Abstract
The induction of a potent humoral and cellular immune response in mucosal tissue is important for the development of an effective HIV vaccine. Most of the current HIV vaccines under development use the intramuscular route for immunization, which is relatively poor in generating potent and long-lived mucosal immune responses. Here, we explore the ability of an oral vaccination with a probiotic organism, Lactococcus lactis (L. lactis), to elicit HIV-specific immune responses in the mucosal and systemic compartments of BALB/c mice. We expressed the HIV-1 Gag-p24 on the tip of the T3 pilus of Streptococcus pyogenes as a fusion to the Cpa protein (LL-Gag). Following four monthly LL-Gag oral immunizations we observed strong Gag-specific IgG and IgA responses in serum, feces, and vaginal secretions. However, the Gag-specific CD8 T cell responses in the blood were at or below our detection limit. Following an intramuscular modified vaccinia Ankara (MVA)/Gag boost, we observed a robust Gag-specific CD8 T cell responses both in systemic and mucosal tissue, including intraepithelial and lamina propria lymphocytes of the small intestine, Peyer's patches, and mesenteric lymph nodes. Consistent with strong immunogenicity, the LL-Gag induced activation of CD11c+ CD11b+ dendritic cells in the Peyer's patches after oral immunization. Our results demonstrate that oral immunization with L. lactis expressing an antigen on the tip of the Group-A streptococcus pilus serves as an excellent vaccine platform to induce strong mucosal humoral and cellular immunity against HIV.
Introduction
According to UNAIDS, about 35 million people were living with HIV-1 at the end of 2012. Most HIV infections occur via genital and rectal mucosal routes (1). Currently, there are no effective vaccines available to prevent HIV infection or disease. Ideally, an HIV vaccine should induce immune responses in both mucosal and systemic compartments and local mucosal immunity is critical for protection against mucosal HIV transmission (2). In addition, it is important to generate both humoral and cellular immunity as both of these responses contribute to the prevention and/or control of infection.
Numerous HIV vaccine strategies including DNA vaccines, recombinant viral vector vaccines, protein immunogens and a combination of these vectors have been developed and some of these are being tested in humans (for listing of candidates in clinical development, see www.iavi.org). Most of the current HIV vaccines under development use the intramuscular (IM) route for immunization, which is relatively poor in generating potent and long-lived mucosal immune responses. Generally, immunization through the mucosal route has elicited far better responses in mucosal tissue than immunization by systemic routes (3-5). For example, the oral route of immunization is the best way to induce a strong immunity in the gut (6). However, most of the HIV vaccine regimens that are being evaluated in humans are not administered through mucosal routes (oral, vaginal or rectal) because either they don't withstand the hostile acidic environment in the stomach when delivered orally or the vaginal and rectal routes are not practical to use. Furthermore, a specific feature of HIV infection is the rapid depletion of CD4 T cells in the gut within days after infection and this happens irrespective of the route of infection (7-9). This early depletion is not reversible following anti-retroviral therapy and contributes to rapid disease progression. Thus, there is a great need for the development of an HIV vaccine that can be delivered orally and is capable of inducing potent anti-viral immunity in the gut with the potential to block or control HIV replication and prevent infection and/or rapid loss of CD4 T cells.
Lactococcus species have been explored as vaccine vectors for generating mucosal immunity against infectious diseases (10, 11). The key advantages of using a Lactococcus vaccine vector are: 1) Lactococcus is a GRAS (Generally Regarded As Safe) organism, 2) it naturally withstands stomach acids and bile (12), 3) it can be administered repeatedly since it survives only temporarily in the intestinal tract and does not colonize humans (12), 4) it has intrinsic adjuvant properties (13), 5) it does not require a cold chain, and 6) it is inexpensive to produce. Also Lactococcus is a Gram-positive bacterium and therefore does not possess endotoxic lipopolysaccharides (LPS), which are associated with commonly used vaccine strain Gram-negative bacteria such as Escherichia coli and Salmonella typhimurium (10, 14-17). Recently, the potential of lactococci as a delivery vector for a DNA vaccine was demonstrated; native noninvasive recombinant lactococcal strains deliver fully functional plasmids to epithelial cells in-vivo and in-vitro (14, 15, 18-20), thus it seemed likely that lactococci would be effective delivery vectors for DNA vaccines.
In the present study, we developed a recombinant L. lactis based vaccine expressing an HIV antigen and tested its potential to induce mucosal immunity following vaccination through different mucosal routes. Specifically, we used an UPTOP (unhindered presentation on tips of pili)(15) system for the expression of the HIV Gag protein on the lactococcal surface. UPTOP utilizes the T3 pilus of Streptococcus pyogenes (the group A streptococcus or GAS) to present a desired antigen to the immune system. The GAS T3 pilus locus encodes the major T3 pilin subunit, two minor pilin subunits, Cpa and OrfB, SipA2 and the pilin specific sortase enzyme SrtC2. Polymerization of the T3 pilus requires SrtC2 and SipA2, and the pilus can be anchored to the cell surface by the pilus specific or housekeeping sortases. T3 pili offer an excellent platform for the presentation of recombinant proteins to the immune system because they have covalent linkages between subunits, and are covalently linked to the cell wall, and extend out from the bacterial surface, providing access to the encoded antigen to the antigen presenting cells or B cells. We engineered a plasmid encoding the GAS T3 pilus with cpa fused to gag P24 an antigen from an HIV-1 clade B (a predominant strain circulating in the USA) and expressed it in L. lactis strain MG1363. We call this strain LL-Gag.
Here we intragastrically immunized BALB/c mice with LL-Gag and observed potent humoral and cellular immune responses in mucosal and systemic compartments. We also tested the effect of an intramuscular modified vaccinia Ankara (MVA) boost, previously used successfully in our lab to boost B and T cell responses(21-23), on the LL-Gag vaccine-generated Gag-specific responses. We then compared the LL-Gag induced gut-associated immune responses to those of an intramuscular DNA primed and MVA boosted vaccine modality that is in clinical studies in the USA and demonstrate that LL-Gag induces stronger humoral immunity in the gut. Finally, we demonstrated a critical role for the GAS pilus in the activation of a specific subset of mucosal dendritic cells (DC) that may be important for the immunogenicity of Gag.
Materials and Methods
Strains and growth conditions
E. coli strains (TOP10 and XL10-Gold) were cultured in LB media supplemented with the appropriate antibiotic, and grown at 37°C with constant shaking. L. lactis wild-type strain MG1363 was cultured without shaking at 30°C in M17 media (BD Biosciences, San Jones, CA, USA) supplemented with 0.5% glucose (GM17). Recombinant L. lactis clones were cultured in GM17 media supplemented with spectinomycin (100ug/ml) at 30°C without shaking.
Construction of L. lactis expressing the HIV-1 clade B Gag (LL-Gag) antigen
The HIV-1 clade B consensus Gag-p24 region was PCR amplified from the pGA1/JS8 vector (GenBank accession number AY531263 http://www.ncbi.nlm.nih.gov/genbank (24) using primers that amplified the region encoding amino acids 132-363 of Gag and subcloned into the cpa region of the FCT-3 GAS pilus encoding amino acids 57-593 of Cpa in the pJRS9550 vector (15). This resulting vector, pJRS9630, was transformed into electrocompetent wild-type L. lacits (strain: MG1363) cells (25) and transformants were selected on GM17 agar plates supplemented with 100 μg/ml spectinomycin at 30°C.
Dot blot analysis.
Dot blot assays were performed as previously described (26). Briefly, 1 ml aliquots of LL-Wt and LL-Gag cultures cultured overnight were washed twice with PBS and resuspended in 100 μl of PBS; 5 μl of these preparations were spotted onto a nitrocellulose membrane and air-dried. After blocking for 1 hour at room temperature with 2% ECL prime blocking agent (GE Healthcare) in PBS + 0.05% Tween 20 (PBST), the membranes were incubated with a 1:4,000 dilution of a rabbit anti-T3 hyper immune serum or a 1:5,000 dilution of a mouse anti-Gag monoclonal antibody (clone H12.5c, NIH AIDS reagent program;). Membranes were then washed with PBST and incubated with a 1:10,000 dilution of anti-rabbit IgG-HRP (Southern Biotech, Birmingham, Alabama, USA) (anti-T3 antibody treated blots) or anti-mouse IgG-HRP (Southern Biotech, Birmingham, Alabama, USA) (anti-Gag antibody treated blots) at 1:10,000 dilutions. The presence of bound antibodies was detected using AEC substrate (BD Biosciences, San Jones, CA, USA).
Preparation of cell wall and protoplast fractions and Western blot analysis
L. lactis cell wall and cell-protoplast fractions were prepared as previously described (15). Briefly, 2 cell units (one cell unit/ml corresponds to an optical density of 2.0 at 600 nm) LL-Wt and LL-Gag bacterial cells cultured overnight were incubated in 150μl of digestion buffer (50 mM Tris-HCl [pH 6.8], 30% raffinose, 4 mg of lysozyme/ml, 400 U of mutanolysin/ml, and Roche complete protease inhibitors) at 37°C for 3 h with gentle rotation. The mixture was centrifuged for 2 min at 13,000 rpm at 4°C and pellet and supernatant were separated carefully. The pellet containing protoplast fraction was resuspended in 100μl of 1 × SDS loading buffer containing DTT. The supernatant containing cell wall fraction was clarified by centrifugation for 4 min at 13,000 rpm at 4°C. Then, 75μl of the supernatant was carefully transferred to a new tube and the composition was adjusted to 1 × SDS loading buffer containing DTT. The proteins in the cell wall and protoplast fractions were separated on SDS-PAGE using a 4-12% gradient gel (Bio-Rad Laboratories, Hercules, CA, USA), and transferred to a nitrocellulose membrane (GE Healthcare, Pittsburgh, PA, USA). After blocking for 1 hour at room temperature with 2% ECL prime blocking agent (GE Healthcare, Pittsburgh, PA, USA) in PBS + 0.05% Tween 20 (PBST), the blots were probed with either rabbit anti-T3 hyper immune serum (1:4,000 dilution) or mouse anti-Gag monoclonal antibody (clone H12.5c; 1:5,000 dilution). Membranes were washed with PBST and incubated with either anti-rabbit IgG-HRP (for anti-T3 antibody treated blots at 1:10,000 dilution) or anti-mouse IgG-HRP (for anti-Gag antibody treated blots at 1:35,000 dilution at 1:10,000 dilution). The presence of bound antibodies was detected using ECL select western blotting reagent system (GE Healthcare, Pittsburgh, PA, USA) according to the manufacturer's instructions.
Animals and animal immunizations
Female 6–8 week old BALB/c mice were purchased from Charles River Laboratories (Willmington, MA, USA). Mice (n=10/group) were immunized intragastrically with either wild type or LL-Gag monthly at weeks 0, 4, 8 and 12. At each immunization, 5×109 cells/ml was delivered on three consecutive days. For some experiments, animals were immunized with LL-Gag intrarectally (1×109 cells/dose), intranasally (1×109 cells/dose), or intraperitoneally (1×106 cells/dose). Where indicated, mice were immunized intramuscularly with MVA (1×106 pfu/mice) expressing HXB2 Gag and ADA Env (27, 28). Similarly, where indicated mice were immunized with DNA plasmid expressing the HIV-1 clade B consensus Gag (24) with 25μg/dose to each quadriceps of the mouse in buffer containing PBS with 1% ethanol, 0.2mM EDTA, pH7.5.
Sample collection
Blood was collected by facial vein puncture. Vaginal wash was obtained by washing the vagina twice with 25ul of PBS. The wash was centrifuged and the clear supernatant was transferred to a sterile microcentrifuge tube and used for in vitro antibody assays. Fecal pellets were added to an extraction buffer consisting of PBS with 5% FBS and Roche complete protease inhibitors. The pellets were then disrupted via vortex and spun down. The supernatant was removed and used for further assays.
Isolation of lamina proprial lymphocytes (LPLs) and Peyer's patch cells
LPLs from small intestines were isolated as described previously (29). Briefly, small intestines were removed and Peyer's patches were excised. The intestine was opened up longitudinally and cleaned of all fecal contents. Intestines were then cut into small pieces and transferred into 50 ml conical tubes and shaken at 250 rpm for 20 min at 37°C in HBSS (Life Technologies, Grand Island, USA) media supplemented with 5% FBS (Cellgro, Mannassas, VA, USA) and 2 mM EDTA (Promega, Madison, WI, USA). After a total of two rounds of EDTA treatment, cell suspensions were passed through a strainer to separate intra epithelial lymphocytes (IELs). The remaining intestinal tissue was washed and shaken for 20 min at 37°C in HBSS media supplemented with 5% FBS and type VIII collagenase (1.5 mg/ml; Sigma). Cell suspensions were passed through a strainer and cells devoid of undigested tissue were pelleted by centrifugation at 1500 rpm. Mononuclear cells were then isolated using a discontinuous density gradient procedure (45 and 70%) with Percoll (GE Healthcare, Pittsburgh, PA, USA). For isolation of Peyer's patch cells, the excised Peyer's patches were incubated for 20 minutes in HBSS media containing 5% FBS, 0.5 mg/ml of Collagenase type II. Tissues were then mashed through a cell strainer, washed with HBSS media, and re-suspended in RPMI media + 10% FBS.
ELISA for anti-Gag IgG and IgA
ELISA assays were performed as previously described (30). Briefly, ELISA plates were coated overnight with HIV-1 Gag-pr55 (1 ug/ml; Immune Technology Corp, New York, USA) at 4°C, blocked with 4% whey and 5% non-fat dry milk in PBST for 1h at room temperature and washed six times with PBST. Plates were then incubated with different dilutions of serum, fecal extract or vaginal wash (diluted with 5% non-fat dry milk in PBST) for 1h at room temperature. Plates were washed six times with PBST and incubated with HRP conjugated anti-mouse IgG (Southern Biotech, Birmingham, Alabama, USA) antibodies at 1:10,000 dilution. Plates were washed and developed using TMB Microwell Peroxidase Substrate System (KPL, Inc. Gaithersburg, Maryland, USA). A standard curve of IgG was generated by coating the plates with anti-mouse IgG (2μg/ml, Sigma-Aldrich Corp, St. Louis, MO, USA) followed by capturing a two-fold dilution series of purified mouse IgG (Sigma-Aldrich Corp, St. Louis, MO, USA) starting from 800ng/ml, and detecting with the same anti-mouse IgG secondary antibody. Similarly, standard curve of IgA was also generated using antibodies obtained from Southern Biotech, Birmingham, Alabama, USA. ELISA plates were read on a SPECTRAmax PLUS 384 spectrophotometer (Molecular Devices, CA). Standard curves were fitted and total Gag-specific IgG or IgA was determined using SOFTmax 2.3 software (Molecular Devices, Sunnyvale, CA, USA). As the concentration of total IgG and IgA isolated from the feces and vaginal wash can vary, the HIV-1 Gag-specific response in these compartments was calculated as a percent specific activity. The HIV-1 Gag-specific activity was calculated as the fraction of Gag-specific IgA/total IgA.
To detect IgG subclasses, the same protocol described above was used with the exception that IgG1, IgG2a, IgG2b or IgG3 subclass-specific secondary antibody conjugated to HRP (Southern Biotech, Birmingham, Alabama, USA) was used at 1:10,000 dilution.
Tetramer assay
Tetramer assay was performed as previously described (28). Briefly, cells were stained using APC-conjugated Gag-tetramer (NIH tetramer core facility), anti-CD4-FITC, anti-CD19-FITC, anti-CD11a-PE and anti-CD8-PerCP in 100ul of complete RPMI at 4°C for 30 min. Cells were washed twice with wash buffer (PBS with 2% FBS) and acquired (approximately 200,000 lymphocytes) on the FACS Calibur (Becton Dickinson) and analyzed using Flowjo software (Tree Star, San Carlos, CA, USA). All antibodies were purchased from BD Pharmingen. CD8+ CD11a+ CD4− CD19− and Gag-tetramer+ cells were scored as tetramer positive cells.
Intracellular cytokines (ICS) assay
Intracellular cytokine staining (ICS) was performed as described before (28), except that here we used 1ug/ml of Gag immunodominant peptide (AMQMLKETI) instead of a complete set of HxB-2 Gag peptide pools.
Statistical analysis
Differences between the experimental groups were analyzed by unpaired t-test using GraphPad Prism 5.0 Mac (GraphPad Software Inc, La Jolla, CA, USA). Graphs represent two to three independent experiments, and error bars represent means ±s.e. Differences in values were considered significant when P was <0.05.
Results
Construction of recombinant L. lactis expressing the HIV-1 Gag P24 antigen fused to a GAS pilus
Previous studies have established a system for effectively expressing proteins of interest on the surface of L. lactis via their attachment to the minor pilin subunit of GAS that forms the tip of theT3 pilus (15). HIV-1 Gag-p24 was cloned into plasmid pJRS9550 (15, 31), which encodes the GAS FCT-3 locus (cpa, sipA2, tee3, and srtC2), resulting in pJRS9630 (Figure 1a). Gag-p24 was fused to Cpa in a way that preserves Cpa's native N- and C- termini, which are essential for proper protein expression, secretion, and incorporation of Cpa at the pilus tip. The mature recombinant pilus will consist of multimers of the T3 major pilin with the Gag-p24-CPA minor pilin attached at the tip of the pilus, furthest from the bacterial surface (Figure 1b). As described in Methods, plasmid pJRS9630 was transformed into L. lactis (strain MG1363), generating MG1363/pJRS9630 (LL-Gag).
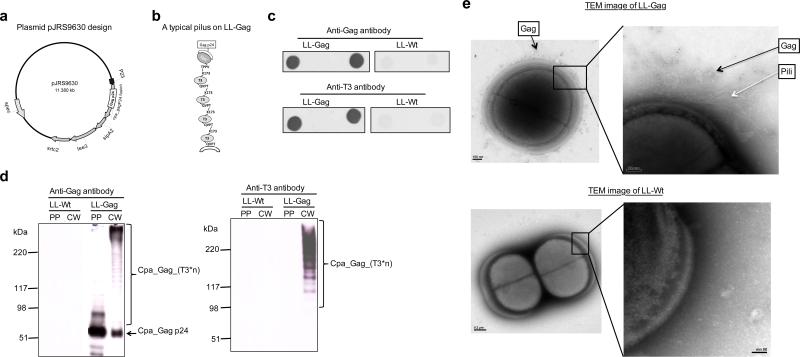
Figure 1. Map of a recombinant T3 pilus operon encoding Cpa-Gagand detection of expressed products in L lactis.
(a) Plasmid map of pJRS9630. HIV-1 Gag-p24 was cloned into pJRS9550, which expresses the FCT-3 gene locus (Cpa, sipA2, tee3, and srtc2). (b) Cartoon of the pilus encoded by pJRS9630, diagraming the probable attachment of the HIV-1 Gag p24-Cpa fusion protein to the pilus. (c) Dot blots analysis using anti-Gag or anti-T3 antibodies demonstrating the presence of the Gag-p24-Cpa fusion protein and T3 on the surface of LL-Gag (L. lactis expressing pJRS9630) but not LL-Wt (wild-type L. lactis) stained. (d) Western blot analysis of protoplast-associated fraction (PP) and cell wall associated fraction (CW) of both LL-Gag and LL-Wt using anti-Gag or anti-T3 antibodies. (e) TEM images of LL-Gag or LL-Wt bacteria after immunogold staining for Gag.
To confirm surface expression of Gag-p24, whole bacteria were spotted onto nitrocellulose membranes, which were then incubated with anti-Gag antibody (see Methods). The anti-Gag antibody bound strongly to the LL-Gag blots, while showing minimal or no reactivity to the wild type L. lactis (LL-Wt) that lacks the plasmid (Figure 1c). This experiment was repeated using a polyclonal anti-T3 antiserum, which confirmed expression of T3 on the surface of LL-Gag and its absence from LL-Wt (Figure 1c). Western blots confirmed the presence of mature Cpa-Gag-p24 (predicted molecular mass of 48 kDa) in both the cell wall fraction (CW) and protoplast fraction (PP) of LL-Gag (Figure 1d) and of the ladder of T3 protein multimers characteristic of T3 pili (15, 31) only in the cell wall fraction of LL-Gag (Figure 1d). Using transmission electron microscopy and immunogold staining using anti-Gag monoclonal antibody, we detected pili containing Gag on LL-Gag cell surface but not on LL-Wt (Figure 1e). Similarly, using anti-T3 antiserum, we detected the presence of T3+ pili on LL-Gag cell surface but not on LL-Wt (data not shown). Together these results established the proper construction of LL-Gag to display the Gag on the pilus.
LL-Gag generates mucosal and systemic Gag-specific antibody responses
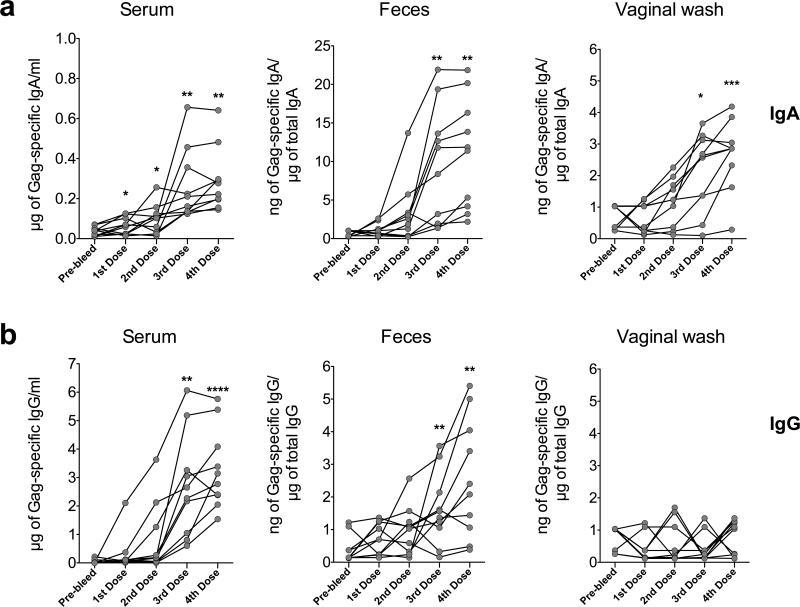
To test for the ability of LL-Gag to induce mucosal immune responses, female BALB/c mice were orally gavaged with LL-Gag in a vaccine regimen of four monthly immunizations, each consisting of three daily doses of LL-Gag. The anti-Gag antibody responses were tracked in the feces, vagina, and serum (Figure 2). Although Gag-specific IgA was detected in the feces of few mice after the second dose, these responses increased significantly after the third and fourth doses (p=0.008 and 0.007 respectively), with most of the mice eliciting Gag-specific antibodies (Figure 2a). The induction of anti-Gag IgA was also seen in the serum and vaginal washes, with the peak response for most of the mice occurring after the third and fourth dose (Figure 2a). Intragastric (IG) immunization with LL-Gag also induced anti-Gag IgG responses in serum and feces but not in vaginal secretions in the majority of the mice by the third and fourth doses (Figure 2b). Taken together, these data suggest that orally delivered LL-Gag induces strong mucosal and systemic Gag-specific IgA and IgG antibody responses upon repeated immunizations.
Figure 2. LL-Gag generates mucosal and systemic anti-Gag antibody responses.
Specific activity (in case of rectal or vaginal) or the concentrations (in case of serum) of HIV-1 Gag-specific (a) IgA or (b) IgG in serum, feces, and vaginal wash at two weeks after each immunization with LL-Gag. The specific activity of Gag-specific IgG and IgA in the fecal and vaginal compartment was calculated as the ratio of Gag-specific IgG or IgA / total IgG isolated from the feces or vaginal wash. Pre-bleed is a pre-vaccination time point. Each line or a dot indicates an individual animal.
The intragastric route of immunization with LL-Gag is the preferred route for generating both mucosal and systemic responses
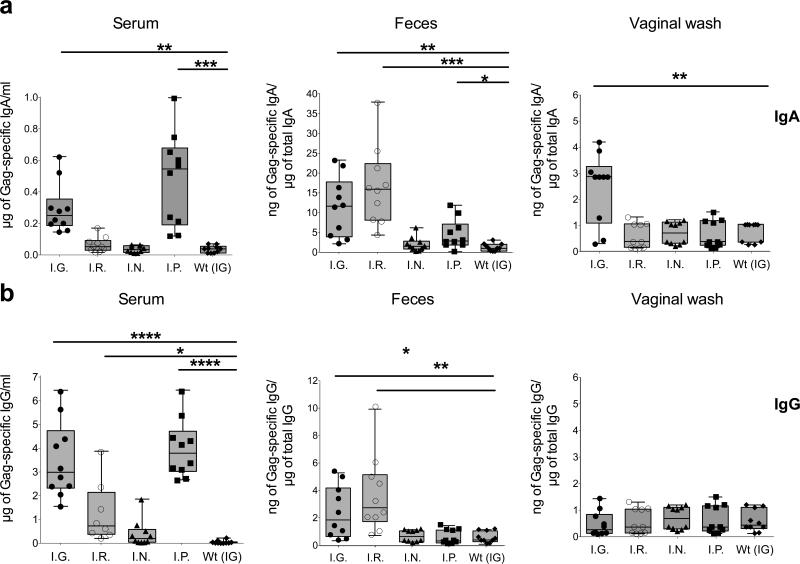
The IG route of vaccine delivery was next compared to other routes of immunization (Figure 3). BALB/c mice were immunized with LL-Gag via the IG, intrarectal (IR), intranasal (IN), or intraperitoneal (IP) route. Mucosal and systemic antibody responses against Gag were then evaluated. Only IG and IP immunizations induced measurable Gag-specific IgA responses in serum (Figure 3a). Similarly and as expected, only IG and IR immunizations induced measurable anti-Gag IgA responses in feces and the responses were comparable between them (Figure 3a). Only the IG immunizations induced measurable anti-Gag IgA responses in vaginal secretions (Figure 3a). A similar pattern was also observed for the Gag-specific IgG responses except that these responses could not be detected in vaginal secretions even in the IG group where Gag-specific IgA was detected (Figure 3b). These results show that while both IG and IR immunizations induced mucosal antibody responses, only the IG route generated both strong systemic and mucosal responses.
Figure 3. Comparison of oral vaccination to other routes of immunization.
Specific activity (rectal or vaginal secretions) or the concentrations (serum) of HIV-1 Gag-specific (a) IgA or (b) IgG induced two weeks after the fourth immunization with LL-Gag delivered intra-gastrically (I.G), intra-rectally (I.R.), intra-nasally (I.N), or intra-peritoneally (I.P). Orally immunization with LL-Wt was used as a negative control.
Oral LL-Gag generates stronger mucosal antibody responses than intramuscular DNA/MVA or MVA/MVA vaccine modalities
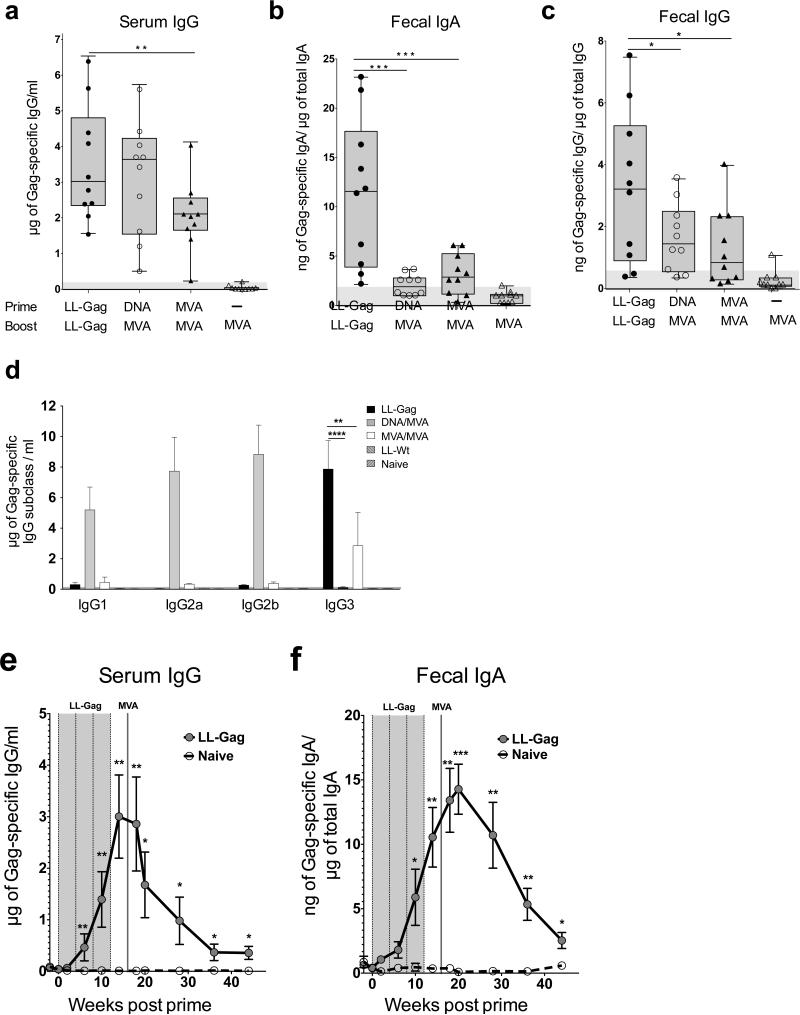
Because prime-boost models of intramuscular vaccination have been shown to generate potent vaccine-specific responses in blood (21), we next compared the systemic and mucosal immune responses generated by LL-Gag oral immunizations with DNA/MVA or MVA/MVA intramuscular immunizations. We selected these two intramuscular modalities since these have completed phase IIa immunogenicity studies in humans (32, 33). As above, the LL-Gag group received four doses of LL-Gag via IG. The DNA/MVA group received DNA vaccine expressing HIV-1 clade B Gag (DNA-Gag) followed by MVA vaccine expressing the same Gag (MVA-Gag). The MVA/MVA group received two doses of MVA-Gag. Both DNA and MVA vaccinations were delivered via IM. Two weeks after the final immunization, the anti-Gag antibodies were quantified in the serum, feces, and vagina (not shown). Impressively, Gag-specific serum IgG induced by LL-Gag vaccination was comparable to that induced by DNA/MVA vaccination and significantly higher than MVA/MVA vaccination (p=0.03) (Figure 4a). A similar trend was seen for the Gag-specific serum IgA responses (data not shown). Importantly, LL-Gag vaccination generated four-fold higher Gag-specific fecal IgA than did the DNA/MVA (p=0.001) or the MVA/MVA (p=0.009) modalities. In addition, the Gag-specific IgG in feces was also about two-fold higher in the LL-Gag group compared to the DNA/MVA (p=0.04) or MVA/MVA (p=0.041) groups (Figure 4c).
Figure 4. Oral immunization with LL-Gag generates stronger mucosal antibody responses than intramuscular DNA/MVA or MVA/MVA immunization.
HIV-1 Gag-specific (a) serum IgG (b) fecal IgA and (c) fecal IgG responses in mice orally immunized with LL-Gag compared with intramuscular DNA/MVA and MVA/MVA vaccines expressing HIV Gag, measured two weeks after the fourth LL-Gag immunization or MVA boost. (d) Concentrations of HIV-1 Gag-specific IgG1, IgG2a, IgG2b, and IgG3 antibodies in the serum of mice immunized with LL-Gag, DNA/MVA, or MVA/MVA vaccines. Longitudinal analysis of (e) Serum IgG and (f) fecal IgA in mice vaccinated with LL-Gag/MVA vaccine.
IgG exists as four different isotypes: IgG1, IgG2a, IgG2b, and IgG3. These isotypes have different effector functions and the specific isotype of IgG induced by vaccination can have implications in the overall immune response generated by the vaccination (34, 35). We found that Gag-specific IgG induced by DNA/MVA vaccination in serum consisted primarily of isotypes IgG1, IgG2a, and IgG2b, with little or no IgG3 (Figure 4d). In sharp contrast to the DNA/MVA modality, LL-Gag induced potent Gag-specific IgG3 and did not induce Gag-specific IgG1, IgG2a, or IgG2b. MVA/MVA also only induced Gag-specific IgG3; however these levels were not as strong as the LL-Gag/MVA IgG3 levels.
To understand the durability of serum and mucosal antibody response induced by LL-Gag/MVA vaccination, we followed the Gag-specific IgG in serum (Figure 4e) and Gag-specific IgA in feces (Figure 4f), and observed measurable responses until 24 weeks after the MVA boost (week 40 in study) demonstrating that LL-Gag/MVA vaccine induces persistent antibody responses in serum and intestinal secretions.
Oral LL-Gag prime, combined with intramuscular MVA boost induce a strong CD8 T cell response in the gut and in systemic compartments
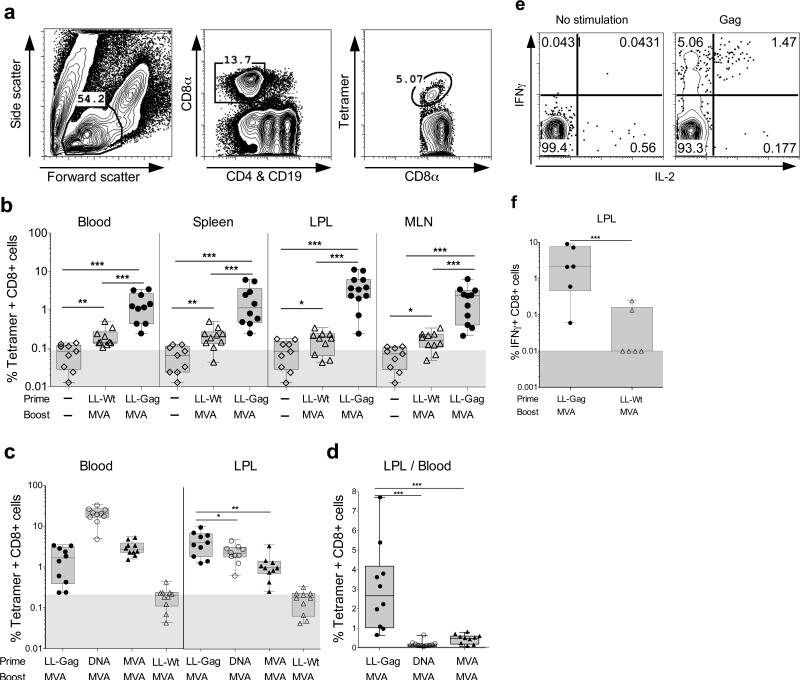
To assess the potential of oral LL-Gag immunizations to elicit Gag-specific CD8 T cell responses in the gut and systemic compartments, we immunized mice with four doses of LL-Gag via the IG route and determined Gag peptide (AMQMLKETI) specific CD8 T cell responses using an MHC class I tetramer. We failed to detect tetramer+ CD8 T cell responses at the end of four LL-Gag immunizations in blood. To determine if there was low level of priming of CD8 T cells, we boosted these mice with a single dose of MVA-Gag intramuscularly. As a control, we included mice that were primed with wild-type L. lactis (LL-Wt) via the IG route and boosted with MVA-Gag intramuscularly. Lymphocytes were identified based on the forward and side scatter, and CD8 T cells (CD8+CD19−CD4−) were checked for binding to the Gag tetramer (Figure 5a). One week after the boost, we observed strong tetramer-specific CD8 responses both in blood, spleen and mucosal compartments (lamina propria lymphocytes from small intestine and mesenteric lymph nodes)(Figure 5b). In addition, we observed strong tetramer-specific CD8 responses in multiple other tissues including intra epithelial lymphocytes of small intestine and lung (data not shown). Responses in LL-Gag-primed/ MVA-Gag boosted animals were significantly higher than in either naïve animals or LL-Wt- primed and MVA-Gag boosted animals. This strongly suggests that low level CD8 T cell responses resulting from oral LL-Gag priming are rapidly boosted by MVA delivered via IM (Figure 5b).
Figure 5. LL-Gag primes vaccine-specific CD8 T cells in the gut.
Mice were immunized orally with LL-Gag or LL-Wt and boosted with MVA four weeks after the fourth L. lactis immunization. (a) Gating strategy to detect HIV-1 Gag-tetramer+ CD8 T cells. (b) Frequencies of tetramer+ CD8 T cells in blood, spleen, lamia propria lymphocytes (LPLs) of small intestine and in mesenteric lymph node lymphocytes (MLN) one week post MVA boost. (c) Comparison of Gag-specific CD8+ T cells in the blood and LPLs of mice immunized with LL-Gag/MVA, DNA/MVA, MVA/MVA, or LL-Wt/MVA vaccine modalities. (d) Ratio of Gag-specific CD8+ T cells in the serum compared to the LPL. (e) Representative FACS plots showing IFNγ and IL-2 production by Gag peptide (AMQMLKETI)-specific CD8 T cells in lamina propria of small intestine. (f) Frequencies of Gag peptide-specific IFNγ+ CD8 T cells in lamia propria lymphocytes (LPLs) of small intestine.
We next determined whether the Gag-specific CD8 T cell responses induced by LL-Gag-prime/ MVA-Gag boost (LL/MVA) immunization regimen were better than the responses in the DNA/MVA and MVA/MVA immunized groups described in Figure 4a. In blood, the tetramer+ CD8 T cell responses in the LL/MVA group were lower than in the DNA primed group (DNA/MVA), but were comparable to those of the MVA-Gag primed group (MVA/MVA)(Figure 5c). In contrast, at mucosal sites, the levels of these tetramer-specific CD8 responses were significantly higher in the LL/MVA group than that of either DNA/MVA or MVA/MVA groups (Figure 5c). In addition, the ratio of tetramer+ CD8 T cells between LPL and blood within each mouse revealed that LL/MVA immunizations elicit about 3 fold higher CD8 T cell responses in the small intestine compared to blood whereas DNA/MVA and MVA/MVA immunizations elicit 10- and 2- fold lower CD8 T cell responses in the small intestine compared to blood (Figure 5d). To understand the functionality of Gag-specific CD8 T cells in the lamina propria, we performed intracellular cytokine assays by stimulating cells with the Gag immunodominant peptide (AMQMLKETI). Consistent with tetramer-specific CD8 T cells, we observed significantly higher frequency of Gag-specific IFNγ producing CD8 T cells in the LL-Gag/MVA group compared to LL-Wt/MVA group (Figure 5e & 5f). A significant fraction of IFNγ+ cells were also positive for IL-2 (Figure 5e).
LL-Gag activates dendritic cells in Peyer's patches
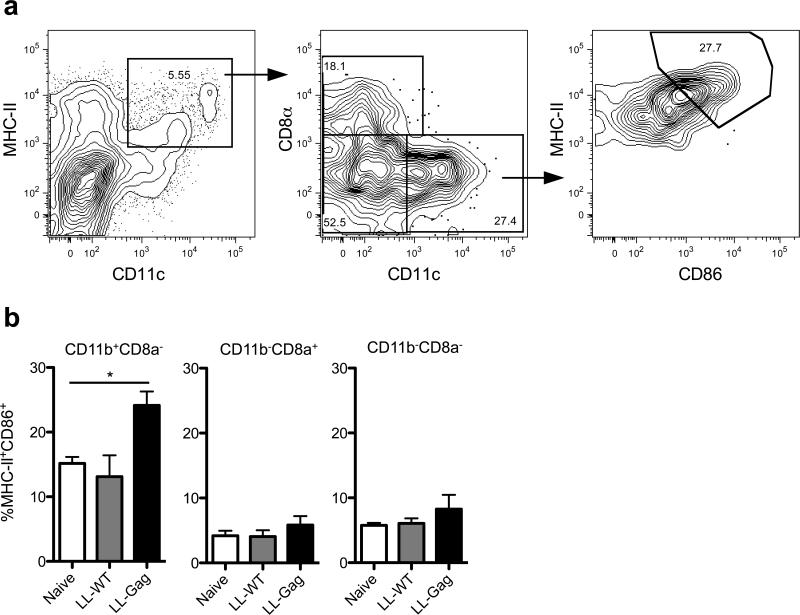
To understand the immunological mechanisms by which oral administration of LL-Gag was able to induce humoral and cellular immunity in the gastrointestinal tract, we determined the activation of gut resident DC after IG immunizations with LL-Wt, or LL-Gag (Figure 6). At 3 hours following the IG administration, mononuclear cells were isolated from Peyer's patches and analyzed by flow cytometry. CD3−CD19−F480−CD103− (36, 37) live cells were gated for MHCII+CD11c+ expression to identify DC (Figure 6a). These DC were then defined into CD11b+ CD8α−, CD11b− CD8α+, and CD11b− CD8α− dendritic cell subsets and were then analyzed for CD86 expression (Figure 6a). As shown in Figure 6b, a higher percentage of CD11b+ CD8α− DC expressed CD86 following vaccination with LL-Gag when compared to the untreated (naïve) or LL-Wt treated mice (Figure 6b). The MFI of MHCII was also higher in the LL-Gag treated mice (data not shown). This enhancement was not observed on CD8α+CD11b− and CD8α−CD11b− DC subsets (Figure 6b). These results demonstrate that L lactis expressing Gag-p24 on the pilus activates CD11b+ CD8α− DC in the small intestine and suggests that this activation may be critical for the ability of LL-Gag IG vaccinations to induce a strong intestinal mucosal immunity.
Figure 6. LL-Gag activates dendritic cells in murine Peyer's patches.
Mice were gavaged with 5×1010 cfu of LL-Wt or LL-Gag. Mice were euthanized 3h post gavage and the duodenum localized Peyer's patch cells were harvested and analyzed via flow cytometry. (a) Gating strategy for identifying dendritic cell subsets. CD3−CD19−−F480− live cells were gated for MHCII+CD11c+ expression. CD8α−CD11b+, CD8α+CD11b− and CD8α−CD11b− dendritic cell subsets were then gated for CD86 expression. (b) Frequency of MHCII+CD86+ cells in distinct dendritic cell subsets as a percent of parent. *, p<0.05.
Discussion
The presence of HIV specific antibodies that can bind, neutralize or clear infected cells via Fc mediated effector functions at the portal of viral entry should dramatically improve the efficacy of HIV vaccines (38). Here, we demonstrate that oral immunization with LL-Gag as a live vaccine vector to deliver the Gag-P24 antigen to the immune system generates strong anti-HIV IgG and IgA responses in the intestinal secretions of vaccinated mice. Importantly, the mucosal antibody responses induced by the LL-Gag vaccine were substantially better than the responses induced by heterologous and homologous immunizations with a strong viral vector such as MVA. In addition, our results show that LL-Gag oral immunizations combined with intramuscular MVA boost induce substantially higher CD8 T cell responses in the mucosal compartment compared to MVA-only immunization. Collectively, these results show that L. lactis can serve as a promising mucosal delivery vector for the induction of strong antibody and T cell responses in the mucosal tissue and highlight the potential of this vector for the development of mucosal HIV vaccines.
The use of pili as a platform to express foreign protein has several advantages for the purpose of eliciting high levels of cellular and humoral immunity: a) Each bacterial cell has numerous pili on its surface, allowing high levels of antigen expression per cell b) pili can activate the innate immune system and thus serve as an adjuvant for the induction of strong cellular and humoral immunity (13) c) expression of an antigen fused to the tip of the pilus allows B cells to recognize the antigen directly and more efficiently relative to intracellular expression (39) d) and pili may increase bacterial adhesion to the intestinal mucosa resulting in better retention of the bacteria in the gut. A previous study used recombinant L. lactis expressing HIV envelope protein (gp120) on the cell wall as an oral vaccine (11). To our knowledge, none of the previous HIV vaccine studies used pili as a platform for antigen expression to elicit antigen-specific cellular and humoral immunity. It is not clear if L. lactis expresses pili so we used a pilus from GAS. Our preliminary data suggested that this pilus from GAS might have contributed for immunogenicity of LL-Gag. One of the limitations for expressing recombinant proteins on the pilus is the size of the protein. In our experience we could express large proteins such as maltose binding protein that is about 54kDa (15). Another limitation could be the proper folding of the protein as a fusion to Cpa. To overcome such limitation, we recently expressed parts of variable loops of HIV envelope glycoprotein on the tip of the pilus without CPA. This protein seems to have folded correctly as it can be detected by antibodies specific to individual variable loops (unpublished data).
One of the interesting findings of our study is that recombinant L. lactis oral immunizations induced a strong IgG3 biased antibody response. This is in sharp contrast to induction of IgG1, IgG2a and IgG2b response by the intramuscular DNA/MVA vaccination. The IgG subclass of the antibody at the mucosa plays an important role in protection against HIV as non-neutralizing effector functions such as ADCC and ADCVI are dictated by the Fc portion of the subclass of the antibody. The unique features of the IgG3 subclass are that they can self-aggregate and bind target antigens in a cooperative manner, which may lead to higher avidity of binding antibody and perform ADCC function in vitro (34, 40). In mice, the IgG3 subclass of antibody response is generally T helper independent and is generally directed against multivalent antigens such as carbohydrate molecules. Thus it is interesting to see the induction of IgG3 response against Gag p24 antigen that is neither multivalent nor glycosylated. However, it is possible that presentation of Gag p24 on hundreds of pili on each bacterial cell could mimic multivalency.
Our attempts to determine the contribution of specific DCs in the intestinal mucosa for inducing mucosal immunity in the gut after oral L. lactis immunizations revealed a possible role for CD11c+ CD8α− CD11b+ DC in the Peyers patches (PPs) of the small intestine. In the GALT, DCs were originally defined simply as CD11c+ MHC II+/− cells and based on their specialized functional roles they are further subdivided into many subsets such as CD8α+, CD11b+ or CD103+ (41). The subepithelial dome of murine PP's house large numbers of these DCs (42). The CD11b- CD8α+ DCs have Th1 polarizing ability, and produce high levels of cytokines IL-12 and lL-10 (43, 44), while CD11b+ CD8α− DCs have Th2 polarizing ability and are involved in IgA class switching; the double negative (CD11b− CD8α−) subsets are mainly involved in Ag uptake and presentation (43, 44). Consistent with strong induction of Gag-specific IgA in fecal extracts, we observed a significant activation of CD11b+ CD8α− DCs in the PPs. Interestingly this activation was dependent on the presence of the pilus. Future studies will investigate the mechanisms by which the GAS T3 pilus is able to induce the activation of these DCs. Previous studies in mice showed that CD11b+ CD8α− PP DCs secrete high levels of IL-6 and promote IgA secretion by B cells (45-47). PP DCs may induce IgA production through IL-10 and/or TGFβ production (48, 49). It is possible that LL-Gag enhances production of these cytokines through activation of CD11b+ DC and thereby induces IgA production.
The mechanism by which LL-Gag was able to induce mucosal immunity is not completely clear and is under active investigation. In general, the PPs and mesenteric lymph nodes (MLN) play a significant role in the induction of mucosal antibody and T cell responses following oral immunization (50). The bacteria can be transported through M cells in the PPs and activate B cells in local follicles. The bacteria can also be sampled by DCs present in the PPs or lamina propria and present the antigen to T cells in the PPs and/or MLN. In addition, to overcome the tolerance these local DCs need to be activated such that they produce cytokines that are associated with T and B cell activation and proliferation, at the same time diminish induction of cells associated with tolerance such as regulatory T cells, Th3 cells and TR1 cells (50). As a first step in the process, our preliminary data showed that LL-Gag vaccination activates CD11b+ DCs in PPs and suggest that the pilus may be critical for this activation. Ongoing experiments will investigate the precise mechanisms by which the LL-Gag vaccination influences the activation and tolerization signals and the respective cells and cytokines involved in this process.
If probiotic bacteria were to colonize the gut epithelium, they could alter the gut ecosystem. In addition, persistent antigen presentation to immune cells could lead to immune cell exhaustion or generation of low avidity antibody response. However, L. lactis doesn't colonize the gut in mice and humans (51, 52). Therefore, we were interested to see whether the presence of a foreign pilus affects gut colonization by the recombinant bacterium. To do this, we determined the colony forming units of spectinomycin resistant bacteria from fecal extracts on a daily basis after vaccination. We could culture LL-Gag for only about 2-3 days after immunization suggesting that the presence of the pilus did not increase persistence in the gastrointestinal tract of the L. lactis strain.
In conclusion, our results clearly demonstrate that oral administration of probiotic L. lactis expressing the antigen on the tip of the GAS pilus induces strong mucosal IgG and IgA antibody responses in the gut of mice. Our results also demonstrate that oral immunization with LL-Gag can be combined with intramuscular MVA boost to induce strong mucosal and systemic CD8 T cell responses. Together these results reveal a promising novel immunization strategy to elicit strong humoral and cellular mucosal immunity against HIV.
Acknowledgements
We thank Dr. Moss ay NIH, Bethesda, DC for providing MVA and Dr. B. Beall at CDC Atlanta, GA, for providing us with anti T3 typing immune serum. Mouse anti-Gag monoclonal antibody (clone H12.5c) was obtained from NIH AIDS reagent program. This work was supported in part by the Robert P. Apkarian Integrated Electron Microscopy Core of Emory University.
1This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grant U19 AI096187.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nature reviews. Immunology. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6:930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov IM, Berzofsky JA. Immunobiology of Mucosal HIV Infection and the Basis for Development of a New Generation of Mucosal AIDS Vaccines. Immunity. 2004;20:247–253. doi: 10.1016/s1074-7613(04)00053-6. [DOI] [PubMed] [Google Scholar]
- 5.Demberg T, Robert-Guroff M. Mucosal Immunity and Protection Against HIV/SIV Infection: Strategies and Challenges for Vaccine Design. International Reviews of Immunology. 2009;28:20–48. doi: 10.1080/08830180802684331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Q, Hidajat R, Peng B, Venzon D, Aldrich MK, Richardson E, Lee EM, Kalyanaraman VS, Grimes G, Gómez-Román VR, Summers LE, Malkevich N, Robert-Guroff M. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIVmac251. Vaccine. 2007;25:8021–8035. doi: 10.1016/j.vaccine.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 8.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. Journal of virology. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4(+) T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 10.Bahey-El-Din M. Lactococcus lactis-based vaccines from laboratory bench to human use: an overview. Vaccine. 2012;30:685–690. doi: 10.1016/j.vaccine.2011.11.098. [DOI] [PubMed] [Google Scholar]
- 11.Xin KQ, Hoshino Y, Toda Y, Igimi S, Kojima Y, Jounai N, Ohba K, Kushiro A, Kiwaki M, Hamajima K, Klinman D, Okuda K. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood. 2003;102:223–228. doi: 10.1182/blood-2003-01-0110. [DOI] [PubMed] [Google Scholar]
- 12.Klijn N, Weerkamp AH, Devos WM. Genetic Marking of Lactococcus-Lactis Shows Its Survival in the Human Gastrointestinal-Tract. Applied and environmental microbiology. 1995;61:2771–2774. doi: 10.1128/aem.61.7.2771-2774.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saez D, Fernandez P, Rivera A, Andrews E, Onate A. Oral immunization of mice with recombinant Lactococcus lactis expressing Cu,Zn superoxide dismutase of Brucella abortus triggers protective immunity. Vaccine. 2012;30:1283–1290. doi: 10.1016/j.vaccine.2011.12.088. [DOI] [PubMed] [Google Scholar]
- 14.Kunji ERS, Slotboom D-J, Poolman B. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2003;1610:97–108. doi: 10.1016/s0005-2736(02)00712-5. [DOI] [PubMed] [Google Scholar]
- 15.Quigley BR, Hatkoff M, Thanassi DG, Ouattara M, Eichenbaum Z, Scott JR. A foreign protein incorporated on the Tip of T3 pili in Lactococcus lactis elicits systemic and mucosal immunity. Infect Immun. 2010;78:1294–1303. doi: 10.1128/IAI.01037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gram GJ, Fomsgaard A, Thorn M, Madsen SM, Glenting J. Immunological analysis of a Lactococcus lactis-based DNA vaccine expressing HIV gp120. Genet Vaccines Ther. 2007;5:3. doi: 10.1186/1479-0556-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pusch O, Kalyanaraman R, Tucker LD, Wells JM, Ramratnam B, Boden D. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. Aids. 2006;20:1917–1922. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 18.Pontes DS, de Azevedo MS, Chatel JM, Langella P, Azevedo V, Miyoshi A. Lactococcus lactis as a live vector: heterologous protein production and DNA delivery systems. Protein expression and purification. 2011;79:165–175. doi: 10.1016/j.pep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Guimaraes VD, Innocentin S, Lefevre F, Azevedo V, Wal JM, Langella P, Chatel JM. Use of native lactococci as vehicles for delivery of DNA into mammalian epithelial cells. Applied and environmental microbiology. 2006;72:7091–7097. doi: 10.1128/AEM.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raha AR, Varma NR, Yusoff K, Ross E, Foo HL. Cell surface display system for Lactococcus lactis: a novel development for oral vaccine. Applied microbiology and biotechnology. 2005;68:75–81. doi: 10.1007/s00253-004-1851-8. [DOI] [PubMed] [Google Scholar]
- 21.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma HL, Grimm BD, Hulsey ML, Miller J, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 22.Kwa S, Lai L, Gangadhara S, Siddiqui M, Pillai VB, Labranche C, Yu T, Moss B, Montefiori DC, Robinson HL, Kozlowski PA, Amara RR. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. Journal of virology. 2014;88:9579–9589. doi: 10.1128/JVI.00975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwa S, Sadagopal S, Shen X, Hong JJ, Gangadhara S, Basu R, Victor B, Iyer SS, LaBranche CC, Montefiori DC, Tomaras GD, Villinger F, Moss B, Kozlowski PA, Amara RR. CD40L-Adjuvanted DNA/MVA SIV Vaccine Enhances Protection Against Neutralization Resistant Mucosal SIV Infection. Journal of virology. 2015;89:4690–4695. doi: 10.1128/JVI.03527-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JM, Amara RR, Campbell D, Xu Y, Patel M, Sharma S, Butera ST, Ellenberger DL, Yi H, Chennareddi L, Herndon JG, Wyatt LS, Montefiori D, Moss B, McClure HM, Robinson HL. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res Hum Retroviruses. 2004;20:1335–1347. doi: 10.1089/aid.2004.20.1335. [DOI] [PubMed] [Google Scholar]
- 25.Holo H, Nes IF. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Applied and environmental microbiology. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas I, Germon P, McDade K, Scott JR. Generation and surface localization of intact M protein in Streptococcus pyogenes are dependent on sagA. Infection and immunity. 2001;69:7029–7038. doi: 10.1128/IAI.69.11.7029-7038.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Hellerstein M, McDonnel M, Amara RR, Wyatt LS, Moss B, Robinson HL. Dose-response studies for the elicitation of CD8 T cells by a DNA vaccine, used alone or as the prime for a modified vaccinia Ankara boost. Vaccine. 2007;25:2951–2958. doi: 10.1016/j.vaccine.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 28.Ganguly S, Liu J, Pillai VB, Mittler RS, Amara RR. Adjuvantive effects of anti-4-1BB agonist Ab and 4-1BBL DNA for a HIV-1 Gag DNA vaccine: different effects on cellular and humoral immunity. Vaccine. 2010;28:1300–1309. doi: 10.1016/j.vaccine.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 30.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infection and immunity. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley BR, Zahner D, Hatkoff M, Thanassi DG, Scott JR. Linkage of T3 and Cpa pilins in the Streptococcus pyogenes M3 pilus. Molecular microbiology. 2009;72:1379–1394. doi: 10.1111/j.1365-2958.2009.06727.x. [DOI] [PubMed] [Google Scholar]
- 32.O'Connell RJ, Kim JH, Corey L, Michael NL. Human immunodeficiency virus vaccine trials. Cold Spring Harbor perspectives in medicine. 2012;2:a007351. doi: 10.1101/cshperspect.a007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munseri PJ, Kroidl A, Nilsson C, Joachim A, Geldmacher C, Mann P, Moshiro C, Aboud S, Lyamuya E, Maboko L, Missanga M, Kaluwa B, Mfinanga S, Podola L, Bauer A, Godoy-Ramirez K, Marovich M, Moss B, Hoelscher M, Gotch F, Stohr W, Stout R, McCormack S, Wahren B, Mhalu F, Robb ML, Biberfeld G, Sandstrom E, Bakari M. Priming with a Simplified Intradermal HIV-1 DNA Vaccine Regimen followed by Boosting with Recombinant HIV-1 MVA Vaccine Is Safe and Immunogenic: A Phase IIa Randomized Clinical Trial. PloS one. 2015;10:e0119629. doi: 10.1371/journal.pone.0119629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 35.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwasaki A. Mucosal dendritic cells. Annual review of immunology. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 37.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature Reviews Immunology. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annual review of immunology. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 39.Ryan EJ, Daly LM, Mills KH. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 2001;19:293–304. doi: 10.1016/s0167-7799(01)01670-5. [DOI] [PubMed] [Google Scholar]
- 40.Yates NL, Lucas JT, Nolen TL, Vandergrift NA, Soderberg KA, Seaton KE, Denny TN, Haynes BF, Cohen MS, Tomaras GD. Multiple HIV-1-specific IgG3 responses decline during acute HIV-1: implications for detection of incident HIV infection. Aids. 2011;25:2089–2097. doi: 10.1097/QAD.0b013e32834b348e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwasaki A, Kelsall BL. Unique Functions of CD11b+, CD8α+, and Double-Negative Peyer's Patch Dendritic Cells. The Journal of Immunology. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 42.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends in immunology. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nature reviews. Immunology. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 44.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. Journal of immunology. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 45.Spalding DM, Williamson SI, Koopman WJ, McGhee JR. Preferential induction of polyclonal IgA secretion by murine Peyer's patch dendritic cell-T cell mixtures. The Journal of experimental medicine. 1984;160:941–946. doi: 10.1084/jem.160.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spalding DM, Griffin JA. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell. 1986;44:507–515. doi: 10.1016/0092-8674(86)90472-1. [DOI] [PubMed] [Google Scholar]
- 47.Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer's Patch Dendritic Cells Secrete IL-6 and Induce IgA Secretion from Naive B Cells. The Journal of Immunology. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 48.Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. The Journal of experimental medicine. 1989;170:1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. The Journal of experimental medicine. 1989;170:1415–1420. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 51.Klijn N, Weerkamp AH, de Vos WM. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Applied and environmental microbiology. 1995;61:788–792. doi: 10.1128/aem.61.2.788-792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klijn N, Weerkamp AH, de Vos WM. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Applied and environmental microbiology. 1995;61:2771–2774. doi: 10.1128/aem.61.7.2771-2774.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]