Abstract
Objectives
Atrasentan, an oral endothelin-A receptor antagonist, demonstrated Phase I activity in patients with renal cell cancer (RCC). A phase II study was undertaken in patients with measurable or bone only metastatic RCC in the pre-VEGF/TKI era.
Methods and Materials
Patients were stratified on disease status and prior immunotherapy. Eligible patients had no prior chemotherapy, 0-1 prior immunotherapies, and ECOG PS 0 - 2. Patients received atrasentan 10 mg/day until progression. The primary endpoint was progression-free (PF) rate at 6 months. Rates of 25% among patients treated with prior immunotherapy and 45% among patients with no prior immunotherapy were considered promising. A two-stage design was used for cohorts without prior immunotherapy.
Results
From 2003 to 2005, 98 patients were registered. Median treatment duration was 9.9 weeks (range, 0.3 - 107 weeks). Toxicities were mild; 71% of patients reported no Grade 3 or higher treatment-related events. Grade 4 events included neutropenia (n=3), dyspnea (n=2), thrombosis, and arrhythmia (n=1 each). Two grade 5 events (dyspnea and constitutional) were possibly treatment-related. Six-month PF rates (90% CI) were 14% (6 - 25%), 0% (0 – 39%), 8% (1 – 23%) and 22% (8 – 44%) respectively for patients with prior immunotherapy/measurable disease (n=44), prior immunotherapy/bone metastases (n=6), no prior immunotherapy/measurable disease (n=25), and no prior immunotherapy/bone metastases (n=18). Median PF survival was 2.3 months (95% CI, 2.0 – 3.5 months).
Conclusions
While well tolerated, atrasentan did not yield 6-month PF rates supporting its use as first-line monotherapy in patients with advanced RCC.
Keywords: kidney cancer, endothelin-A receptor antagonist, bone metastases
INTRODUCTION
Renal cell carcinoma is the third most common genitourinary cancer with approximately 61,560 new cases and 14,080 deaths projected for 20151. At the time of diagnosis, 30% of patients have distant metastases and 25% have locally advanced disease. Since this study was conducted, improvements in overall survival have been reported using temsirolimus in patients with poor prognosis metastatic RCC.2 Improvements in progression-free survival (PFS) using vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors (TKIs) have also been reported, and have been adequate for FDA approval3,4,5. However, the search for new and potentially active agents continues. Aside from recent promising studies with immune checkpoint inhibitors, VEGF TKIs and mTOR inhibitors are the mainstay agents. Targeting other pathways may have a role in RCC.
The endothelins, a family of amino acid peptides, are produced in a variety of tissues, including the kidney. Among other functions, they act as modulators of cell proliferation and may contribute to the morbidity and mortality of advanced cancers, such as prostate, renal and ovarian cancers.6,7,8,9 Atrasentan (Abbott Laboratories, Abbott Park, IL) is an orally available selective endothelin receptor antagonist that was found to be an active mitogen in prostate and other cancer cell lines. Differential expression of ET receptors (ETA and ETB) as well as the endothelin-converting enzymes (ECE-1 and ECE-2) were noted in a 2004 article By Douglas et al10. In this manuscript, the endothelin axis was elevated in clear cell renal cancer, but impaired in papillary renal cancer. These results were confirmed in a report by Pflug et al11 and attributed to methylation of the ETB receptor promoter as a possible mechanism to enhance ETA receptor activation in renal cancer. A comprehensive summary of the role of endothelin receptor antagonists in cancer therapy has been reported by Lalich et al.12 After phase I studies demonstrated some responses in patients with RCC13,14, this study was proposed.
Additionally, it was hypothesized that atrasentan might provide palliative therapeutic benefit in treating bone pain due to metastatic cancer. 15 In an accrual expansion added during the course of the study, we also sought to investigate the potential benefit of atrasentan in patients with bone metastases from RCC. Toxicity in prior studies was manageable and would support the use of atrasentan in this setting.
Based on the phase I data in patients with RCC and the well-tolerated toxicity profile, this study was undertaken.
MATERIALS AND METHODS
Eligibility Criteria
Between July 2003 and July 2005, 98 patients were recruited from 21 sites. Eligible patients had histologically proven advanced RCC, defined as locally recurrent or metastatic disease not amenable to resection. Clear cell and papillary histologies were allowed. While no prior chemotherapy was allowed, patients may have had prior nephrectomy, immunotherapy, bisphosphonate therapy, or radiation for local control or palliation of painful bony lesions. (This study was conducted before the advent of routine use of VEGF TKIs.) Patients must have had ECOG performance status of 0, 1, or 2 and adequate bone marrow, liver and renal function. No concurrent infection, pulmonary disease, cardiovascular disability, current or prior history of brain metastases, recent history of prior malignancy, or intercurrent illness interfering with the patient’s safety were permitted.
Study Design
Patients were accrued separately to four cohorts based on prior immunotherapy and whether they had measurable disease by RECIST criteria or non-measurable disease manifested solely by bone metastases. The cohorts for patients with bone metastases only were added after the study had been active for about 7 months, based on results from a parallel study of patients with prostate cancer.15 Institutional review boards governed sites that registered patients, and all patients provided signed, written informed consent. The study is registered in clinicaltrials.gov (NCT00039429).
Patients were treated with atrasentan 10 mg per day administered orally. The drug was to be taken at the same time each day, at least 30 minutes prior to the patient’s first meal. Missed doses were not replaced. Patients completed a pill calendar, recording missed doses, side effects, and other medications. A study team member also conducted pill counts at each follow-up visit (every 4 weeks).
There were no dose reductions. Doses were held for hematologic toxicity of grade 3 or 4. If counts did not recover to ≤ grade 2 within 4 weeks, the patient discontinued treatment. Doses were also held for non-hematologic toxicity of grade 3. If toxicities did not resolve to ≤ grade 1 or baseline within 2 weeks, the patient discontinued treatment. Occurrence of a second clinically significant grade 3 toxicity or any grade 4 non-hematologic toxicity resulted in permanent treatment discontinuation. Additional criteria for holding treatment for nausea, neurotoxicity, and hepatic toxicity were specified in the protocol. Patients received supportive measures consistent with optimal patient care.
Patients were treated until there was unacceptable toxicity, evidence of disease progression, or the patient withdrew consent. Response evaluation was performed following every 2 cycles (8 weeks).
Outcome Measures
RECIST criteria (version 1.0)16 were used to determine response and progression. For patients with bone metastases only, RECIST criteria for progression in non-target lesions (new lesions or unequivocal progression in bone lesions) applied. Time to progression was defined as the time from registration to documentation of progression. For the primary endpoint (proportion progression-free at 6 months), patients alive and free from progression at least 6 months from registration were considered progression-free and all others were not. For the secondary endpoint of PFS, patients without progression but who died within 2 months of the last disease evaluation were considered failures at the time of death. Patients alive 2 months after the last evaluation showing freedom from progression were censored at the last disease evaluation. Patients with no follow-up disease evaluations were considered failures if they died within 2 months of study entry and were censored at the time of the first scheduled disease evaluation (~56 days) otherwise.
Survival was defined as time from study entry to death from any cause. Patients alive at last follow-up were censored on that date.
Statistical Analysis
The primary objective was to determine the proportion of patients with measurable disease or bone metastases only, with or without prior immunotherapy, who were alive and progression-free (PF) at 6 months. In patients with prior immunotherapy, a PF rate at 6 months of 25% was considered promising, while a rate of 10% was not. Among patients with no prior immunotherapy, a PF rate at 6 months of 45% was considered promising, while a rate of 25% was not, and a two-stage design was used. The rules led to a study with 10% Type I and Type II error.
Exact 90% binomial confidence intervals (CI) were formed on the PF rates. The Kaplan-Meier17 method was used to illustrate PFS and overall survival (OS). Differences in proportions PF at 6 months by gender and ethnicity were evaluated using Fisher’s exact test. Differences in OS and PFS were estimated using the log rank test18. Adverse events were assessed using CTCAE Version 319. P-values are two-sided.
A modification of risk factors developed by Motzer et al20 at Memorial Sloan-Kettering Cancer Center (MSKCC) was used to characterize patients. A point was assigned for each of the following: performance status > 0, corrected calcium ≥ 10 mg/dL, hemoglobin less than the institution’s lower limit of normal, and no prior nephrectomy. Patients were classified as low risk (0), intermediate risk (1) or high risk (≥2 points).
RESULTS
Patient Characteristics
The study enrolled 98 patients and terminated before all cohorts had been accrued, after sorafenib and sunitinib were approved in this setting. Four patients were ineligible (baseline disease evaluations were inadequate or outside the required timeframe). One patient withdrew before treatment. Efficacy analyses include 93 eligible, treated patients. Table 1 provides demographic and disease characteristics of patients. The median age of patients was 62 years (range, 37 to 86 years). Eighty-two patients (88%) had undergone prior nephrectomy. Most patients had 0 or 1 risk factors according to MSKCC criteria. No patients had more than 2 risk factors, but corrected calcium was only available for 52 patients. Most common sites of metastases at entry were lung, liver, bone, and lymph nodes.
TABLE 1.
Demographics and Disease Characteristics
| Prior Immunotherapy | No Prior Immunotherapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurable Disease |
Bone Mets Only |
Measurable Disease |
Bone Mets Only |
||||||
| N | % | N | % | N | % | N | % | ||
| Eligible Treated Patients | 44 | 6 | 25 | 18 | |||||
| Age, years | median (range) | 63 (42 - 86) | 62.5 (50 – 70) | 68 (48 - 81) | 58 (37 – 84) | ||||
| Months from Diagnosis to Registration |
median (range) | 30.1 (2.9 – 238.2) |
51.3 (15.1 – 134.3) |
5.6 (0.7 – 265.9) |
8.9 (1.2 – 221.2) |
||||
| Gender | Male | 30 | 68.2 | 3 | 50.0 | 17 | 68.0 | 14 | 77.8 |
| Female | 14 | 31.8 | 3 | 50.0 | 8 | 32.0 | 4 | 22.2 | |
| Race | White | 42 | 95.5 | 5 | 83.3 | 23 | 92.0 | 18 | 100.0 |
| Black | 1 | 2.3 | - | - | 2 | 8.0 | - | - | |
| Other | 1 | 2.3 | 1 | 16.7 | - | - | - | - | |
| ECOG | 0 | 22 | 50.0 | - | - | 15 | 60.0 | 3 | 16.7 |
| Performance | 1 | 21 | 47.7 | 2 | 33.3 | 9 | 36.0 | 12 | 66.7 |
| Status | 2 | 1 | 2.3 | 4 | 66.7 | 1 | 4.0 | 3 | 16.7 |
| Histology | Clear Cell | 27 | 61.4 | 2 | 33.3 | 18 | 72.0 | 13 | 72.2 |
| Other | 4 | 9.1 | 1 | 16.7 | 1 | 4.0 | 2 | 11.1 | |
| MSKCC Risk | 0 | 21 | 47.7 | - | - | 12 | 48.0 | 3 | 16.7 |
| Factors | 1 | 21 | 47.7 | 5 | 83.3 | 11 | 44.0 | 13 | 72.2 |
| 2 | 2 | 4.5 | 1 | 16.7 | 2 | 8.0 | 2 | 11.1 | |
| Prior Treatment | Nephrectomy | 41 | 93.2 | 5 | 83.3 | 20 | 80.0 | 16 | 88.9 |
| Immunotherapy | 44 | 100.0 | 6 | 100.0 | 2 | 8.0 | 1 | 5.6 | |
| Hormonal Therapy | 2 | 4.5 | - | - | - | - | 1 | 5.6 | |
| Radiation Therapy | 6 | 13.6 | 5 | 83.3 | 4 | 16.0 | 12 | 66.7 | |
| Metastatic Sites at Entry |
Bone Liver |
10 12 |
22.7 27.3 |
6 - |
100.0 - |
3 6 |
12.0 24.0 |
18 - |
100.0 - |
| Lung | 33 | 75.0 | - | - | 20 | 80.0 | - | - | |
| Lymph Nodes | 20 | 45.5 | - | - | 13 | 52.0 | - | - | |
| Other | 18 | 40.9 | 1 | 16.7 | 10 | 40.0 | - | - | |
Treatment
Patients were treated until progression or unacceptable toxicity. Median treatment duration was 9.9 weeks (range, 0.3 – 107 weeks), slightly longer than the interval to the first scheduled disease evaluation. For 81% of patients, treatment was discontinued due to progression. Other reasons for discontinuation included adverse events (5%), death (2%), patient withdrawal/refusal (5%), other complicating disease (1%), and other reasons (5%).
Safety
One untreated patient had no follow-up adverse event (AE) assessments. For 68 of the remaining 97 patients (70%), no AEs greater than grade 2 were reported. While atrasentan was generally well tolerated, two grade 5 AEs possibly related to treatment were reported. One patient with a history of chronic obstructive pulmonary disease was admitted to the hospital for hip replacement, declined, and died later in hospice care. Attributions to disease, atrasentan, COPD and concurrent medications were all considered “possible”. Another patient was admitted to the hospital in declining health and found to have progressive disease. The patient was discharged to hospice care and died shortly thereafter. Death was considered definitely related to disease progression and possibly to atrasentan. Five other patients died within 30 days of the end of treatment from disease progression. Treatment-related AEs of ≥ grade 3 appear in Table 2. Commonly reported events include those related to atrasentan’s function as a vasodilator, including anemia, edema, and dyspnea. About a third of patients experienced nasal stuffiness (reported as “allergic rhinitis”), but it was not reported at grade 3 severity. Ten patients experienced weight gain of less than 10% of body weight.
TABLE 2.
Treatment-Related Adverse Events
| Grade | |||
|---|---|---|---|
| 3 (%) |
4 (%) |
5 (%) |
|
| Hemoglobin | 3 | - | - |
| Neutrophils | 1 | 3 | - |
| Transfusion: PRBCs | 5 | - | - |
| Supraventricular Arrhythmias | 1 | 1 | - |
| Cardiac-Left Ventricular Function | 1 | - | - |
| Edema | 4 | - | - |
| Thrombosis/Embolism | - | 1 | - |
| Fatigue | 3 | - | - |
| Constitutional | - | - | 1 |
| Anorexia | 1 | - | - |
| Dehydration | 1 | - | - |
| Nausea | 1 | - | - |
| Hypoalbuminemia | 2 | - | - |
| SGOT | 1 | - | - |
| SGPT | 1 | - | - |
| Hyperglycemia | 1 | - | - |
| Hypocalcemia | 1 | - | - |
| Hyponatremia | 5 | - | - |
| Headache | 1 | - | - |
| Dyspnea | 4 | 2 | 1 |
| Hypoxia | 1 | - | - |
| Pleural Effusion | 2 | - | - |
| Creatinine | 1 | - | - |
|
| |||
| Worst Degree Toxicity by Patient | 21 | 5 | 2 |
Proportion Alive and Progression-Free at 6 Months
Table 3 shows rates of patients alive and PF for each cohort with 90% confidence intervals. Among all patients with prior immunotherapy, 12.0% were PF at 6 months (90% exact binomial CI, 5.4 – 22.5%). Among all patients without prior immunotherapy, the proportion was 14.0% (90% CI, 6.3 – 25.7%). Overall the rate was 12.9% (90% CI, 7.6 – 20.1%). The upper bounds of the CIs were generally less than the target rates of interest (25% and 45%, respectively, for patients with/without prior immunotherapy). Six patients without follow-up scans and one patient whose last disease assessment was less than 6 months from registration were considered not to be PF at 6 months and were included in the negative category.
TABLE 3.
Proportion Alive and Progression-Free at 6 Months
| Prior Immunotherapy | No Prior Immunotherapy | |||||||
|---|---|---|---|---|---|---|---|---|
| Measurable Disease |
Bone Mets Only |
Measurable Disease |
Bone Mets Only |
|||||
| N | % | N | % | N | % | N | % | |
| No | 38 | 86.4% | 6 | 100.0% | 23 | 92.0% | 14 | 77.8% |
| Yes | 6 | 13.6% | 0 | 0.0% | 2 | 8.0% | 4 | 22.2% |
| 90% Confidence | ||||||||
| Interval | 6.1 – 25.2% | 0.0 – 39.3% | 1.4 – 23.1% | 8.0 – 43.9% | ||||
There were no complete or partial responses. Approximately 28% of patients with measurable disease had periods of stable disease. Thirty-three percent of patients with bone metastases after prior immunotherapy had stable disease, and 44% of patients with bone metastases and no prior therapy had stable disease as best response.
Progression-free Survival
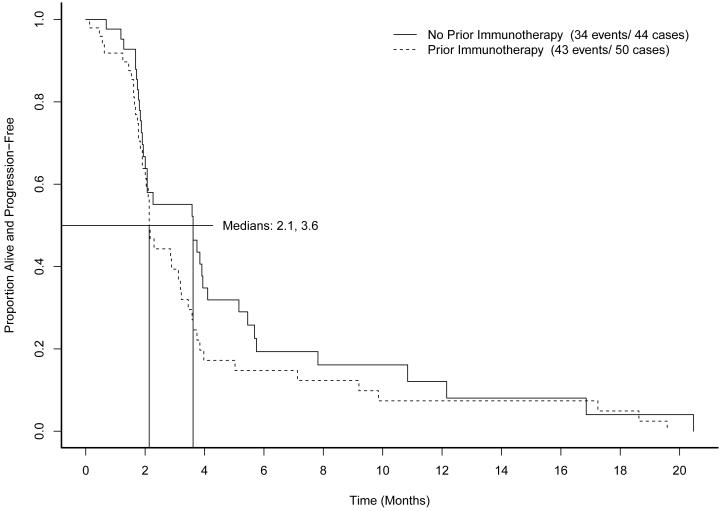
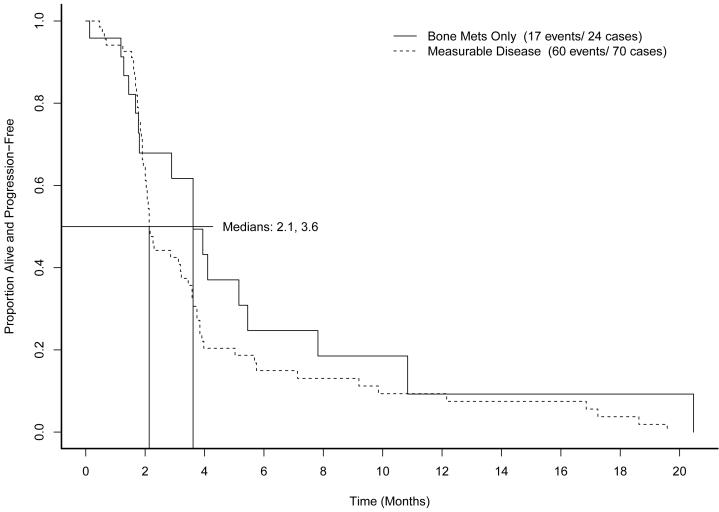
Across all cohorts, median PFS was 2.3 months (95% CI, 2.1 to 3.6 months). Figure 1 shows PFS by prior immunotherapy cohort. Figure 2 shows PFS by measurable disease cohort. No differences in PFS were found to be associated with MSKCC risk category (p=0.43). The supplemental table shows median survival and hazard ratios for subsets of patients defined by metastatic site, prior treatment, sex, histology, and risk category.
Figure 1.
Progression-free survival by prior immunotherapy
Figure 2.
Progression-free survival by measurable disease status
Survival
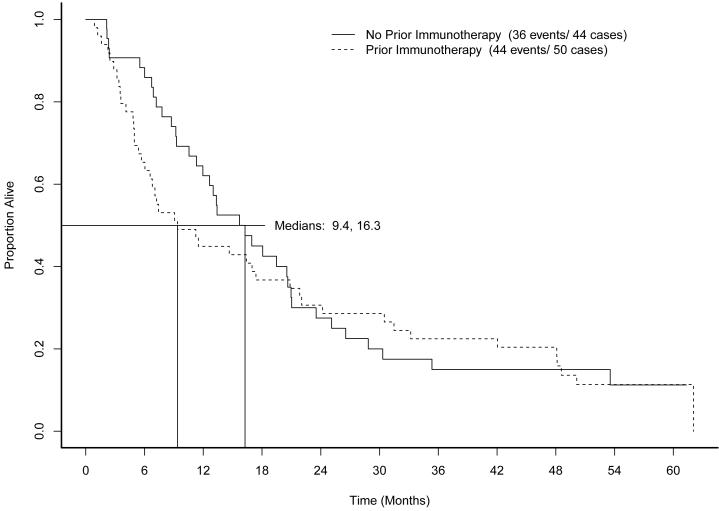
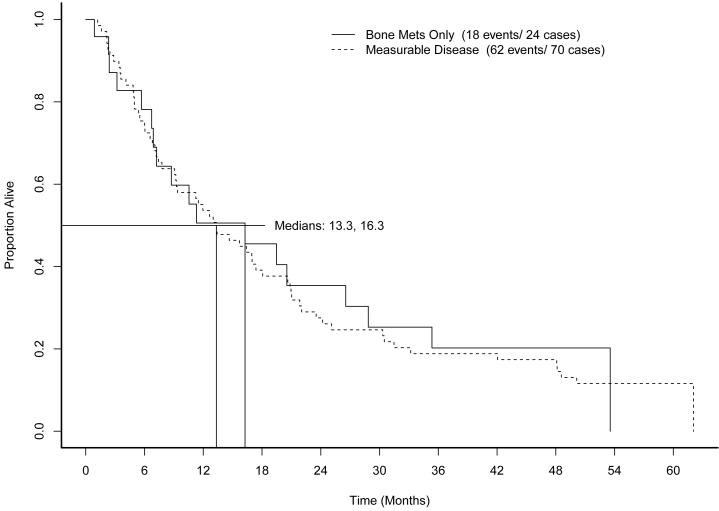
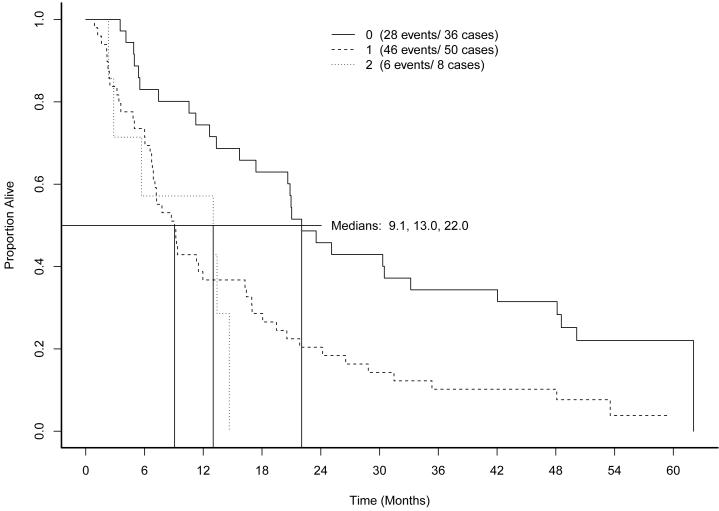
As of May 2009, when follow-up was discontinued, 74 of the 93 patients had died. Median OS was 13.3 months. Figure 3 shows OS by prior immunotherapy cohort. Figure 4 shows overall survival by measurable disease cohort. Figure 5 shows OS by MSKCC risk category. Low risk patients demonstrated superior OS (22 months compared to 9.1 months for intermediate risk and 13.0 months for high risk patients, log rank p=0.002).
Figure 3.
Overall survival by prior immunotherapy
Figure 4.
Overall survival by measurable disease status
Figure 5.
Overall survival by MSKCC Risk Status
Changes in Tumor Burden over Time in Patients with Measurable Disease
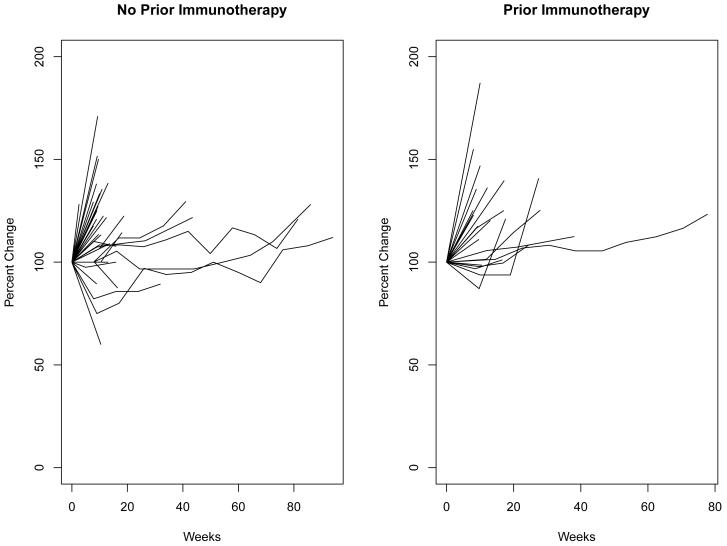
In 2004, Yang21 illustrated the effect of bevacizumab for patients with metastatic RCC by showing changes in tumor volume over time during treatment. Figure 6 shows changes in tumor burden over time among patients with measurable disease, here expressed as a percent change from baseline in the sum of the longest diameters of target lesions. Few patients demonstrated long-term reduction in tumor burden.
Figure 6.
Changes in tumor burden over time among patients with measurable disease
DISCUSSION
This phase II study of atrasentan was conducted just prior to the advent of targeted therapies for RCC, when biologic response modifiers such as interferon and interleukin were the only therapies shown to have consistent responses. Based on early observation of responses among RCC patients in Phase I studies, this study’s hypothesis was rational. As evidence emerged among prostate cancer patients that atrasentan might provide palliative relief from bone pain, the addition of cohorts with bone metastases was also reasonable. The outcome of the study provides evidence that single agent atrasentan is not an effective therapy for advanced RCC.
Since the agent was not expected to elicit measurable responses, the endpoint of PF status at 6 months was assessed. In none of the cohorts did the PF rate approach the targeted rate. Accrual to the cohort of patients with bone metastases and prior immunotherapy closed early, as interest in sorafenib, sunitinib, bevacizumab, and temsirolimus increased. Patients on the study had relatively short courses of therapy, typically discontinuing treatment due to disease progression. There is a sharp decline in PFS at the first follow-up disease assessment. Yang7 pointed out that with bevacizumab, some lesions might be progressing while at the same time others were stable or decreasing. A similar speculation could be applied to atrasentan, but the median OS of 13.3 months is not encouraging. Examination of changes in tumor burden over time provides no evidence of declines in tumor burden relative to baseline. We conclude that further study of atrasentan as single agent treatment for advanced RCC is not warranted.
Since the study completed, results from other studies of atrasentan in renal cancer in renal cancer have been reported. A phase I study of atrasentan and Interferon-α showed one partial response and inconsistent changes in pharmacodynamic parameters such VEGF and ET-1 levels22. -Given the preclinical hypothesis that endothelin contributed to the morbidity and mortality of prostate cancer, this was the target population for several trials23,24,25,26. Ultimately, all studies failed to achieve their primary endpoint and further development of atrasentan has stopped. Similar results were observed for zibotentan, another endothelin antagonist27,28,29. Our negative study in RCC confirms the lack of significant benefit in another tumor type.
The safety profile that emerged from the phase III studies strengthened evidence for the role of endothelin-A as a “vasodilator”. Peripheral edema, nasal congestion, dyspnea, and headache were all attributed to this role. In the metastatic disease study, some incidents of heart failure were also noted among patients with a history of cardiovascular disease. Anemia, weight gain, and decreases in white blood count were attributed to plasma volume expansion resulting in hemodilution. These effects stabilized after 2 weeks and returned to normal after treatment discontinuation.
The short PFS (median 2.3 months) observed in this study was disappointing. Motzer et al. reported 11 months median PFS for single-agent sunitinib in a trial comparing it to interferon alfa.5 Temsirolimus alone demonstrated median PFS of 3.8 months among patients with intermediate to poor prognosis according to the MSKCC criteria. We did not find differences in PFS associated with MSKCC criteria, but the study was not powered to detect such differences. PFS among patients on atrasentan was somewhat shorter than reported for temsirolimus, even among a more favorable risk cohort of patients. Since the time this study was conducted, several targeted agents have been added to the arsenal of treatments for RCC 30,31,32.
CONCLUSIONS
We conclude that single-agent atrasentan does not contribute to the collection of agents that can be used to treat advanced RCC. While the study was unable to completely accrue to the cohorts of patients with bone metastases only, the description of outcomes in patients with and without prior immunotherapy and with measurable disease may be useful in directing future research toward more fruitful mechanisms.
Supplementary Material
CLINICAL PRACTICE POINTS.
Atrasentan is an orally available selective endothelin receptor antagonist that was found to be an active mitogen in prostate and other cancer cell lines. In phase 1 studies, some responses were observed among patients with renal cell cancer.
This study showed that atrasentan did not prolong progression-free survival in patients with advanced RCC. As a result of this and other studies, atrasentan is no longer being developed.
Aside from recent promising studies with immune checkpoint inhibitors, VEGF TKIs and mTOR inhibitors are the mainstay agents for treating this disease.
ACKNOWLEDGMENTS
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA 180794, CA180820, CA180802, CA180799, CA189863, CA189859, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Investigational agent for the study was provided by Abbott, Inc. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
References
- 1.American Cancer Society Cancer Facts & Figures. 2015 [Google Scholar]
- 2.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet. 2007;370:2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomized, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Tang R, Wang B, et al. Localization of endothelin receptors in the human prostate. J Urol. 1994 Mar;151(3):763–766. doi: 10.1016/s0022-5347(17)35083-8. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JB, Hedican SP, George DJ, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995 Sep;1(9):944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JB, Chan-Tack K, Hedican SP, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- 9.Rosano L, Spinella F, Salani D, DiCastro V, Venuti A, Nicotra MR, Natali PG, Bagnato A. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res. 2003;63:2447–2453. [PubMed] [Google Scholar]
- 10.Douglas ML, Richardson MM, Nicol DL. Endothelin axis expression is markedly different in the two main subtypes or renal cell carcinoma. Cancer. 2004;100:2118–2124. doi: 10.1002/cncr.20222. [DOI] [PubMed] [Google Scholar]
- 11.Pflug BR, Zheng H, Udan MS, D’Antonio JM, Marshall FF, Brooks JD, Nelson JB. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2007;246:139–148. doi: 10.1016/j.canlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Lalich M, McNeel DG, Wilding G, Liu G. Endothelin receptor antagonists in cancer therapy. Cancer Invest. 2007 Dec;25(8):785–794. doi: 10.1080/07357900701522588. [DOI] [PubMed] [Google Scholar]
- 13.Carducci MA, Nelson JB, Bowling MK, et al. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetic. J Clin Oncol. 2002;20:2172–80. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Zonnenberg BA, Groenewegen G, Janus TJ, et al. Phase I dose-escalation study of the safety and pharmacokinetics of atrasentan: an endothelin receptor antagonist for refractory prostate cancer. Clin Cancer Res. 2003;9:2965–72. [PubMed] [Google Scholar]
- 15.Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21(4):679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A. 1972;135:185–206. [Google Scholar]
- 19.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events. Version 3.0, DCTD, NCI, NIH, DHHS, March 31, 2003 ( http://ctep.cancer.gov/MajorInitiatives/Common_Terminology_Criteria.htm), [accessed August 14, 2013]
- 20.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22(3):454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC. Bevacizumab for patients with metastatic renal cancer: an update. Clinical Cancer Res. 2004;10:6367s–6370s. doi: 10.1158/1078-0432.CCR-050006. [DOI] [PubMed] [Google Scholar]
- 22.Groenewegen G, Walraven M, Vermaat J, deGast B, Witteveen E, Giles R, Haanen K, Voest E. Targeting the endothelin axis with atrasentan, in combination with IFN-alpha, in metastatic renal cell carcinoma. Br J Cancer. 2012;106:284–289. doi: 10.1038/bjc.2011.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaelson MD, Kaufman DS, Kantoff P, Oh WK, Smith MR. Randomized phase II study of atrasentan alone or in combination with zoledronic acid in men with metastatic prostate cancer. Cancer. 2006;107:530–535. doi: 10.1002/cncr.22043. [DOI] [PubMed] [Google Scholar]
- 24.Carducci MA, Saad F, Abrahamsson P-A. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 25.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate Cancer. Cancer. 2008;113:2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (S0421): a randomized phase 3 trial. Lancet Oncol. 2013;14:893–900. doi: 10.1016/S1470-2045(13)70294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fizazi KS, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–1747. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 28.Miller K, Moul JW, Gleave M, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Disease. 2013;16:187–192. doi: 10.1038/pcan.2013.2. [DOI] [PubMed] [Google Scholar]
- 29.Nelson JB, Fizazi K, Miller K, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012;118:5709–5718. doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 30.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 31.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 32.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomized phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.