Abstract
Emotionally arousing events are typically better attended to and remembered than neutral ones. Current theories propose that arousal-induced increases in norepinephrine during encoding bias attention and memory in favor of affectively salient stimuli. Here, we tested this hypothesis by manipulating levels of physiological arousal prior to encoding and examining how it influenced memory for emotionally salient images, particularly those that are negative rather than positive in valence. We also tested whether sex steroid hormones interact with noradrenergic activity to influence these emotional memory biases in women. Healthy naturally cycling women and women on hormonal contraception completed one of the following physiological arousal manipulations prior to viewing a series of negative, positive and neutral images: 1) Immediate handgrip arousal – isometric handgrip immediately prior to encoding, 2) Residual handgrip arousal – isometric handgrip 15 min prior to encoding, or 3) No handgrip. Sympathetic arousal was measured throughout the session via pupil diameter changes. Levels of 17β-estradiol and progesterone were measured via salivary samples. Memory performance was assessed approximately 10 minutes after encoding using a surprise free recall test. The results indicated that handgrip successfully increased sympathetic arousal compared to the control task. Under immediate handgrip arousal, women showed enhanced memory for negative images over positive images; this pattern was not observed in women assigned to the residual and no-handgrip arousal conditions. Additionally, under immediate handgrip arousal, both high estradiol and progesterone levels attenuated the memory bias for negative over positive images. Follow-up hierarchical linear models revealed consistent effects when accounting for trial-by-trial variability in normative International Affective Picture System valence and arousal ratings. These findings suggest that heightened sympathetic arousal interacts with estradiol and progesterone levels during encoding to increase the mnemonic advantage of negative over positive emotional material.
Keywords: Sympathetic arousal, emotional memory, hormones, pupil dilation, BANE, GANE
1. Introduction
People are more likely to attend to and remember emotionally arousing events or stimuli compared to neutral ones (Markovic et al., 2014, LaBar and Cabeza, 2006). Current theories of arousal-cognition interactions positthat the release of norepinephrine (NE) during emotionally arousing experiences contributes to these attention and memory effects (Mather et al., in press, McGaugh, 2002, Markovic et al., 2014). In particular, the Biased Attention via Norepinephrine (BANE; Markovic et al., 2014) and Glutamate Amplifies Noradrenergic Effects (GANE; Mather et al., in press) models both propose that increased norepinephrine under arousal should enhance affect-biased attention and memory.
According to BANE, emotionally salient stimuli effectively bias attention and memory to boost their own processing; however, this model focuses exclusively on how NE biases mental processing in favor of the emotionally salient stimuli that induced its release (Markovic et al., 2014). In contrast, the GANE model covers a broader range of salience and arousal types to account for arousal's simultaneous ability to enhance memory of affective stimuli at the expense of memory for distracting or mundane information. Specifically, GANE predicts that memory of salient stimuli should benefit from NE release around the time of encoding, regardless of whether NE release was stimulated by the emotionally arousing stimulus or not.
The prediction that higher levels of NE enhance emotional memory effects is consistent with recent studies using genotyping technology. These studies examined carriers of a deletion variant of the ADRA2b gene, which codes for the α2b-adrenoreceptor and is associated with greater extracellular NE availability (Todd et al., 2014). Compared to non-carriers, carriers of the deletion variant not only show a greater capacity for emotionally enhanced memory of images (de Quervain et al., 2007), but also greater amygdala activation when viewing negatively valenced scenes (Rasch et al., 2009); deletion carriers also show enhanced perceptual encoding of negative words over both positive and neutral words (Todd et al., 2013). These findings suggest that increased NE availability biases attention towards affectively salient stimuli, particularly those of negative valence.
However, there are issues with drawing broad conclusions about the mechanisms of emotional memory from this genotyping approach. The previous genotyping studies focused exclusively on the α2b-adrenoreceptor, but other adrenoreceptors (e.g., β-adrenoreceptor) have significant contributions to noradrenergic influences on emotional memory (Cahill et al., 1994, Chamberlain et al., 2006, Strange et al., 2003)and likely play a role in the modulatory effects of NE on biased attention and memory for emotionally salient stimuli (Mather et al., in press; Tully and Bolshakov, 2010). Additionally, these genotyping studies have only examined how NE increases induced by emotional stimuli themselves modulate subsequent emotional memory. They have not addressed whether NE increases induced by an external physiologically arousing stimulus affect memory for emotionally salient stimuli. Separating the stimulus that induces arousal from the memoranda eliminates any potential confounds created due to differences in the perceptual qualities or semantic nature of emotional versus neutral stimuli.
Thus, our first aim was to build upon the genotyping findings by experimentally manipulating levels of physiological arousal prior to encoding and examine how the resulting changes in sympathetic arousal (and NE level) affect memory for emotionally salient images. Intriguingly, the noradrenergic genotyping studies found a valence-specific effect of NE, such that individuals with ostensibly higher NE availability showed selectively enhanced processing of only negative stimuli. Therefore, we assessed memory for negative and positive images in this study to examine how stimulus valence interacts with sympathetic arousal state. To induce sympathetic arousal and increase NE levels, we utilized an isometric handgrip paradigm (Nielsen and Mather, 2015). GANE predicts that arousal-induced NE increases will bias memory in favor of whatever stimulus is prioritized, or highly salient. Previous research indicates that young adults generally have a negativity bias such that they process and attend to negative stimuli more deeply than positive stimuli (Baumeister et al., 2001; Rozin & Royzman, 2001). Given this greater attentional salience of negative over positive valenced stimuli, GANE predicts that increased physiological arousal will amplify the effects of priority and enhance memory of negative stimuli more than positive stimuli.
Our second aim was to examine the role of sex steroid hormones in modulating the influence of NE activity on emotional memory biases. Steroid hormones, specifically estradiol and progesterone, affect autonomic nervous system activity in various ways. For example, in a study using static isometric handgrip with young women, levels of estradiol during the mid-luteal phase of the menstrual cycle were negatively correlated with muscle sympathetic nerve activity whereas changes in progesterone trended toward a significant positive relationship with muscle sympathetic nerve activity (Carter et al., 2013). In another study, estrogen replacement therapy decreased sympathetic activity in postmenopausal women (Vongpatanasin et al., 2001), indicating that estradiol may be sympathoinhibitory.
However, other studies provide evidence to the contrary. For example, one study showed that women in the mid-luteal phase (when estradiol and progesterone are elevated) exhibited increased sympathetic baroreflex sensitivity, increased resting muscle sympathetic nerve activity, and increased resting plasma NE levels compared to women in the early follicular phase (when estradiol and progesterone are low), indicating that hormone fluctuations across the menstrual cycle alter sympathetic nervous activity (Minson et al., 2000). In addition, hormonal contraception users (characterized by reduced levels of endogenous estradiol and progesterone) exhibited blunted sympathetic activity in response to both cardiovascular exercise (Otterstetter et al., 1999) and emotional images (Nielsen et al., 2013b). Even though these studies did not assess the specific relationship between sex hormone levels and noradrenergic activity, the results suggest that together, lower levels of progesterone and estradiol are associated with reduced sympathetic responses.
In women, sex steroid hormones levels are associated not only with sympathetic activity but also with emotional memory (Andreano and Cahill, 2009). Looking first at the role of progesterone, some studies found that in naturally cycling women, higher progesterone levels predicted enhanced memory for negative emotional images (Ertman et al., 2011) and increased intrusive memories for negative emotional events (Ferree et al., 2011, Soni et al., 2013); in these studies, there was no relationship between estradiol and memory. Other work showed that, under sympathetic arousal, women on hormonal contraception exhibited enhanced long-term memory for negative, but not positive, images compared to their non-aroused counterparts (Nielsen et al., 2013b). Thus, both high and low levels of endogenous progesterone have been associated with enhanced memory for negative stimuli.
Other research suggests that estradiol attenuates memory for negative emotional stimuli. For example, in a recent study of intrusive memories, women with lower estradiol levels showed stronger intrusive memories for violent film clips over a three-day period (Wegerer et al., 2014). These data suggest that estradiol may promote fear extinction processes such that intrusive memories are less likely to be extinguished when estradiol levels are low. Other studies testing the effects of sex steroid hormones on fear extinction processes support this notion; in both rodents and humans, estrogens, but not progesterone, were necessary for fear extinction (Graham and Milad, 2013, Milad et al., 2010, Glover et al., 2012). Thus, our second aim here was to test how levels of both estradiol and progesterone interact with sympathetic arousal at encoding to modulate emotional memory in women.
To address our two aims, we recruited women on and off of hormonal contraception so we could assess the effects of high and low levels of sex steroid hormones on arousal and memory (Kirschbaum et al., 1999). Although the mixed findings in the literature made it difficult to predict overall sympathetic activity differences by hormone group, previous evidence does suggest specific predictions regarding differences in emotional memory. Our primary prediction was that under immediate physiological arousal induced by isometric handgrip, or increased NE, women would show enhanced memory for negative, but not positive, relative to the residual handgrip arousal (handgrip 15 min before encoding) and no handgrip arousal (no-handgrip control task) conditions. We also predicted that, under immediate handgrip arousal, enhanced memory for negative images would occur predominantly in women in a low estradiol and progesterone state.
2. Materials and methods
2.1 Participants
This memory study was nested within a larger behavioral study with two recruitment phases. In the first phase, we compared the effectiveness of two isometric handgrip protocols on eliciting arousal in follicular, luteal, and women on hormonal contraception. Although all of these women also completed the study described in the current paper, we found that one of the handgrip squeeze conditions was ineffective in eliciting arousal, as we reported in a methods-focused paper (Nielsen and Mather, 2015). Thus, in the second recruitment phase, to maximize the power for the memory study, we dropped the ineffective squeeze condition and collected data from 56 more women. Ninety-six women between the ages of 18-34 participated in the present study, which was approved by the University of Southern California's Institutional Review Board. The participants received course credit or payment for their participation.
Participants were asked to refrain from alcohol, caffeine, and cardiovascular exercise for twenty-four hours prior to their experimental session to control for outside influences on baseline salivary alpha-amylase levels (as described below, this measure was collected as part of the methods study). To avoid contamination of salivary samples, participants were asked to fast one hour prior to each experimental session as well as refrain from brushing teeth and chewing gum within the hour before their appointment. Their compliance with these criteria was verified upon their arrival.
Naturally cycling women were recruited in the “follicular” phase (1-15 days from the start of menstruation) and the “luteal” phase of the menstrual cycle (15-30 days from the start of menstruation; Azziz et al., 1999, Franklin et al., 2008, Sakaki and Mather, 2012, De Bondt et al., 2013). We used a forward day count from the first self-reported day of menstruation to determine menstrual phase. One woman on hormonal contraception was excluded from final analyses for using a progestin-only form of contraception. In the final analyses, we included 27 women in the follicular phase, 37 women in the luteal phase, and 31 women on hormonal contraception. Of the women on hormonal contraception, all participants were on combined contraceptive formulations that had both ethinyl estradiol and a synthetic progestin; three women on hormonal contraception reported using triphasic formulations, 28 women used monophasic formulations. The women in the final analyses self-identified as the following ethnicities: 11.6% as Hispanic, 85.3% as Non-Hispanic, and 3.2% declined to state. In addition, 7.4% identified as African American, 46.3% as Asian, 6.3% as Bi-racial, 33.7% as Caucasian, 1.1% as Pacific Islander/Native Hawaiian, 1.1% as other, and 4.2% declined to state. We also collected health and stress ratings using a 1 – 9 scale. For health ratings, “1” represented “very poor health” and “9” represented the “excellent health” and for the stress ratings, “1” represented “very low” and “9” represented “very high.” The women included in the final analyses had an average health rating (± SEM) of 7.5 ± 0.09, an average stress rating (± SEM) of 4.39 ± 0.2, and a stress compared to usual rating (± SEM) of 4.38 ± 0.18.
2.2 Procedures
All experimental sessions were conducted between the hours of 1200 and 1830h to control for the effects of circadian rhythm on stress hormone levels. Upon arrival, each participant rinsed their mouth by drinking an 8-oz. bottle of water; they also completed a demographic information packet. Approximately 10 min after their arrival, participants provided a 1-mL saliva sample using the “passive drool” collection method. Following collection of the baseline saliva sample, participants completed a 5-pt. calibration on the iView X RED eye-tracking system (SensoMotoric Instruments). We obtained seven saliva samples throughout the experiment, which we later analyzed for sex steroid hormone levels (described in more detail below). These samples were also used to assess levels of alpha-amylase for the larger methods study; these data will not be discussed here(see Nielsen and Mather, 2015).
To induce physiological arousal and manipulate NE levels, we employed an isometric handgrip paradigm that has been shown to reliably increase sympathetic nervous system activity and other indices (e.g., pupil dilation) of NE (Nielsen and Mather, 2015, Topolovec et al., 2004). After successful calibration, participants were randomly assigned to one of three physiological arousal conditions for their first experimental block (see Figure 1): 1) No Handgrip Arousal – 3-min control task, 2) Residual Handgrip Arousal – 18-s maximum isometric handgrip, or 3) Immediate Handgrip Arousal – 18-s control task(see Nielsen and Mather, 2015). In the first experimental block, the 18-s isometric handgrip task was implemented to induce physiological arousal 15 min prior to the encoding task (residual arousal). It is important to note that the term “residual handgrip arousal” refers to the relative timing of the arousal induction to the memory encoding phase. Studies have shown that NE increases in response to stress for up to 15 min post-stressor, soto account for any residual effects of NE on encoding, this experimental condition is referred to as “residual handgrip arousal.” In contrast, the immediate arousal condition participants completed the handgrip in the second experimental block, just before the slideshow since NE levels and sympathetic arousal should be increased and/or sustained across most of the slideshow. In the initial methods study, sympathetic arousal was effectively induced in women using this 18-s paradigm, even in the absence of calibrating a maximum voluntary contraction (Nielsen and Mather, 2015). All tasks were completed with the right hand. Isometric handgrip tasks were completed with a Gaiam hand therapy exercise ball of medium resistance, and for the control task, participants rested their fingertips on an empty, upright 8-oz water bottle (Nielsen and Mather, 2015).
Fig 1.
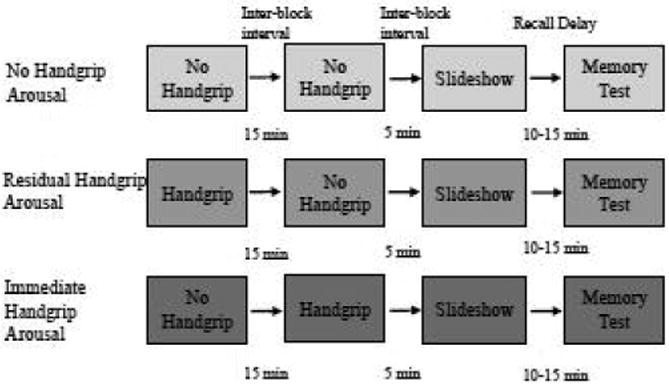
Experiment timeline by physiological arousal condition.
For the experimental block, participants were presented with a grayscale screen with a yellow or a blue circle in the center (normalized for luminance). During the yellow circle “rest” periods, participants were asked to rest and relax while maintaining their gaze on the screen. During the blue circle “squeeze” periods, participants were asked to either squeeze the hand therapy exercise ball maximally (for 18 s), or they were asked to gently rest their fingertips on the top of an empty water bottle. All conditions started with a 10-s yellow circle period to determine baseline pupil diameter. For the 18-s conditions, there were three cycles of the 18-s squeeze (blue) and 60-s rest (yellow), and participants were asked to maintain the maximum squeeze or water bottle fingertip rest for the full 18-s (Topolovec et al., 2004). For the 3-min control condition (No Handgrip Arousal), participants saw the blue circle for three minutes and were asked to maintain the water bottle fingertip rest for the full three minutes (Nielsen and Mather, 2015). Pupil diameter was measured continuously throughout the block as a measure of noradrenergic activity (Murphy et al., 2014).
During the recovery period, participants rested and provided 1-mL samples at 7- and 15-min post-task. After the 15-min post-task sample, participants completed a second 5-pt. calibration on the iView X RED eye-tracking system (SensoMotoric Instruments) and underwent the same experiment format as in the first block. However, in the second experiment block, each participant who completed the handgrip task in the first block now performed the control task and vice versa. The one exception was the third condition in which participants performed the 3-min control task for a second block. For the 3-min control condition (No Handgrip Arousal), participants saw the blue circle for three minutes and were asked to maintain the water bottle fingertip rest for the full three minutes (Nielsen and Mather, 2015).
Immediately after the second block, participants provided a fifth 1-mL saliva sample. Shortly after the fifth saliva sample, participants underwent a third 5-pt. calibration on the iView X RED eye-tracking system. Upon successful calibration, each participant was also shown a series of 80 images in a random order for each participant. Prior to encoding, the participants were told to pay attention to the images as they would be asked questions about them later in the session. The slideshow was composed of 16 highly arousing, positive images (valence rating = 7.32 ± 0.48; arousal rating = 6.11 ± 0.74), 16 low arousing, positive images (valence rating = 7.04 ± 0.08; arousal rating = 4.63 ± 0.26), 16 highly arousing, negative images (valence rating = 2.24 ± 0.39; arousal rating = 6.27 ± 0.74, 16 low arousing, negative images (valence rating = 3.30 ± 0.46; arousal rating = 4.55 ± 0.17) and 16 neutral images (for 14 IAPS images: valence rating = 4.97 ± 0.07; arousal rating = 3.18 ± 1.82). All means are ± SEM. Arousal and valence ratings represent mean normative IAPS ratings ± SEM. Of these, 78 were from the International Affective Picture System (IAPS; Lang and Bradley, 2007), and 2 were neutral images from other sources(see Mather and Knight, 2005 Experiment 2 for details) Positive and negative emotional images in the highly arousing sets did not differ significantly in their arousal ratings, nor did those in the low-arousal sets (p > .5).
We also assessed whether the negative and positive valence ratings differed in the high and low arousing images. The negative ratings significantly differed between the high and low arousing images, with highly arousing negative images being rated as significantly more negative than low arousing negative images (p <.001). The positive valence ratings did not significantly differ between the high and low arousing images (p >.1). Each image was presented for 2 s, and the presentation order of the images was randomized for each participant. The total duration of the slideshow was 2 minutes and 40 seconds. Following the slideshow, saliva samples were provided at 7- and 15-min post-handgrip/control task.
After completing the final saliva sample, participants were asked to freely recall as many of the images from the slideshow as possible; this memory test occurred approximately 10-15 min after the start of the encoding task. They were instructed to state a brief description of each image out loud while the experimenter recorded their recalled images. Participants were given as much time as they needed. Following the memory test, participants were debriefed and compensated with course credit or payment. For the recall test, an item was scored as correctly recalled if the description of the slide unequivocally matched an image that was presented. Recalled images were classified by arousal and valence based on the standard IAPS ratings.
2.3 Eye Movements and Pupil Dilation
Pupil dilation was measured using the iView X RED eye-tracking software at a sampling rate of 120 Hz. We selected this measure for analysis because pupil dilation is considered a reliable measure of sympathetic arousal (Einhäuser et al., 2008, Gilzenrat et al., 2010). Also, recent studies with isometric handgrip showed pupil diameter increased in response to a 2-min isometric handgrip task (Hayashi and Someya, 2011) and the 18-s task used in this study (Nielsen and Mather, 2015). Fixation events and pupil dilation data for each participant were exported using the eye-tracking analysis software program BeGaze 2 (SensoMotoric Instruments). To calculate the pupil diameter change in response to the handgrip and control tasks, we examined pupil diameter values across the entire task, and we adopted methods from Hayashi and Someya (2011) and Nielsen and Mather (2015). We determined the 20 fixations with the greatest pupil diameter during each 1-min interval in the 3-min task and during each 18 s interval in the 18-s tasks to obtain the top-20 pupil diameter values. Therefore, each task had three experimental intervals. In order to normalize the data between participants, we calculated a percent of the baseline pupil diameter for each handgrip and control interval in each task, as follows:
These average top-20 pupil responses were then averaged across the three intervals for handgrip and control blocks during the 3-min and 18-s tasks, and this value was the top-20 pupil response used for subsequent analyses.
2.4 Saliva Samples
Saliva samples were immediately frozen, and kept frozen for a minimum of 24 hours to allow mucins to precipitate. Prior to the assays, they were thawed and centrifuged at 3,000 × g for 15 min to extract particulates from saliva. Clear supernatant was decanted into microtubes. The saliva samples were then analyzed for 17β-estradiol and progesterone levels using Salimetrics, LLC (State College, PA) ELISA kits and optical measurements acquired from a Molecular Devices, LLC SpectraMax M3 Multi-mode Microplate Reader (Sunnyvale, CA). We assayed the first and the last saliva samples for 17β-estradiol and progesterone; from these samples, we determined the average levels of these hormones. We used the assays to determine whether there were overall group differences between the women, and mean ± SEM values of 17β-estradiol and progesterone were within the expected ranges of the used assays for women (Salimetrics, LLC, State College, PA). The observed ranges from the assay of 17β-estradiol (M = 2.27, SD = 0.86) and progesterone (M = 90.54, SD = 68.7) were also similar to the expected ranges for women. Salivary alpha-amylase levels were measured as part of the methods-focused study (Nielsen and Mather, 2015) and will not be discussed in the current study.
3. Results
3.1 Participants
Participants' mean age was 21.1 years (SD = 2.4), and women on hormonal contraception, luteal women, and follicular women did not differ significantly in age, F(2, 91) = 0.43, p>.1, ratings of current health status, F(2, 91) = 0.35, p> .1, current stress status, F(2, 91) = 0.9, p> .1, and current stress compared to usual, F(2, 91) = 0.13, p> .1.
Menstrual cycle position in naturally cycling women was determined by self-report. We used salivary assays to determine the average levels of progesterone and 17β-estradiol in all participants. Follicular (N = 27), luteal (N = 37), and hormonal contraception (N = 31) groups did not differ in their measured levels of progesterone, F(2, 92) = 0.03, p> .1, or 17β-estradiol, F(2, 92) = 0.13, p> .1. Since these women did not differ in their levels of sex steroid hormones, we collapsed them into one cohort to assess the effects of 17-β estradiol and progesterone on arousal modulation of memory for negative and positive images.
In the subsequent tests with hormone groups, we used median splits to generate high and low groups for both estradiol and progesterone. One-way Analysis of Variances(ANOVAs) confirmed that after the median split, women in the high estradiol group (M = 3.05pg/mL ± .08) had significantly higher levels of estradiol, F(1, 93) = 156.9, p < .001, compared to the low estradiol women (M = 1.56pg/mL ± .08), and women in the high progesterone group (M = 145.4pg/mL ± 6.9) had significantly higher levels of progesterone, F(1, 93) = 122.4, p < .001, compared to low progesterone women (M = 38.6pg/mL ± 6.8). Estradiol levels positively correlated with progesterone levels across all women, r(93) = .41, p< .001.
3.2 Handgrip manipulation – physiological manipulation check
Before turning to the primary focus of the study (i.e., the impact of arousal and sex hormones on emotional memory), we examined the efficacy of the handgrip arousal manipulation. To assess sympathetic arousal, we measured pupil diameter changes for women in the immediate handgrip arousal condition (i.e., those who completed the handgrip task immediately before encoding, N = 33), the residual handgrip arousal condition (i.e., those who completed the handgrip task 15 min before encoding, N = 30), and the no handgrip arousal (i.e., those who completed the control task in both experimental blocks prior to encoding, N = 32).
We first assessed the pupil response data from all women who completed the handgrip task using the handgrip-specific top-20 pupil diameter value method described above (Hayashi and Someya, 2011, Nielsen and Mather, 2015). Using a repeated-measures ANOVA with Physiological Arousal Condition (immediate handgrip arousal v. residual handgrip arousal) as the between-subjects factor and task block (handgrip v. control) as the within-subjects factor, we found a significant main effect of handgrip on pupil dilation, F(1,61) = 19.5, p< .001; in women who completed the handgrip, pupil diameter increased more to the handgrip task (M = 106.1% ± 0.71) compared to the control (M = 1028% ± 0.47), regardless of physiological arousal condition. There were no significant interactions between condition and task block, F(1,61) = 1.5, p> .1. In a test of the block order effect, women in the control-control block showed no difference in their pupil responses to control block 1 and control block 2, t(31) = .05, p > .1.
The previous analyses show that the handgrip task elicited sympathetic arousal in women. However, this does address whether arousal levels were equated between these two conditions prior to when the participants viewed the emotional pictures. To demonstrate this, we conducted an ANOVA with Physiological Arousal Condition (immediate handgrip arousal vs. residual handgrip arousal vs. no handgrip arousal) as the between-subjects factor on the pupil dilation response to the block immediately before encoding (experiment block 2). The ANOVA revealed a significant effect of physiological arousal condition on the pupil dilation response immediately prior to encoding, F(2, 92) = 3.1, p< .05 (see Fig 2); follow-up pairwise comparisons showed that the immediate handgrip arousal group had significantly greater pupil dilation prior to encoding compared to the residual handgrip group (p < .05) and the no handgrip group (p < .05). There were no significant differences between the residual handgrip group and the no handgrip group (p > .5). These data indicate that NE levels prior to encoding, as indexed by pupil dilation, were highest in the immediate handgrip arousal group and so most likely to influence memory encoding.
Fig 2.
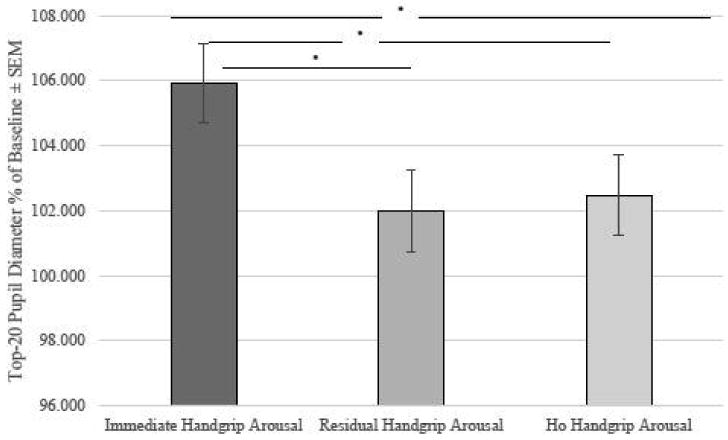
Pupil dilation response to task immediately prior to image encoding. The increase in pupil dilation was significantly greater in the immediate handgrip condition compared to either the residual or no-handgrip conditions.*p <.05
We next tested whether the pupil dilation response to the handgrip task varied as a function of sex steroid hormones. To do so, we conducted a 2 (Estradiol Level: high vs. low) × 2 (Progesterone Level: high vs. low) ANOVA within women who completed the handgrip task (i.e., combined immediate handgrip arousal and residual handgrip arousal groups). Within this analysis, there was no significant interaction between the hormone levels, F(1, 47) = 0.28, p> .5, indicating that levels of estradiol and progesterone did not modulate the pupil dilation responses to our handgrip manipulation. To confirm this, we also ran correlation analyses examining the relationship between estradiol and pupil dilation responses in the handgrip conditions as well as between progesterone and pupil dilation responses in the handgrip conditions. Both analyses revealed no significant relationship between the sex steroid hormones (estradiol r = .05, progesterone: r = .07) and pupil dilation responses to handgrip (p> .05).
In summary, our manipulation of sympathetic arousal significantly increased mean pupil dilation, and more importantly, the immediate handgrip group exhibited significantly greater sympathetic arousal prior to encoding compared to the residual and no handgrip groups, which did not differ from one another. Based on these indices, we conclude that our handgrip manipulation successfully induced physiological arousal and noradrenergic activity in women. Furthermore, this occurred regardless of their sex steroid hormone levels.
3.3 Memory Recall Performance
Having established that our manipulation of physiological arousal was effective, we next turned to the two primary aims of the current study. First, did immediate or residual sympathetic arousal induced by the handgrip enhance emotional memory, specifically for the negatively valenced stimuli? Second, were the effects of enhanced NE activity on emotional memory modulated by estradiol and/or progesterone levels?
3.3.1 Effects of handgrip and emotional image arousal and valence on memory
To assess how handgrip influenced memory performance by image arousal and valence, we performed a repeated-measures ANOVA with Physiological Arousal Condition (immediate vs. residual vs. no handgrip arousal) as the between-subjects factor and Image Arousal (arousing vs. non-arousing) and Image Valence (positive vs. negative) as the within-subjects factors. Memory performance was operationalized as the proportion of correctly recalled images from each of the four arousal × valence categories (16 in each category).
The results revealed a significant main effect of image arousal such that highly arousing images (M = 0.28 ± .011) were significantly better recalled than non-arousing images (M = 0.23 ± .008), F(1,92) = 16.21, p < .001. There was also a significant arousal × valence interaction effect on memory, F(1,92) = 223.38, p< .001. Plotting the means revealed that this interaction effect was driven by high arousing negative (M = 0.37 ± .012) and low arousing positive (M = 0.31 ± .011) images being better recalled than low arousing negative (M = 0.16 ± .011) and high arousing positive (M = 0.19 ± .013) across all handgrip conditions. There was no significant two-way interaction between image arousal and physiological arousal condition, F(1,92) = 0.12, p > .5. However, as predicted, there was a significant two-way interaction between physiological arousal condition and image valence, F(2, 92) = 7.69, p = .001; Fig. 3.
Fig 3.
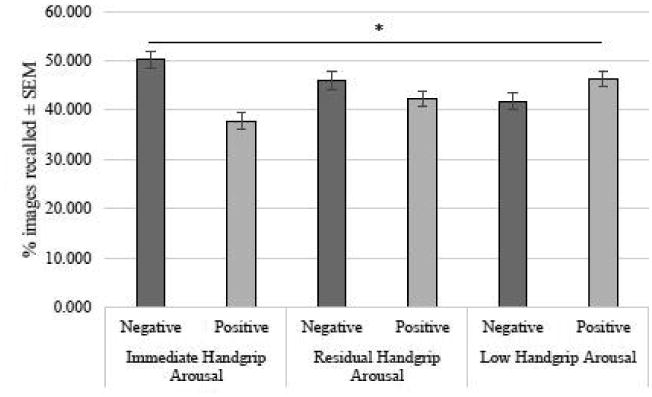
The relationship between physiological arousal conditions and memory for negative and positive images. Only women in the immediate handgrip-induced physiologicalarousal showed enhanced memory for negative images over positive images, *p < .01
We next conducted follow-up paired samples t-tests to examine how recall differed between the positive and negatively-valenced images within each of the three handgrip arousal conditions. Results revealed that under immediate handgrip arousal, women recalled significantly more negative than positive images, t(32) = 3.7, p< .01; this memory enhancement for negative over positive images was not found for participants in the residual handgrip arousal, t(29) = 1.4, p> 0.1, or no handgrip arousal conditions, t(31) = -1.4, p> 0.1. Furthermore, a one-way ANOVA with Physiological Arousal Condition (immediate vs. residual vs. no handgrip arousal) on memory for only negative valenced images revealed a significant main effect of physiological arousal condition, F(2, 92) = 5.8, p < .01. This result indicated that as sympathetic arousal was increased by handgrip immediately before encoding, memory for negative images also increased.
3.3.2 Interactions between handgrip arousal and trial-by-trial effects of image arousal and valence on memory
We next used a hierarchical linear model (HLM) analysis to examine how handgrip modulated the trial-by-trial influence of image arousal and valence on memory performance. Normative IAPS image arousal and valence ratings were analyzed using linear mixed-effects regressions fit by the maximum likelihood in R (Team, 2012) with the glmer function in the lme4 library (Baayen et al., 2008). Memory recall was modeled as the dependent variable with a binomial (logit) distribution (1 = recalled, 0 = not recalled). The arousal and valence ratings were group-centered and modeled as the level-1 trial-by-trial predictors, which were nested within a grand-mean-centered level-2 predictor, Physiological Arousal Condition (the two extremes: immediate handgrip arousal = 1, no handgrip arousal = -1).
Consistent with the prior ANOVA, there was a significant main effect of image arousal on memory performance (z = 4.11, p< .001), with higher arousing images being recalled better than less arousing images. There was also a significant main effect of image valence on memory, such that positive images were better recalled than more neutral and negative images overall (z = 5.08, p< .001). In addition, there was a significant image arousal by image valence interaction effect on overall memory (z = -15.94, p < .001), with negative high arousal and positive low arousal images being best recalled. Supporting our main hypothesis, we also found a significant interaction effect between physiological arousal condition and trial-by-trial image valence on memory recall (z = -2.91, p = .0037). This result indicates that participants who had the most elevated NE activity (i.e., those who squeezed immediately before the slideshow) showed better memory for negative than neutral and positive images.
3.3.3 Interaction effects between sex hormones, condition, image arousal and image valence
Our second aim was to determine whether sex hormone levels modulated the effects of physiological arousal on memory for images of varying arousal and valence. We ran a series of analyses using the median split groups for estradiol and progesterone levels. However, we only included women in the high progesterone-high estradiol state (N = 35) and low progesterone-low estradiol state (N = 36); the mixed high-low hormone groups were underpowered (smallest bin size = 1, largest bin size = 6).
Here, we conducted a mixed-factor ANOVA with Physiological Arousal Condition (immediate vs. residual vs. no-handgrip arousal) and Hormone State (high vs. low) as between-subjects factors and Image Arousal (high vs. low) and Image Valence (negative vs. positive) as within-subjects factors. As in previous analyses, we observed a main effect of image arousal, F(1, 65) = 8.8, p < .01. We also observed significant interactions between image arousal and image valence, F(1, 65) = 143.9, p < .001, image arousal and hormone state, F(1, 65) = 5.0, p < .05, and image valence and physiological arousal condition, F(2, 65) = 3.2, p < .05. Finally, we found a significant four-way interaction between condition, hormone state, image arousal, and image valence, F(2, 65) = 6.6, p < .01. Based on the means, women in the immediate handgrip group appeared to be driving the overall interaction. In the immediate handgrip group, women with high hormone levels exhibited enhanced recall for high arousing negative images (M = 0.39 ± .03) and low arousing positive images (M = 0.34 ± .03); however, women with low hormone levels showed enhanced recall for the high arousing negative images (M = 0.39 ± .03), but not high arousing (M = 0.19 ± .03) or low arousing (M = 0.23 ± .03) positive images, These means suggest that under immediate handgrip arousal, the negative memory bias was present only in women with low levels of estradiol and progesterone.
To better understand this four-way interaction, we next conducted mixed-factor ANOVAs in the residual and immediate handgrip arousal conditions to assess how physiological arousal influenced the relationship between hormones and memory for images of varying arousal and valence. These mixed-factor ANOVAs used Hormone State (high vs. low) as the between-subjects factor and Image Arousal (high vs. low) and Image Valence (negative vs. positive) as within-subjects factors. For the residual handgrip group, we found a significant interaction between image arousal and image valence, F(1, 18) = 47.7, p < .001. No other significant main effects or interactions were found.
For the immediate handgrip group, our main group of interest, we found a main effect of image arousal, F(1, 27) = 8.7, p < .01 and image valence, F(1, 27) = 10.4, p < .01. Women had enhanced recall for the high arousal images compared to the low arousal images, and better recall for the negative images than for the positive images. We also observed significant interactions between image valence and hormone state, F(1, 27) = 8.1, p < .01, image arousal and image valence, F(1, 27) = 56.2, p < .001, and image arousal, image valence, and hormone state, F(1, 27) = 7.1, p < .05. This was because the women with low levels of progesterone and estradiol tended to have enhanced recall of the high arousal negative images relative to the other images. This same pattern did not emerge for women with high levels of progesterone and estradiol.
To confirm that increases in progesterone and estrogen levels were associated with an attenuation of the handgrip arousal-enhanced memory of negative images, we next ran a series of ANOVAs in each physiological arousal condition using a difference score (percentage negative images recalled – percentage positive images recalled) as our index of memory negativity bias. Consistent with our previous analyses, under immediate handgrip arousal, women with low progesterone and estrogen (M = 18.03 ± 4.4) exhibited a greater negativity bias in their recall than women with high progesterone and estrogen levels (M = 1.6 ± 3.95), F(1, 27) = 7.7, p < .05. This effect was not observed under conditions of residual handgrip arousal (p >.5) or no-handgrip arousal (p > .5). This pattern suggests that when both progesterone and estrogen are elevated, there is an attenuation of the handgrip arousal-induced memory enhancement of negative images.
3.3.4 Interaction between sex steroid hormones and trial-by-trial image valence and arousal on memory
Next, we ran a second HLM to determine whether sex hormones levels (assessed here as continuous rather than dichotomized variables) modulated the effects of trial-by-trial image valence and arousal on memory performance. This linear regression analysis was conducted only in the immediate handgrip arousal group, since we anticipated that the effects of sex hormones on emotional memory would be strongest under high sympathetic arousal (Andreano and Cahill, 2009; see Section 3.2 for pupil dilation analyses demonstrating that sympathetic arousal was highest for women in the immediate handgrip arousal group for this study). A diagnostic linear regression indicated that progesterone and estradiol levels in the high arousal group were not highly collinear (VIF = 1.29); thus, we included both sex hormones in the HLM in order to examine their interaction effect on arousal, valence, and memory. Another advantage of this HLM approach was that it allowed us to dissociate the unique variance accounted for by each individual sex hormone. This could not be examined in the prior median split analyses as they focused on how with women high levels of both hormones differed from women with low levels of bot hormones. As in the first HLM, memory recall was coded as the binomial dependent variable. Normative IAPS valence and arousal ratings for each image were group-centered and modeled as the level-1 trial-by-trial predictors, which were nested within two grand-mean-centered level-2 predictors, estradiol and progesterone levels.
The HLM analysis revealed that in the immediate handgrip arousal condition, there was a significant interaction between estradiol and trial-by-trial arousal rating on memory recall (z = -2.03, p = .043), such that higher estradiol diminished the memory bias for highly arousing images. Furthermore, we found a significant three-way interaction effect between estradiol, image arousal and image valence on memory performance (z = -2.26, p = .024), which was driven by higher estrogen levels biasing memory more towards low positive arousal images. Furthermore, consistent with the earlier ANOVA (see section 3.3.3), there was a significant four-way interaction between progesterone, estradiol, image valence and image arousal on memory recall (z = 3.42, p = .00064), indicating that women with high levels of both sex hormones showed an even more exaggerated memory bias toward low positive arousal images than women with low levels of both sex hormones.
4. Discussion
In this study, we aimed to: 1) examine the relationship between sex steroid hormone levels and sympathetic arousal; 2) determine how sympathetic arousal induced by isometric handgrip before encoding affects subsequent memory for negative and positive images in healthy young women; and 3) determine how sympathetic arousal effects on emotional memory were modulated by levels of progesterone and estradiol in women. To examine the influence of varying degrees of physiological arousal on memory, we either implemented isometric handgrip immediately before (immediate handgrip arousal) or approximately 15 min (residual handgrip arousal) prior to the encoding of images. Other women were assigned to a control task of equal duration (no handgrip arousal) before the slideshow.
Overall, we did not find a relationship between estradiol or progesterone levels and our index of sympathetic nervous activity, mean pupil dilation, in response to handgrip. Previous research indicated that in young women, estradiol may be sympathoinhibitory and progesterone may be sympathoexcitatory (Carter et al., 2013). This relationship was identified specifically when muscle sympathetic nerve activity was the index of sympathetic nervous activity; however, estradiol and progesterone levels were unrelated to sympathetic nervous activity when it was operationalized as heart rate and mean arterial blood pressure (Carter et al., 2013). Based on these data, the lack of a relationship between sex steroid hormones and pupil dilation in our study is not surprising since arousal-related pupil diameter changes are similar to those observed in skin conductance (Bradley et al., 2008) and blood pressure (Tassorelli et al., 1995, Tavernor et al., 2000). Thus, it may be that like other measures, pupil dilation is less influenced by estradiol and progesterone levels than muscle sympathetic nerve activity.
In spite of the fact that, in this study, pupil diameter changes in response to the isometric handgrip task were similar across women with different sex hormone statuses, there was still reason to suspect that the handgrip task would influence memory outcomes differently as a function of sex hormone status (i.e., the second aim of this study). Previous studies with pupil dilation as an index of arousal have shown women in different hormone states to have equivalent pupil diameter responses to emotionally arousing narratives (Nielsen et al., 2013a, Nielsen et al., 2014) but different memory patterns for these narratives.
More specifically, we had several predictions about how physiological arousal state, sex hormones, and image valence would affect memory. Based on previous findings indicating an attentional bias for negative stimuli (Baumeister et al., 2001, Rozin and Royzman, 2001), we predicted that women in the immediate handgrip physiological arousal condition would show enhanced memory for negative over positive stimuli. Our findings were consistent with this hypothesis, as external arousal induced by isometric handgrip immediately before the encoding slideshow enhanced memory for negative images over positive emotional images; this mnemonic advantage of negative stimuli was not observed in women assigned to the residual (handgrip 15 min before the slideshow) and no handgrip arousal (no handgrip) conditions.
According to both the BANE and GANE models of arousal-cognition interactions (Markovic et al., 2014; Mather et al., in press), arousal amplifies the effects of affective salience in perception, attention, and memory via a noradrenergic mechanism. Thus, the negativity bias in memory should be most apparent when physiological arousal and NE levels are highest. Indeed, our results indicated that women in the immediate handgrip showed a greater negativity bias in their free recall performance. This finding aligns with earlier genotyping studies of the ADRA2b gene, in which participants with a genetic variant associated with higher NE availability noticed and remembered more negative than neutral stimuli (Todd et al., 2013, Todd et al., 2014). These findings also align with previous research demonstrating that norepinephrine enhances memory for negative, but not positive, stimuli in young adults (Todd et al., 2014, de Quervain et al., 2007, Rasch et al., 2009).
We were also interested in whether sex steroid hormone levels modulate the effects of high physiological arousal on encoding emotionally salient information. Research has demonstrated that sex steroid hormones influence the noradrenergic system by acting on the locus coeruleus (LC; primary noradrenergic nucleus) and regions in arousal-related brain circuits. The LC and hypothalamic-pituitary-gonadal axis activity are functionally interconnected (Helena et al., 2006). LC neurons are targets for estradiol (Heritage et al., 1980) and express mRNA for estrogen (Shughrue et al., 1997) and progesterone receptors (Curran-Rauhut and Petersen, 2002).
Fluctuating levels of estradiol and progesterone also exert a profound influence on other brain regions that process emotional information. For example, human neuroimaging studies indicate that progesterone increases amygdala and hippocampus reactivity to emotional stimuli (Van Wingen et al., 2008, Andreano and Cahill, 2010), whereas estradiol inhibits reactivity in these same regions (Goldstein et al., 2005, Goldstein et al., 2010). While the interaction effects between trial-level image arousal and sex hormones on memory were only marginally significant in the current study, our results were consistent with the notion that estrogen and progesterone play opposing roles in modulating amygdala-mediated consolidation of emotional memories. Together these findings suggest that estradiol and progesterone are important in regulating central noradrenergic activity and activity in the amygdala and hippocampus, regions that are critical for the consolidation of emotional memories (McGaugh, 2000).
The fact that sex steroid hormones interacted with arousal and valence to impact memory may seem surprising given that sex hormone levels were unrelated to levels of physiological arousal (as assessed via pupil diameter changes). However, this pattern suggests that while sex hormone status did not modulate how much arousal women experienced from the isometric handgrip task, it did have a role in moderating the effects of physiological arousal on encoding and consolidation processes.
With respect to estradiol, our findings supported our original predictions: under immediate handgrip arousal, women in a low estradiol state showed a greater memory bias for negative images over positive images. This is consistent with previous literature showing that estradiol attenuates learning and memory effects associated with aversive stimuli (Wegerer et al., 2014, Nielsen et al., 2013b). For progesterone, the results supported our apriori predictions of enhanced negative memory in a low estradiol and progesterone state. However, based on the hormone and emotion literature, women with higher levels of progesterone typically exhibit enhanced memory for negative images under high arousal (Ertman et al., 2011, Ferree et al., 2011, Soni et al., 2013). Instead, we found that under immediate handgrip arousal, women with high levels of progesterone had reduced memory for negative relative to positive images. There are potential explanations for these results. First, it is important to note that our results are consistent with other work demonstrating that women on hormonal contraception (e.g. lower levels of progesterone and estradiol) exhibit enhanced recall of negative material (Nielsen et al., 2013b). Thus, it may be the case that the combination of low progesterone and low estradiol leads to an enhanced negativity bias under arousal.
Secondly, whereas the aforementioned studies (Ertman et al., 2011, Ferree et al., 2011, Soni et al., 2013) utilized long-term memory paradigms (i.e., 1-week retention interval), we used a retention interval of approximately 10-15 minutes. It may be the case that progesterone enhances memory for negative material only when interacting with a higher physiological arousal state over a long-term consolidation window. In the short-term, progesterone may instead have an anxiolytic effect (Van Wingen et al., 2007) that counteracts conditions of high physiological arousal, and this could potentially attenuate the memory bias towards negative stimuli. Although we did not find an effect of progesterone levels on the sympathetic arousal response to isometric handgrip, there may be an effect of progesterone on the phasic arousal specifically elicited by the negative and positive images. For instance, women with higher progesterone levels may have experienced less phasic arousal in response to negative images compared to women with lower levels of progesterone. Future studies should investigate the attenuating effect of progesterone on short-term and long-term memory for negatively and positively valenced emotional stimuli.
The present findings are not only consistent with the BANE and GANE models of how arousal interacts with affective salience but also suggest that sex steroid hormone levels modulate emotional memory in young women. Specifically, in our study, a combination of low estradiol and progesterone hormone states were associated with negative emotional memory biases. We anticipate that other arousal manipulations (e.g., social stress tests, pharmacological manipulations) prior to encoding would yield similar memory patterns in young women, given that such manipulations also modulate sympathetic and noradrenergic activity (Cahill et al., 1994, Gordis et al., 2006). The emotional memory findings here support the notion that sex hormones modulate brain regions involved in emotional memory processes, such as the hippocampus and the amygdala (Andreano and Cahill, 2009). Additional research into these arousal-hormone interactions may provide further insight into the neurobiology underlying disorders of emotional memory that disproportionately affect women (i.e., depression, anxiety, post-traumatic stress disorder; Breslau et al., 1991, Breslau et al., 1997, Kessler et al., 1995).
Future work should test whether the arousal modulation of the negativity bias in memory is observable in men. This study was designed to test this phenomenon in women; however, in order to generalize these findings, it is important to examine whether these effects are consistent across women and men. Another question for future research is whether these effects are maintained over longer retention intervals, such as 48 hours or one week after the encoding session. Finally, the hormone analyses in the present study were limited by the fact that women in the follicular and luteal menstrual cycle phases did not have significantly different hormone levels; it may be the case that anthropometric characteristics (i.e. body mass index; BMI) affected hormone production and subsequent levels in the naturally cycling women (Lukanova et al., 2004). Also, more fine-grained characterization of menstrual cycle phases (e.g., early follicular, late follicular, luteal) might have revealed clearer hormone differences between groups (see Supplementary Materials for data). Therefore, future studies of arousal and cognition across the menstrual cycle should record anthropometric characteristics to account for possible BMI effects on hormone production and use a finer menstrual cycle phase classification.
The present results support the notion that physiological arousal and levels of estradiol and progesterone at encoding have different effects on memory for negative and positive emotional material. Further investigation of how these factors influence learning and memory is warranted for a full understanding of the underlying neurobiology of physiological arousal and emotional memory processing in women of varying sex hormone statuses.
Supplementary Material
Fig 4.
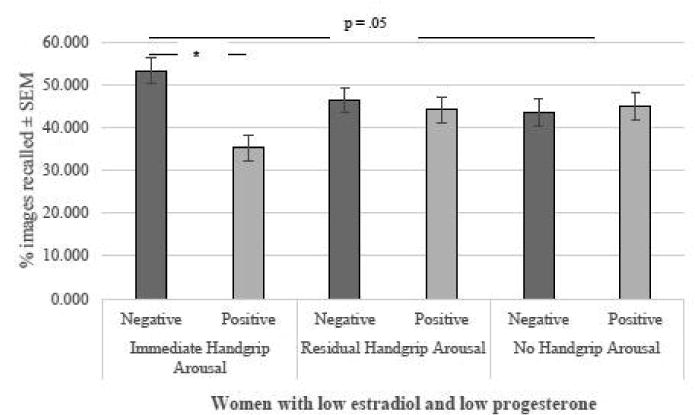
Recall of positive and negative images as a function of estradiol, progesterone, and physiological arousal. There was a negativity bias in women with low levels of estradiol and progesterone. Under immediate physiological arousal, but not residual or no handgrip-related physiological arousal, women with low levels of estradiol and progesterone recalled significantly more negative compared to positive images. *p < .05.
Fig 5.
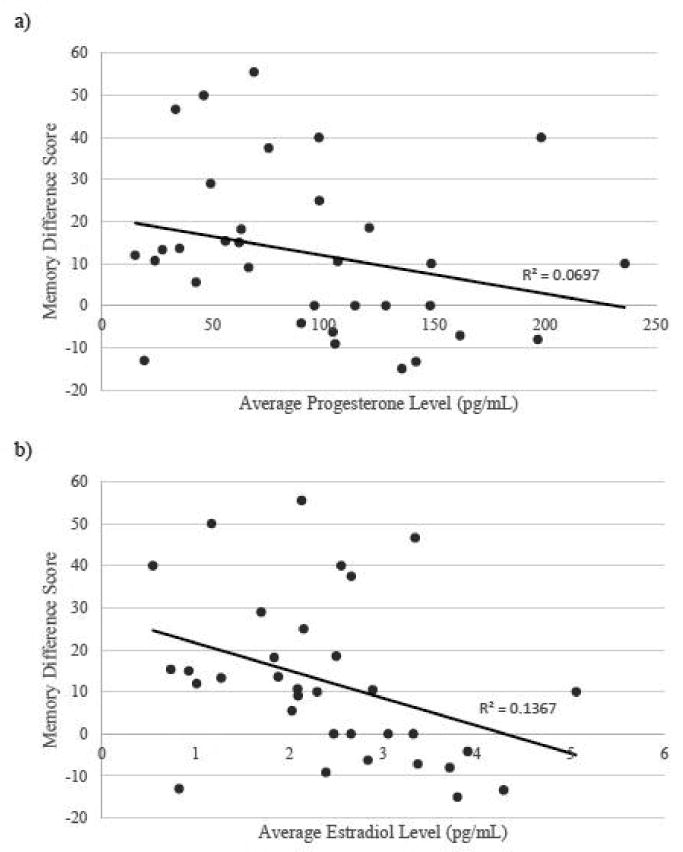
Correlations between sex steroid hormone levels and memory difference score (% negative images recalled - % positive images recalled) in the immediate handgrip condition. a) Average progesterone levels trended toward a significant negative correlation with memory difference score. b) Average estradiol levels were significantly negatively correlated with memory difference score; higher levels of estradiol a predicted greater negativity bias in memory performance.
Table 1.
Hierarchical linear modeling (HLM) analyses.
| Predictors | Estimate | SE | z | p | |
|---|---|---|---|---|---|
| (a) Analysis of Arousal Condition and Trial-by-Trial Arousal and Valence Rating Effects on Memory | |||||
| Intercept | -1.29 | 0.04 | -30.05 | < 2 × e−16*** | |
| Arousal Rating (IAPS) | 0.09 | 0.02 | 4.11 | 3.96 × e−05*** | |
| Valence Rating (IAPS) | 0.08 | 0.016 | 5.08 | 3.81 × e−07*** | |
| Physiological Arousal Condition | -0.04 | 0.052 | -0.82 | 0.41 | |
| Arousal Rating × Valence Rating | -0.197 | 0.012 | -15.9 | < 2 × e−16*** | |
| Condition × Valence Rating | -0.06 | 0.02 | -2.91 | .00367** | |
| Condition × Arousal Rating | -0.02 | 0.03 | -0.59 | 0.558 | |
| Arousal Rating × Valence Rating × Condition | 0.003 | 0.015 | 0.19 | 0.851 | |
| (b) Analysis of Estradiol Levels and Trial-by-Trial Arousal and Valence Rating Effects on Memory in Immediate Handgrip Arousal Condition | |||||
| Intercept | -1.27 | 0.076 | -16.77 | < 2 × e−16*** | |
| Arousal Rating (IAPS) | 0.15 | 0.035 | 4.26 | 2.04× e−16*** | |
| Valence Rating (IAPS) | -0.12 | 0.022 | -5.5 | 3.71 × e−16*** | |
| Estradiol Level | 0.037 | 0.071 | 0.517 | 0.605 | |
| Valence Rating × Estradiol | 0.084 | 0.026 | 3.186 | .00144** | |
| Arousal Rating × Estradiol | -0.053 | 0.034 | -1.559 | .119 | |
| Arousal Rating × Valence Rating × Estradiol | -0.061 | 0.019 | -3.165 | 0.00155** | |
| (c) Analysis of Progesterone Levels and Trial-by-Trial Arousal and Valence Rating Effects on Memory in Immediate Handgrip Arousal Condition | |||||
| Intercept | -1.27 | 0.0752 | -16.88 | < 2 × e−16*** | |
| Arousal Rating (IAPS) | 0.149 | 0.0353 | 4.23 | 2.34 × e−05*** | |
| Valence Rating (IAPS) | -0.121 | 0.0225 | -5.371 | 7.84 × e−08*** | |
| Progesterone Level | -0.0008 | 0.00136 | -0.591 | 0.554 | |
| Valence Rating × Progesterone | 0.00146 | 0.00051 | 2.869 | 0.00412** | |
| Arousal Rating × Progesterone | 0.00036 | 0.00065 | 0.550 | 0.582 | |
| Arousal Rating × Valence Rating × Progesterone | -0.00117 | 0.00037 | -3.147 | 0.00165** | |
p < .05;
p < .001
Highlights.
Emotionally arousing events are better remembered than neutral events
Estradiol and progesterone modulate emotional memory
We tested if arousal and hormones modulate memory for negative over positive images
Under immediate arousal, memory was enhanced for negative over positive images
Estradiol and progesterone reduce negative recall under immediate arousal
Acknowledgments
This work was supported by R01AG03804, R01AG025340, and 1F32AG047840-01A1. We would like to thank the laboratory of Dr. Pinchas Cohen for providing space and equipment to processes the saliva samples.
Funding: These funding sources had no involvement in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Footnotes
Conflict of Interest: There are no conflicts of interest to declare.
Author Contributions: All authors contributed significantly to the experimental design and the manuscript. SEN, SJB, and MM designed and generated the experiment. SEN and AC executed the experiment and collected all data. SEN, DVC, and MM contributed significantly to the theoretical framework of the manuscript. SEN, SJB, AC, DVC, and MM all participated in data analysis and the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning & Memory. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Hincapie LA, Knochenhauer ES, Dewailly D, Fox L, Boots LR. Screening for 21-hydroxylase–deficient nonclassic adrenal hyperplasia among hyperandrogenic women: a prospective study. Fertility and sterility. 1999;72:915–925. doi: 10.1016/s0015-0282(99)00383-0. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language. 2008;59:390–412. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of general psychology. 2001;5:323. [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of general psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, Mcgaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61:395–399. doi: 10.1161/HYPERTENSIONAHA.112.202598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, Robbins TW, Sahakian BJ. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology. 2006;188:397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor MRNA in rat brainstem. Gene Expression Patterns. 2002;1:151–157. doi: 10.1016/s1567-133x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- De Bondt T, Van Hecke W, Veraart J, Leemans A, Sijbers J, Sunaert S, Jacquemyn Y, Parizel PM. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. European radiology. 2013;23:57–64. doi: 10.1007/s00330-012-2572-5. [DOI] [PubMed] [Google Scholar]
- De Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nature neuroscience. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- Einhäuser W, Stout J, Koch C, Carter O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proceedings of the National Academy of Sciences. 2008;105:1704–1709. doi: 10.1073/pnas.0707727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertman N, Andreano JM, Cahill L. Progesterone at encoding predicts subsequent emotional memory. Learning & Memory. 2011;18:759–763. doi: 10.1101/lm.023267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree NK, Kamat R, Cahill L. Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Consciousness and cognition. 2011;20:1154–1162. doi: 10.1016/j.concog.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O'Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. Journal of Women's Health. 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. The Journal of Neuroscience. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. The Journal of Neuroscience. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological psychiatry. 2013;73:371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Someya N. Muscle metaboreflex activation by static exercise dilates pupil in humans. European journal of applied physiology. 2011;111:1217–1221. doi: 10.1007/s00421-010-1716-z. [DOI] [PubMed] [Google Scholar]
- Helena CV, De Oliveira Poletini M, Sanvitto GL, Hayashi S, Franci CR, Anselmo-Franci JA. Changes in alpha-estradiol receptor and progesterone receptor expression in the locus coeruleus and preoptic area throughout the rat estrous cycle. J Endocrinol. 2006;188:155–65. doi: 10.1677/joe.1.06268. [DOI] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Labar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley MM. The International Affective Picture System (IAPS) in the study of emotion and attention. Handbook of emotion elicitation and assessment. 2007;29 [Google Scholar]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. European Journal of Endocrinology. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, Todd RM. Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural brain research. 2014;259:229–241. doi: 10.1016/j.bbr.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults' emotional memory. Psychology and aging. 2005;20:554. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mcgaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mcgaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in neurosciences. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the Menstrual Cycle on Sympathetic Activity, Baroreflex Sensitivity, and Vascular Transduction in Young Women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Murphy PR, O'Connell RG, O'Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp. 2014;35:4140–54. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiology of learning and memory. 2013a;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Post learning stress differentially affects memory for emotional gist and detail in naturally cycling women and women on hormonal contraceptives. Behavioral neuroscience. 2014;128:482. doi: 10.1037/a0036687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Mather M. Comparison of two isometric handgrip protocols on sympathetic arousal in women. Physiology & behavior. 2015;142:5–13. doi: 10.1016/j.physbeh.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological psychology. 2013b;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterstetter R, Szymanski LM, Kamimori GH, Kessler CM, Goldb MR, Fernhall B. Hemostatic responses to maximal exercise in oral contraceptive users. American journal of obstetrics and gynecology. 1999;181:958–963. doi: 10.1016/s0002-9378(99)70332-7. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, De Quervain DF. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proceedings of the National Academy of Sciences. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Personality and social psychology review. 2001;5:296–320. [Google Scholar]
- Sakaki M, Mather M. How reward and emotional stimuli induce different reactions across the menstrual cycle. Social and personality psychology compass. 2012;6:1–17. doi: 10.1111/j.1751-9004.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and-β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Soni M, Curran VH, Kamboj SK. Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiology of learning and memory. 2013;104:32–38. doi: 10.1016/j.nlm.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Strange B, Hurlemann R, Dolan R. An emotion-induced retrograde amnesia in humans is amygdala-and β-adrenergic-dependent. Proceedings of the National Academy of Sciences. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassorelli C, Micieli G, Osipova V, Rossi F, Nappi G. Pupillary and cardiovascular responses to the cold-pressor test. Journal of the autonomic nervous system. 1995;55:45–49. doi: 10.1016/0165-1838(95)00026-t. [DOI] [PubMed] [Google Scholar]
- Tavernor S, Abduljawad K, Langley R, Bradshaw C, Szabadi E. Effects of pentagastrin and the cold pressor test on the acoustic startle response and pupillary function in man. Journal of Psychopharmacology. 2000;14:387–394. doi: 10.1177/026988110001400407. [DOI] [PubMed] [Google Scholar]
- TEAM, R. C. R: A language and environment for statistical. Computing. 2012;14:12–21. [Google Scholar]
- Todd R, Müller D, Palombo D, Robertson A, Eaton T, Freeman N, Levine B, Anderson A. Deletion variant in the ADRA2B gene increases coupling between emotional responses at encoding and later retrieval of emotional memories. Neurobiology of learning and memory. 2014;112:222–229. doi: 10.1016/j.nlm.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Todd RM, Müller DJ, Lee DH, Robertson A, Eaton T, Freeman N, Palombo DJ, Levine B, Anderson AK. Genes for emotion-enhanced remembering are linked to enhanced perceiving. Psychological science. 2013;24:2244–2253. doi: 10.1177/0956797613492423. [DOI] [PubMed] [Google Scholar]
- Topolovec JC, Gati JS, Menon RS, Shoemaker JK, Cechetto DF. Human cardiovascular and gustatory brainstem sites observed by functional magnetic resonance imaging. J Comp Neurol. 2004;471:446–61. doi: 10.1002/cne.20033. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Molecular brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wingen G, Van Broekhoven F, Verkes R, Petersson K, Bäckström T, Buitelaar J, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Molecular psychiatry. 2007;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Van Wingen G, Van Broekhoven F, Verkes R, Petersson KM, Bäckström T, Buitelaar J, Fernandez G. Progesterone selectively increases amygdala reactivity in women. Molecular psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of learning and memory. 2014;116:145–154. doi: 10.1016/j.nlm.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


