Abstract
Rupture of abdominal aortic aneurysm (AAA) is a lethal event. No oral medicine has been available to prevent or treat AAA. We have recently identified a novel mechanism of eNOS uncoupling by which AAA develops, in Angiotensin II (Ang II) infused hyperphenylalaninemia 1 (hph-1) mice. Using this unique model we investigated effects on AAA formation of the L-type calcium channel blocker nifedipine, in view of the unclear relationship between hypertension and AAA, and unclear mechanisms of aneurysm protective effects of some blood pressure lowering drugs. Six-month old hph-1 mice were infused with Ang II (0.7 mg/kg/day) for 2 weeks, and fed nifedipine chow at two different doses (5 and 20 mg/kg/day). While the high dose of nifedipine reduced blood pressure, the lower dose had no effect. Interestingly, the incidence rate of AAA dropped from 71% to 7 and 12.5% for low and high dose nifedipine, respectively. Expansion of abdominal aorta, determined by ultrasound imaging, was abolished by both doses of nifedipine, which recoupled eNOS completely to improve NO bioavailability. Both also abrogated aortic superoxide production. Of note, Ang II activation of NADPH oxidase in vascular smooth muscle cells and endothelial cells, known to uncouple eNOS, was also attenuated by nifedipine. Although low dose was a sub-pressor while the high dose reduced blood pressure via inhibition of calcium channels, both doses were highly effective in preventing AAA by preserving eNOS coupling activity to eliminate sustained oxidative stress from uncoupled eNOS. These data demonstrate that oral treatment of nifedipine is highly effective in preserving eNOS function to attenuate AAA formation. Nifedipine may be used for AAA prevention either at low dose to AAA risk group, or at high dose to patients with co-existing hypertension.
Keywords: abdominal aortic aneurysm, hph-1 mice, angiotensin II, nifedipine, blood pressure, eNOS recoupling, NADPH oxidase (NOX)
INTRODUCTION
The rupture of the abdominal aortic aneurysm (AAA) is a lethal event, with a prevalence of around 2 percent in the United States [1]. The incidence of AAA has increased during the past 2 decades. There is currently no effective prophylaxis or oral medication for AAA, due to the fact that molecular mechanisms of AAA development are not fully understood. Only AAA of larger size is treated with surgical correction with considerable risk.
It has been reported that, in a well characterized murine model of AAA, namely angiotensin II (Ang II) infused apoE null mice, AAA formation is independent of blood pressure elevation [2–4], and some potential treatments against AAA usually do not alter blood pressure in animal models [5]. However, these data do not completely rule out a potential link between blood pressure and AAA in general or in different animal models. Recent studies have shown that, in humans with AAA, elevated aortic blood pressure is a considerable risk factor for AAA progression [6]. Therefore, it remains a clinically relevant question to examine the role blood pressure plays in AAA formation and progression. Of note, some anti-hypertensive drugs have been shown to prevent AAA formation [7–9], although this is far from being conclusive. We have recently observed that Ang II infusion into hyperphenylalaninemia (hph-1) mice results in rapid development of severe AAA. In these animals 79% developed AAA in two weeks, with 14% died suddenly of rupturing aneurysm. We have further showed that eNOS uncoupling, consequent to an endothelial deficiency in eNOS cofactor salvage enzyme dihydrofolate reductase (DHFR), plays a causal role in AAA formation in this model [10], and in the classic model of Ang II infused apoE null mice [11]. In this novel AAA model, blood pressure went up equally in Ang II infused WT and hph-1 mice before AAA ruptured, and it declined when AAA started to rupture in Ang II infused hph-1 mice.
Calcium channel blockers (CCB) are a diverse class of drugs that are used extensively as anti-hypertensives. CCBs can be broadly categorized into dihydropyridines and nondihydropyridines. While different CCBs have different potencies and pharmacokinetics, they all reduce blood pressure via vasodilation from the blockade of calcium channels in vascular smooth muscle. Some previous studies have shown that CCBs may be effective in the prevention of AAA [7, 8, 12], while others found the opposite [13–15]. Further, these studies did not dissect out an anti-hypertensive effect of CCBs as a potential cause of AAA protection. Hence, whether CCBs are effective against AAA, and the potential underlying mechanism for this protection, remain to be elucidated.
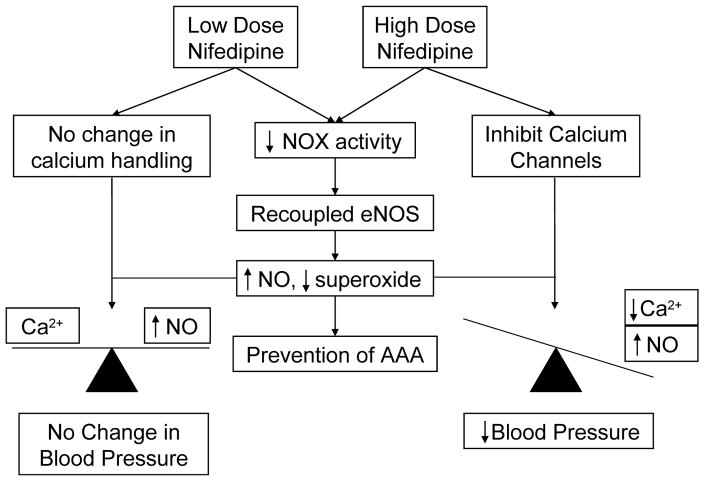
In view of the unclear relationships among hypertension, anti-hypertensives and AAA, clarification of which would define efficacy of anti-hypertensives for the treatment of AAA, we fed Ang II infused hph-1 mice with two different doses of nifedipine, a commonly used CCB for the treatment of hypertension, to delineate the related or unrelated effects of the drug on AAA prevention and blood pressure control. The results suggest that nifedipine prevents development of AAA independent of its effect on blood pressure, by recoupling of eNOS to attenuate oxidative stress and improve NO bioavailability. The overall mechanisms are summarized in Figure 6. These data suggest that nifedipine can serve as a therapeutic agent for AAA development, either given at lose dose to AAA risk group, or given at high dose to patients also having elevated blood pressure.
Figure 6. Mechanisms of action for AAA preventive effects of Nifedipine at both low and high doses.
Whereas high dose of Nifedipine blocks vascular smooth muscle calcium channel to reduce vasoconstriction to lead to reduced blood pressure, low dose of Nifedipine is ineffective on this pathway. Nonetheless, both doses of the drug are able to inhibit NOX activity to recouple eNOS, resulting improved NO bioavailability, diminished superoxide production, and consequent prevent of AAA development.
METHODS
Animals, Ang II Infusion, and Blood Pressure Measurements
Hph-1 male mice at 6 months of age were infused with Ang II (0.7 mg/kg per day) using subcutaneously implanted osmotic pumps (Alzet, 2002)[10]. Incidence of AAA was determined by the increase in the maximal diameter of the abdominal aorta, as measured via ultrasound or histology of the aorta after euthanasia. During the 14-day infusion, blood pressure was monitored by an intracarotid telemetry method. Wireless blood pressure probes were implanted into the animals 10 days before the implantation of the osmotic pumps. The catheter of the blood pressure probe was inserted into the left carotid artery, whereas the body of the probe was inserted into the right flank. Animals were given 1 week to recover from the surgery. After this period, blood pressure was measured for 3 days to obtain a baseline. The osmotic pumps were then implanted on day 10 after surgery. Measurements were made daily from 10:00 AM to 4:00 PM at a 250-Hz sampling rate. Average blood pressure was calculated daily as the average of the entire recording period. The use of animals and experimental procedures were approved by the institutional animal care and usage committee at the University of California Los Angeles.
Oral Administration of nifedipine
The hph-1 mice were started on two doses of continuous oral administration of nifedipine (5 [16] and 20 [17, 18] mg/kg/day) 2 days prior to Ang II infusion, with the dosage determined based on previous studies as cited, and our own preliminary data. The mice were monitored twice per day during the infusion period of 2 weeks, and aortas were harvested for assessment of AAA formation, vascular superoxide production and eNOS uncoupling activity by day 14. The abdominal aorta size was also monitored using ultrasound on day 0 and 14.
Ultrasound Determination of Abdominal Aorta Size
Animals were anesthetized with isoflurane and placed on a temperature-controlled table. Hair was removed from the abdomen using a hair removal cream, and preheated ultrasound transmission gel was applied onto the abdomen area. An ultrasound probe (Velvo 2100, echocardiograph, MS-400) was placed on the gel to visualize aorta transversely. The aorta was identified using Doppler measurement for the presence of pulsatile flow. Consistent localization of image acquisition was insured by visualizing the aorta immediately superior to the branch of the left renal artery in all of the animals. Images were recorded and saved onto a PC computer for offline area analysis.
Tissue Collection
Animals were euthanized with CO2 2 weeks after the osmotic pump surgery. The aorta was rapidly removed from the body, rinsed with ice cold PBS, and cleaned of connective tissue and fat, prior to ESR analyses of NO and superoxide production.
For histology, small sections (~2 mm) of abdominal aorta was removed and fixed in 10% formalin overnight, incubated in 30% sucrose for 24 hr, and embedded in paraffin. Sections were sliced at 5 μm.
Electron Spin Resonance Determination of eNOS Uncoupling State
To examine potential effects of oral Nifidipine treatment on eNOS uncoupling state in Ang II infused hph-1 mice, freshly isolated aortas were homogenized in lysis buffer (0.2 mol/L Tris, 1.5 mol/L NaCl, 10 mmol/L EDTA, 10 mmol/L EGTA, 25 mmol/L sodium pyrophosphate, 10 mmol/L β-glycerophosphate, 10mmol/L Na3VO4, 1 mmol/L PMSF, 2 μmol/L leupeptin and 10% Triton, pH7.4), centrifuged at 12,000 g for 15 min, and protein supernatant was collected. After determination of protein concentration using a protein assay kit (Bio-Rad, #500-0113), 5 μg of protein was loaded into ice-cold and nitrogen bubbled modified Krebs/HEPES buffer (KHB, in mmol/L: NaCl 99; KCl 4.7; MgSO41.2; KH2PO4 1.0; CaCl2 1.9; NaHCO3 25; glucose 11.1, NaHEPES 20) containing diethyldithiocarbamic acid (5 μmol/L), deferoxamine (25 μmol/L), and the superoxide specific spin trap methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH, 500 μmol/L, Alexis) in the presence or absence of 1 mmol/L L-NAME. The solution was loaded into a glass capillary (Kimble, 71900-50) for analysis using the electron spin resonance (ESR) spectrometer (eScan, Bruker) at 37°C. To remove background noise, measurements were subtracted from measurements made with the addition of SOD (100 U/mL). The eNOS uncoupling activity was represented by L-NAME sensitive superoxide production, which is the difference between the measurement taken with and without L-NAME. When eNOS is coupled and producing NO, its inhibition with L-NAME will result in higher measured superoxide production, resulting in a negative difference in L-NAME sensitive superoxide production. However, if eNOS is uncoupled and producing superoxide, its inhibition with L-NAME will result in lower measured superoxide production, resulting in a positive difference in L-NAME sensitive superoxide production.
Electron Spin Resonance Determination of Aortic Nitric Oxide Bioavailability
Freshly isolated aortic rings were incubated with freshly prepared NO specific spin trap Fe2+(DETC)2 (0.5 mmol/L) in modified Krebs/HEPES buffer at 37°C for 60 min, in the presence of calcium ionophore A23187 (10 μmol/L). After the incubation, the aorta in Krebs/HEPES buffer was snap frozen in liquid nitrogen and loaded into a finger Dewar for analysis with ESR spectrophotometer (eScan, Bruker). The instrument settings were as the followings: bio-field 3,280, field sweep 77.54 G (1 G = 0.1 mT), microwave frequency 9.78 GHz, microwave power 4 dB (40 mW), modulation amplitude 10 G, 4,096 points of resolution, and receiver gain 900.
Dihydroethidium Detection of Vascular Superoxide Production
The fluorescent dye dihydroethidium (DHE) was used to detect total superoxide production in the vessels in situ. DHE is a cell permeable dye that is oxidized by superoxide to ethidium bromide, which subsequently intercalates with DNA and is trapped within the nuclei of cells. Ethidium bromide is excited at 488 nm and emits light at 610 nm. Aortas were harvested from Ang II infused hph-1 mice with or without nifedipine diet at day 14, and rings of arteries were embedded in OCT compound at −80°C. Seven μm thick-frozen sections were cut on a cryostat and mounted on glass slides. Slides were then rinsed briefly in KHB to remove OCT compound. Tissue sections were covered in DHE (2 μmol/L), cover-slipped, and incubated in a lightproof humidified container at 37°C for 30 min. Slides were washed with KHB 3 times, mounted by ProLong Gold antifade reagent (Invitrogen) and then viewed with a Nikon TE2000-U fluorescent microscope.
Measurement of NADPH Oxidase Activity
Rat aortic smooth muscle cells (Lonza, R-ASM-580) between passage 4 and 10 were cultured in DMEM containing 10% fetal bovine serum (FBS) until confluence, and then starved with media containing 5% FBS overnight. The cells were pretreated with either 25 or 100 nmol/L nifedipine for 30 min prior to exposure to Ang II (100 nmol/L) for 30 min. The cells were harvested and suspended in homogenization buffer (Tris 50 mmol/L, EDTA 0.1 mol/L, EGTA 0.1 mmol/L, protease inhibitor cocktail, pH 7.4), sonicated 5 sec for 10 times on ice. Then the cell lysate was centrifuged at 3,000 rpm for 5 min at 4°C. The supernatant was again centrifuged at 50,000 rpm for 90 min at 4°C. The pellet was collected and resuspended in lysis buffer. Superoxide production was measured from this membrane fraction in the presence or absence of freshly prepared 100 mmol/L NADPH, using ESR. NADPH oxidase activity was calculated as the difference between the two measurements.
Statistical Analysis
All statistical analysis was performed on the Prism graphical software package. After testing for normality and equal variance, one way ANOVA test was used to compare between multiple groups, followed by the Newman-Keuls posthoc post-hoc test. For the comparison of incidence, 2 by 2 contingency table with the Fisher exact probability test was used. For the analysis of blood pressure data, two-way ANOVA was used, followed by the Bonferroni post hoc test. Statistical threshold was set to be p<0.05.
RESULTS
Nifedipine’s effect on blood pressure in Ang II infused hph-1 mice
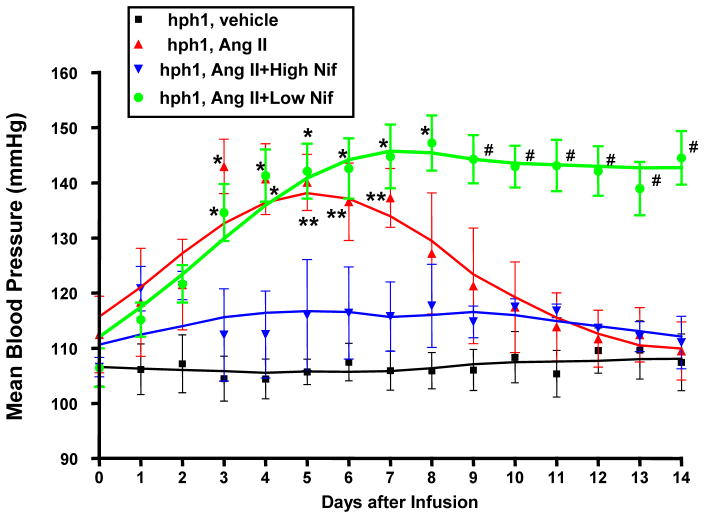
In a pervious study, we observed equal increase in blood pressure in WT and hph-1 mice before AAA rupture [10]. In the present study, to inhibit Ang II induced hypertension, hph-1 mice were started on a high dose of oral nifedipine (20 mg/kg/day) 2 days before Ang II infusion (0.7 mg/kg per day), and treated throughout the study period of 14 days. The mean blood pressure (MBP) was measured continuously throughout the study period using telemetry. As showed in Figure 1, this high dose of nifedipine was effective in preventing Ang II induction of hypertension from the very beginning, and MBP remained stable throughout 2 weeks of Ang II infusion.
Figure 1. Changes in mean blood pressure (MBP) in Ang II–infused hph-1 mice with or without Nifedipine treatment.
The hph-1 mice were pretreated with two doses of Nifedipine (5 or 20 mg/kg/day) for 2 days prior to being infused with Ang II (0.7 mg/kg per day) for 2 weeks, during which blood pressure was monitored by an intracarotid telemetry method (Data Sciences International) continuously. #p<0.05 vs. all others *p<0.05 vs. hph-1+high nif and hph-1; **p<0.05 vs. hph-1; n=3–5.
In a separate group of animals, we fed animals a low dose (5 mg/kg/day) of nifedipine, which did not significantly reduced blood pressure prior to AAA development (Figure 1, green). Interestingly, these animals did not experience the decrease in blood pressure after day 6, as compared to untreated animals, and instead, remained elevated up to day 14 post-infusion. These data seem to indicate prevention of AAA formation, as it prevented AAA rupture associated blood pressure decline. This AAA preventive and blood pressure stabilization effects share similarity with our previous observations with oral administration of folic acid, which completely prevented AAA formation and blood pressure decline in Ang II infused hph-1 mice [10].
Nifedipine prevents AAA formation in Ang II infused hph-1 mice
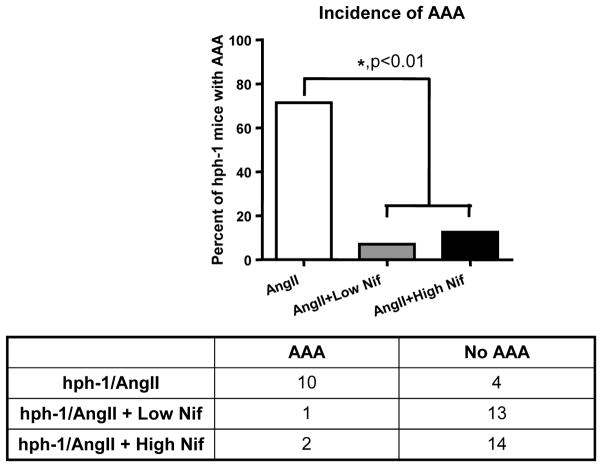
The previous blood pressure data suggest that nifedipine treated animals did not develop AAA. Here, we examined the incidence of AAA in both low and high dose nifedipine treated animals to see the effect of nifedipine on AAA development. Incidence of AAA, summarized in Figure 2, was determined using either ultrasound measurement of aortic diameter or visual inspection of the aortic ring after tissue harvest. Interestingly, this oral treatment of nifedipine was highly effective in reducing incidence rate of AAA from 71% to 7% and 12.5% for low and high dose treated animals, respectively. Statistical analysis using a 2 × 2 contingency table shows that this reduction in AAA development was significant (p<0.01), demonstrating that nifedipine is effective in the prevention of AAA formation in Ang II infused hph-1 mice, regardless of its effect on blood pressure.
Figure 2. Nifedipine greatly reduces incidence of AAA in Ang II infused hph-1 mice.
The hph-1 mice were pretreated with two doses of Nifedipine (5 or 20 mg/kg/day) for 2 days prior to being infused with Ang II (0.7 mg/kg per day) for 2 weeks. Top panel shows the percent of animals that developed AAA, while the bottom panel shows the actual number of animals. Both doses of Nifedipine significantly reduced the incidence of AAA when compared to non-treated animals (71% to 7 and 12.5%) (p<0.01, 2 × 2 contingency table).
Nifedipine reduces enlargement of abdominal aorta and prevents vascular remodeling in Ang II Infused hph-1 mice
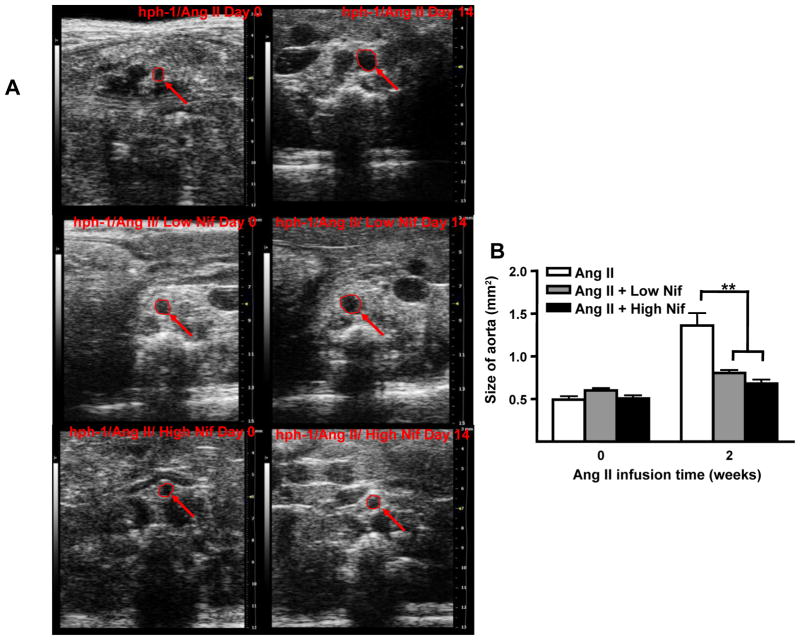
Expansion of abdominal aorta is one of the major characteristics of AAA development. On days 0 and 14, sizes of abdominal aortas were monitored using ultrasound. Ang II infusion induced a marked expansion of abdominal aorta in hph-1 mice (0.47±0.05 to 1.45±0.14 mm2), which was substantially reduced by both doses of nifedipine treatment (0.60±0.03 to 0.68±0.05 and 0.51±0.04 to 0.68±0.05 mm2 for low and high dose, respectively, Figure 3A&B)
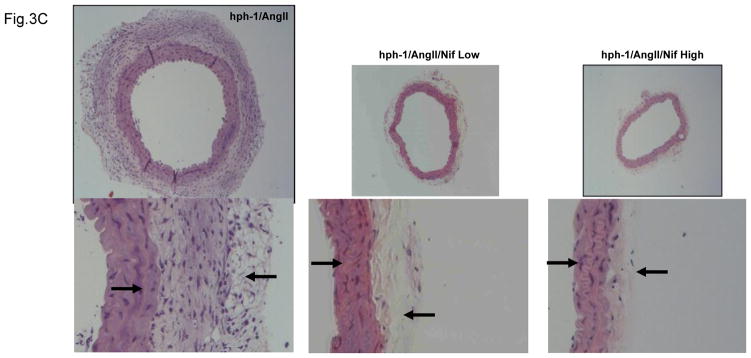
Figure 3. Nifedipine treatment reduces enlargement of abdominal aorta and prevents vascular remodeling in Ang II-Infused hph-1 mice.
A) Ultrasound determination of AAA development in Nifedipine treated, Ang II infused hph-1 mice. Representative images of ultrasound taken from Ang II infused (0.7 mg/kg per day, 2 weeks) hph-1 animals without (top row), and with low dose (5 mg/kg/day, middle row) or high dose (20 mg/kg/day, bottom row) of Nifedipine treatment. B) Summarized data from ultrasound measurements of abdominal aorta sizes. Nifedipine significantly prevented expansion of abdominal aorta at 2 weeks. **p<0.05, n=5–10. C) Nifedipine prevents vascular remodeling in Ang II infused hph-1 mice. Ang II infused hph-1 mice were treated with Nifedipine (5 or 20 mg/kg/day) 2 days before Ang II infusion and throughout the study period of 14 days, after which aortas were harvested for hematoxylin-eosin (H&E) staining. Left arrow on the left panel shows the flattening of the elastin fibers, while the right arrow on the left panel show the enlargement of the adventitial layer, both of these features were completely attenuated in the Nifedipine treated animals as in the middle (low dose) and right panels (high dose).
Formation of AAA is known to be associated with extensive vascular remodeling. Freshly isolated aortas were sectioned and examined by hematoxylin-eosin (H&E) staining. As showed in Figure 3C, oral administration of either dose of nifedipine abrogated medial degradation characterized by elastin breakdown and flattening (left arrows), and adventitial hypertrophy (right arrows) in Ang II-infused hph-1 mice.
Nifedipine recouples eNOS and improves NO bioavailability in Ang II infused hph-1 mice
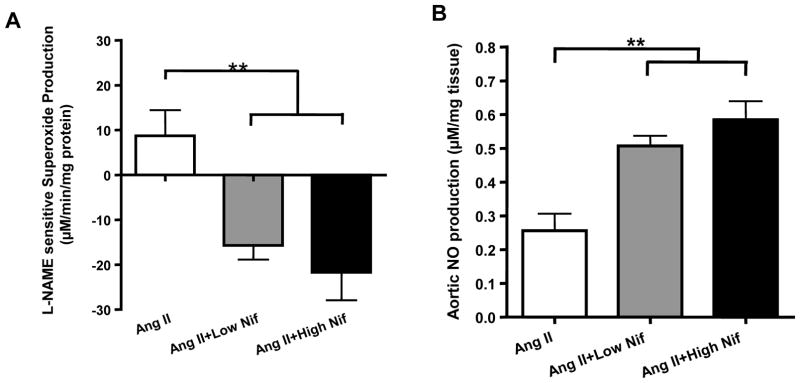
We have recently shown that uncoupled eNOS plays a causal role in AAA development in Ang II infused hph-1 mice, and that folic acid diet prevents AAA formation via recoupling of eNOS [10]. Therefore, eNOS function was examined here to determine whether eNOS recoupling is involved in nifedipine induced inhibition of AAA formation. Aortas were harvested and subjected to ESR detection of superoxide in the presence or absence of L-NAME, an inhibitor of NOS. If eNOS is functional and coupled, its inhibition by L-NAME will increase the measured superoxide. However, if eNOS is dysfunctional and uncoupled, it is producing superoxide and therefore, inhibition with L-NAME will reduce measured superoxide. Hence, the difference between the superoxide values measured with and without L-NAME reflects the state of eNOS coupling, with positive values indicating uncoupled eNOS. As is obvious in Figure 4A, L-NAME-sensitive superoxide production, reflective of eNOS uncoupling activity, was completely attenuated by both doses of nifedipine. Since nifedipine restored eNOS function, we next measured NO levels in isolated aortas from treated hph-1 mice. The results, shown in Figure 4B, demonstrate that both doses nifedipine markedly improved NO bioavailability in Ang II infused hph-1 mice.
Figure 4. Nifedipine recouples eNOS and restores NO bioavailability in Ang II infused hph-1 mice, and restored DHFR protein level in endothelial cells.
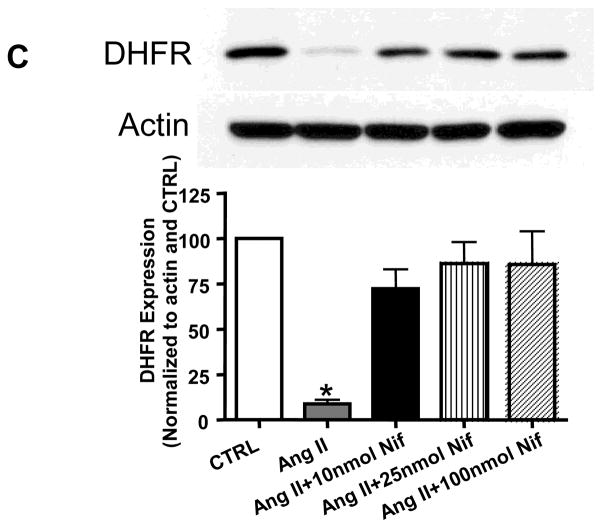
The hph-1 mice were pretreated with 2 different doses of Nifedipine for 2 days (5 or 20 mg/kg/day) prior to being infused with Ang II (0.7 mg/kg per day) for 2 weeks, after which aortas were harvested for electron spin resonance (ESR) determination of superoxide and NO production. A) Aortic eNOS uncoupling activity, indicated by NG-nitro-L-arginine methylester (L-NAME)-sensitive superoxide production. Results shown as the difference between measurements of superoxide production made with and without the addition of L-NAME, where positive values indicate that eNOS is uncoupled **<0.01, n=5–6; B) Aortic NO production determined by ESR. **p<0.01, n=5–8; C) DHFR protein level in endothelial cells as measured with Western blotting. *<0.001 vs all others, n=5 each.
Nifedipine restores DHFR protein levels in endothelial cells
In a previous studies in mice, we observed that folic acid restoration of eNOS activity was accompanied by a restoration of DHFR[10, 11]. Here, we examine whether nifedipine restoration of eNOS activity uses a similar pathway. As shown in Figure 4C, Ang II significantly suppressed the protein level of DHFR in endothelial cells. However, when the cells were co-treated with different doses of nifedipine, the protein level of DHFR was restored.
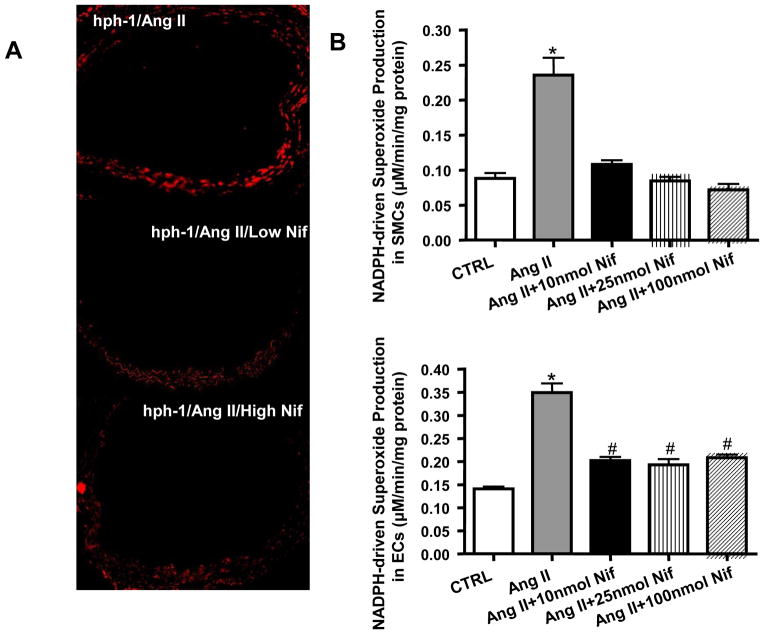
Nifedipine reduces vascular ROS production and attenuates Ang II induced NADPH oxidase activity in vascular smooth muscle cells and endothelial cells
Recent studies have suggested that some CCB (i.e. amlodipine) have additional vascular protective effect through stimulation of NO release and inhibition of ROS production that is independent of its effect on calcium channels [19–22]. Here, DHE staining was used to investigate the efficacy of nifedipine on overall vascular ROS production in situ. As shown in Figure 5A, in Ang II infused hph-1 mice with nifedipine diet, aortic production of total ROS was significantly attenuated compared with mice treated with normal diet. It is known that NADPH-driven generation of ROS plays a critical role in Ang II-induced hypertension [23], which could also lead to uncoupling of eNOS [24, 25]. Therefore, we then investigated the effect of nifedipine on NADPH oxidase (NOX) activation by Ang II in cultured vascular smooth muscle cells (VSMC, n=5–7) and endothelial cells (EC, n=5). Different doses of nifedipine were used to show that any effect on NOX activity was independent of its effects on calcium channels. As shown in Figure 5B, Ang II induced NOX activity was attenuated by all doses of nifedipine treatment in both cell types.
Figure 5. Nifedipine reduces vascular ROS production and attenuates Ang II activation of NADPH oxidase activity in VSMCs and ECs.
A) Vascular superoxide production was determined by DHE staining in Ang II infused hph-1 mice with or without low (5 mg/kg/day) or high (20 mg/kg/day) Nifedipine treatment; B) Membrane fractions of VSMC (top panel, n=4–9) or EC (lower panel, n=5) exposed to Ang II in the presence or absence of different doses of Nifedipine (10, 25 and 100 μM) were subjected to ESR determination of NADPH-driven superoxide production. *p<0.001 vs all, #p<0.01 vs CTRL
DISCUSSION
The most significant finding of the present study is that oral administration with L-type CCB nifedipine effectively prevents AAA formation given at low dose that does not lower blood pressure, or given at high dose that reduces blood pressure. Expansion of abdominal aorta and vascular remodeling were both abolished by nifedipine treatment. Moreover, nifedipine completely recoupled eNOS in Ang II infused hph-1 mice to markedly improve NO bioavailability, while it also attenuated total vascular superoxide production, and inhibited Ang II induced NADPH oxidase activity in vascular smooth muscle. These data reveal a novel mechanism by which nifedipine prevents AAA formation, namely via recoupling of eNOS. This response seem to be independent of blood pressure regulation. Low dose of nifedipine, although not sufficient to prevent blood pressure increase in response to Ang II due to lack of sufficient inhibition of calcium, it remains effective in preventing AAA via preservation of eNOS function. These pathways have been summarized in Figure 6. These findings are clinically relevant to indicate that nifedipine can be used as a valuable protective agent against AAA formation, given at lower dose to AAA risk group, or at higher dose to patients with co-existing hypertension.
The risk factors of AAA are somewhat different from other aortic aneurysm diseases, such as thoracic aortic aneurysm, where past studies have consistently shown family history and certain genetic markers to be critical determinants [26, 27]. In contrast, family history is a relatively weak predictor for AAA[28], and the only consistently observed genetic link with AAA is the male gender [29]. Although, recent studies indicate family history maybe indicative of growth speed of smaller AAAs [30]. Instead, studies have shown that environmental factors are more associated with the development of AAA, such as diet and life style [31]. The major risk factor that is consistently observed across studies is smoking [32], where in one study almost all of the patients with AAA were smokers [33]. In a previous study in animals, it was shown that infusion of nicotine and Ang II promoted AAA formation through the activation of AMPKα2 by ROS, ultimately leading to increased MMP activation [34]. In the current work, treatment with both doses of nifedipine reduced ROS production from NOXs. Taken together, this suggests that nifedipine exerts its AAA protective effect via the inhibition of downstream effectors of ROS/uncoupled eNOS, such as MMP activation.
It is interesting to note that in the Ang II-infused hph-1 model of AAA, blood pressure initially rises after Ang II infusion, but then declines after ~5–6 days, which was seen both in this study (Fig. 1) as well as in our previous study [10]. This effect is attributed to the development of AAA in these animals, as prevention of AAA with folic acid completely attenuated the blood pressure decline [10]. A high dose of nifedipine largely eliminated the rise in blood pressure from Ang II infusion. Interestingly, low dose of the drug, which did not prevent the initial blood pressure increase, also prevented the decline in blood pressure after 5–6 days. Both doses of nifedipine were effective in the prevention of the development of AAA. Taken together, these data show that nifedipine’s action on AAA development is independent of its effects on blood pressure, rather, dependent on its ability to recouple eNOS.
In this study, we have shown that both high and low dose of nifedipine prevented the expansion of the abdominal aorta as measured by ultrasound (Fig. 3A&B), as well as remodeling of the aorta typically occurring in AAA (Fig. 3C). This was accompanied by improved NO bioavailability and the coupled activity of eNOS (Fig. 4). These results are comparable to our previous findings in this model, where folic acid diet prevented AAA formation [10]. While the treatment with folic acid in the previous more directly targets the coupling state of eNOS, the use of nifedipine in this study targets a seemingly unrelated pathway of calcium handling in the cell. The fact that these unrelated treatments both lead to the recoupling of eNOS and the prevention of AAA suggests that the coupling state of eNOS is a critical factor in the development of AAA. Of note, nifedipine recouples eNOS via its additional effect on VSMC, namely inhibition of NOX activity. Further, we showed that nifedipine improves the protein level of dihydrofolate reductase (DHFR, Figure 4C), which can salvage the critical eNOS cofactor tetrahydrobiopterin to recouple eNOS. This is in agreement with a previous study which showed nifedipine improving the expression of DHFR [18]. This mechanism is similar to the improvement in DHFR that was observed in the prevention of AAA by folic acid [10, 11].
Calcium channel antagonists have been shown in the past to have anti-inflammatory effects[35, 36] and hence, seem to be an attractive candidate for AAA treatment. However, there are conflicting results on the efficacy of these CCBs on preventing AAA. It was shown that Verapamil was not able to block elastase release from neutrophils in aneurysm patients [14]. Amlodipine was ineffective in preventing AAA in a rat model [13], and was further found to enhance elastin degradation in a pig model [15]. However, in other studies Amlodipine [7], or in combination with Atorvastatin [37], was found effective in preventing AAA in experimental mice. Several other CCBs, such as Diltiazem [8] and Azelnidipine [9], were also found to prevent AAA in mice. Nifedipine has been shown to inhibit AAA formation in a rat model through a NF-κB and MMP-9 dependent manner [12]. However, in that study blood pressure was not controlled, and hence could not be excluded as a possible cause for the prevention. In our present study, the effect of blood pressure was delineated from nifedipine’s AAA protective effect by the use of two different doses of the drug, which had differential effects on blood pressure while both retaining its protection against AAA. Further, our data show that the enhanced superoxide production and uncoupling of eNOS in the AAAs were prevented in nifedipine treated animals. The reduction in oxidative stress has been shown in the past to be upstream of NF-κB and MMP-9 activity. Taken together, this would suggest improvement in eNOS coupling activity and reduction of superoxide production observed in this study lead to changes in NF-κB and MMP-9 activities to mediate nifedipine’s AAA protective effect.
It is interesting to note that while both doses of nifedipine improved aortic NO production to comparable levels (Fig. 4B), they had very different blood pressure responses to Ang II (Fig. 1). Given that NO is a potent vasodilator, one would expect that the improvement in NO with the low dose of nifedipine would also suppress the increase in blood pressure due to Ang II. The likely explanation for this would be that while NO is improved with both doses of nifedipine treatment, its absolute levels are still too low to affect the change in blood pressure. The difference in blood pressure between the two doses is more related to nifedipine’s inhibition of calcium channels and subsequent inhibition of vasoconstriction, where the low dose was a sub-pressor dosage. Hence, in the case of the high dose of nifedipine, the inhibition of calcium channels, combined with the increase in NO, results in reduction of blood pressure. In the case of the low dose of nifedipine, calcium channels are insufficiently inhibited and hence, the increase in NO alone was not sufficient to change blood pressure. These pathways are summarized in Figure 6. This would also suggest that this increase in NO production and eNOS coupling was enough to prevent the development of AAA, once again showing the importance of the coupling state of eNOS in the development of the disease. Rescuing eNOS from its uncoupled state that produces ROS to deteriorate oxidative stress proves to be a central protective strategy.
In humans, nifedipine is commonly given as immediate release (10 mg, 3 times daily) or extended release systems (30–60 mg, once daily) for the treatment of hypertension. While the comparison between the doses used in humans with those of mice in this study are difficult, the data in this study shows that even a sub-pressor dose of nifedipine can prevent the development of AAA. Therefore further studies into the anti-AAA effect of nifedipine in humans are warranted.
In conclusion, this study shows that oral treatment with nifedipine is highly effective in reducing the incidence of AAA in Ang II infused hph-1 mice, which is independent of its effects on blood pressure. This AAA protective effect can be at least partially attributed to an inhibition of Ang II driven NOX activation in vascular smooth muscle, which in turn prevents eNOS uncoupling. These confirm that eNOS uncoupling is an important mediator of AAA formation, and that nifedipine can serve as a powerful protective agent against development of AAA, given at low dose to AAA risk group, or at higher dose to patients with co-existing hypertension. These findings are highly translational to support guided usage of nifedipine as an effective therapeutic for AAA, based on well defined molecular mechanisms.
Highlights.
Nifedipine prevents AAA at both a dose that lowers BP and one that has no effect on BP
Nifedipine prevents the pathological expansion and remodeling of the abdominal aorta
Nifedipine recouples eNOS, improves NO bioavailability, and improves DHFR expression
Nifedipine reduces NOX derived superoxide production in both ECs and SMCs
Nifedipine may be used to prevent AAA in AAA risk group or in higher dose in hypertensive patients
Acknowledgments
This study was supported by National Institute of Health National Heart, Lung and Blood Institute (NHLBI) Grants HL077440 (HC), HL088975 (HC), HL108701 (HC, DGH), HL119968 (HC), and an American Heart Association (AHA) Established Investigator Award (EIA) 12EIA8990025 (HC).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURES CITED
- 1.Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population - a meta-analysis. PLoS One. 2013;8:e81260. doi: 10.1371/journal.pone.0081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi DB, Loftin CD, Clark J, Myers P, DeGraff LM, Cheng J, et al. beta-Arrestin-2 deficiency attenuates abdominal aortic aneurysm formation in mice. Circ Res. 2013;112:1219–29. doi: 10.1161/CIRCRESAHA.112.280399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, et al. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–5. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, et al. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–13. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosokawa Y. Effects of angiotensin receptor blocker and calcium channel blocker on experimental abdominal aortic aneurysms in a hamster model. Kurume Med J. 2010;57:1–8. doi: 10.2739/kurumemedj.57.1. [DOI] [PubMed] [Google Scholar]
- 6.Ruegg G, Mason RH, Hardinge M, Perkins J, Husmann M, Russi EW, et al. Augmentation index and central aortic blood pressure in patients with abdominal aortic aneurysms. J Hypertens. 2010;28:2252–7. doi: 10.1097/HJH.0b013e32833e1187. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Morris AJ, et al. Amlodipine reduces AngII-induced aortic aneurysms and atherosclerosis in hypercholesterolemic mice. PLoS One. 2013;8:e81743. doi: 10.1371/journal.pone.0081743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mieth A, Revermann M, Babelova A, Weigert A, Schermuly RT, Brandes RP. L-type calcium channel inhibitor diltiazem prevents aneurysm formation by blood pressure-independent anti-inflammatory effects. Hypertension. 2013;62:1098–104. doi: 10.1161/HYPERTENSIONAHA.113.01986. [DOI] [PubMed] [Google Scholar]
- 9.Kurobe H, Matsuoka Y, Hirata Y, Sugasawa N, Maxfield MW, Sata M, et al. Azelnidipine suppresses the progression of aortic aneurysm in wild mice model through anti-inflammatory effects. J Thorac Cardiovasc Surg. 2013;146:1501–8. doi: 10.1016/j.jtcvs.2013.02.073. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Siu KL, Chalupsky K, Nguyen A, Chen P, Weintraub NL, et al. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid. Hypertension. 2012;59:158–66. doi: 10.1161/HYPERTENSIONAHA.111.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu KL, Miao XN, Cai H. Recoupling of eNOS with folic acid prevents abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E null mice. PLoS One. 2014;9:e88899. doi: 10.1371/journal.pone.0088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita N, Yamasaki K, Izawa K, Kunugiza Y, Osako MK, Ogihara T, et al. Inhibition of experimental abdominal aortic aneurysm progression by nifedipine. Int J Mol Med. 2008;21:239–44. [PubMed] [Google Scholar]
- 13.Huang W, Alhenc Gelas F, Osborne-Pellegrin MJ. Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ Res. 1998;82:879–90. doi: 10.1161/01.res.82.8.879. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JR, Faust G, Tenenbaum N, Sarfati I, Rogowsky P, Wise L. The calcium messenger system and the kinetics of elastase release from human neutrophils in patients with abdominal aortic aneurysms. Ann Vasc Surg. 1990;4:570–4. doi: 10.1016/S0890-5096(06)60841-8. [DOI] [PubMed] [Google Scholar]
- 15.Boyle JR, Loftus IM, Goodall S, Crowther M, Bell PR, Thompson MM. Amlodipine potentiates metalloproteinase activity and accelerates elastin degradation in a model of aneurysmal disease. Eur J Vasc Endovasc Surg. 1998;16:408–14. doi: 10.1016/s1078-5884(98)80008-7. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Iwai M, Inaba S, Tomono Y, Kanno H, Mogi M, et al. Attenuation of monocyte chemoattractant protein-1 expression via inhibition of nuclear factor-kappaB activity in inflammatory vascular injury. Am J Hypertens. 2007;20:1170–5. doi: 10.1016/j.amjhyper.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Sakurada T, Ishizawa K, Imanishi M, Izawa-Ishizawa Y, Fujii S, Tominaga E, et al. Nitrosonifedipine ameliorates angiotensin II-induced vascular remodeling via antioxidative effects. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:29–39. doi: 10.1007/s00210-012-0810-7. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto E, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, Fukuda M, et al. Nifedipine prevents vascular endothelial dysfunction in a mouse model of obesity and type 2 diabetes, by improving eNOS dysfunction and dephosphorylation. Biochem Biophys Res Commun. 2010;403:258–63. doi: 10.1016/j.bbrc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y, Vaziri ND. Nifedipine and diltiazem but not verapamil up-regulate endothelial nitric-oxide synthase expression. J Pharmacol Exp Ther. 2000;292:606–9. [PubMed] [Google Scholar]
- 20.Berkels R, Egink G, Marsen TA, Bartels H, Roesen R, Klaus W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension. 2001;37:240–5. doi: 10.1161/01.hyp.37.2.240. [DOI] [PubMed] [Google Scholar]
- 21.Chen XN, Xu J, Feng Z, Fan M, Han JY, Yang Z. Simvastatin combined with nifedipine enhances endothelial cell protection by inhibiting ROS generation and activating Akt phosphorylation. Acta Pharmacol Sin. 2010;31:813–20. doi: 10.1038/aps.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu B, Xiao-hong L, Lin G, Queen L, Ferro A. Amlodipine, but not verapamil or nifedipine, dilates rabbit femoral artery largely through a nitric oxide- and kinin-dependent mechanism. Br J Pharmacol. 2002;136:375–82. doi: 10.1038/sj.bjp.0704753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–73. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 24.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–61. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–9. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bee KJ, Wilkes DC, Devereux RB, Basson CT, Hatcher CJ. TGFbetaRIIb mutations trigger aortic aneurysm pathogenesis by altering transforming growth factor beta2 signal transduction. Circ Cardiovasc Genet. 2012;5:621–9. doi: 10.1161/CIRCGENETICS.112.964064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–16. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–23. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 29.Mani K, Lees T, Beiles B, Jensen LP, Venermo M, Simo G, et al. Treatment of abdominal aortic aneurysm in nine countries 2005–2009: a vascunet report. Eur J Vasc Endovasc Surg. 2011;42:598–607. doi: 10.1016/j.ejvs.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Roddy SP. Family history of aortic aneurysm is an independent risk factor for more rapid growth of small abdominal aortic aneurysms in Japan. J Vasc Surg. 2015;61:566. doi: 10.1016/j.jvs.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–48. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 32.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 33.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Zhang C, Zhang M, Liang B, Zhu H, Lee J, et al. Activation of AMP-activated protein kinase alpha2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat Med. 2012;18:902–10. doi: 10.1038/nm.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii N, Matsumura T, Kinoshita H, Fukuda K, Motoshima H, Senokuchi T, et al. Nifedipine induces peroxisome proliferator-activated receptor-gamma activation in macrophages and suppresses the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:1598–605. doi: 10.1161/ATVBAHA.109.202309. [DOI] [PubMed] [Google Scholar]
- 36.Matsumori A, Nishio R, Nose Y. Calcium channel blockers differentially modulate cytokine production by peripheral blood mononuclear cells. Circ J. 2010;74:567–71. doi: 10.1253/circj.cj-09-0467. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Matsumoto Y, Do e Z, Kanazawa M, Satoh K, Shimizu T, et al. Combination therapy with atorvastatin and amlodipine suppresses angiotensin II-induced aortic aneurysm formation. PLoS One. 2013;8:e72558. doi: 10.1371/journal.pone.0072558. [DOI] [PMC free article] [PubMed] [Google Scholar]