcium-dependent mechanism--a model of in vitro differentiated cardiomyocytes to study molecular effects of tachycardia. Biochim Biophys Acta. 2006;1762:608–15. doi: 10.1016/j.bbadis.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuramochi Y, Guo X, Sawyer DB, Lim CC. Rapid electrical stimulation induces early activation of kinase signal transduction pathways and apoptosis in adult rat ventricular myocytes. Exp Physiol. 2006;91:773–80. doi: 10.1113/expphysiol.2006.033894. [DOI] [PubMed] [Google Scholar]
- 38.Mateo F, Vidal-Laliena M, Canela N, Busino L, Martinez-Balbas MA, Pagano M, et al. Degradation of cyclin A is regulated by acetylation. Oncogene. 2009;28:2654–66. doi: 10.1038/onc.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–74. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 40.Qian MX, Pang Y, Liu CH, Haratake K, Du BY, Ji DY, et al. Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell. 2013;153:1012–24. doi: 10.1016/j.cell.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Fang C, Zong NC, Liem DA, Cadeiras M, Scruggs SB, et al. Regulation of acetylation restores proteolytic function of diseased myocardium in mouse and human. Mol Cell Proteomics. 2013;12:3793–802. doi: 10.1074/mcp.M113.028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djakovic SN, Schwarz LA, Barylko B, DeMartino GN, Patrick GN. Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2009;284:26655–65. doi: 10.1074/jbc.M109.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nayak MK, Kumar K, Dash D. Regulation of proteasome activity in activated human platelets. Cell Calcium. 2011;49:226–32. doi: 10.1016/j.ceca.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Park JY, Jang SY, Shin YK, Suh DJ, Park HT. Calcium-dependent proteasome activation is required for axonal neurofilament degradation. Neural Regen Res. 2013;8:3401–9. doi: 10.3969/j.issn.1673-5374.2013.36.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue N, Ohkusa T, Nao T, Lee JK, Matsumoto T, Hisamatsu Y, et al. Rapid electrical stimulation of contraction modulates gap junction protein in neonatal rat cultured cardiomyocytes: involvement of mitogen-activated protein kinases and effects of angiotensin II-receptor antagonist. J Am Coll Cardiol. 2004;44:914–22. doi: 10.1016/j.jacc.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 46.Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G. Optimization of electrical stimulation parameters for cardiac tissue engineering. J Tissue Eng Regen Med. 2011;5:e115–25. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barash Y, Dvir T, Tandeitnik P, Ruvinov E, Guterman H, Cohen S. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods. 2010;16:1417–26. doi: 10.1089/ten.TEC.2010.0068. [DOI] [PubMed] [Google Scholar]
- 48.Miragoli M, Kadir SH, Sheppard MN, Salvarani N, Virta M, Wells S, et al. A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart. Hepatology. 2011;54:1282–92. doi: 10.1002/hep.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duverger JE, Boudreau-Béland J, Duc Le M, Comtois P. Multicellular automaticity of cardiac cell monolayers: effects of density and spatial distribution of pacemaker cells. New J Phys. 2014;16:113046. [Google Scholar]
- 50.Bien H, Yin L, Entcheva E. Calcium instabilities in mammalian cardiomyocyte networks. Biophys J. 2006;90:2628–40. doi: 10.1529/biophysj.105.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epstein AE, Kay GN, Plumb VJ, Dailey SM, Anderson PG. Gross and microscopic pathological changes associated with nonthoracotomy implantable defibrillator leads. Circulation. 1998;98:1517–24. doi: 10.1161/01.cir.98.15.1517. [DOI] [PubMed] [Google Scholar]
- 52.Mase H, Tamura K, Hiromoto A, Hotta M, Hotomi S, Togashi M, et al. Histopathological study of tissue reaction to pacemaker electrodes implanted in the endocardium. J Nippon Med Sch. 2005;72:52–9. doi: 10.1272/jnms.72.52. [DOI] [PubMed] [Google Scholar]
- 53.Kontogeorgis A, Kaba RA, Kang E, Feig JE, Gupta PP, Ponzio M, et al. Short-term pacing in the mouse alters cardiac expression of connexin43. BMC Physiol. 2008;8(8) doi: 10.1186/1472-6793-8-8. 6793-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.