Abstract
Tumor-associated macrophages (TAM) with an M2-like phenotype have been linked to tumor-elicited inflammation, immunosuppression, and resistance to chemotherapies in cancer, thus representing an attractive target for an effective cancer immunotherapy. Here, we demonstrate that particulate yeast-derived β-glucan, a natural polysaccharide compound, converts polarized M2 macrophages or immunosuppressive TAM into an M1-like phenotype with potent immuno-stimulating activity. This process is associated with macrophage metabolic reprograming with enhanced glycolysis, krebs cycle and glutamine utilization. In addition, particulate β-glucan converts immunosuppressive TAM via the C-type lectin receptor dectin-1-induced Syk-Card9-Erk pathway. Further in vivo studies show that oral particulate β-glucan treatment significantly delays tumor growth, which is associated with in vivo TAM phenotype conversion and enhanced effector T cell activation. Mice injected with particulate β-glucan-treated TAM mixed with tumor cells have significantly reduced tumor burden with less blood vascular vessels compared to those with TAM plus tumor cell injection. In addition, macrophage depletion significantly reduced the therapeutic efficacy of particulate β-glucan in tumor-bearing mice. These findings have established a new paradigm for macrophage polarization and immunosuppressive TAM conversion and shed the light on the action mode of β-glucan treatment in cancer.
Introduction
Macrophages are the major tumor-infiltrating leukocytes and play a critical role in cancer-related inflammation (1, 2). Depending on the different activation signals, macrophages may undergo polarized activation (3). Classically activated macrophages (M1) are characterized by elevated expression of MHC class II, expression of IL-12 and TNF-α, generation of reactive oxygen species (ROS) and nitric oxide (NO), and have tumoricidal activity. In contrast, macrophages alternatively activated (M2) have potent tumor-promoting activity. Macrophage polarization is also linked to differential metabolic programming (4). In the tumor microenvironment, most macrophages have an M2-like phenotype (5); they express low levels of MHC class II, IL-12 and TNF-α while expressing high levels of vascular endothelial growth factor (VEGF), arginase-1, and cyclo-oxygenase (COX-2)-derived prostaglandin E2 (PGE2) as well as the anti-inflammatory cytokine IL-10. Tumor immune evasion has been linked to a switch from M1 activation in the early stages of tumor initiation towards an M2-like phenotype during tumor progression, a process that highlights the heterogeneity and plasticity of macrophage activation and which offers a possible therapeutic target directed against repolarizing the TAM phenotype in the tumor (6, 7). Although using the M1/M2 model to describe macrophage polarization is a question of debate (7), clinical studies have demonstrated that high macrophage density correlates with poor patient prognosis (8–10). In human non-small cell lung carcinoma (NSCLC) patients, there is a strong association between poor survival and increased macrophage infiltration within the tumor microenvironment (11, 12). TAM also limit the efficacy of chemotherapeutic agents (13–15). These findings collectively suggest that targeting macrophages within the tumors may provide effective immunotherapy for cancer.
Natural product β-glucans have been investigated for their anti-tumor and anti-infective activity (16). Most β-glucans, which are derived from yeast, fungi, bacteria, or barley have a backbone structure of linear β-1, 3-linked D-glucose subunits (β-1,3-D-glucan). As a pattern recognition molecule, fungal β-glucans have been shown to trigger phagocytosis, generation of superoxide by the NADPH oxidase, and inflammatory cytokine production on macrophages (17–20). In addition, fungal β-glucan binds to its receptor dectin-1 to form a phagocytic synapse thus initiating direct cellular anti-microbial responses (21). On the contrary, dectin-1 activation by fungal ligand zymozan induces regulatory macrophage phenotype (22). However, it is unknown whether β-glucan has any effect on polarized macrophages in tissues such as TAM. In addition, it is unclear whether β-glucan stimulation alters macrophage metabolism.
In this study, we demonstrated that yeast-derived particulate β-glucan treatment converts polarized M2 bone marrow-derived macrophages (BMM) and immunosuppressive TAM to an M1-like phenotype leading to reduced tumor progression. This effect is associated with macrophage metabolic reprograming and mediated through the dectin-1-dependent canonical Syk-Card9-Erk pathway. Further in vivo studies showed that tumor-bearing mice orally administered with particulate β-glucan had significantly reduced tumor burden with converted TAM phenotype and enhanced effector T cell activation. These findings reveal an unprecedented effect of natural compound β-glucan on immunosuppressive macrophage conversion and tumor microenvironment modulation.
Materials and Methods
Mice and in vivo tumor models
Wildtype (WT) C57Bl/6 mice were purchased from the National Cancer Institute (NCI). Dectin-1-knockout (KO), CD11b KO, and Card9 KO mice were described previously (23, 24). OT-I and OT-II mice were purchased from Taconic. For WGP β-glucan treatment protocol, mice were implanted s.c. with LLC cells (2×105/mouse) or EO771 cells (6×105/mouse). On day 8 after palpable tumors formed, mice were treated with WGP β-glucan orally (800 µg/mouse) or 100 µL of PBS given every day using an intragastric gavage needle. WGP β-glucan was from Biothera (Eagan, MN). In some experiments, LLC cells were mixed with TAM (2.5:1) treated with or without WGP β-glucan and then injected into mice. For macrophage depletion protocol, mice were injected intravenously with 100 µl Clodronate (5 mg/ml, Clodrosome) one day prior to LLC subcutaneous inoculation. Mice were then injected with Clodronate weekly during the experiment. Tumor diameters were measured every third day and mice were euthanized when tumors reached 15 mm in diameter. Tumor volume was calculated by the following formula: length× wide2/2. For an in vivo imaging analysis, TAM were labeled with XenoLight DiR dye (PerkinElmer) while LLC were labeled with Vivo Track 680 (PerkinElmer). Mice were imaged with Spectral Ami (Spectral Instrument Imaging, LLC). The murine tumor protocols were performed in compliance with all relevant laws and institutional guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville.
Immunohistochemistry staining and Immunofluorescence staining
Formalin fixed, paraffin embedded (FFPE) human breast cancer tissue blocks corresponding to the reviewed cases were pulled from the University of Louisville’s Department of Pathology archives. Five micron sections from the FFPE tissue blocks were placed on glass slides. The slides were immunostained for anti-human CD68 (KP1; eBioscience), anti-CD163 (1:400; 10D6; Novacastra, Buffalo Grove, IL) or anti-HLA-DR (1:50; LN-3; Novacastra, Buffalo Grove, IL) via an automated system (Bond Max, Leica) using both the DAB-Bond Polymer Refine Detection System and the Red-Bond Polymer Red Detection System (Leica, Buffalo Grove, IL) according to manufacturer’s instructions. Appropriate positive and negative controls were used throughout the study.
Cryosections from fresh human lung cancer tissues or mouse tumor tissues were fixed with ice-cold acetone for 20 min. The slides were blocked with 5% BSA in PBS for 1 h and were subjected to incubation at 4°C overnight with the following primary Ab mixtures: biotin-anti-human CD68 (1:100), biotin-anti-CD163, Alexa 647-anti-HLA-DR (1:100) or biotin-anti-CD31 (1:100). Slides were washed and then incubated with streptavidin-Alexa fluor 488 conjugate (1:200) or streptavidin Alexa fluor 594 conjugate (1:200) for 90 min. The slides were co-stained with 4’,6-diamidino-2-phenylindole (DAPI) and mounted with fluoro-gel ( Electron microscopy science). Confocal images were acquired by Leica TCS SP5 confocal microscope system and quantitated by ImageJ software.
Macrophage polarization and TAM purification
BM cells were isolated from the femurs and tibias and resuspended in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were washed, counted, and then added (4×106) into 60 mm2 petri dishes. GM-CSF (50 ng/ml, BD) or M-CSF (100 ng/ml, Peprotech) was added to polarize the M1 or the M2 macrophages, respectively. The medium was changed on day 4. On day 7, adherent cells were used for the subsequent experiments. For some experiments, BM cells were cultured in complete DMEM containing 10 ng/ml M-CSF. The medium was changed on day 3 and cells were cultured for additional 3 days to generate M0 macrophages. On day 6, medium was changed with fresh DMEM containing 20 ng/ml IL-4 and IL-13. The cells were cultured for 2 days. These cells were designated as IL-4/IL-13-polarized M2 BMM.
For TAM purification, tumors (12–15 mm) were minced and then digested with buffer containing collagenase IV, hyaluronidase and DNase-I at 37°C for 30 min. Single cell suspensions were separated using 60% and 30% Percoll and the middle layer of cells was collected, washed, and resuspended in MACS running buffer. Cells were first blocked with Fc-blocking mAb for 15 min on ice and then stained with biotin-anti-mouse F4/80 Ab. The cells were washed and incubated with streptavidin microbeads on ice for 15 min. TAM were purified by AutoMACS separator (Miltenyi Biotec). These cells were CD11b+F4/80hi and the purity was ≥90% assessed by flow cytometry. In some experiments, these cells were further sorted based on Ly6C and MHC class II expression. Ly6C−MHCclassII−TAM were further sorted by FACSAria III.
In vitro TAM-T cell co-culture assay
TAM purified from LLC-bearing mice were treated with or without the Erk inhibitor PD98059 (30 µM) for 2 h and then washed and stimulated with WGP for 24 h. TAM were collected and co-cultured with CFSE-labeled splenocytes from OT-I or OT-II mice in the presence of OVA for 3 days. Cells were restimulated with PMA+ionomycin for 6 h and then stained with CD4 or CD8 mAbs, fixed and permeabilized for intracellular cytokine staining.
RNA microarray analysis and quantitative real-time PCR (qRT-PCR)
RNAs were extracted from polarized M2 BMM stimulated with or without WGP β-glucan for 6 h with a QIAGEN RNeasy kit (QIAGEN). Agilent oligonucleotide arrays were performed and analyzed at the James Graham Brown Cancer Center Microarray core facility, University of Louisville. Complete array data were deposited in a public database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE71814). For qRT-PCR analysis, RNA samples were transcripted into cDNA with a Reverse Transcription Kit (Bio-Rad). qRT-PCR was then performed on MyiQ single color RT-PCR detection system with SYBR Green Supermix (Bio-Rad). Primer sequences for each gene were as follows. IL-10, forward: 5’-AGTGGAGCAGGTGAAGAGTG-3’, reverse: 5’-TTCGGAGAGAGGTACAAACG-3’; Arginase, forward: 5’-CAGAAGAATGGAAGAGTCGA-3’, reverse: 5’-CAGATATGCAGGGAGTCACC-3’; IL-1β, forward: 5’-CCCAACTGGTACATCAGCAC-3’, reverse: 5’-TCTGCTCATTCACGAAAAGG-3’, IL-6, forward: 5’-TCCCCTCCAGGAGCCCAGCTA-3’, reverse: 5’-CAGGGCTGAGATGCCGTCGAG-3’; TNF-α, forward: 5’-ACCCACGGCTCCACCCTCTC-3’, reverse: 5’-CCCTCTGGGGGCCGATCACT-3’; iNOS, forward: 5’-AATAGAGGAACATCTGGCCAGG-3’, reverse: 5’-ATGGCCGACCTGATGTTGC-3’; IL-12p35, forward: 5’-CAGAATCACAACCATCAGCAG-3’, reverse: 5’-CACCCTGTTGATGGTCACGAC-3’. Gene expression was measured by the change-in-threshold (ΔΔCT), where ΔCt= Ct target gene−Ct House keeping gene and ΔΔCt= ΔCt induced−ΔCt reference. We normalized gene expression levels to house keeping genes as indicated.
Western blot (WB) analysis
For immunoblot analysis, BMM and TAM stimulated with or without WGP β-glucan were lysed in Triton X-100 lysis buffer containing protease and phosphatase inhibitors. In some experiments, the Syk inhibitor piceatannol (30 µg/ml, Sigma) was added. The whole cell extracts were separated by SDS-PAGE and electro-transferred to PDVF membrane. After blocking, the membranes were probed overnight at 4°C with appropriate primary Abs and then secondary Ab. The primary Abs included p-Erk1/2 (Thr202/Tyr204, Cell Signaling), Erk1/2 (MK1, Santa Cruz), p-Stat3 (Tyr705, Cell Signaling), p-AKT (Ser473, Cell Signaling), p-p38 (Thr180/Tyr182, Cell Signaling), and pZap/Syk (Try352, Cell Signaling). The blots were developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare).
Tracer treatment and SIRM Analyses
M2 BMM treated with or without WGP β-glucan (100 µg/ml) and M1 BMM were maintained for 25 h in DMEM supplemented with either 2 mM 13C5,15N2-Gln or 10 mM 13C6-Glc. Aliquots of the medium were taken at 0 and 25 h. The cells were rinsed in cold PBS, quenched in cold acetonitrile, extracted for metabolites and prepared for GC-MS, FT-ICR-MS, and NMR analysis described previously (25). NMR spectra were recorded at 14.1 T under standard acquisition conditions using 1D proton and 1D 1H{13C}-HSQC for isotopomer analysis. GC-MS was performed on a Thermo Finnigan Polaris™ instrument. Peaks were assigned and quantified as previously described (25).
Flow cytometry
Single cell suspensions were blocked in the presence of anti-CD16/CD32 for cells from mice at 4°C for 15 min and stained on ice with the appropriate antibodies and isotype controls in PBS containing 1% FBS. Flurochrome-labeled CD11b, CD45, F4/80, CD4, CD8, IFN-γ, and Foxp3 mAbs were purchased from Biolegend or eBiosciences. The samples were acquired using FACSCalibur or Canto II cytometer (BD Bioscience, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Data are expressed as means ± s.e.m. The unpaired Student t test was used to determine the significance of differences between M1 versus M2 or M2+WGP versus M2 datasets. To correct for multiple testing, the false discovery rate (FDR) q values were calculated according to the formula:
qi = pi*N/rank(i)
where pi is the uncorrected p value for the ith metabolite, N is the number of metabolites tested and rank(i) is the ordinal rank order of the p values. Significance was assumed to be reached at p<0.05. Statistical analysis was performed using Prism 5.0 (GraphPad Software).
Results
Macrophages with the M2 phenotype are the major constitutes of leukocytes within the tumor microenvironment
Previous studies have shown that macrophage infiltration in human breast cancer and NSCLC is associated with tumor invasion and progression (9, 12). As shown in Supplemental Fig. 1A, there were abundant macrophages infiltrated within the human breast cancer tissue (CD68+, brown). In humans, HLA-DR and CD163 are used to co-stain with CD68 to differentiate between M1 (CD68/HLA-DR double positive) and M2 macrophages (CD68/CD163 double positive) (26, 27). In human breast cancer tissues, macrophages co-stained with CD68 and CD163 (brown and red, respectively) but not with HLA-DR (Supplemental Fig. 1A). Similarly, we observed accumulation of macrophages with the M2 phenotype in the human NSCLC tumors (Supplemental Fig. 1B). Additionally, three murine tumor models were established including Lewis lung carcinoma (LLC), mammary carcinoma EO771 and B16 melanoma. Among CD45+ leukocytes infiltrated within the tumors, CD11b+ myeloid cells constituted the predominant population and F4/80+ macrophages were the significant infiltrated leukocytes (Supplemental Fig. 1C). These macrophages showed the typical M2 phenotype with potent immune suppressive function (Supplemental Fig. 1D and data not shown).
β-Glucan converts in vitro polarized M2 BMM into an M1-like phenotype via dectin-1 receptor
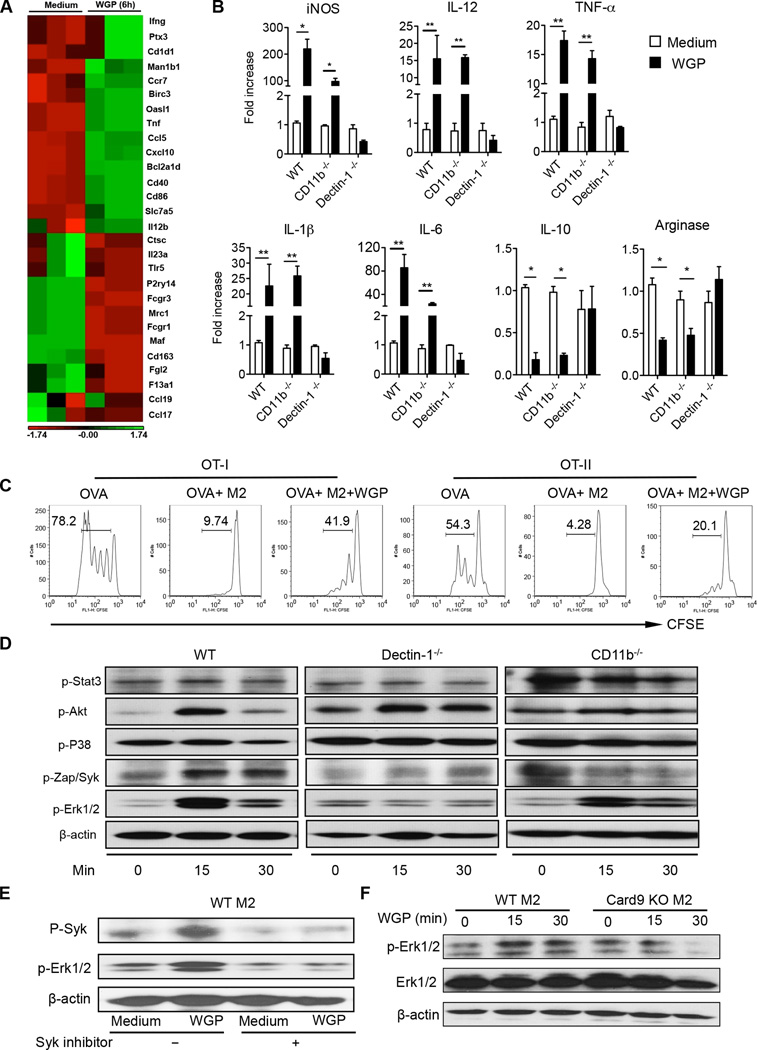
Because the majority of TAM is of the M2 phenotype, we first examined whether β-glucan treatment would alter the M2 macrophage phenotype. To this end, BMM were polarized into the M2 phenotype (28). We used a gene microarray to determine the gene expression changes in the M2 BMM after stimulation with yeast-derived particulate β-glucan WGP (whole glucan particles). As depicted in Fig. 1A, the expression of the conventional M2 marker genes including Cd163, Mrc1, Il-23a, Fcgr, Ccl17, Ccl19, and Maf were significantly downregulated upon WGP treatment. Conversely, the M1 markers, such as Il-12, Ifng, Cd40 and Cd86 were up-regulated after stimulation. β-Glucan treatment also decreased the mRNA levels of arginase I and IL-10 whereas it increased mRNA levels of iNOS, IL-12, TNF-α, IL-1β and IL-6 (Fig. 1B). We also used IL-4/IL-13 protocol to polarize M2 BMM. Similarly, β-glucan treatment promoted M1 signature gene expression including iNOS, IL-12p35, TNF-α, IL-1β and IL-6 while it down-regulated IL-10 and arginase mRNA expression levels (data not shown). These effects were mediated through the dectin-1 pathway and were independent of CD11b. Next, we examined whether β-glucan treatment alters M2 suppressive function. To this end, splenocytes from OT-I and OT-II mice were CSFE labeled and then stimulated with OVA in the presence of M2 BMM treated with or without particulate β-glucan. As shown in Fig. 1C, M2 indeed showed potent immunosuppressive activity on both CD4 and CD8 T cell proliferation. Particulate β-glucan treatment significantly abolished M2-mediated immunosuppression, suggesting that M2 macrophages are converted both phenotypically and functionally upon β-glucan in vitro treatment. In contrast, WGP treatment had minimal effect on M1 BMM (Supplemental Fig. 1E).
Figure 1. β-Glucan treatment converts M2 macrophage characteristic gene expression and its immunosuppressive function and activates Syk/Erk in a dectin-1-dependent manner.
(A) Polarized M2 BMM from WT mice (n=3) were treated with WGP β-glucan for 6 h. The total RNAs were extracted for the microarray analysis. (B) mRNA expression levels of specific genes as indicated in polarized M2 BMM from WT, CD11b−/− or dectin-1−/− mice upon stimulation with WGP β-glucan by quantitative real-time (qRT) PCR. (C) Splenocytes from OVA Tg OT-I and OT-II mice were labeled with CFSE and then stimulated with OVA in the presence of polarized M2 BMM (ratio 1:20) with or without WGP β-glucan treatment. Histogram shows cell proliferation. (D) Polarized M2 BMM from WT, CD11b−/− or Dectin-1−/− mice were stimulated with WGP β-glucan at indicated time points. Cells were lysed and extracted proteins were probed with Abs to p-Stat3, p-Akt, p-P38, pZap/Syk, p-Erk1/2, and β-actin. (E) M2 BMM were stimulated with WGP β-glucan in the presence or absence of the Syk inhibitor. The expression of p-Syk and p-Erk1/2 was determined by WB. (F) M2 BMM from WT or Card9 KO mice were treated with or without WGP β-glucan at indicated time points. Lysates were immunoblotted with p-Erk1/2, Erk1/2, and β-actin Abs. Data are representative of three independent experiments with similar results. *P<0.05, **P<0.01.
Previous studies have shown that the dectin-1 pathway signals via Syk kinase to recruit and activate CARD9/Bcl10 and subsequently the p65/p50 pathway and Malt1 (29). WGP stimulation induced phosphorylation of Syk, Akt, and Erk1/2 dependent of dectin-1 receptor but did not induce phosphorylation of STAT3 or p38 (Fig. 1D). To investigate whether Erk is downstream of the canonical dectin-1-Syk/Card9 pathway, the Syk inhibitor and Card9 KO BMM were used. The phosphorylation of Syk and Erk1/2 stimulated by WGP β-glucan was abolished by the Syk inhibitor, suggesting Erk phosphorylation is downstream of Syk activation (Fig. 1E). In addition, β-glucan-induced Erk phosphorylation was abolished in Card9 KO mice (Fig. 1F). These data suggest that particulate β-glucan is capable of converting M2 BMM into an M1-like phenotype via the dectin-1/Syk/Card9/Erk signaling pathway.
M1 versus M2 BMM display distinct metabolism and β-glucan-treated M2 BMM exhibit M1-like metabolic activity
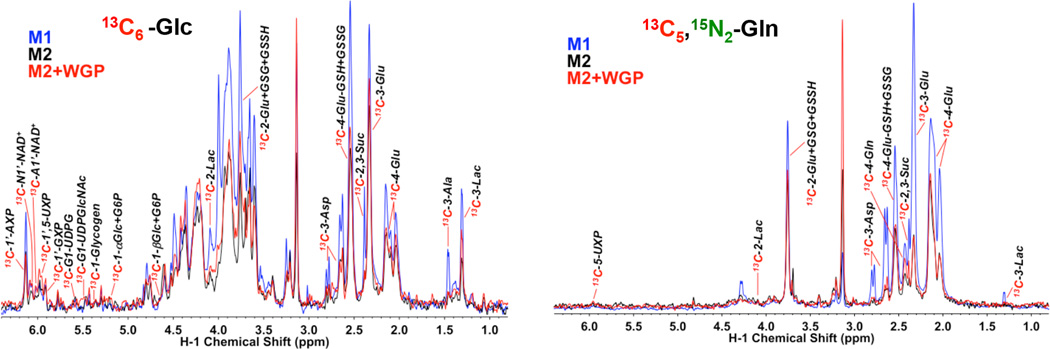
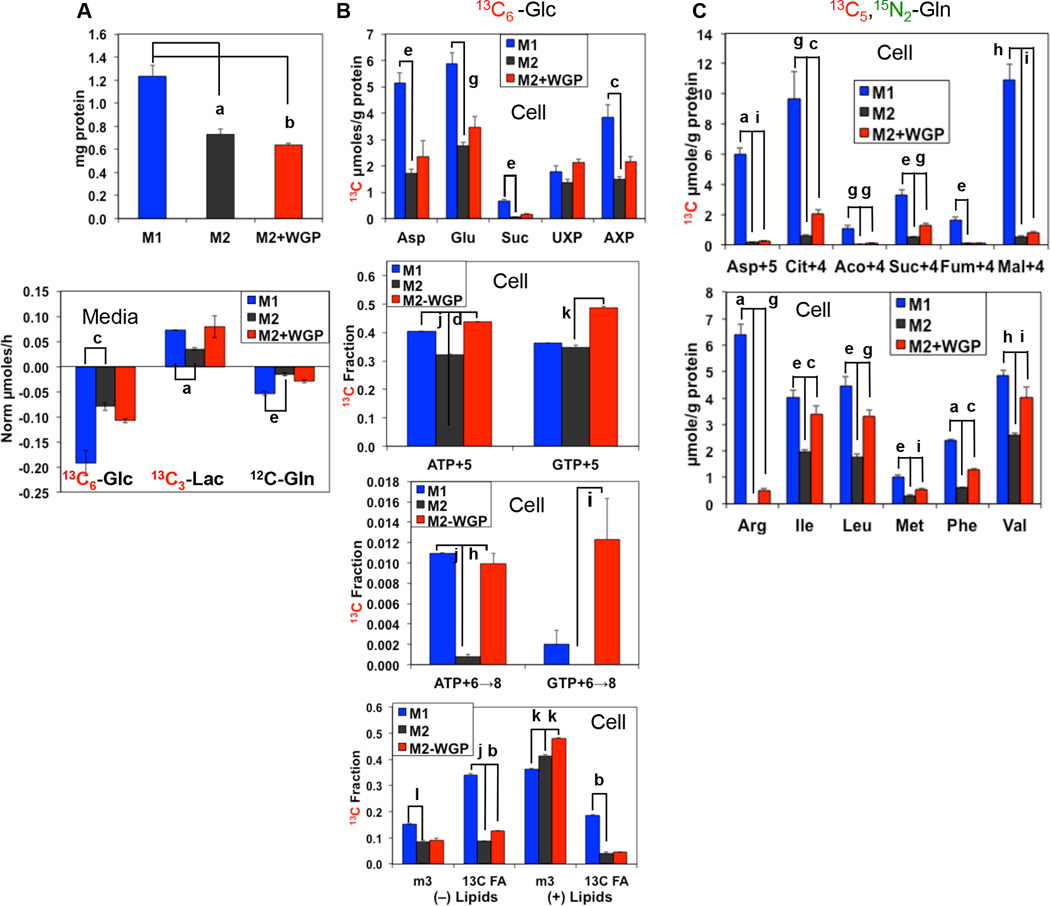
Increasing evidence indicates that macrophage activation and function are controlled by cellular metabolism (30). As the M1 and M2 macrophages are functionally distinct and may involve altered expression of genes that regulate metabolism, we thus employed the Stable Isotope Resolved Metabolomics (SIRM) approach by using uniformly 13C-labeled glucose (13C6-Glc) and 13C-/15N-labeled glutamine (13C5,15N2-Gln) as tracers, followed by nuclear magnetic resonance (NMR) and mass spectrometry (MS) analysis of isotopic labeling patterns of various metabolites to enable the reconstruction of altered metabolic networks induced by M2/M1 polarization and β-glucan activation (31, 32). M1 and M2 BMM exhibited distinct Glc and Gln metabolism via glycolysis, the Krebs cycle, the pentose phosphate pathway (PPP), and the nucleotide/glutathione/lipid biosynthetic pathways (Fig. 2). Compared to the M2 BMM, M1 exhibited elevated Glc metabolism, based on the 1H NMR analysis of increased consumption of medium 13C6-Glc and increased release of triply 13C-labeled lactate (13C3-Lac) into the medium; the latter indicates elevated glycolytic activity (Fig. 3A, bottom panel). Gln consumption was also accelerated in M1 macrophages. Subsequent 13C6-Glc oxidation by the Krebs cycle and incorporation into nucleotides and glutathiones was enhanced in M1 macrophages, as revealed by the heteronuclear single quantum coherence (HSQC) NMR analysis (Fig. 2 and Fig. 3B, top panel). Moreover, gas chromatograph (GC)-MS and fourier transform ion cyclotron (FT-ICR)-MS analysis of the same sets of cell extracts both corroborated and complemented the NMR analysis in revealing enhanced PPP/nucleotide biosynthesis activity (Fig. 3B, middle two panels) and increased lipid biosynthesis (Fig. 3B, bottom panel) as well as enhanced oxidation of 13C5,15N2-Gln via glutaminase and the Krebs cycle (Fig. 3C, top panel and cf. Supplemental Fig. 2 for pathway tracing). These metabolic activation events are consistent with a higher capacity of M1 macrophages for expansion, as evidenced by their elevated protein levels (Fig. 3A, top panel) and essential amino acid content (Fig. 3C, bottom panel). Although WGP β-glucan treatment did not stimulate M2 macrophage proliferation, it activated many of the metabolic events as occurring in M1 macrophages (Fig. 3A–C). In addition, the accumulation of arginine in M1 and WGP β-glucan-treated M2 BMM (Fig. 3C, lower panel) is consistent with the suppression of arginase in these macrophages (Fig. 1B). Thus, it is likely that these events are important to M1 activation or converting the immunosuppressive property of M2 macrophages by β-glucan.
Figure 2. M1 and WGP β-glucan activated M2 BMM display elevated 13C incorporation into glycolytic and Krebs cycle metabolites from labeled Glc and Gln.
M1, M2, or WGP-treated M2 BMM extracts were analyzed by 1D 1H{13C} HSQC NMR as described in the Materials and Methods. The HSQC analysis compared the peak intensity of protons attached to 13C atoms (akin to 13C abundance) at specific positions of various metabolites. Compared to M2 BMM, M1 BMM exhibited elevated activity of glycolysis, Krebs cycle, glutathione synthesis, and nucleotide synthesis, as evidenced respectively by the increased 13C abundance of cellular lactate (Lac; cf. also Figure 3 for medium lactate), Asp/Glu/succinate (Suc), adenine nucleotides (AXP), and glutathione (GSH)/glutathione disulfide (GSSG) derived from these pathways using Glc or Gln as precursor (cf. Figure S2). WGP-treated M2 macrophages also displayed elevated activity of glycolysis and Krebs cycle over untreated M2 macrophages but not in nucleotide and glutathione biosynthesis. 1’-AXP, -GXP, and -UXP: 1’-ribose of adenine, guanine, and uracil nucleotides, respectively.
Figure 3. Glycolysis, Krebs cycle and associated anabolic activities are enhanced in M1 and β-glucan-activated M2 macrophages.
M1, M2, or WGP-treated M2 BMM were co-cultured with labeled tracers, and the polar extracts of both cells and media prepared before analysis by 1D 1H NMR (A, bottom panel), 1H{13C} HSQC NMR (B, top panel), GC-MS (C), and FT-ICR-MS (B, lower three panels), as described in the Materials and Methods. The total soluble proteins were also analyzed in these cells (A, top panel). The medium metabolite data were calculated as µmoles/h and normalized to that of Val, the HSQC and GC-MS quantification of cellular metabolites was expressed as µmoles/g protein, and the FT-ICR-MS data were calculated as fraction of the total metabolite level. ATP+5 and GTP+5 represent 13C5-isotopologues of ATP and GTP, i.e. fully 13C labeled in the ribose unit; ATP+6→8 and GTP+6→8 represent 13C6→8-isotopologues of ATP and GTP, i.e. fully 13C labeled in the ribose unit plus 1–3 13C in the ring; m3 and 13C FA are lipids containing 13C3 (fully 13C labeled glycerol backbone) and 13C labeled fatty acyl chains, respectively; Asp+5, Cit+4, Aco+4, Suc+4, Fum+4, and Mal+4 are respectively the 13C415N1 isotopologue of Asp and the 13C4 isotopologues of citrate, cis-aconitate, succinate, fumarate, and malate. Fractional enrichment in 13C FA of lipids was obtained by summing the fractions of lipid isotoplogues with even number (FA only) and >3 odd number (FA+glycerol backbone) of 13C atoms; (−) charged lipids include phosphatidylserines, phosphatidylglycerols, and phosphatidylinositols while (+) lipids are composed of phosphatidylethanolamines (PE), phosphatidylcholines (PC), plasmalogens of PE and PC, sphingomyelins, and triacylglycerides. Data for M1 versus M2 or M2+WGP versus M2 were statistically compared using unpaired t-test with correction for FDR (q values). a: q≤0.0005; b: q≤0.0001; c: q<0.02; d: q<0.00001; e: q<0.005; f: q<0.00002; g: q≤0.01; h: q<0.002; i: q≤0.05; j: q<0.000005; k: q≤0.001; l: q<0.0002.
β-Glucan treatment repolarizes immunosuppressive TAM phenotypically and functionally
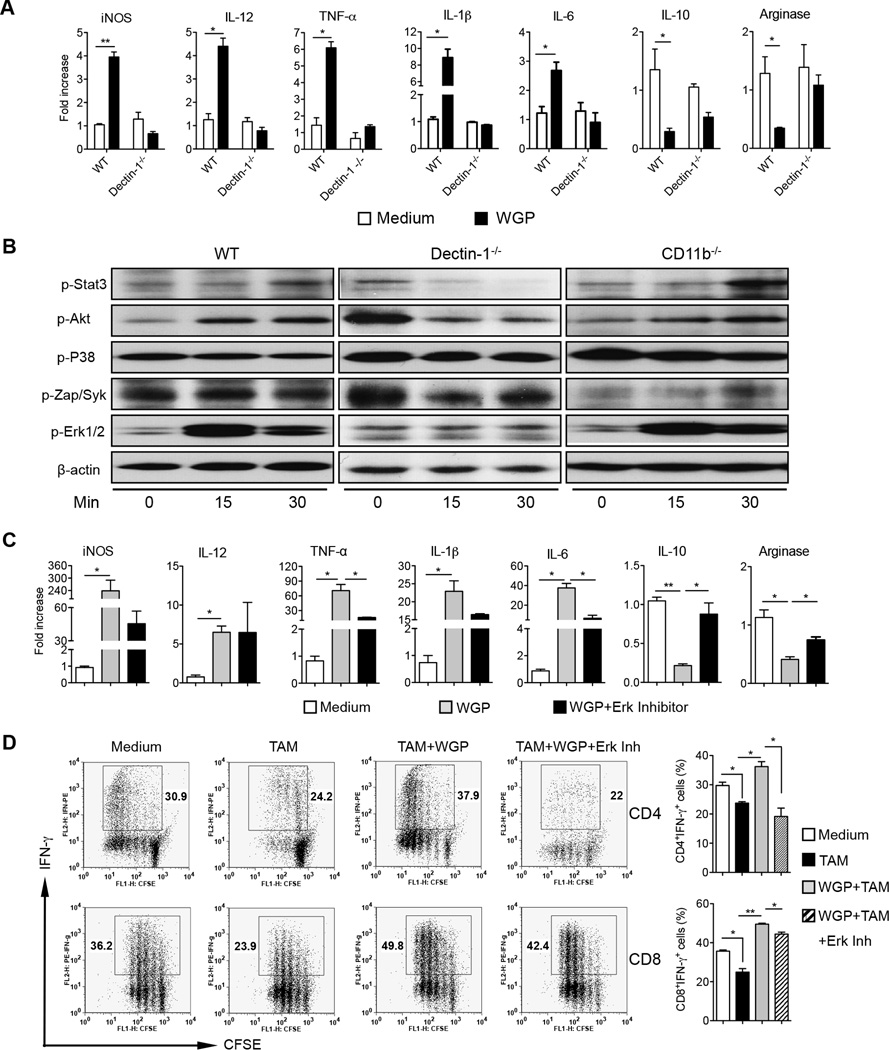
We next analyzed whether β-glucan treatment is capable of modulating TAM to reverse their immunosuppressive activity. F4/80hi TAM obtained from LLC-bearing mice (WT and dectin-1−/−) were stimulated with WGP β-glucan. Similar to polarized M2 BMM, β-glucan treatment significantly downregulated the mRNA levels of arginase and IL-10 while it upregulated the mRNA levels of iNOS, IL-12, TNF-α, IL-1β and IL-6 in a dectin-1 receptor dependent manner (Fig. 4A). Since TAM are heterogeneous populations, we further sorted F4/80+MHC classII−Ly6C−CD11b+PDL-1lo TAM which previously were shown to be associated with enhanced M2 marker gene expression (33). WGP treatment significantly decreased the mRNA levels of IL-10 and arginase while it upregulated iNOS, IL-12, TNF-α, IL-1β and IL-6 mRNA expression levels (Supplemental Fig. 3). In addition, WGP stimulated Erk1/2 phosphorylation via the dectin-1 receptor (Fig. 4B). To further study the Erk signaling pathway in β-glucan-mediated TAM repolarization, TAM were treated with or without Erk inhibitor in the presence of WGP β-glucan treatment. The mRNA levels of TNF-α, IL-6, IL-10, and arginase were significantly affected by the Erk inhibitor treatment (Fig. 4C). However, the Erk inhibitor did not show any effect on IL-12 mRNA expression levels.
Figure 4. WGP β-glucan treatment alters TAM phenotype and immunosuppressive activity on T cell responses partly through the dectin-1-Erk pathway.
(A) TAM purified from LLC tumors in WT or dectin-1 KO mice were stimulated with WGP β-glucan (150 µg/mL) for 24 h. Total RNAs were extracted and qRT-PCR analysis were performed. (B) TAM from LLC-tumor bearing WT, CD11b−/− or dectin-1−/− mice were stimulated with WGP β-glucan at indicated time points. Cells were lysed and extracted proteins were probed with Abs to p-Stat3, p-Akt, p-P38, pZap/Syk, p-Erk1/2, and β-actin. Data are representative of three experiments. (C) TAM purified from LLC-bearing mice were treated with or without the Erk inhibitor PD98059 for 2 h and then stimulated with WGP β-glucan for 24h. RNAs were extracted and qRT-PCR was performed for the indicated genes. (D) TAM isolated from LLC-bearing mice were treated with or without Erk inhibitor PD98059 for 2 h and then stimulated with WGP β-glucan for 24 h. Cells were harvested and co-cultured with CFSE-labeled splenocytes from CD4 or CD8 OVA Tg mice in the presence of OVA. Splenocytes alone with the Erk inhibitor were used as controls. Graphs show CFSE dilution versus intracellular IFN-γ on day 3 of culture. Percent of CD4+IFN-γ+ or CD8+IFN-γ+ cells is shown. * P<0.05, ** P<0.01.
To determine the functional activity of β-glucan-treated TAM, TAM treated with or without WGP β-glucan were co-cultured with CFSE-labeled splenocytes from OVA CD4 or CD8 TCR Tg mice. As depicted in Fig. 4D, TAM significantly inhibited IFN-γ production by CD4 and CD8 T cells. β-Glucan in vitro treatment completely abrogated TAM-induced CD4 and CD8 T cell suppression and even induced augmented CD4 and CD8 T cell proliferation and IFN-γ production as compared to the Ag alone-stimulated CD4 and CD8 T cell responses (Fig. 4D). The Erk inhibitor completely abrogated β-glucan-induced CD4 T cell response while partly inhibited CD8 T cell activation. Taken together, these data suggest that particulate β-glucan WGP not only converts TAM phenotypically but also alters TAM functionally to induce potent Ag-specific T cell responses partly via the dectin-1-Erk pathway.
WGP β-glucan in vivo treatment reduces tumor burden and polarizes the TAM phenotype
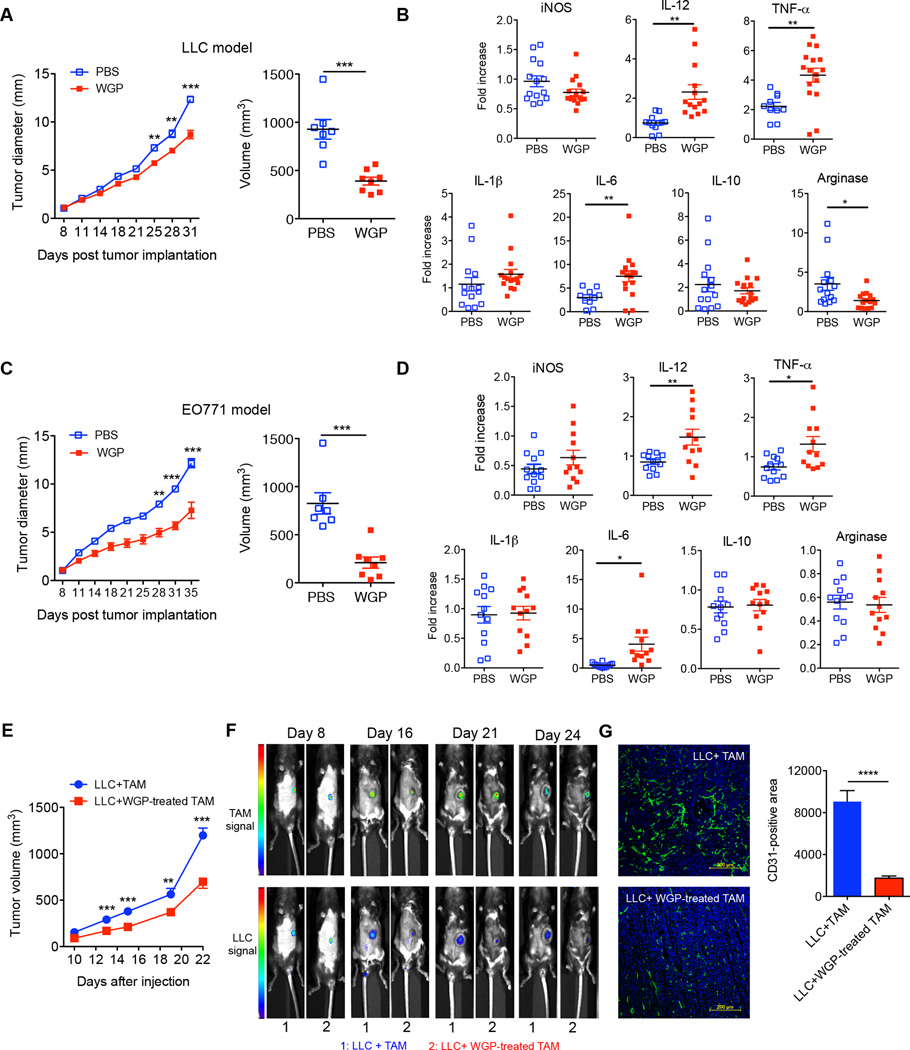
To determine the in vivo effect of β-glucan treatment on TAM polarized activation, tumor-bearing mice were daily administered orally either with PBS or WGP. Mice treated with WGP had a significantly decreased tumor burden compared with the mice treated with PBS (Fig. 5A, 5C). We next analyzed the phenotype of freshly isolated TAM. In the LLC tumor model, the mRNA level of arginase in TAM was significantly decreased in β-glucan-treated mice while the mRNA levels of IL-12, TNF-α, and IL-6 were significantly increased (Fig. 5B). The mRNA level of IL-10 showed decreasing trend in TAM of WGP-treated mice. Similarly, the mRNA levels of IL-12, TNF-α, and IL-6 in TAM significantly increased in EO771 tumor model upon β-glucan treatment (Fig. 5D) although the mRNA levels of arginase and IL-10 were not significantly altered. We further examined T cell responses in these mice and found that the frequency of CD4+Foxp3+Treg cells both in tumor and spleen tended to decrease in WGP treated mice whereas IFN-γ-producing CD4 T cells were significantly increased (Supplemental Fig. 4A). WGP treatment also significantly promoted CD8+ IFN-γ–producing T cells in the tumor milieu (Supplemental Fig. 4B). These results suggest that the anti-tumor efficacy of β-glucan could be mediated via its effect on the TAM.
Figure 5. WGP β-glucan treatment significantly reduces tumor burden with altered TAM phenotype.
(A) Groups of WT mice (n=7,8) were implanted s.c. with LLC tumor cells. When tumors were palpable, mice were fed with WGP β-glucan or PBS control for 3 wks. Tumor diameter was recorded at the indicated time. Tumor volumes for the last timepoint were also shown. (B) TAM purified from LLC tumor-bearing mice treated with or without WGP β-glucan from two independent protocols were assayed for specific gene mRNA expression levels determined by qRT-PCR. Each dot corresponds to one TAM sample sorted from one tumor. (C) Groups of WT mice (n=7, 8) were implanted s.c. with EO771/OVA tumor cells. After palpable tumors formed, mice were treated daily with or without WGP β-glucan for 3 wks. Tumor diameter was recorded at the indicated time. Tumor volumes for the last timepoint were also shown. (D) TAM isolated from EO771-tumor bearing mice treated with or without WGP β-glucan from two independent protocols were assayed for specific gene mRNA expression levels determined by qRT-PCR. (E) TAM treated with or without WGP β-glucan were mixed with LLC tumor cells and then injected into mice (n=6, 7). Tumor progression was monitored. (F) Tumor-bearing mice were imaged for tumor or TAM signals using different wavelengths at indicated time points. (G) Tumors from LLC plus TAM treated with or without WGP β-glucan were sectioned and stained with anti-CD31 (green) and DAPI to reveal nuclei. CD31+ areas (n=6) were quantitated by ImageJ software. Scale bar: 200 µm. * P<0.05, ** P<0.01, **** P<0.0001.
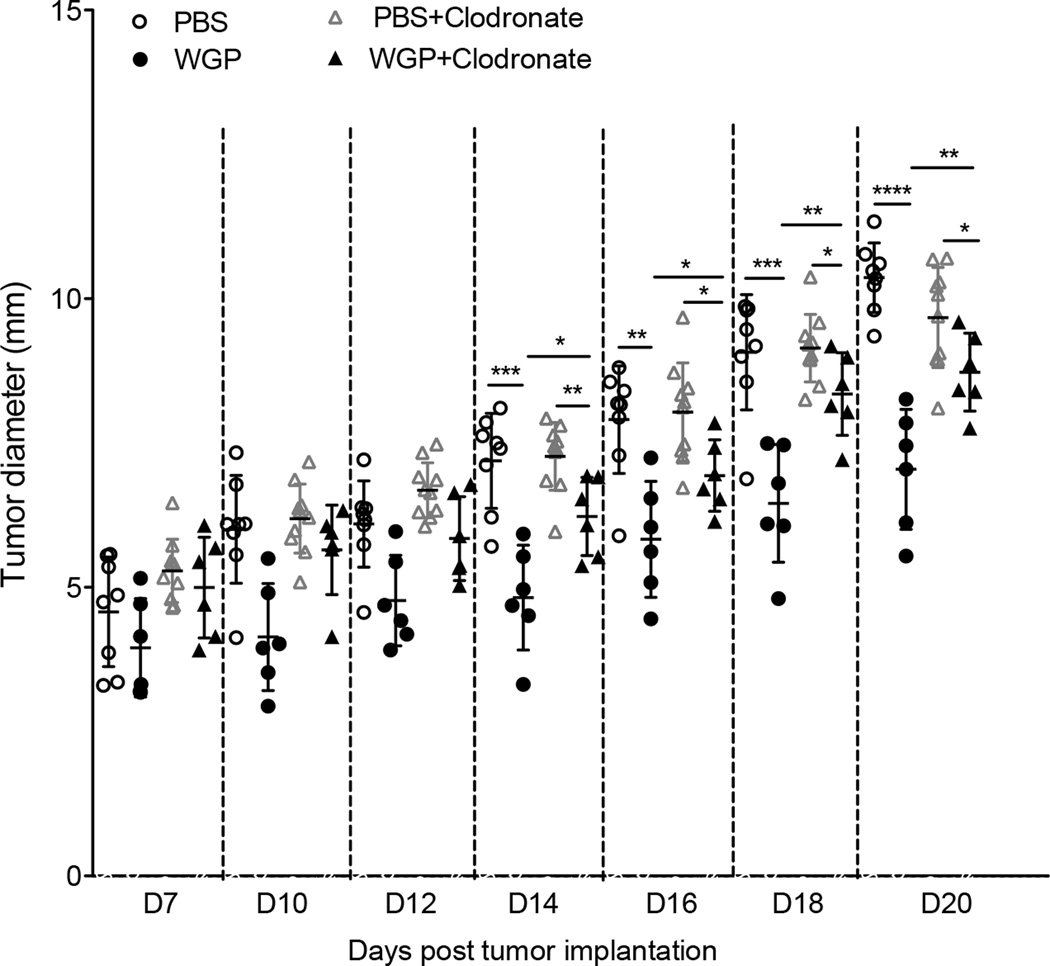
To further support this notion, we mixed LLC tumor cells with TAM treated with or without β-glucan and then injected into mice. Mice injected with LLC plus TAM had significant tumor progression as compared to tumors in mice injected with LLC and β-glucan-treated TAM (Fig. 5E). This was also shown by an in vivo imaging analysis (Fig. 5F). We also observed that tumors from LLC plus TAM had more vascular structure as revealed by CD31 staining (Fig. 5G). Since previous studies have shown that particulate β-glucan treatment also activates DCs and reverses myeloid-derived suppressor cells (MDSC) (23, 34), we thus examined the relative contribution of TAM conversion in the setting of β-glucan treatment by depleting macrophages. Mice were injected with Clodronate to deplete macrophages prior tumor inoculation. As shown in Fig. 6, tumor-bearing mice received WGP treatment showed significant reduced tumor progression regardless of macrophage depletion. However, mice with macrophage depletion showed significant increased tumor burden upon WGP treatment compared to those without macrophage depletion (Fig. 6). Overall, these results are consistent with the view that converting TAM phenotype and function is, at least in part, responsible for the antitumor activity of β-glucan.
Figure 6. Depletion of macrophages significantly reduces WGP β-glucan-mediated therapeutic efficacy.
Groups of C57Bl/6 mice (n=6–8) were injected intravenously with 100 µl Clodronate (5mg/ml) one day prior to LLC subcutaneous inoculation. Mice were then injected with Clodronate weekly during the experiment. Mice without Clodronate injection were used as controls. When the tumor sizes reached 5 mm in diameter, mice were treated with WGP β-glucan or control PBS daily via oral gavage. Tumor diameters were measured every other day and mice were euthanized when tumors reached 15 mm in diameter. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001.
Discussion
Macrophages can be polarized into extreme M1 or M2 phenotype depending on the environmental cues. Although macrophage phenotype within the tumor microenvironment is more complicated, it is clear that TAM phenotypically more resemble M2 macrophages with potent immunosuppressive activity, thus representing attractive targets for cancer immuno-therapeutics (35, 36). However, efforts to steer TAM functions are still in their infancy (37). Currently, strategies to target TAM include depletion or blocking of recruitment (38–40) and decreasing M2-like TAM via re-education (41–43). These approaches employ chemotherapeutic drugs, Abs, or small molecule inhibitors, which may cause unwanted adverse effect. In addition, some of these approaches may impact all macrophage subsets including M1-like macrophages. Here, we showed that a natural product yeast-derived β-glucan treatment converts the immunosuppressive M2 and TAM toward the M1 anti-tumor phenotype. Particulate β-glucan can be administered orally with proven safety profile and low cost.
We found that in vitro treatment of M2 BMM with β-glucan reverses M2 phenotype characterized as increased expression of IL-12, iNOS, TNF-α, IL-6, and IL-1β and downregulation of IL-10 and arginase in mice. This is regardless of different M2 polarization protocols. In addition, this conversion is accompanied by metabolic reprogramming to the M1 metabotype, particularly in terms of enhanced glycolysis, Krebs cycle, and glutamine utilization, which can fuel both energy and anabolic demands for activating macrophages. Although a previous study showed that macrophages with different activation signals exhibited different metabolic profiles using a [1,2-13C2]glucose tracer-based metabolomics approach (44), our studies demonstrated that glutamine uptake and subsequent metabolism to glucose and glutathione were enhanced when M2 BMM were converted into an M1-like phenotype using the SIRM technology. Stable isotope tracing at the atomic level enables the determination of the flow of atoms and groups of atoms from a precursor such as 13C glucose or 13C,15N glutamine through metabolic pathways, thereby discriminating which branches of a pathway differ between different cells such as M1 versus M2 macrophages. We found that glycolysis and associated anabolic activities are enhanced in M1 and β-glucan activated M2 BMM. As tumor cells are the major nutrient consumer within the tumor microenvironment (45), this limits nutrients for other cells such as macrophages. This may be one of the mechanisms by which tumors polarize TAM into M2-like phenotype. Indeed, a recent study showed that lactic acid secreted by tumors via anaerobic glycolysis induces M2-like TAM (46).
Similarly, we demonstrated that immunosuppressive TAM from tumor-bearing mice are also phenotypically and functionally altered upon β-glucan treatment. We showed that TAM-mediated inhibition of CD4 and CD8 T cell responses is completely reversed upon β-glucan treatment in vitro. Further in vivo studies demonstrate that β-glucan treatment significantly decreases tumor burden and alters TAM phenotype, which correlates with more IFN-γ production by T cells and less Treg infiltration. It is worth noting that TAM contain heterogeneous populations and the relative percentage of different TAM subsets changes as tumor progresses (33). Clearly, we showed that β-glucan treatment significantly alters M2-like TAM phenotypically and functionally. However, it needs further investigation whether different subsets of TAM could be differentially affected by β-glucan treatment.
C-type lectin receptor dectin-1 has been identified as the main receptor for particulate β-glucan binding and signaling (29). We showed that WGP β-glucan-mediated M2 and TAM conversion is dependent on the dectin-1 receptor. Upon β-glucan stimulation, dectin-1 can directly recruit and activate Syk kinase (47, 48). Subsequently Syk triggers Card9 recruitment to form Card9/Bcl10/Malt-1 complex that activates the IκB kinase complex for NF-κB signaling (29). We found that the Erk inhibition partly abolishes β-glucan-mediated TAM phenotype conversion. Interestingly, glucose assumption and lactate production in macrophages are also associated with the Erk1/2 signaling (49). Although the Erk inhibitor completely abrogates β-glucan-treated TAM-mediated CD4 T cell proliferation and IFN-γ production, CD8 T cell activation is only partially affected by the Erk inhibitor, suggesting differential TAM cytokine profiles regulated by the dectin-1-Erk signaling. Previous studies have demonstrated that the dectin-1 signaling activates p38, Erk and JNK cascade and NFAT (50, 51). Although activation of p38 and JNK via the receptor Nod2 has been associated with Card9 (52), it is unknown whether Erk activation is dependent on Card9. We showed that Erk phosphorylation induced by β-glucan is dependent on Card9 as Erk activation by β-glucan is completely abolished in Card9-deficient mice. These data suggest that the canonical dectin-1-Syk-Card9-Erk pathway is partially involved in β-glucan-mediated M2 BMM and TAM conversion. It still needs to be explored whether other pathway(s) is involved in this effect.
Previous studies have shown that β-glucan treatment activates DCs (23) or reduces myeloid-derived suppressor cells (34) in tumor-bearing mice, thus eliciting enhanced anti-tumor immune responses. Since TAM are the major constitutes within the tumor milieu, findings that β-glucan treatment converts TAM immunosuppressive function are significant. Indeed we found that macrophage depletion in tumor-bearing mice significantly reduced β-glucan-mediated anti-tumor therapeutic efficacy. There are several important clinical implications of this study. First, our data have established a new paradigm for macrophage polarization and immunosuppressive TAM conversion by a natural compound β-glucan. As TAM limit chemotherapeutic drug efficacy, oral β-glucan administration can be used as immuno-adjuvant therapy for cancer patients to modulate TAM phenotype and then be combined with chemotherapeutic agents. This may turn chemo-resistant tumors into chemo-sensitive status. Second, since TAM are an important player in establishing tumor immunosuppressive network, targeting TAM by β-glucan will provide additional benefit to improve the efficacy of other cancer immunotherapy such as adoptive T cell therapy or cancer vaccines. Collectively, this study provides a novel way to engage immunosuppressive TAM for maximizing cancer therapeutic efficacy.
Supplementary Material
Acknowledgement
We thank J. Tan and R. Balasubramaniam for performing protein measurement and assistance in the GC-MS analysis.
This work was supported by the NIH R01CA150947, P01CA163223, 1U24DK097215, NSF/EPSCoR EPS-0447479, and the Kentucky Lung Cancer Research Program.
References
- 1.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 2.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, Lin A, Adeyanju OO, Li S, Gong C, McGrath M, Olopade OI, Esserman LJ. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dannenmann SR, Thielicke J, Stockli M, Matter C, von Boehmer L, Cecconi V, Hermanns T, Hefermehl L, Schraml P, Moch H, Knuth A, van den Broek M. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. doi: 10.4161/onci.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, Chou CL, Chen HC, Lin SM, Kuo HP. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131:E227–E235. doi: 10.1002/ijc.27403. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, Liu S, Qin Y, Chen H. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. 2011;74:188–196. doi: 10.1016/j.lungcan.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 13.De Palma M, Lewis CE. Cancer: Macrophages limit chemotherapy. Nature. 2011;472:303–304. doi: 10.1038/472303a. [DOI] [PubMed] [Google Scholar]
- 14.Denardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 Blocks CD8(+) T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Gunn L, Hansen R, Yan J. Combined yeast-derived beta-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp Mol Pathol. 2009;86:208–214. doi: 10.1016/j.yexmp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J Exp Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF, Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One. 2013;8:e60086. doi: 10.1371/journal.pone.0060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, Qian K, Vasilakos J, Saijo S, Iwakura Y, Yannelli JR, Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived {beta}-glucans. Blood. 2011;117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu W, Hsu YM, Bi L, Songyang Z, Lin X. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat Immunol. 2009;10:1208–1214. doi: 10.1038/ni.1788. [DOI] [PubMed] [Google Scholar]
- 25.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ, Liebler DC, Slebos RJ, Lorkiewicz PK, Higashi RM, Fan TW, Dang CV. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell metabolism. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011;187:1157–1165. doi: 10.4049/jimmunol.1100889. [DOI] [PubMed] [Google Scholar]
- 28.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 29.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traves PG, de Atauri P, Marin S, Pimentel-Santillana M, Rodriguez-Prados JC, Marin de Mas I, Selivanov VA, Martin-Sanz P, Bosca L, Cascante M. Relevance of the MEK/ERK signaling pathway in the metabolism of activated macrophages: a metabolomic approach. J Immunol. 2012;188:1402–1410. doi: 10.4049/jimmunol.1101781. [DOI] [PubMed] [Google Scholar]
- 31.Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM) Molecular cancer. 2009;8:41. doi: 10.1186/1476-4598-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan TW, Lane AN, Higashi RM, Yan J. Stable isotope resolved metabolomics of lung cancer in a SCID mouse model. Metabolomics : Official journal of the Metabolomic Society. 2011;7:257–269. doi: 10.1007/s11306-010-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 34.Tian J, Ma J, Ma K, Guo H, Baidoo SE, Zhang Y, Yan J, Lu L, Xu H, Wang S. beta-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol. 2013;43:1220–1230. doi: 10.1002/eji.201242841. [DOI] [PubMed] [Google Scholar]
- 35.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015 doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bronte V, Murray PJ. Understanding Local Macrophage Phenotypes In Disease: Modulating macrophage function to treat cancer. Nat Med. 2015;21:117–119. doi: 10.1038/nm.3794. [DOI] [PubMed] [Google Scholar]
- 38.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, Nebuloni M, van Rooijen N, Mortarini R, Beltrame L, Marchini S, Fuso Nerini I, Sanfilippo R, Casali PG, Pilotti S, Galmarini CM, Anichini A, Mantovani A, D’Incalci M, Allavena P. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Ruttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 45.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis ESC. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traves PG, de Atauri P, Marin S, Pimentel-Santillana M, Rodriguez-Prados JC, Marin de, Mas I, Selivanov VA, Martin-Sanz P, Bosca L, Cascante M. Relevance of the MEK/ERK Signaling Pathway in the Metabolism of Activated Macrophages: A Metabolomic Approach. J Immunol. 2011 doi: 10.4049/jimmunol.1101781. [DOI] [PubMed] [Google Scholar]
- 50.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 51.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 52.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.