Abstract
Objectives
Left ventricular (LV) assist device (LVAD) support reduces pathological loading. However, load-induced adaptive responses may be suppressed. Pathological loading dysregulates cardiac G protein-coupled receptor (GPCR) signaling. Signaling through G proteins is deleterious, whereas beta (β)-arrestin-mediated signaling is cardioprotective. We examined the effects of pathological LV loading/LV dysfunction and treatment via LVAD, on β-arrestin-mediated signaling, and genetic networks downstream of load.
Methods
An ovine myocardial infarction (MI) model was employed. Sheep underwent either sham thoracotomy (n=3), mid-left anterior descending coronary artery ligation to produce MI (n=3), or MI with placement of a small platform catheter-based LVAD (n=3). LVAD support was continued for 2 weeks. Animals were maintained for a total of 12 weeks. Myocardial specimens were harvested and analyzed.
Results
MI induced β-arrestin activation. Increased interactions between the EGF receptor and β-arrestins were observed. LVAD support inhibited these responses to MI (p < 0.05). LVAD support inhibited activation of cardioprotective signaling effectors Akt (p < 0.05), and to a lesser extent, ERK1/2 (p = NS). However, MI resulted in regional activation of load-induced GPCR signaling via G proteins, as assessed by induction of atrial natriuretic peptide (ANP) mRNA expression in the MI-adjacent zone relative to the MI-remote zone (p < 0.05). MI-adjacent zone ANP expression was renormalized with LVAD support.
Conclusions
LVAD support inhibited cardioprotective β-arrestin-mediated signaling. However, net benefits of normalization of load-induced GPCR signaling were observed in the MI-adjacent zone. These findings may have implications for the optimal extent and duration of unloading, and adjunctive medical therapies.
Introduction
The mortality and morbidity of acute myocardial infarction (MI) have been vastly improved with coronary arterial revascularization. However, many patients present or are treated in a delayed fashion. Consequently, left ventricular (LV) systolic dysfunction frequently complicates proximal coronary artery occlusion with large-territory MI. It is estimated that ~30% of MI results in heart failure (HF) (1), and that 5-8% of MI results in overt cardiogenic shock (2).
Mechanical circulatory support (MCS) is now well-validated as a therapy for both chronic and acute LV dysfunction when heart failure (HF) is present (3-4). However, it is less clear whether LV MCS at or around the time of MI actually attenuates pathologic LV remodeling and reduces the extent/severity of LV dysfunction. To address this question, we previously reported the development of a large animal model of MI, and investigated the effects of short-term MCS via the Abiomed Impella small-platform LV assist device (LVAD) on subsequent regional and global pathologic remodeling and systolic function (5). LVAD support for 2 weeks following MI resulted in reduced peri-infarct zone diastolic strains assessed 12 weeks following MI, with improved (increased) peri-infarct zone systolic strains and LV ejection fraction. Another group has reported similar findings using ventricular restraint therapy (6).
Regional myocardial and global LV contractility are consequences of myofilament function (7-9). In turn, myofilament physiological function is directly controlled by calcium handling within the cardiomyocyte. Consistent with these concepts, we found that deranged calcium handling in the MI-adjacent zone cardiomyocytes was substantially improved by LV MCS. However, the mechanisms – cell surface receptors and coupled intracellular signaling pathways – that link cardiomyocyte mechanical loading conditions to alterations in calcium handling, and how MCS in turn alters mechanotransduction - remain to be elucidated. The purpose of these studies was to investigate specific intracellular signaling pathways, outlined below, that are increasingly appreciated to play important roles in cardioprotective responses to cellular stresses such as mechanical loading.
Seven-transmembrane receptors (7-TMRs), also termed G protein-coupled receptors (GPCRs), are the receptors generally thought to be the most important in regulating cardiac and vascular function (10); 7-TMR/GPCR pathways are the targets for the overwhelming majority of cardiovascular drug therapies (11). Numerous lines of evidence suggest that GPCRs, and in particular Gαq-coupled receptors, are critically involved in mechanotransduction and may even serve as direct mechanosensors (12-14).
Canonical GPCR signaling is mediated by G protein activation, resulting in coupled second messenger molecule activation. This conventional pathway is antagonized by GPCR cytoplasmic tail phosphorylation, which is catalyzed by GRKs, of which 6 family members exist. β-arrestins (of which there are 2 types, β-arrestin1 and β-arrestin2) bind to the tails, preventing further G protein coupling; this process is termed desensitization (15). However, β-arrestins not only antagonize G protein activation, but couple GPCRs to downstream effector molecules (16). These effectors are distinct from those that the paired G protein/second messengers stimulate. In the heart, β-arrestin signaling appears to enhance both cardiomyocyte function (17) and survival (18), whereas G protein-mediated signaling enhances cardiomyocyte function acutely at the expense of long-term function and survival. LVAD support has been shown to inhibit chronic GPCR activation, specifically of β-adrenergic receptors (β-ARs) (19-20). However, it is unclear whether β-arrestin-mediated signaling is affected by this. The principal aim of these studies was to investigate the effects of MI and subsequent short-term LV MCS on β-arrestin-mediated signal transduction.
Methods
Large animal model of MI, LV dysfunction, and LVAD support
These are described in detail in (5). In brief, adult Dorsett hybrid sheep (53.2 ± 0.9 kg) were used. Sheep either underwent sham thoracotomy, creation of large territory MI by permanent ligation of the mid-left anterior descending (LAD) coronary artery, or creation of MI followed by implantation of the Impella 5.0 (Abiomed, Danvers, MA) small-platform catheter-based LVAD at the same setting (2 to 3 hours following infarction). Pilot studies previously demonstrated that this results in infarction of 25% of the LV mass. Sonomicrometry crystal arrays were implanted subepicardially in all animals, to determine regional LV strains and global LV dimensions. In animals assigned to LVAD implantation, pump speed was set between 20,000 and 24,000 rpm to achieve ~50% unloading of the total cardiac output. Support was continued for a 2 week period, and the animals were followed for an additional 10 weeks. All animals were monitored for 12 weeks post-MI creation, at which point they were sacrificed, and hearts harvested. All the animals were treated in compliance with the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health publication 85-23, revised 1996). All surgical procedures and postoperative care were approved by the Institutional Animal Care and Use Committee of the University of Maryland-Baltimore. For the purposes of the studies outlined below, 3 animals in each group (control, MI alone, or MI + LVAD) had tissue samples available for analyses.
Lysate preparation and immunoblotting
At the time of termination, regional myocardium in the MI-adjacent zones (defined as ≤ 2 cm from the infarct border) and the remote zones were harvested and snap-frozen in liquid nitrogen, and stored at −80°C for future analysis. Lysates from cytosolic and membrane fractions were prepared using a cellular fraction and lysis kit (Pierce Thermo Scientific, Waltham, MA). To determine phospho- and total protein expression levels, cardiac lysates were subjected to gel electrophoresis using a 4–15% Tris–HCl ready gel (Bio-Rad Laboratories, Hercules CA) were prepared with cell fractionation kit (Pierce Thermo Scientific, Waltham, MA) with necessary modification. Gels were transferred to membranes, and immunoblotting was performed. Analyses were performed on the following proteins, using antibodies (Abs) directed against: phospho-Akt (T-308), total Akt, phospho-ERK1/2, total ERK1/2, β-arrestin1/2, and EGFR (all primary antibodies from Cell Signaling Technology, Danvers, MA). Immunoreactivities were detected using enhanced chemiluminescence (ECL) (Hyglo, Denville Scientific Inc. Metuchen, NJ) and photographic film (Hyblot CL, Denville Scientific Inc. Metuchen, NJ). Densitometric quantification of the digitized immunoreactive bands was performed using the UN-SCAN-IT Gel 5.1 software (Silk Scientific, Orem, UT). Immunoblotting for the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) was used to confirm equal loading conditions for cytosolic protein expression.
Immunoprecipitation reactions were performed using per the protocol from the Pierce Crosslink Immunoprecipitation kit (Pierce Thermo Scientific, Waltham, MA). Myocardial tissue cytosolic extracts were incubated with either anti-β-arrestin1/2 or anti-EGFR antibody (Ab) at 1:50 dilution (Cell Signaling Technology, Danvers, MA; and Santa Cruz Biotechnology, Santa Cruz, CA, respectively) overnight at 4 °C, for assessment of β-arrestin-ubiquitin and β-arrestin-EGFR binding, respectively. Ab-bound immune complexes were retrieved as immunoprecipitates with DSS (disuccinimidyl suberate)-crosslinked protein A/G-agarose column. Immunoprecipitates were prepared in reducing sample buffer and subjected to gel electrophoresis. Gels were transferred to membranes, and immunoblotted with anti-ubiquitin Ab (Cell Signaling Technology, Danvers, MA) at 1:500 dilution for β-arrestin-ubiquitin interaction assessment, or anti-β-arrestin1/2 at 1:500 dilution for EGFR-β-arrestin interaction assessment. ECL was captured on photographic film, and densitometry was used to quantify band intensity.
RNA isolation and sequencing
RNA was isolated from 30 mg of snap-frozen heart tissues using a kit (Qiagen Fibrous tissue kit; Qiagen, Hilden, Germany) with few modifications. RNA was eluted with 25 μl of RNAse free water and each sample was kept in −80C freezer after aliquot. The RNA integrity number (RIN values) of the extracted RNA and their concentration (ng/μl) were measured by on-chip-electrophoresis using the BioAnalyzer (Agilent RNA Nano 6000 Lab Chip kit and BioAnalyzer 2100 Expert, Agilent Technologies, Santa Clara, CA).
RNA sequencing studies were performed at the University of Maryland-Baltimore RNA sequencing facility. Illumina RNAseq libraries were prepared with the TruSeq RNA Sample Prep kit (Illumina, San Diego, CA) per manufacturer's protocol. Adapters containing 6 nucleotide indices were ligated to the cDNA. cDNA was purified between enzymatic reactions and the size selection of the library was performed with AMPure XT beads (Beckman Coulter Genomics, Danvers, MA). Libraries were assessed for concentration and fragment size using the DNA High Sensitivity Assay on the LabChip GX (Perkin Elmer, Waltham, MA). The libraries were pooled and sequenced on a 100PE Illumina HiSeq 2500 run (Illumina, San Diego, CA). Data were analyzed through the use of IPA (Ingenuity® Systems, www.ingenuity.com<http://www.ingenuity.com/>).
Statistical analyses
All data are presented here as means ± standard errors of the mean (SEM). One-way repeated measures analysis of variance (ANOVA) in GraphPad Prism was used to compare changes in protein expressions on immunoprecipitation and Western blot data. All ANOVAs were followed by multiple comparisons with the least significant difference (Bonferroni) correction. The significance level (p) was set at 0.05.
Results
MI reduces cytosolic β-arrestin levels; attenuation by LVAD support
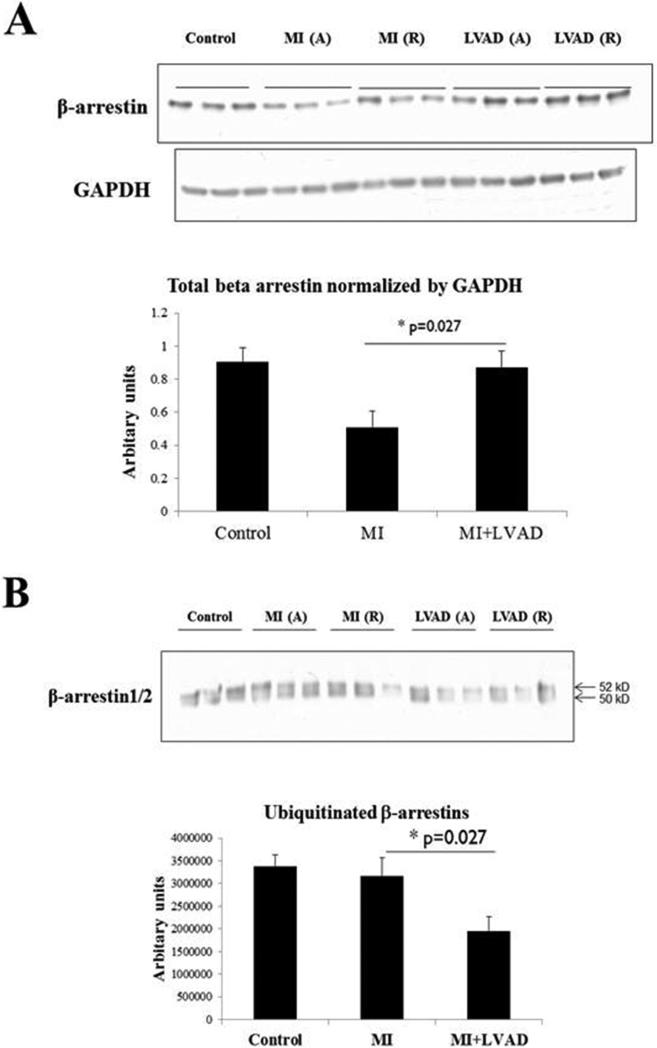
To determine the effects of MI, with or without LVAD support, on β-arrestin-mediated signaling, we initially examined cytosolic levels of β-arrestins, and post-translational modification of β-arrestins by ubiquitination. GPCR signaling through β-arrestins is known to be inversely related to β-arrestin levels. Recruitment of β-arrestins to phosphorylated GPCR tails results not only in recruitment of additional signaling molecules to the complex, but also results in β-arrestin ubiquitination, internalization of the multi-molecular complex, and receptor/β-arrestin degradation (21). However, measurement of ubiquitinated proteins may be complicated by their rapid degradation. As shown in Figure 1, MI resulted in decreased levels of β-arrestins; LVAD support restored normal levels of cytosolic β-arrestins. Although MI did not result in an increase in detectable ubiquitinated β-arrestins relative to control specimens, LVAD support resulted in a reduction in the levels of ubiquitinated β-arrestins, consistent with normalization (increase relative to MI) of overall cytosolic β-arrestin levels.
Figure 1.
Effects of MI and LVAD support on β-arrestin levels and ubiquitination. A = adjacent, R = remote. A. MI reduces β-arrestin1/2 levels, consistent with increased β-arrestin-mediated signaling; LVAD support restores β-arrestin1/2 levels, consistent with relative suppression of β-arrestin-mediated signaling. Immunoblotting was performed as described in Methods. Both β-arrestins as present as a single band due to whole cell lysate samples run for a shorter duration than immunoprecipitate samples (e.g., panel B and Figure 2). B. LVAD support decreases levels of ubiquitinated β-arrestins, consistent with reduced GPCR internalization and β-arrestin-mediated signal transduction. Immunoprecipitations and immunoblotting were performed as described in Methods.
Increased epidermal growth factor receptor (EGFR) interaction with β-arrestins in response to MI, and suppression by LVAD support
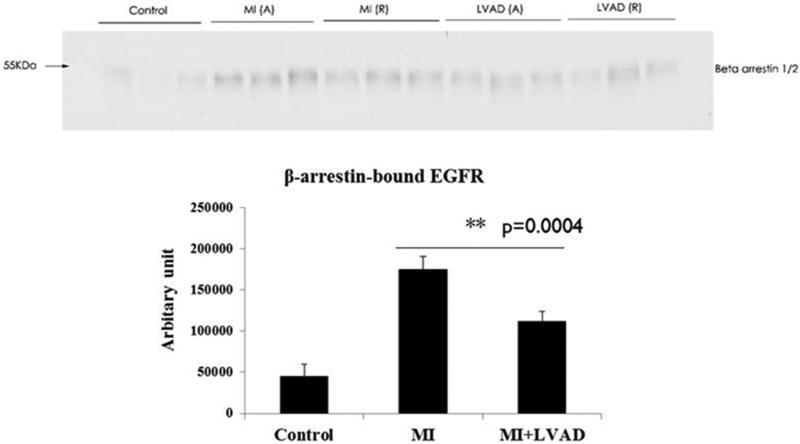
Cardiovascular mechanical load has previously been shown to activate GPCRs, most notably Gαq-coupled receptors. Strain-induced activation of the angiotensin II type 1 receptor (AT1R), which along with the β/α adrenergic (ARs) and endothelin A/B receptors (ETRs) constitute the most important cardiac GPCRs, results in β-arrestin2 activation. β-arrestin2 in turn couples the AT1R to trans-activation of the EGFR (22). MI resulted in increased interaction between β-arrestins and the EGFR (Figure 2), consistent with increased diastolic myocardial strain regionally and LV end-diastolic volume globally. LVAD support decreased interaction between β-arrestins and the EGFR, consistent with relatively reduced diastolic myocardial strain and LV end-diastolic volume (Figure 2).
Figure 2.
Effects of MI and LVAD support on interactions between the EGFR and β-arrestins. A = adjacent, R = remote. MI increased the amount of EGFR-bound β-arrestin1/2. Immunoprecipitations and immunoblotting were performed as described in Methods.
LVAD support impairs activation of Akt
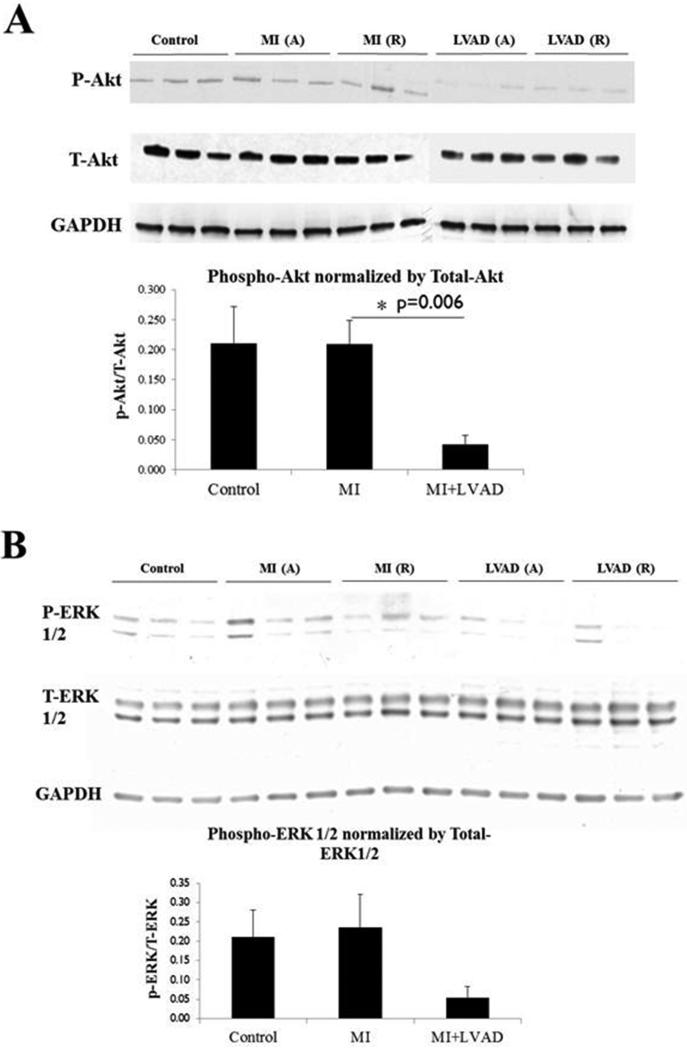
As previously discussed, numerous studies suggest that in the heart, chronic G protein activation is deleterious to cardiomyocyte function and survival. Conversely, β-arrestin-mediated signaling appears to exert beneficial effects on cardiomyocyte function and survival. These effects are in large part via the kinases Akt and ERK (23), which are frequently activated in an apparently adaptive feedback response to various pathological cellular stress stimuli. MI did not induce activation (phosphorylation) of Akt or ERK relative to control levels of Akt/ERK activation, as shown in Figure 3. However, MCS via LVAD reduced Akt phosphorylation; similar results were observed for ERK, although these were not statistically significant. Thus, LV volume unloading via MCS suppresses activation of the cardioprotective effector Akt, and also may suppress ERK activation.
Figure 3.
Effects of MI and LVAD support on phosphorylation (activation) of Akt and ERK. A = adjacent, R = remote. MI increased the amount phospho-Akt and phospho-ERK, while LVAD support relatively reduced phospho-Akt and phospho-ERK levels. Immunoblotting was performed as described in Methods.
Regional effects of MI and post-MI LVAD support on intracellular signaling networks
The biochemical studies above suggest that activation of β-arrestin-mediated signaling in response to MI is particularly heightened in the MI-adjacent zone, and that LVAD support partially reverses these effects. However, due to limited numbers of animal subjects and tissue samples for analysis, the presence or absence of possible relative differences in β-arrestin-mediated signaling between the MI-adjacent and remote zones could not be determined reliably. RNA sequencing is a highly sensitive approach that can be used to determine large differences in signal transduction pathway activation or suppression based upon changes in downstream target gene (mRNA) levels, even with limited sample numbers (24). The purposes of performing such studies in our experimental system were: (1) to identify molecular genetic signatures that could be tightly correlated to specific upstream receptor-driven signal transduction pathways, and (2) to provide more robust signal transduction profiling with limited sample numbers.
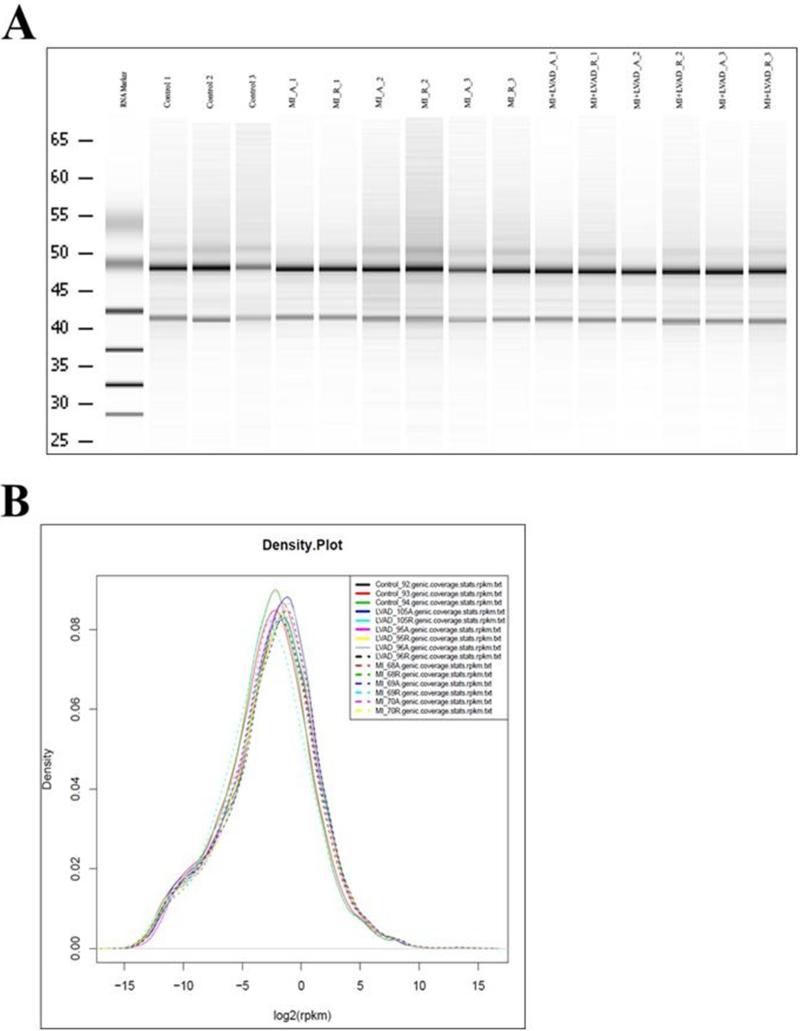
RNA samples were prepared from control LV apices, and the MI-adjacent and remote zones of LVs subjected to MI +/− LVAD support. Figure 4 illustrates the quality of RNA isolation from the specimens; RNA quality was found to be homogeneous between the samples. RNA sequencing was then performed. The following comparisons were of particular biological interest: (1) MI-adjacent zone versus control, (2) MI-adjacent versus remote zone, (3) MI-adjacent zone without or with LVAD support, and (4) MI-adjacent versus remote zone in the setting of LVAD support. Four transcripts were found to vary consistently in a physiologically relevant fashion. The results are displayed in Table 1. mRNA levels for NPPA, the gene that encodes atrial natriuretic peptide (ANP) (25), were found to be substantially increased in the MI-adjacent zone relative to the remote zone (as well as relative to control animal specimens); this constitutes a highly stringent internally controlled difference, in that inter-animal heterogeneity cannot explain the finding. LVAD support not only reduced NPPA expression in the MI-adjacent zone relative to the MI-adjacent zone in the absence of LVAD support (i.e., between animals subjected to MI alone versus MI plus LVAD support), but also reduced NPPA expression in the MI-adjacent relative to remote zones (i.e., internally controlled within the same animal). A nearly identical expression profile was observed for F2RL1, which encodes the protease-activated receptor-2 (PAR-2), a GPCR that has been implicated in regulation of inflammatory signaling that has complex effects on cardiac function (26). Differences were also demonstrated in expression profiles for osteopontin (SPP1) (27), and several myosin light chain (MYL) isoforms, but these did not exhibit a tight correlation with the presence or absence of MI with or without LVAD support.
Figure 4.
Quality of RNA isolation for RNA sequencing studies. RNA isolation was performed as described in Methods. A. Gel electrophoresis characterization of RNA integrity. Numbers denote animal numbers. A = adjacent; R = remote. B. RNA density plot demonstrating similar distribution profiles between samples from different animals and tissue regions.
Table 1.
Expression of specific gene transcripts (mRNAs) found to have biologically relevant patterns of expression variation as functions of experimental conditions. Log-fold ratios (LFCs) are listed, using base 2 logarithm (mRNA expression ratio of 1st condition/2nd condition = 2LFC). NPPA = atrial natriuretic peptide (ANP). F2RL1 = protease-activated receptor-2 (PAR-2). SPP1 = osteopontin. MYL = myosin light chain. NPPA and F2RL1 were increased in MI-adjacent zone (MI_A) relative to control samples, and importantly, the increase in MI_A was preserved relative to the internally controlled (same animals) remote (MI_R) zones. LVAD support normalized NPPA and F2RL1 expression in the adjacent zone (LVAD_A), such that NPPA and F2RL1 expression were both decreased relative to MI_A and the internally controlled remote zone (LVAD_R).
| Gene | MI_A vs Control | MI_A vs MI_R | MI_A vs LVAD_A | LVAD_A vs LVAD_R |
|---|---|---|---|---|
| NPPA | 1.779 | 4.674 | 4.408 | - |
| F2RL1 | 3.248 | 2.185 | 2.713 | - |
| SPP1 | - | 1.542 | 2.071 | - |
| MYL4 | - | 3.007 | 1.247 | - |
| MLY9 | - | 1.045 | - | - |
| MYL1 | - | 3.098 | 3.421 | - |
| MYL7 | 1.413 | - | - | - |
NPPA is induced in response to ventricular and atrial mechanical loading, as well as biochemical stresses. Signal transduction through cardiac GPCRs and non-GPCR growth factor receptors are thought to be the principal regulators of transcription. Specifically, previous studies have demonstrated that positive (agonist) G protein-mediated signaling through the AT1R (28), α1-AR (29), and ETAR (30) increases NPPA transcription; conversely, positive signaling through the β-ARs results in decreased NPPA transcription. IPA assessment (see Methods) of the RNA sequencing data found that differential signaling through the AT1R and α1-AR, but not β-ARs or ETRs, could account for the findings observed.
Regulation of F2LR1 expression is not well-understood; however, IPA results are consistent with those for NPPA, suggesting regulation by α1-AR or AT1R signaling upstream of gene transcription or post-transcriptional control.
Correlative effects of LVAD support on MI-adjacent zone diastolic strain
Our previous study (5) focused on the effects of LVAD support on regional LV remodeling. Specifically, partial LV unloading with the Impella 5.0 LVAD for only 2 weeks post-MI was found to reduce diastolic strain (i.e., local cardiomyocyte stretch/preloading) in the MI-adjacent zone at up to 12 weeks following MI. The signaling analyses performed in this study were from a subset of these aforementioned animals with sufficient specimens to conduct the signal transduction studies. Sonomicrometry measurement-derived regional diastolic strain data from these animals are displayed in Table 2. In brief, no statistically significant differences in MI-adjacent zone diastolic strain were observed with LVAD support between the 3 animals subjected to unsupported MI versus the 3 animals that were LVAD-supported post-MI. However, this appears to be due to one animal in the MI group that had much lower adjacent zone diastolic strain values than the other two animals, such that LV diastolic strain was not statistically significantly higher in the adjacent versus remote zones within the MI group animals, which previous studies including ours with larger animal numbers than the subset analyzed (5) have demonstrated. Moreover, the levels of the β-arrestin-activated effectors phospho-Akt and phospho-ERK1/2 (Figure 3) are directly proportional to diastolic strain; the second MI group sample that displays lower levels of phospho-Akt and phospho-ERK1/2 was from the LV/animal with the lowest diastolic strain in the group. This is consistent with mechanical load-induced stimulation of β-arrestin-mediated signal transduction.
Table 2.
Diastolic strains in MI-adjacent (A) versus MI-remote (R) LV myocardial zones. Sonomicrometry crystals were implanted as described in Methods. Post-LAD ligation/pre-LVAD implantation (in the MI and MI+LVAD groups respectively) and 12-week A and R zone diastolic strains are listed, as well as changes in these values. One control animal (#2) did not have interpretable sonomicrometry data. A and R zones for control animals correspond to territories in which MI would have been created.
| Pre | 12 Weeks | Change | |
|---|---|---|---|
| Control | |||
| 1 A | 0 | 7.95 | 7.95 |
| 3 A | 6.33 | 14.77 | 8.44 |
| Mean ± SD | 3.17 ± 4.48 | 11.36 ± 4.82 | 8.20 ± 0.35 |
| 1 R | 0.6 | 18.72 | 18.12 |
| 3 R | 2.73 | 17.12 | 14.39 |
| Mean ± SD | 1.67 ± 1.51 | 17.92 ± 1.13 | 16.26 ± 2.64 |
| MI | |||
| 1 A | 3.94 | 45.18 | 41.24 |
| 2 A | 5.39 | 27.21 | 21.82 |
| 3 A | 0.29 | 50.92 | 50.63 |
| Mean ± SD | 3.21 ± 2.63 | 41.10 ± 12.37 | 37.90 ± 14.69 |
| 1 R | 5.67 | 26.56 | 20.89 |
| 2 R | 12.69 | 11.27 | −1.42 |
| 3 R | 1.88 | 19.09 | 17.21 |
| Mean ± SD | 6.75 ± 5.48 | 18.97 ± 7.65 | 12.22 ± 11.96 |
| MI + LVAD | |||
| 1 A | 11.70 | 33.65 | 21.95 |
| 2 A | 5.25 | 34.73 | 29.48 |
| 3 A | 6.55 | 28.21 | 21.66 |
| Mean ± SD | 7.83 ± 3.41 | 32.20 ± 3.49* | 24.36 ± 4.43* |
| 1 R | 3.57 | 15.91 | 12.34 |
| 2 R | 0.75 | 14.46 | 13.71 |
| 3 R | 1.76 | 19.8 | 18.04 |
| Mean ± SD | 2.03 ± 1.43 | 16.72 ± 2.76 | 14.70 ± 2.98 |
denotes p < 0.05 for diastolic strains between the A and R zones for MI+LVAD group animals. Because of one apparent outlier in the MI group (animal 2), not only were differences between the MI and MI+LVAD group A zone diastolic strains observed, but even intra-animal A versus R zone diastolic strain differences in the MI group were not statistically significant.
Discussion
In this study, we used a post-MI model of LV systolic dysfunction and investigated the effects of LVAD support on: (1) β-arrestin-mediated signaling, and (2) activity of signaling networks as a function of myocardial region. We found that LVAD support suppressed cardioprotective β-arrestin-mediated signal transduction. However, this appeared to occur in the overall context of normalization of signaling networks in the LVs of LVAD-supported hearts, particular in the MI-adjacent zones (border-zones) of myocardium. These findings suggest that although LV MCS has beneficial effects on myocardial signal transduction as a whole, which translates to improved calcium handling and cardiomyocyte function, cytoprotective signaling may be suppressed in addition to cytotoxic signaling.
MCS for chronic LV failure is now a well-established treatment with validated benefits over HF pharmacotherapy alone. In contrast, short-term MCS is comparatively less well-accepted in the treatment of acute HF/cardiogenic shock, although recent evidence and even consensus documents support more widespread application. LVAD support as a mode of therapy to actually prevent LV pathologic remodeling and enhance LV function and recovery is even more controversial. Our group's studies, as well as those of others, suggest that this novel application of MCS is reasonable and effective. Yet, MCS has inherent procedural risk, and its benefits may be further limited. Pathological stimuli exert directly deleterious effects through pathologically activated or suppressed signaling pathways, but also induce adaptive cytoprotective signals. In MI-induced LV dysfunction, pathological loading conditions are present regionally in the MI-adjacent zone. LVAD support normalizes loading conditions in the adjacent zone, and while this suppresses Gαq-mediated α1-AR/AT1R signaling, it also suppresses β-arrestin-mediated signaling. These concepts are displayed in Figure 5. It is possible that differential effects on each pathway may be related to changes in the extent and duration of mechanical unloading, and that an optimal point for extent and/or duration of LVAD support exists. However, it is also possible that suppression of cytoprotective signaling is an unavoidable side effect of inhibiting cytotoxic Gαq-mediated signaling. Adjunctive medical therapies, such as selective GPCR ligands (“biased agonists”) that only activate β-arrestin-mediated signaling, may have utility in treating LV dysfunction. One such ligand for the AT1R is currently in clinical trials for usage in acutely decompensated HF.
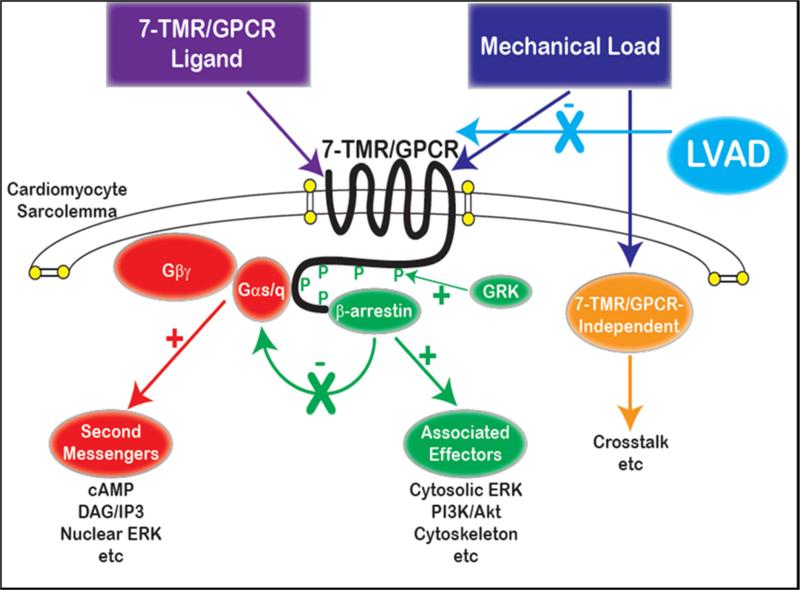
Figure 5.
Cartoon diagram of the hypothesized effects of mechanical loading, with or without LVAD support, on cardiac 7-TMR/GPCR signal transduction. Mechanical load is depicted as acting like a ligand in facilitating cell-surface receptor (GPCR) activation. This activates both G protein-mediated signaling as well as β-arrestin-mediated signaling, the latter of which also suppresses G protein coupling to the receptor (desensitization). As discussed in the text, chronic G protein and second messenger activation is cytotoxic, whereas several lines of evidence demonstrate that β-arrestin-mediated signaling is cytoprotective. As a result, it is suggested that load reduction may not selectively reduce G protein activation, and may also reduce β-arrestin activation. The effects of extent of loading/unloading and timing of LVAD support on differential pathway activity are unclear.
Important methodological limitations are present. First, the MI model employed did not incorporate reperfusion, which is normally performed when feasible. Thus, the results should be interpreted in the context of completed large territory MI without revascularization, which is a minority of MI. Second, standard HF pharmacotherapies were not employed. However, these therapies such as β-AR antagonists, and ACE inhibitors or AT1R antagonists, themselves non-selectively suppress both G protein- and β-arrestin-mediated signaling, not substantially different from those of LVAD support alone. Moreover, the roles of standard HF pharmacotherapies in the setting of LVAD support are not validated. Third, the model as developed is one of LV dysfunction and not necessarily clinical HF; we did not ascertain whether the end-organ features of HF are present in this model. Finally, the initial biochemical studies are limited by sample numbers, which was one of the reasons that the statistically robust RNA sequencing approach was used for subsequent analyses.
In conclusion, these studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective β-arrestin-mediated signal transduction, while normalizing the signaling networks of Gαq-coupled cardiac GPCRs in the MI-adjacent zone. Further studies are required to elucidate the roles of specific GPCRs in directed β-arrestin-mediated signaling in post-MI LV dysfunction, and to define potential optimal titration of LVAD support based upon signaling and genetic markers in addition to standard LV functional endpoints.
Acknowledgments
This research was funded in part by the Norman E. Shumway Career Development Award of the International Society for Heart and Lung Transplantation to K.R., and by National Institutes of Health/National Heart, Lung, and Blood Institute Grant R01-HL118372 to B.P.G..
The authors thank Sean Daugherty and Amol Shetty of the University of Maryland Institute of Genome Sciences for processing Ingenuity IPA pathway analyses.
Funding Sources: This work was funded by the Norman E. Shumway Career Development Award of the International Society for Heart and Lung Transplantation to KR, and National Institutes of Health (National Heart, Lung, and Blood Institute) Grants 1R01 HL 124170 to ZJW and 1R01 HL 118372 to BPG.
List of Abbreviations
- 7-TMR/GPCR
7-transmembrane receptor/G protein-coupled receptor
- β–AR
β-adrenergic receptor
- AT1R
angiotensin II type 1 receptor
- EGFR
epidermal growth factor receptor
- ERK
extracellular regulated kinase
- ETR
endothelin receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HF
heart failure
- LVAD
left ventricular assist device
- MCS
mechanical circulatory support
- MI
myocardial infarction
- LAD
left anterior descending
- LV
left ventricle/ventricular
- RNA
ribonucleic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interests pertaining to any of this work.
References
- 1.Hellermann JP, Goraya TY, Jacobsen SJ, Weston SA, Reeder GS, Gersh BJ, et al. Incidence of heart failure after myocardial infarction: is it changing over time? Am J Epidemiol. 2003;157(12):1101–7. doi: 10.1093/aje/kwg078. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117(5):686–97. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. New Engl J Med. 2009;361(23):2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 5.Wei X, Li T, Hagen B, Zhang P, Sanchez PG, Williams K, et al. Short-term mechanical unloading with left ventricular assist devices after acute myocardial infarction conserves calcium cycling and improves heart function. JACC Cardiovasc Interv. 2013;6(4):406–15. doi: 10.1016/j.jcin.2012.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom AS, Pilla JJ, Arkles J, Dougherty L, Ryan LP, Gorman JH, 3rd, et al. Ventricular restraint prevents infarct expansion and improves borderzone function after myocardial infarction: a study using magnetic resonance imaging, three-dimensional surface modeling, and myocardial tagging. Ann Thorac Surg. 2007;84(6):2004–10. doi: 10.1016/j.athoracsur.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 7.de Tombe PP. Cardiac myofilaments: mechanics and regulation. J Biomech. 2003;36(5):721–30. doi: 10.1016/s0021-9290(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 8.Tibayan FA, Lai DT, Timek TA, Dagum P, Liang D, Daughters GT, et al. Alterations in left ventricular torsion in tachycardia-induced dilated cardiomyopathy. J Thorac Cardiovasc Surg. 2002;124(1):43–49. doi: 10.1067/mtc.2002.121299. [DOI] [PubMed] [Google Scholar]
- 9.Yankey GK, Li T, Kilic A, Cheng G, Satpute A, Savai K, et al. Regional remodeling strain and its association with myocardial apoptosis after myocardial infarction in an ovine model. J Thorac Cardiovasc Surg. 135(5):991–998. doi: 10.1016/j.jtcvs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopal K, Lefkowitz RJ, Rockman HA. When 7 transmembrane receptors are not G protein-coupled receptors. J Clin Invest. 2005;115(11):2971–4. doi: 10.1172/JCI26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–86. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6(6):499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 13.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103(42):15463–8. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488(7411):394–8. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodall MC, Ciccarelli M, Woodall BP, Koch WJ. G protein-coupled receptor kinase 2: a link between myocardial contractile function and cardiac metabolism. Circ Res. 2014;114(10):1661–70. doi: 10.1161/CIRCRESAHA.114.300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24(5):643–52. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, et al. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci USA. 2006;103(44):16284–9. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303(8):H1001–10. doi: 10.1152/ajpheart.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, et al. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45(5):668–76. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, et al. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12(5):360–8. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy SK, 1, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001 Nov 9;294(5545):1307–13. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009;284(18):11953–62. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3(125):ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, et al. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014;129(9):1009–21. doi: 10.1161/CIRCULATIONAHA.113.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houweling AC, van Borren MM, Moorman AF, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc Res. 2005;67(4):583–93. doi: 10.1016/j.cardiores.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Antoniak S, Pawlinski R, Mackman N. Protease-activated receptors and myocardial infarction. IUBMB Life. 2011;63(6):383–9. doi: 10.1002/iub.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingel K, Kandolf R. Osteopontin: a biomarker to predict the outcome of inflammatory heart disease. Semin Thromb Hemost. 2010;36(2):195–202. doi: 10.1055/s-0030-1251504. [DOI] [PubMed] [Google Scholar]
- 28.Majalahti T, Suo-Palosaari M, Sármán B, Hautala N, Pikkarainen S, Tokola H, et al. Cardiac BNP gene activation by angiotensin II in vivo. Mol Cell Endocrinol. 2007;273(1-2):59–67. doi: 10.1016/j.mce.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Knowlton KU, Baracchini E, Ross RS, Harris AN, Henderson SA, Evans SM, et al. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991;266(12):7759–68. [PubMed] [Google Scholar]
- 30.Shubeita HE, McDonough PM, Harris AN, Knowlton KU, Glembotski CC, Brown JH, et al. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990;265(33):20555–62. [PubMed] [Google Scholar]